Abstract
Behavioural studies underpin the weight of experimental evidence for the existence of a magnetic sense in animals. In contrast, studies aimed at understanding the mechanistic basis of magnetoreception by determining the anatomical location, structure and function of sensory cells have been inconclusive. In this review, studies attempting to demonstrate the existence of a magnetoreceptor based on the principles of the magnetite hypothesis are examined. Specific attention is given to the range of techniques, and main animal model systems that have been used in the search for magnetite particulates. Anatomical location/cell rarity and composition are identified as two key obstacles that must be addressed in order to make progress in locating and characterizing a magnetite-based magnetoreceptor cell. Avenues for further study are suggested, including the need for novel experimental, correlative, multimodal and multidisciplinary approaches. The aim of this review is to inspire new efforts towards understanding the cellular basis of magnetoreception in animals, which will in turn inform a new era of behavioural research based on first principles.
Keywords: magnetoreception, magnetite, navigation, iron, neurobiology, sense
1. Introduction
Despite a wealth of behavioural evidence that provides compelling support for magnetic field perception in animals, the cellular and molecular basis for this sense remains to be discovered and characterized [1,2]. The reasons why the location, structure and function of magnetoreceptor cells have remained hidden to this point are many, but with the advent of new characterization methods and innovative experimental approaches many research tools now exist for resolving this long-standing biological question.
1.1. The magnetic sense
The Earth's magnetic field provides a relatively stable and globally pervasive reference frame that animals can exploit for short- or long-distance orientation and navigation across the entire biosphere. Such a sense therefore provides a primary or ancillary mechanism for maintaining course in situations where other navigational mechanisms are compromised or across landscapes devoid of landmarks. Magnetosensory systems are thought to use various components of the Earth's magnetic field, including its intensity, polarity and inclination (figure 1), which can be incorporated into two distinctly different sense types, being either a compass- or map-like sense, capable of detecting directional or positional information, respectively.
Figure 1.
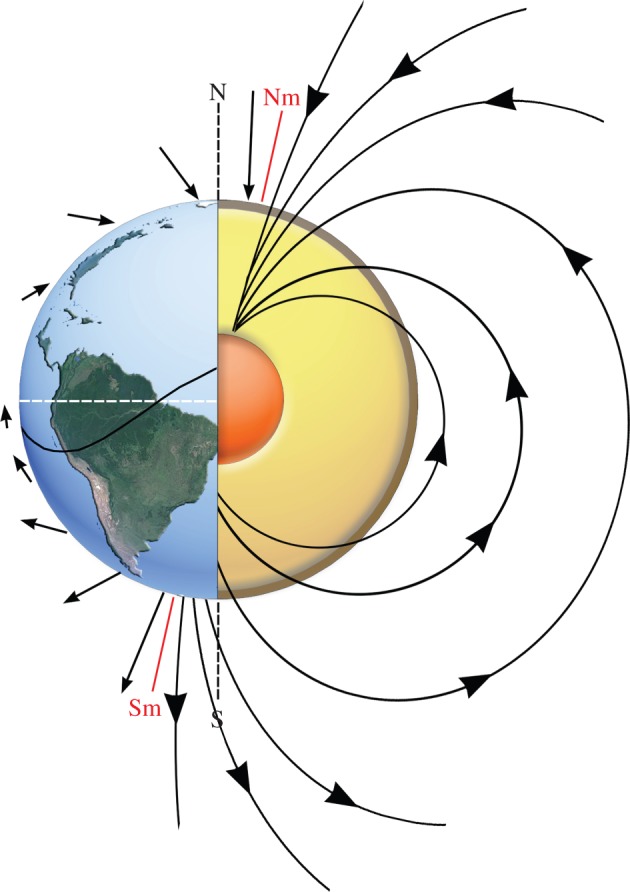
Diagrammatic representation of the Earth's geomagnetic field detailing the main components thought to be available to animals for magnetoreception. Lines of magnetic flux (shown on right), emanate from the Earth's iron core at the south magnetic pole (Sm) in the Southern Hemisphere (S) and travel to the north magnetic pole (Nm) in the Northern Hemisphere (N). As for a standard dipole bar magnet, this force can provide directional information for a compass sense. Lines of magnetic flux are closer together at the poles compared to the magnetic equator (black curving line) resulting in increased magnetic intensity (represented by arrow length on left), which is potentially useful for a map-like sense. The inclination angle of the magnetic flux lines as they leave or enter the Earth's surface change consistently from ±90° at the magnetic poles to 0o at the magnetic equator (relative to gravity), which could also be used for a compass or map type sense. White dashed line represents geographical equator.
The two sense types are thought to comprise two separate and specialized receptor systems [3]. In general, the compass sense is hypothesized to rely on magnetic polarity and/or inclination and the map on inclination and/or intensity. This aspect of magnetoreception has been investigated primarily in behavioural or electrophysiological studies of a broad range of animal groups, resulting in complex and often controversial interpretations, and the subject of various reviews [3–7]. Of specific relevance to magnetic particle-mediated magnetoreceptor (MPM) systems are studies involving the so-called Kalmijn–Blakemore re-magnetization experiment [8], which involves the application of a short strong magnetic pulse that should disrupt MPM and not radical pair-mediated magnetoreceptor systems (RPM; see below). These pulse experiments have been used most extensively on birds, resulting in compromised navigation in pigeons and long-distance migratory species [9–11], but insects [12], reptiles [13] and mammals [14] also exhibit pulse-mediated behavioural responses.
1.2. The radical pair and magnetic particle hypotheses
The field studying magnetoreception in animals is divided concerning the exact mechanistic basis of the sense, with RPM and MPM being the primary hypotheses to have been developed. RPM is based on a light-driven electron transfer reaction in photoreceptor molecules (cryptochrome), where the degree of neuronal activation is a function of the biochemical activity of the molecule subject to different magnetic field conditions (figure 2) [15–18]. As light is a prerequisite for a functioning RPM, the sensory system is thought to be anatomically localized to the eyes, although, it has been proposed that alternative locations accessible to light should not be discounted [19]. The MPM hypothesis is based on the premise of cell depolarization and neuronal activation in response to a deflection of magnetite nanoparticles that are anchored to the cell membrane of specialized neural cells (figure 3) [20–24]. Notably, the RPM and MPM systems are not mutually exclusive, and it has been suggested that some interaction between the two systems may exist [18,25–27].
Figure 2.
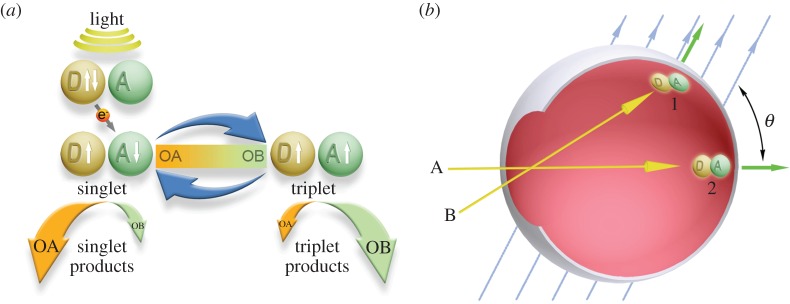
Diagrammatic representations of the hypothesized radical pair mechanism of magnetoreception. (a) In specialized photoreceptor molecules (crytochromes), light drives an electron transfer between donor (D) and acceptor (A) molecules generating a radical pair in the singlet (arrows ↑↓) or triplet (arrows ↑↑) state. The interconversion between singlet and triplet states (blue arrows) changes under different magnetic field conditions (e.g. at orientation A (OA) or B (OB)) that, in turn, changes the ratio of singlet to triplet products (denoted by the size of the bottom arrows). (b) Light entering the eye (e.g. rays A and B) drives radical pair formation in cryptochrome molecules oriented normal to the retina surface (green arrows) at sites 1 and 2, which are oriented at different angles (θ) relative to the external magnetic field (blue lines). The anisotropy of radical pair production across the retina surface may result in the addition of a superimposed impression of the magnetic field to the animal's sight (adapted from [15,16]).
Figure 3.
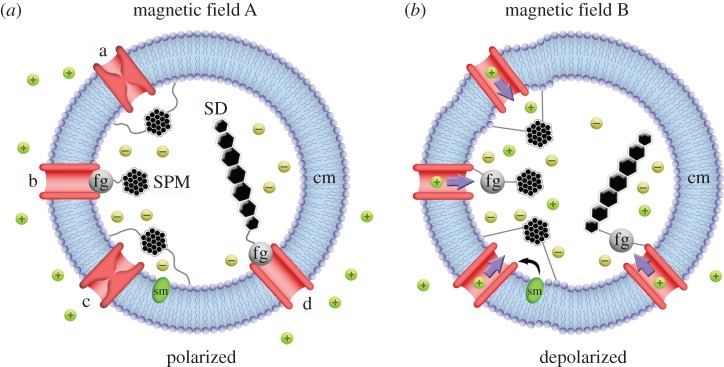
(a,b) Diagrammatic representation of various hypothesized magnetite particle-based magnetoreceptor systems under two differing magnetic field conditions. Three separate magnetoreceptor systems based on clusters of superparamagnetic (SPM) particles are shown on the left hemisphere of each cell (a, b and c) and an example of a single domain system is provided on the right hemisphere (d). Under magnetic field condition A, all systems are shown in the resting, polarized state, where particles are connected to closed mechanosensitive ion channels via cytoskeletal filaments to the cell membrane (cm) or to force-gated ion channels (fg). The change to magnetic field condition B, results in movement of the SPM clusters, which distorts the cell membrane in example a and opens a force-gated ion channel in example b. Example c is similar to a, but in this case ion channel activation is mediated by a secondary messenger (sm). In the single domain system (d), shown as a chain of single crystals, the change from magnetic field condition A to B applies torque on the chain and again results in the opening of a force-gated ion channel. In all cases, cell depolarization leads to an action potential, which, travelling via afferent nerves, leads to neuronal activation in the brain. Scenarios modelled on [20].
Although cryptochrome expression is not limited to the retina of animals [28], the eyes have received the most attention in the literature with respect to the role of this protein family in magnetoreception [16]. In the case of the RPM system, the challenge of describing magnetoreceptor function falls more towards describing the role of these proteins at the molecular and atomic level. The major challenge concerning the elucidation of a magnetic particle-based system is determining the anatomical location of the sensor, which, in the case of an MPM system, could be located anywhere in the body. Owing to the disparity between these two hypothetical systems, this review will only consider studies related to the existence of the MPM system, as each will require very different experimental approaches to unravel their structure and function.
1.3. Iron's role in biology and magnetoreception
Iron is a reactive element, known for the ease with which it cycles between its ferrous (Fe2+) and ferric (Fe3+) states, otherwise known as its redox potential [29]. This reactivity is a double-edged sword as, while it is ideal for catalysing useful biochemical reactions, if left unregulated, it also has the ability to generate toxic free radicals that can damage cells. As such, all organisms have developed various mechanisms for managing the uptake, transport and storage of iron that, in some cases, has been exploited for specialized functional purposes, such as orientation or structural reinforcement (figure 4).
Figure 4.
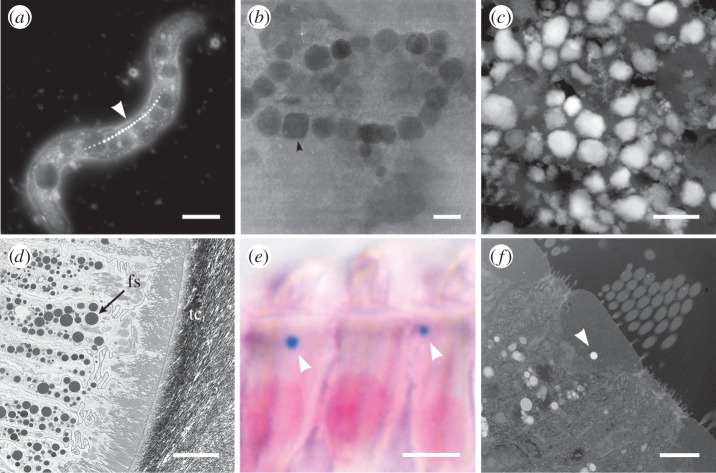
A selection of electron and optical micrographs of biogenic iron minerals formed by organisms. (a) Dark-field scanning transmission electron micrograph (DF-STEM) of magnetotactic bacterium Magnetospirillum magnetotacticum clearly showing the chain of nanoparticulate magnetite forming the magnetosome (arrowhead), scale bar, 1 µm. (b) Bright-field TEM (BF-TEM) micrograph of chains of magnetite isolated from ethmoid tissue from the salmon Oncorhynchus nerka (reproduced from refrence [30]), scale bar, 50 nm. (c) DF-STEM micrograph of iron granules extracted from trophocyte cells in the abdominal fat layer of the honeybee Apis mellifera, scale bar, 500 nm. (d) BF-TEM micrograph of the mass accumulation of ferritin siderosomes (fs) in the epithelial tissue surrounding the magnetite mineralized tooth cusp (tc) of the chiton Acanthopleura hirtosa, scale bar, 5 µm. (e) Optical micrograph of a Perls' Prussian blue stained section and (f) a DF-STEM micrograph both showing a single iron-rich cuticulosome (arrowheads) in the cuticular plate of inner ear hair cells from the pigeon Columbia livia, scale bars, 5 µm (e), 2 µm (f). Note: bright regions in DF-STEM images correspond to regions of high mass.
Although various mineral phases of iron exist in biology, magnetite (Fe3O4) is hypothesized to be the most likely phase for forming a functioning magnetoreceptor mechanism [22,24]. Experimental evidence for this is, in part, provided by the pulse re-magnetization experiments discussed above, and the various studies that have either directly or indirectly observed magnetite as discussed below. The two types of particles that are thought to exist include superparamagnetic (SPM) and single domain (SD) magnetite. The small size of SPM particles (approx. less than 30 nm), makes them unable to retain a stable magnetic moment of their own relative to background thermal energy, but will align in the direction of an externally applied magnetic field [31]. For SD magnetite, the single crystals are large enough (approx. 60 nm) to possess their own permanent magnetic moments (which is amplified in the case of particle chaining), such that they behave in a fashion similar to that of a compass needle.
2. Magnetotactic bacteria—the smoking gun
When the structure, modelled behaviour or evolution of MPM systems are discussed in the literature, references are commonly made to the existence of magnetotactic bacteria [22,32–35]. This group of micro-aerobes has been studied extensively owing to their ability to form single crystals of nanoparticulate magnetite (and greigite in some species), which are often arranged in chains forming a structure termed the magnetosome [36–38]. The magnetosome is used to align the bacterial cell with the Earth's magnetic field, thereby aiding these bacteria in maintaining, or moving towards, regions more favourable for growth [39]. Together with chitons, (Mollusca: Polyplacophora), magnetotactic bacteria were the first organisms in which biogenic magnetite formation was demonstrated, and are possibly the single-most important element in the argument for the existence of an MPM system in eukaryotic organisms [21,37].
Given the existence of these bacteria, it is perhaps unsurprising that MPM models predict that similar magnetite particles possess the biophysical properties necessary to mediate the detection of Earth-strength magnetic fields in vertebrates [23,24]. While the bacterial magnetosome provides us with a logical example for a functional receptor system, the strong focus on this model has perhaps precluded the pursuit of alternative, as yet unconsidered, mechanisms of magnetosensory perception. While we do not presume to know what these alternative mechanisms might be, exploring the magnetic properties of biogenic iron oxides, other than magnetite, under different structural configurations may give rise to new biophysical possibilities.
Magnetotactic bacteria have also been used as controls for studying MPM systems. Some studies have used the magnetosome to make comparisons against particulates extracted from animal tissues [40] and have been used to test the efficacy of techniques [41–45]. These natural biogenic magnetite particles represent the ideal model with which to test a host of practical methods, including extraction protocols, instrument detection limits, the behaviour of isolated particles and confirm that the chemical/crystal structure of the particles remains unchanged by such procedures.
3. The ‘needle-in-a-haystack’ problem
The discovery of an MPM has been impeded by biophysical limitations inherent to the hypothesized nature of the receptor itself. In this review, two factors are identified as major obstacles that must be overcome in order to locate an MPM system. These factors have proven to be a significant barrier to scientific enquiry, where researchers are confronted with a search for a potentially rare cell type of unknown location and structure; the classic ‘needle-in-a-haystack’ problem, or as one researcher has phrased a ‘needle in a haystack of needles’, owing to the presence of other iron oxide materials in biology [32].
(1) Anatomical location and rarity—as magnetic fields freely penetrate biological tissue, the receptor is not restricted to a specific location in the body as for other senses. Additionally, MPMs are hypothesized to be a rare cell type with relatively few cells being needed for the sense to function.
(2) Composition—subcellular nanoparticulates (≤100 nm) of magnetite are commonly considered to be the most likely candidate structures for an MPM system. This is due to the fact that magnetite is known to be the most magnetic iron oxide formed biogenically. Finding these small particles, given that iron is common in physiological processes, is extremely difficult as many common analytical tools cannot differentiate between MPM particles and the iron compounds used in other metabolic activities. In addition, with iron being a highly abundant element in the Earth's crust, there is a high potential for environmental or laboratory contamination [46].
4. Finding a magnetic particle-mediated magnetoreceptor system
There are currently two experimental approaches used to narrow down the anatomical location of an MPM. A recent and innovative method studies magnetic field-induced neuronal activation in the brain, making it possible to identify new target sensory structures by following the afferent nerves connected to these activated regions [47,48]. This has been partly achieved in pigeons were the inner ear has been implicated in magnetoreception following the detection of magnetic field-induced neuronal activation in the birds vestibular system. Notably, the fact that these experiments were conducted in the dark adds support for an MPM over the light-dependent radical pair hypothesis.
The alternative approach for elucidating the anatomical location of an MPM is to take advantage of the only distinctive feature that separates these cells from others, which is the expected presence of the magnetic particles. This approach, which aims to characterize the particles either by direct observation or indirectly by studying their magnetic signature, forms the basis of the vast majority of published studies trying to identify the magnetic properties and location of an MPM system. Direct and indirect observations have been made on various animal species using a broad range of techniques from fields such as microscopy or magnetics (table 1) and are the primary focus of this review.
Table 1.
Acronyms and abbreviated terms for direct and indirect techniques applied to the study of magnetic particle-mediated magnetoreception.
| direct observation techniques |
indirect observation techniques |
||
|---|---|---|---|
| optical beam-based | magnetometry-based | ||
| LM | light microscopy | Cryo mag | cryogenic magnetometry |
| MagScrn | magnetic screening optical microscope | EPR | electron paramagnetic resonance |
| PB | Perls' Prussian blue (stain) | FMR | ferromagnetic resonance |
| electron beam-based | Möss | Mössbauer spectroscopy | |
| EDS | energy dispersive X-ray microanalysis | MR | magnetic resonance |
| EELS | electron energy loss spectroscopy | Pulse mag | pulse magnetometry |
| EFTEM | energy-filtered TEM | Spinner mag | spinner magnetometry |
| HRTEM | high-resolution TEM | SQUID | super quantum interference device magnetometry |
| SAED | selected area electron diffraction | VSM | vibrating sample magnetometry |
| SEM | scanning electron microscopy | ||
| TEM | transmission electron microscopy | ||
| ion beam-based | |||
| PIXE | particle-induced X-ray emission | ||
| TOF-SIMS | time of flight secondary ion mass spectroscopy | ||
| scanning probe-based | |||
| AFM | atomic force microscopy | ||
| MFM | magnetic force microscopy | ||
| X-ray beam-based | |||
| micro-CT | X-ray micro-computed tomography | ||
| micro-SXRF | micro-scanning X-ray fluorescence microscopy | ||
| micro-XANES | micro-X-ray absorption near edge structure | ||
| XRD | X-ray diffraction | ||
| others | |||
| Elect. | electrophysiology | ||
| MRI | magnetic resonance imaging | ||
5. Direct observations
Microscopic characterization techniques that can resolve the structure of tissues, cells or particles and/or their chemical/mineral composition, form the basis of all direct observations in the field (table 2) [30,41–43,45,49–62]. The most widely used approach has been to combine light microscopy with the histological stain Perls' Prussian blue, which renders ferric iron (Fe3+) blue in colour [51,52,62]. Staining is conducted on either paraffin or epoxy resin-embedded tissue sections cut with a microtome onto glass slides. However, Prussian blue is not a specific stain for magnetite, and there have been instances where false-positives have led to misidentifications. For example, in the case of homing pigeons, the long-held claim that Prussian blue positive structures in the upper beak are putative magnetoreceptor cells [59,63,64] was overturned by the more recent finding that they are in fact iron-rich macrophages [19,60].
Table 2.
List of published articles using direct methods for characterizing magnetic particles in various animal groups/species and the techniques applied. A list of acronyms and abbreviated terms for specific techniques is provided in table 1. SD, single domain; An, antennae; H, head; Th, thorax; Ab, abdomen; JO, Johnston's organ.
| species | reported particle characteristics | material observed | techniques | location | reference |
|---|---|---|---|---|---|
| insects | |||||
| Pachycondyla marginata | 37 × 26 nm | magnetite, maghaemite | TEM, EFTEM, SAED | H, Th, Ab | [49] |
| Apis mellifera | 20–100 nm | magnetite, maghaemite, haematite, goethite, AlSi, Fe/Ti/O composites | LM, PB, TEM, SAED, EDS | An, JO, H, Th, Ab | [50] |
| 100–320 nm | non-crystalline Fe, Ca, P | LM, PB, TEM, EDS, Elect., PIXE | Ab | [51,52] | |
| 10–20 nm rod and ring structures, 300 nm granules | no analysis | LM, TEM, Elect. | Ab—dendrites | [53] | |
| 600 nm with sub-10 nm inclusions | magnetite | TEM, HRTEM, SEM | Ab | [54] | |
| aggregations of 4 nm holoferritin | Fe, Ca, P | TEM, EDS, EFTEM | Ab—queens | [39] | |
| Scaptotrigona postica | aggregations of 2 nm holoferritin | Fe, Ca, P, Mg | TEM, EDS | Ab—queens | [55] |
| Vespa affınis | 100–700 nm | Fe, Ca, P | LM, PB, TEM, EDS | Ab | [56] |
| fish | |||||
| Oncorhynchus mykiss | 50 nm (chains) | Fe (but see 42) | LM, TEM, EDS, AFM, MFM, Elect. | olfactory epithelium | [41,45] |
| micrometre-sized iron-rich crystals | LM, MagScrn, SEM, EDS | olfactory epithelium | [42,43] | ||
| Oncorhynchus nerka | 48 nm (SD chains) | magnetite | TEM, HRTEM, EDS, SAED | ethmoid tissue | [30] |
| Oncorhynchus keta | submicrometre | Fe | SEM, EDS, TOF-SIMS | H | [57] |
| birds | |||||
| Columba livia | iron deposits, SPM crystals in 1–2 µm clusters, ferritin-like granules | Fe, magnetite, goethite, ferrihydrite (but see 42) | LM, PB, TEM, EELS, EFTEM, EDS, MRI, micro-CT, micro-SXRF, micro-XANES | upper beak cuticular plate—hair cells | [42,58–62] |
| Sylvia borin | iron deposits | Fe | LM, PB, micro-SXRF, micro-XANES | upper beak | [58] |
| Erithacus rubecula | |||||
| Gallus gallus domesticus | iron deposits, cuticulosome | Fe | LM, PB, micro-SXRF, micro-XANES | upper beak, cuticular plate—hair cells | [35,58,61] |
| Taeniopygia guttata | iron deposits, cuticulosome | Fe | LM, PB | upper beak, cuticular plate—hair cells | [46,61] |
| Emberizia c. citrinella | iron deposits | Fe | LM, PB | upper beak | [46] |
| Melothrus ater | |||||
| Anas platyrhynchos domesticus | cuticulosome | ferrihydrite | LM, PB, TEM, EELS, EFTEM, SAED | cuticular plate of inner ear hair cells | [61] |
| Struthio camelus | |||||
| Melopsittacus undulatus |
The potential for generating false-positives with the Prussian blue technique highlights the MPM composition problem (i.e. iron is common in biology and the environment) and stresses the need for complementary imaging and analytical approaches to verify the structure and composition of target particles. Conventionally, this has been done by resorting to the higher-resolution capabilities of transmission electron microscopy (TEM). However, it is generally not possible to transfer samples that have been sectioned for Prussian blue identification into the TEM for direct correlative imaging and analysis. To overcome this, investigators have developed a semi-correlative Prussian blue and TEM approach which involves taking alternate semi-thin and ultra-thin sections for optical and electron microscopy. This method has been used to characterize iron-rich macrophages in the pigeon beak [19,60] and a newly discovered iron-rich organelle in the inner ear hair cells of birds [61]. While this has proven to be a powerful tool for characterizing candidate structures, preparation and analysis is labour intensive and is subject to the MPM location and rarity problem, as a well-defined region of interest must first be known.
A novel and highly innovative study recently took advantage of the inherent magnetic properties of putative MPMs by screening populations of dissociated cells with a specialized inverted microscope that possessed a rotating magnetic probe around the objective lens [43]. This technology allowed researchers to collect magnetically responsive (spinning) cells from trout olfactory epithelia, which could then be examined using scanning electron microscopy (SEM) combined with X-ray microanalysis. This approach addresses issues with MPM location and rarity, but suffers from false-positives, with the spinning behaviour of cells being attributed to contamination, such as the extracellular attachment of particles from environmental or laboratory sources [42]. While the use of this technique for finding an MPM is currently in question, it may prove to be useful in the future as a tool for examining the magnetic properties of an MPM once it is discovered.
6. Indirect observations
Magnetic-based techniques, such as super quantum interference device (SQUID) magnetometry, magnetic resonance and electron paramagnetic resonance, are highly sensitive and seek to detect the presence of magnetic material and describe the size, arrangement and mineral form of the particulates. In general, living things are predominantly made up of diamagnetic materials, which generate a very weak opposing moment in a magnetic field. While the most common ‘magnetically active’ element in biological material is iron, most forms of iron in metabolic processes have only weak magnetic states (paramagnetic or antiferromagnetic). In contrast, the magnetic particles thought to be associated with an MPM system are strongly magnetic (ferrimagnetic). As such, magnetic measurements could provide a way of identifying MPM sites.
Notably, 11 of the 14 studies found in the literature that solely use indirect methods used insects as model systems (table 3) [65–78]. This is likely due to the fact that these animals are small, and whole samples or body parts can be analysed easily with minimal preparation. Insects are also available in large numbers, making it possible to analyse multiple individuals simultaneously in order to amplify the magnetic signal.
Table 3.
List of published articles using indirect methods for characterizing magnetic particles in various animal groups/species and the techniques applied. A list of acronyms and abbreviated terms for specific techniques is provided in table 1. SD, single domain; SPM, superparamagnetic magnetite; An, antennae; H , head, Th, thorax; Ab, abdomen.
| species | reported particle characteristics | material observed | techniques | location | reference |
|---|---|---|---|---|---|
| insects | |||||
| Pachycondyla marginata | clusters of 3 × 13 nm SPM | magnetite-based | EPR | Ab | [65] |
| pseudo-single or multi-domain | magnetite | SQUID | whole | [66] | |
| total saturation magnetization data only | highest % in An | SQUID | An, H, Th, Ab | [67] | |
| Solenopsis substituta | 12.5 nm (Ab) 11.0 nm (H) chains or ellipsoids | magnetite | FMR | H, Th, Ab | [68] |
| Rhodnius prolixus | no ferromagnetic material found | VSM | whole | [69] | |
| Neocapritermes opacus | 18.5 nm particles organized in a film-like system | magnetite | MR | Th+Ab | [70] |
| Apis mellifera | transversely oriented magnetic material | n.a. | SQUID | whole, Ab | [71] |
| 3 × 102 nm3 and 103 nm3 volume nanoparticles | Fe3+ , amorphous FeOOH, magnetite | EPR | Ab | [72] | |
| ca 30 nm, ferritin | magnetite, 98% iron atoms as ferrihydrite | SQUID | Ab | [73] | |
| time evolution of magnetic nanoparticles varies with bee age, preserving solution and body part | FMR | An, H, Th, Ab | [74] | ||
| Schwarziana quadripunctata | 220 nm in Ab estimated | magnetic material (highest % in An) | SQUID, FMR | An, H, Th, Ab | [75] |
| fish | |||||
| Oncorhynchus nerka | SD | magnetite | SQUID | ethmoid tissue | [76] |
| birds | |||||
| Dolichonyx oryzivorus | partially SD | magnetite | SQUID, spinner mag | H, nasal cavity to orbit | [77] |
| Passerculus sandwichensis | |||||
| Passerine cyanea | |||||
| mammals | |||||
| Miniopterus fuliginosus | SPM | magnetite | SQUID, pulse and cryomagnetometer | H, brain | [78] |
| Chaerephon plicata | |||||
| Nyctalus plancyi | |||||
| Hipposideros armiger | |||||
| Myotis ricketti | |||||
| Rhinolophus ferrumequinum |
The segmented insect body plan lends itself to dissection into head, thorax and abdomen, which is a useful strategy for screening individual body parts separately and provides a way to define regions of interest, thereby addressing the MPM location and rarity problem. The ability to study intact body parts, where their anatomical orientation is known relative to the applied magnetic field, also allows information about how the magnetic material is organized within the body [66,70,71].
The highly sensitive nature of most magnetic techniques also makes this range of analytical methods subject to the composition problem, especially in the case of ground dwelling or subterranean species such as ants or termites. Environmental sources of iron contamination can be found in the joints, hairs and digestive tracts of insects [50,79]. For example, iron particles extracted from ants cleaned by sonication were suggested to be derived from soil and were shown to be similar to those extracted from soil controls [50]. Particulates attached to the hairs of bumblebees have also been demonstrated to be contaminants from their environmental surroundings [80].
As shown in attempts to isolate spinning cells, a background level of magnetic particle contamination can be expected, despite great efforts to exclude such material [42]. Researchers have made attempts to mitigate environmental contamination by taking advantage of the fact that honeybees, ants and termites can be bred in the laboratory. Laboratory-reared insects are fed on artificial diets for the purpose of purging the digestive tract, such as pure cellulose in the case of termites [81,82] or sucrose solutions in the case of bees [52]. Notably, the vast majority of indirect studies have not used this approach, which could be problematic when interpreting magnetics data. It would be advisable to obtain baseline information on the presence of particulates in the gut contents of model species prior to further studies.
Certainly, there are advantages to having control over animal husbandry and the conditions under which animals are reared. However, consideration should also be given to the fact that these artificial environments could lead to experimental artefacts, such as abnormal development/physiology or induced stress. For example, while magnetotactic bacteria are vastly different from animals, it is known that they grow well in micro-anaerobic culture media, but will not form chains of magnetite particles (magnetosomes) unless the media is maintained under very specific oxygen saturation conditions [83].
7. Combined direct and indirect observations
Several studies have combined direct and indirect approaches, such as optical and electron microscopy with SQUID, in the search for an MPM system (table 4) [64,80,82,84–96]. Owing to the disparate sample preparation requirements for microscopic versus magnetic analysis, samples are typically generated from different individuals, but some studies were able to make direct and indirect observations on the same individual or sample. The closest example that could be found for this review is where the presence of magnetosome-like particles was confirmed in subsamples prepared from human brains for respective TEM and SQUID analysis [95]. However, these particles have not been implicated in magnetoreception.
Table 4.
List of published articles using both direct and indirect methods for characterizing magnetic particles in various animal groups/species and the techniques applied. A list of acronyms and abbreviated terms for specific techniques is provided in table 1. SD, single domain; SPM, superparamagnetic magnetite; P-SD, pseudo-single domain; H, head; Th, thorax; Ab, abdomen.
| species | reported particle characteristics | material observed | techniques | location | reference |
|---|---|---|---|---|---|
| insects | |||||
| Apis mellifera | 100–900 nm granules | hydrous iron oxide (ferrihydrite) | LM, PB, TEM, EDS, SAED, Möss | Ab | [84] |
| 100–600 nm granules with SPM inclusions, an average density of 1.25 g cm−3 | hydrous iron oxide with magnetite inclusions | LM, TEM, EDS, SQUID, EPR, AFM, MFM, ESCA | Ab | [85] | |
| Bombus terrestris | SPM | magnetite, wuesite | SEM, EDS, VSM, Möss, XRD | H, wing | [80] |
| Nasutitermes exitiosus | 10 nm SPM | ferrimagnetic material | TEM, Cryo mag | H, Th + Ab | [82] |
| Amitermes meridionalis | |||||
| fish | |||||
| Thunnus albacares | SD, mean size 45 × 38 nm ± 5 nm SE | magnetite | TEM, XRD, EDS, SQUID | H (dermethmoid tissue) | [86,87] |
| Oncorhynchus tshawytscha | SD chains | magnetite | TEM, XRD, SQUID | H (dermethmoid cartilage) | [88] |
| Salmo salar | SD 60–100 nm and 340–380 nm | magnetite | TEM, EDS, SQUID | lateral line | [89] |
| Anguilla anguilla | SD, mean 90 nm ± 40 nm | magnetite | TEM, EDS, SQUID | lateral line (mandibular) | [90] |
| birds | |||||
| Columba liva | SD | magnetite | XRD, EDS, SQUID | H | [91] |
| 1–5 nm SPM particles in 1–3 µm clusters that form 200 µm elongate aggregates | magnetite | LM, PB, TEM, SAED, SQUID | upper beak | [64,92] | |
| SPM, 1–4 µm aggregates | magnetite | LM, PB, SQUID | upper beak | [93] | |
| Dolichonyx oryzivorus | SD, P-SD | magnetite, other iron material present | LM, PB, TEM, SEM, EDS, spinner mag | H (various) | [94] |
| mammals | |||||
| Homo sapiens | SD in 50–100 clumps | magnetite (5 M particles per g tissue estimated) | TEM, SAED, SQUID | brain | [95] |
| nematode | |||||
| Caenorhabditis elegans | 10 s of nm | magnetite | TEM, EELS, EFTEM, EDS, SQUID | whole | [96] |
8. Four putative magnetic particle-mediated magnetoreceptor systems
While magnetoreceptors have been sought in a wide variety of animals and their various body parts, four main morphological structures emerge from the literature as the most extensively studied and perhaps most likely location for putative MPM systems. These include the insect abdomen, the upper beak of birds, the inner ear of birds and the nasal tissues of fish. It is curious to note that, despite these well-defined anatomical locations, which in some cases were even narrowed down to specific tissues or subcellular levels, the magnetoreception field arguably considers the question of MPM location, structure and function unresolved.
8.1. The insect abdomen
The insect abdomen has been the focus of numerous studies, and the presence of several SPM and SD iron oxide particles have been confirmed by direct and indirect methods (tables 2–4). In particular, the abdomen of the honeybee Apis mellifera has received substantial scientific attention. Two different anatomical sites have been proposed as possible locations for an MPM: (i) the anterior dorsal region of the abdomen, where evidence suggests SPM and SD magnetite particles are present [71,97] and (ii) iron granules located in sheets of trophocyte cells in the subcuticular fat layer of the ventral abdomen [54,85].
Using SQUID, transversely oriented SPM magnetite was indirectly detected in the anterior dorsal region of the abdomen [71,98] and direct TEM observations have revealed the presence of 15–30 nm (SPM/SD) and 3–5 nm (SPM) magnetite in digested tissue extracts of newly hatched worker honeybees [99]. In behavioural studies, where magnetic wires were attached to the anterior dorsal abdomen of honeybees, magnetic field discrimination was found to be impaired compared with animals with non-magnetic wire attached as controls [100]. Evidence has also been provided for the existence of a specialized band of SPM containing hairs in the anterior dorsal part of the abdomen [53]. As no further studies have been conducted on the anterior dorsal abdomen, it is unknown whether a connection exists between the particles identified by Gould and Schiff or whether these are responsible for the behavioural changes observed by Walker and Bitterman.
The iron granules within the trophocyte cells of the subcuticular fat layer have been studied in some detail and have been implicated in magnetoreception (tables 2–4). Insect fat layer trophocyte cells are present beneath the abdominal cuticle and are involved in various metabolic activities, including energy storage, the production of yolk proteins, heavy metal detoxification and the storage of excess dietary iron [101,102]. Using the Prussian blue stain on whole mounts of the abdomen, the granules were found to be distributed in bands beneath the cuticle in all abdominal segments [84]. Although no systematic stereological studies have so far been conducted using TEM, the granules are reported to range in diameter from 100 to 900 nm and are known to be primarily composed of iron, oxygen, phosphorous and calcium [52,55,84,85]. While these hydrous iron oxide particles are only weakly magnetic, it has been suggested that only 0.33% of the granules would need to be reduced to magnetite to explain previous measurements of the magnetic moment in the abdomen and be sufficient for an MPM to function [84].
The potential role of these granules for magnetoreception has been debated. Ventrally located trophocyte cells were claimed to be innervated by axons connected to the ganglia that exist along the ventral nerve cord in the abdomen, and that the iron granules contained an SPM magnetite component [54]. These findings were later challenged, and the interpretation and validity of these results were questioned on a number of fronts [103]. Specifically, given that the granules are comprised 7.5 nm particulates, consistent with the iron storage proteins ferritin or haemosiderin, a role in dietary iron storage was not considered. It was also suggested that trachea and excitatory nerves were mistaken for sensory nerves in trophocyte tissue. If correct, the findings of Hsu and Li were also inconsistent with magnetics data collected from the honeybee abdomen. Persisting with the notion that SPM magnetite particles were present in iron granules within ventrally located trophocytes, a follow-up multimodal study by the same authors claimed that granule distortion in response to an applied magnetic field resulted in a cytoskeletally triggered release of calcium [85].
8.2. The upper beak of birds
Numerous studies have implicated the head, nasal and upper beak regions of birds as the physical location for a magnetoreceptor system. A range of SD and SPM magnetite particles have been reported (tables 2–4) and have been reviewed recently [20]. Specifically, the ophthalmic branch of the trigeminal nerve in the pigeon's upper beak has been studied in detail. It has been claimed that it is associated with a specialized magnetic sense system comprised six discrete clusters of sensory neurons containing complex arrangements of SPM magnetite and maghaemite (γ-Fe2O3) platelets, acting to elicit a mechanosensory neuronal response [58,59,64,104]. The involvement of these structures in MPM was the dominant and accepted theory for magnetoreception in birds for over 10 years. However, this was challenged by recent findings using the semi-correlative optical, Prussian blue and analytical TEM approach, which demonstrated that the previously identified Prussian blue positive structures are iron-rich macrophages, and not magnetosensory neurons [19,60]. Notably, neurobiological evidence continues to emerge for the involvement of the trigeminal nerve in magnetoreception [105]. As the tissues of the upper beak of birds have arguably been the most thoroughly explored region of tissue with respect to finding an MPM system, the fact that an MPM remains undiscovered in the beak, despite strong behavioural and neurobiological evidence, raises the possibility that a light-based non-ocular RPM system may exist at this site.
8.3. The inner ear of birds
Due to the fact that the brain must ultimately process the incoming information from a magnetosensory stimulus, immunohistochemical markers (i.e. c-Fos) could be used to pinpoint such neuronal activity. Dickman and co-workers used this technique to examine neuronal expression in the brain of pigeons and determined that activation occurs in vestibular brainstem neurons when the animals are exposed to varying magnetic field conditions [47,48]. This region of the brain is linked by afferent nerves to the inner ear lagena. In birds subjected to ablation of the lagena, this vestibular activation was greatly diminished [48].
Although such experiments are challenging, the approach is highly suitable for addressing the anatomical location and rarity problem as new, well-defined regions of interest can be established. These findings have since sparked a search for putative magnetoreceptors in the inner ear of birds. Using correlative light and electron microscopy, a previously undescribed iron-rich organelle has been discovered in the cuticular plate of avian hair cells [61]. The organelle ranges from 300 to 600 nm in size and is comprised of ferritin-like granules. The structure is conspicuous given that almost 100% of hair cells contain only a single iron-rich organelle and seems to be limited to birds. Although the location of these iron-rich organelles partly coincides with the region of interest indicated by neuronal expression experiments, selected area electron diffraction of the ‘cuticulosome’ produces patterns that are consistent with the mineral ferrihydrite, a mineral that exhibits only weak magnetic properties (but see [106]). However, its discovery has generated interest in exploring magnetoreception mechanisms beyond traditional torque-based MPM systems [107].
8.4. The nasal tissues of fish
Single domain magnetite particles similar to those found in the magnetosome of magnetotactic bacteria have been observed from the olfactory tissues in a number of fish, including species of tuna, salmon and trout (tables 2–4). Early studies used SQUID to describe the magnetic properties of frozen samples and revealed the presence of 40–100 nm-sized SD magnetite particles arranged in chains [30,86,88]. These samples were then macerated and chemically digested to extract putative particles for observation using other analytical methods, such as TEM, to confirm the existence of these particulates and chains.
Subsequent studies in trout used laser scanning confocal microscopy and TEM to locate these materials in situ at the tissue and subcellular level, respectively, and fluorescence microscopy to demonstrate their connection to the nervous system [41,45]. Reflective particles, which were suggested to be chains of magnetite, were found to reside within the lamina propria layer of the olfactory lamellae.
9. Summary of magnetic particle-mediated magnetoreceptors in insects, birds and fish
Whether an MPM system exists in these three animal groups remains an open question. In all cases, an anatomically well-defined region exists that could be scrutinized further. It is unclear why no further studies have attempted to clarify the existence of an MPM in the anterior dorsal region of the abdomen in honeybees since it was last proposed as a suitable location more than 20 years ago. Recent studies on the upper beak of pigeons have raised the possibility that an MPM system may not exist at this location, or perhaps remains hidden owing to it being structured differently than current models suggest. The alternative hypothesis that olfactory rather than magnetic cues are required for successful homing in pigeons also remains a possibility [108–110]. In the case of fish, the composition problem has foiled recent attempts to isolate spinning cells in trout, and 15 years have passed since the last report of magnetite particulates in the olfactory tissues of these animals. It is important to also note that there have been no attempts to determine whether an MPM system exists in reptiles, amphibians or crustaceans, despite the strong evidence for a magnetic map sense in turtles, newts and spiny lobsters [111–113]. Additionally, the recent implication of AFD sensory neurons in geomagnetic orientation in Caenorhabditis elegans [114] provides a clear opportunity to microscopically examine a well-defined region of interest for putative magnetoreceptive cells. Further scrutiny could be given to each of these groups in future studies.
Direct and indirect techniques have provided tantalizing evidence for the existence of an MPM system, but have thus far proven to be inconclusive. Clearly, new methods of investigation are required. Additionally, the publication of negative results could be of great benefit to the magnetoreception field where the absence of particulates may be equally as informative as their presence given the location and rarity problem.
10. Future prospects
Despite a substantial amount of published work, the major goal in magnetoreception remains to fully describe the fine structure of MPM cells, as well as the magnetic particle–cell interactions. While electron microscopy is the only suitable technique for achieving this goal, no single approach is capable of finding the anatomical location of MPM cells or elucidating their function and proving a connection to the nervous system. Future studies should incorporate multimodal approaches of imaging and analysis and involve collaborative multidisciplinary teams with expertise in the biological, physical and technical aspects that the magnetoreception field demands.
The following approaches should be considered
(1) Novel techniques need to be developed that address the location and rarity problem and resolve the issue of the ubiquity of iron.
(2) Correlative approaches are needed as research conducted to this point has revealed that no single technique is capable of resolving this problem.
(3) A reproducible ultrastructural approach must be used to finally demonstrate the particle/cell relationship.
11. Addressing the location, rarity and composition problems
11.1. Particle extraction—potential uses and problems
Assuming inorganic particles form the basis of an MPM system, it should be possible to isolate them from tissues and characterize them separately, prior to finding the functional structures in situ. This approach has been used previously on insects and fish, with most studies using a combination of tissue homogenization, chemical digestion and filtration [30,49,85,86,88]. This strategy can be used to initially define regions of interest by screening different body parts or organs for inorganic particulates.
It must be emphasized that methods aiming to extract rare particulates are highly prone to the composition problem as contaminants are naturally concentrated during the process. The extent of the problem has been outlined explicitly in previous studies [40,115], the authors of which state that virtually all laboratory plasticware contains ferromagnetic contamination, which can be exacerbated when techniques such as centrifugation are used. This can most likely be extended to other equipment and materials, such as reagent grade chemicals, pure water supplies, tools, slides or TEM grids, which in very few cases are prepared or manufactured with the exclusion of nanoparticulates in mind. At all stages of sample preparation the risk of introducing contaminants should be of primary concern.
The issue of contamination was clearly demonstrated using leucocyte culture medium to rinse a range of commonly used plasticware, where magnetics data revealed the equivalent of 160 ng of magnetite had been collected in 50 ml of medium following the rinsing process [46]. Using appropriate controls or blanks, it may be possible to characterize baseline levels of contamination in a given system, thereby amplifying the signal to noise ratios during imaging or analysis. A comparative approach using a negative control tissue structure would help to distinguish background noise from a real signal. Furthermore, work should be done on larger sample sizes and replicates to confirm reproducibility.
In order to mitigate the problem, researchers have developed specialized clean rooms that are free of magnetic particle contamination, used non-ferrous tools and acid washed glassware [33,43,60]. However, these do not account for the fact that the biological material inherently contains various iron minerals [33]. This iron can be ingested, trapped in body tissues such as skin or mucous membranes or be formed biogenically, where a variety of iron minerals have been described, including magnetite, ferrihydrite, goethite and lepidocrocite [116].
Given the ubiquity of iron in both laboratory settings and the animal systems under investigation, it may be necessary to simply acknowledge the presence of iron rather than go to extraordinary lengths to exclude it. A major omission from many of the studies involving particle extraction is the use of appropriate controls. For example, if extraction steps are used that include homogenization, chemical digestion and filtration, the same procedure should be conducted in the absence of the sample or with other tissue samples that do not contain putative MPM structures. Controls are a basic element of the scientific method, and it is imperative that they are adopted in future studies involving particle extraction.
While the removal of the tissue fraction significantly reduces the volume of target material, even these small sample extracts can present problems for detailed analysis. Subsampling homogenized liquid extracts to prepare TEM samples or passing material through physical filters, as done previously [49,85,88], may result in type 1 errors owing to the handling of material. Remarkably, little is known about how such magnetic materials behave once removed from tissue, and particles may aggregate to form much larger structures that do not remain suspended in solution or become trapped/bound to filtration media. A significant body of literature now exists on magnetite nanoparticles produced for medical imaging and therapeutic treatments due to the fact that they must be both effective and non-cytotoxic for applications in human health [117,118]. Future studies aimed at characterizing particle extracts for identifying an MPM system could therefore draw upon the analogous physical properties, chemistry and behaviour of these synthetic materials.
Although magnetism can be both a blessing and a curse in terms of particle extraction, it is surprising that magnetic methods have not been exploited more widely for extracting particles. With the exception of a single attempt to isolate particles in suspension using an electromagnetic filter (Frantz separator) [71], or the isolation of spinning cells [42,43], the majority of studies use simple magnets attached to the side of vials to isolate the magnetic fraction in a sample of interest [30,50,86,88]. There would seem to be broad scope for the development of novel magnetic screening methods. For example, high-field gradient magnetic fractionation systems have been used successfully to concentrate malaria parasites, thereby improving their detection [119], suggesting similar approaches could help to isolate low concentrations of magnetoreceptive cells containing magnetite.
Owing to the location and rarity problem, large sample sizes may be necessary to increase the number of particles to a detectible level. For practical reasons, such methods may be constrained to insects. The number of particles needed to form a functioning MPM system remains uncertain, although estimates in the range of 107 SD magnetite particles per animal have been suggested in birds and fish [31,88,91]. Even at a fraction of this number, if enough individuals are sampled, it should be feasible to reliably and repeatedly extract particles for examination and distinguish them from a background of contaminants. Correlative methods that can probe the entire volume of the extracted material at different length scales must be developed.
11.2. Imaging
There seems agreement in the field that an ultrastructural approach is needed to determine the structure and function of magnetoreceptive cells [1,79,99]. However, techniques capable of resolving subcellular detail remain impractical without first identifying small target regions. If we consider that a piece of tissue prepared for TEM is typically no larger than approximately 1 mm3, and standard approximately 100 nm ultra-thin 300 µm2 sections are cut from the block face, close to 100 000 sections would need to be generated to examine the sample in full. While TEM will be critical for finally characterizing an MPM system, it is clearly unfeasible to use TEM for a systematic blind search.
New developments in optical imaging could provide ways to screen larger volumes of tissue to define regions of interest for subsequent analysis. Techniques such as single-plane illumination microscopy can provide three-dimensional fluorescent data on relatively large samples [120] and is emerging as a powerful tool in neurobiology. For example, functional light sheet microscopy has been used to image neural activity in the brain of live zebrafish [121] and could also be used for mapping neural networks and imaging neuronal activation in magnetically stimulated regions of tissue.
Other three-dimensional imaging platforms such as high-resolution magnetic resonance imaging (MRI) or X-ray micro-computed tomography (micro-CT) may also be advantageous by providing a means for investigating relatively large (mm–cm) volumes of tissue. High-field MRI systems that are capable of achieving near cellular resolution could potentially be used to scan for magnetic anomalies in tissue. While MRI may not be capable of resolving individual nanoparticles, they may influence the proton relaxation rates in the surrounding tissue enough to generate susceptibility effects far larger than the particles themselves [122]. Many X-ray micro-CT systems can now achieve submicrometre resolution, which could reveal aggregations of dense material, such as magnetite. An advantage of both MRI and X-ray micro-CT is that they are non-destructive, thereby allowing regions targeted with these techniques to be examined using other correlative imaging methods.
In electron microscopy, there have also been a number of technical advances in the automation of serial sectioning. Serial block face sectioning, using a microtome built into a scanning electron microscope (SEM) chamber, and focused ion beam milling of biological tissue in the SEM are both becoming mainstream techniques for the acquisition of detailed three-dimensional data at the tissue and subcellular level [123]. Both involve the incremental removal of layers of resin-embedded tissue to expose a new surface for imaging. Confined to reasonably small fields of view, these SEM-based approaches would be more useful for characterizing an MPM in detail once found.
The microscopy field is increasingly looking towards the integration of any number of these multimodal platforms to provide correlative or semi-correlative data across a broad range of length scales in two and three dimensions [123]. While each animal model has specific limitations, such as sample size, structure and availability, and will require different experimental approaches, the above-mentioned methods and techniques are broadly applicable. The four main anatomical locations identified above for insects, birds and fish warrant further investigation in order to categorically rule on whether an MPM system is truly located at these sites. In each case, the regions of interest are small enough to attempt a range of these new correlative imaging and analytical approaches.
12. Conclusion
Behavioural research continues to generate phenotypic evidence for the existence of a magnetic sense in animals. However, progress in determining the anatomical location, structure and function of an MPM system has been frustratingly slow. Unravelling the mechanistic basis of the magnetic sense will underpin a new era of behavioural research based on this fundamental knowledge. A wide range of existing and emerging characterization and analysis options are now available that can be adapted and exploited in the search for an MPM system. The search will require correlative methodologies and the development of novel and innovative experimental approaches that combine conventional direct and indirect techniques with modern research tools.
Acknowledgements
The authors acknowledge the facilities, and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility and National Imaging Facility, The University of Western Australia, a facility funded by the University, State and Commonwealth Governments. Thanks also to the four anonymous referees for their positive and constructive feedback on the manuscript.
Authors' contributions
J.S. drafted the manuscript, all other authors have made substantial contributions to manuscript conception, design, drafting and revision of intellectual content.
Competing interests
We have no competing interests.
Funding
J.S. is funded by the Australian Research Council (ARC) under the Discovery Early Career Researcher Award (DECRA) fellowship scheme, grant no. DE130101660
References
- 1.Kirschvink JL, Winklhofer M, Walker MM. 2010. Biophysics of magnetic orientation: strengthening the interface between theory and experimental design. J. R. Soc. Interface 7(Suppl 2), S179–S191. ( 10.1098/rsif.2009.0491.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winklhofer M. 2010. Magnetoreception. J. R. Soc. Interface 7(Suppl 2), S131–S134. ( 10.1098/rsif.2010.0010.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiltschko R, Wiltschko W. 2013. The magnetite-based receptors in the beak of birds and their role in avian navigation. J. Comp. Physiol. 199, 89–98. ( 10.1007/s00359-012-0769-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freake MJ, Muheim R, Phillips JB. 2006. Magnetic maps in animals: a theory comes of age? Q. Rev. Biol. 81, 327–347. ( 10.1086/511528) [DOI] [PubMed] [Google Scholar]
- 5.Gould JL. 2014. Animal navigation: a map for all seasons. Curr. Biol. 24, R153–R155. ( 10.1016/j.cub.2014.01.030) [DOI] [PubMed] [Google Scholar]
- 6.Holland RA. 2014. True navigation in birds: from quantum physics to global migration. J. Zool. 293, 1–15. ( 10.1111/jzo.12107) [DOI] [Google Scholar]
- 7.Walker MM, Dennis TE, Kirschvink JL. 2002. The magnetic sense and its use in long-distance navigation by animals. Curr. Opin. Neurobiol. 12, 735–744. ( 10.1016/S0959-4388(02)00389-6) [DOI] [PubMed] [Google Scholar]
- 8.Kalmijn AJ, Blakemore R. 1978. The magnetic behaviour of mud bacteria. In Animal migration, navigation, and homing (eds Schmidt-Koenig K, Keeton WT), pp. 354–355. Berlin, Germany: Springer. [Google Scholar]
- 9.Holland RA. 2010. Differential effects of magnetic pulses on the orientation of naturally migrating birds. J. R. Soc. Interface 7, 1617–1625. ( 10.1098/rsif.2010.0159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holland RA, Helm B. 2013. A strong magnetic pulse affects the precision of departure direction of naturally migrating adult but not juvenile birds. J. R. Soc. Interface 10, 20121047 ( 10.1098/rsif.2012.1047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munro U, Munro JA, Phillips JB, Wiltschko W. 1997. Effect of wavelength of light and pulse magnetisation on different magnetoreception systems in a migratory bird. Aust. J. Zool. 45, 189–198. ( 10.1071/ZO96066) [DOI] [Google Scholar]
- 12.Kirschvink JL, Kirschvink AK. 1991. Is geomagnetic sensitivity real? Replication of the Walker–Bitterman magnetic conditioning experiment in honey bees. Am. Zool. 31, 169–185. [Google Scholar]
- 13.Irwin W, Lohmann K. 2005. Disruption of magnetic orientation in hatchling loggerhead sea turtles by pulsed magnetic fields. J. Comp. Physiol. A 191, 475–480. ( 10.1007/s00359-005-0609-9) [DOI] [PubMed] [Google Scholar]
- 14.Holland RA, Kirschvink JL, Doak TG, Wikelski M. 2008. Bats use magnetite to detect the Earth's magnetic field. PLoS ONE 3, e1676 ( 10.1371/journal.pone.0001676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ritz T, Adem S, Schulten K. 2000. A model for photoreceptor-based magnetoreception in birds. Biophys. J. 78, 707–718. ( 10.1016/S0006-3495(00)76629-X) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritz T, Ahmad M, Mouritsen H, Wiltschko R, Wiltschko W. 2010. Photoreceptor-based magnetoreception: optimal design of receptor molecules, cells, and neuronal processing. J. R. Soc. Interface 7(Suppl 2), S135–S146. ( 10.1098/rsif.2009.0456.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maeda K, Henbest KB, Cintolesi F, Kuprov I, Rodgers CT, Liddell PA, Gust D, Timmel CR, Hore PJ. 2008. Chemical compass model of avian magnetoreception. Nature 453, 387–390. ( 10.1038/nature06834) [DOI] [PubMed] [Google Scholar]
- 18.Mouritsen H, Hore PJ. 2012. The magnetic retina: light-dependent and trigeminal magnetoreception in migratory birds. Curr. Opin. Neurobiol. 22, 343–352. ( 10.1016/j.conb.2012.01.005) [DOI] [PubMed] [Google Scholar]
- 19.Treiber CD, Salzer M, Breuss M, Ushakova L, Lauwers M, Edelman N, Keays DA. 2013. High resolution anatomical mapping confirms the absence of a magnetic sense system in the rostral upper beak of pigeons. Commun. Integr. Biol. 6, e24859 ( 10.4161/cib.24859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cadiou H, McNaughton PA. 2010. Avian magnetite-based magnetoreception: a physiologist's perspective. J. R. Soc. Interface 7(Suppl 2), S193–S205. ( 10.1098/rsif.2009.0423.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirschvink JL, Gould JL. 1981. Biogenic magnetite as a basis for magnetic field detection in animals. Biosystems 13, 181–201. ( 10.1016/0303-2647(81)90060-5) [DOI] [PubMed] [Google Scholar]
- 22.Kirschvink JL, Walker MM, Diebel CE. 2001. Magnetite-based magnetoreception. Curr. Opin. Neurobiol. 11, 462–467. ( 10.1016/S0959-4388(00)00235-X) [DOI] [PubMed] [Google Scholar]
- 23.Walker MM. 2008. A model for encoding of magnetic field intensity by magnetite-based magnetoreceptor cells. J. Theor. Biol. 250, 85–91. ( 10.1016/j.jtbi.2007.09.030) [DOI] [PubMed] [Google Scholar]
- 24.Winklhofer M, Kirschvink JL. 2010. A quantitative assessment of torque-transducer models for magnetoreception. J. R. Soc. Interface 7(Suppl 2), S273–S289. ( 10.1098/rsif.2009.0435.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen KK. 2010. Light-dependent orientation responses in animals can be explained by a model of compass cue integration. J. Theor. Biol. 262, 129–141. ( 10.1016/j.jtbi.2009.09.005) [DOI] [PubMed] [Google Scholar]
- 26.Phillips JB, Jorge PE, Muheim R. 2010. Light-dependent magnetic compass orientation in amphibians and insects: candidate receptors and candidate molecular mechanisms. J. R. Soc. Interface 7(Suppl 2), S241–S256. ( 10.1098/rsif.2009.0459.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phillips JB, Muheim R, Jorge PE. 2010. A behavioral perspective on the biophysics of the light-dependent magnetic compass: a link between directional and spatial perception? J. Exp. Biol. 213, 3247–3255. ( 10.1242/jeb.020792) [DOI] [PubMed] [Google Scholar]
- 28.Chaves I et al. 2011. The cryptochromes: blue light photoreceptors in plants and animals. Annu. Rev. Plant Biol. 62, 335–364. ( 10.1146/annurev-arplant-042110-103759) [DOI] [PubMed] [Google Scholar]
- 29.Aisen P, Enns C, Wessling-Resnick M. 2001. Chemistry and biology of eukaryotic iron metabolism. Int. J. Biochem. Cell Biol. 33, 940–959. ( 10.1016/S1357-2725(01)00063-2) [DOI] [PubMed] [Google Scholar]
- 30.Mann S, Sparks NH, Walker MM, Kirschvink JL. 1988. Ultrastructure, morphology and organization of biogenic magnetite from sockeye salmon, Oncorhynchus nerka: implications for magnetoreception. J. Exp. Biol. 140, 35–49. [DOI] [PubMed] [Google Scholar]
- 31.Kirschvink JL, Walker MM. 1985. Particle-size considerations for magnetite-based magnetoreceptors. In Magnetite biomineralization and magnetoreception in organisms (eds Kirschvink JL, Jones DS, MacFadden BJ), pp. 243–254. New York, NY: Plenum Press. [Google Scholar]
- 32.Johnsen S, Lohmann KJ. 2008. Magnetoreception in animals. Phys. Today 61, 29–35. ( 10.1063/1.2897947) [DOI] [Google Scholar]
- 33.Kirschvink JL. 1989. Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendations for future study. Bioelectromagnetics 10, 239–259. ( 10.1002/bem.2250100304) [DOI] [PubMed] [Google Scholar]
- 34.Kirschvink JL. 1997. Homing in on vertebrates. Nature 390, 339–340. ( 10.1038/36986) [DOI] [Google Scholar]
- 35.Posfai M, Dunin-Borkowski RE. 2009. Magnetic nanocrystals in organisms. Elements 5, 235–240. ( 10.2113/gselements.5.4.235) [DOI] [Google Scholar]
- 36.Blakemore R. 1975. Magnetotactic bacteria. Science 190, 377–379. ( 10.1126/science.170679) [DOI] [PubMed] [Google Scholar]
- 37.Frankel RB, Blakemore RP, Wolfe RS. 1979. Magnetite in freshwater magnetotactic bacteria. Science 203, 1355–1356. ( 10.1126/science.203.4387.1355) [DOI] [PubMed] [Google Scholar]
- 38.Schüler D. 1999. Formation of magnetosomes in magnetotactic bacteria. J. Mol. Microbiol. Biotechnol. 1, 79–86. [PubMed] [Google Scholar]
- 39.Blakemore RP, Frankel RB. 1981. Magnetic navigation in bacteria. Sci. Am. 245, 58–65. ( 10.1038/scientificamerican1281-58) [DOI] [Google Scholar]
- 40.Kirschvink JL, Brassart J, Nesson MH. 1998. Magnetite-based biological effects in animals: biophysical, contamination, and sensory aspects, 118 Palo Alto, CA: Electric Power Research Institute. [Google Scholar]
- 41.Diebel CE, Proksch R, Green CR, Neilson P, Walker MM. 2000. Magnetite defines a vertebrate magnetoreceptor. Nature 406, 299–302. ( 10.1038/35018561) [DOI] [PubMed] [Google Scholar]
- 42.Edelman NB, et al. 2015. No evidence for intracellular magnetite in putative vertebrate magnetoreceptors identified by magnetic screening. Proc. Natl Acad. Sci. USA 112, 262–267. ( 10.1073/pnas.1407915112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eder SH, Cadiou H, Muhamad A, McNaughton PA, Kirschvink JL, Winklhofer M. 2012. Magnetic characterization of isolated candidate vertebrate magnetoreceptor cells. Proc. Natl Acad. Sci. USA 109, 12 022–12 027. ( 10.1073/pnas.1205653109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green CR, Holloway H, Walker MM. 2001. Detection of submicroscopic magnetite particles using reflectance mode confocal laser scanning microscopy. Cell Biol. Int. 25, 985–990. ( 10.1006/cbir.2001.0773) [DOI] [PubMed] [Google Scholar]
- 45.Walker MM, Diebel CE, Haugh CV, Pankhurst PM, Montgomery JC, Green CR. 1997. Structure and function of the vertebrate magnetic sense. Nature 390, 371–376. ( 10.1038/37057) [DOI] [PubMed] [Google Scholar]
- 46.Kobayashi AK, Kirschvink JL, Nesson MH. 1995. Ferromagnetism and EMFs [2]. Nature 374, 123 ( 10.1038/374123a0) [DOI] [PubMed] [Google Scholar]
- 47.Wu LQ, Dickman JD. 2012. Neural correlates of a magnetic sense. Science 336, 1054–1057. ( 10.1126/science.1216567) [DOI] [PubMed] [Google Scholar]
- 48.Wu L-Q, Dickman JD. 2011. Magnetoreception in an avian brain in part mediated by inner ear lagena. Curr. Biol. 21, 418–423. ( 10.1016/j.cub.2011.01.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Acosta-Avalos D, Wajnberg E, Oliveira PS, Leal I, Farina M, Esquivel DMS. 1999. Isolation of magnetic nanoparticles from Pachycondyla marginata ants. J. Exp. Biol. 202, 2687–2692. [DOI] [PubMed] [Google Scholar]
- 50.de Oliveira JF, Wajnberg E, Esquivel DM, Weinkauf S, Winklhofer M, Hanzlik M. 2010. Ant antennae: are they sites for magnetoreception? J. R. Soc. Interface 7, 143–152. ( 10.1098/rsif.2009.0102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuterbach DA, Walcott B. 1986. Iron-containing cells in the honey-bee (Apis mellifera). II. Accumulation during development. J. Exp. Biol. 126, 389–401. [DOI] [PubMed] [Google Scholar]
- 52.Kuterbach DA, Walcott B. 1986. Iron-containing cells in the honey-bee (Apis mellifera). I. Adult morphology and physiology. J. Exp. Biol. 126, 375–387. [DOI] [PubMed] [Google Scholar]
- 53.Schiff H. 1991. Modulation of spike frequencies by varying the ambient magnetic field and magnetite candidates in bees (Apis mellifera). Comp. Biochem. Physiol. A 100, 975–985. ( 10.1016/0300-9629(91)90325-7) [DOI] [PubMed] [Google Scholar]
- 54.Hsu CY, Li CW. 1994. Magnetoreception in honeybees. Science 265, 95–97. ( 10.1126/science.265.5168.95) [DOI] [PubMed] [Google Scholar]
- 55.Keim CN, Cruz-Landim C, Carneiro FG, Farina M. 2002. Ferritin in iron containing granules from the fat body of the honeybees Apis mellifera and Scaptotrigona postica. Micron 33, 53–59. ( 10.1016/S0968-4328(00)00071-8) [DOI] [PubMed] [Google Scholar]
- 56.Hsu CY. 2004. The processes of iron deposition in the common hornet (Vespa affinis). Biol Cell 96, 529–537. ( 10.1016/j.biolcel.2004.05.001) [DOI] [PubMed] [Google Scholar]
- 57.Yano A, Aoyagi S. 2008. TOF-SIMS analysis of magnetic materials in chum salmon head. Appl. Surf. Sci. 255, 1100–1103. ( 10.1016/j.apsusc.2008.05.085) [DOI] [Google Scholar]
- 58.Falkenberg G, Fleissner G, Schuchardt K, Kuehbacher M, Thalau P, Mouritsen H, Heyers D, Wellenreuther G, Fleissner G. 2010. Avian magnetoreception: elaborate iron mineral containing dendrites in the upper beak seem to be a common feature of birds. PLoS ONE 5, e9231 ( 10.1371/journal.pone.0009231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fleissner G, Holtkamp-Rotzler E, Hanzlik M, Winklhofer M, Fleissner G, Petersen N, Wiltschko W. 2003. Ultrastructural analysis of a putative magnetoreceptor in the beak of homing pigeons. J. Comp. Neurol. 458, 350–360. ( 10.1002/cne.10579) [DOI] [PubMed] [Google Scholar]
- 60.Treiber CD, et al. 2012. Clusters of iron-rich cells in the upper beak of pigeons are macrophages not magnetosensitive neurons. Nature 484, 367–370. ( 10.1038/nature11046) [DOI] [PubMed] [Google Scholar]
- 61.Lauwers M, et al. 2013. An iron-rich organelle in the cuticular plate of avian hair cells. Curr. Biol. 23, 924–929. ( 10.1016/j.cub.2013.04.025) [DOI] [PubMed] [Google Scholar]
- 62.Williams MN, Wild JM. 2001. Trigeminally innervated iron-containing structures in the beak of homing pigeons, and other birds. Brain Res. 889, 243–246. ( 10.1016/S0006-8993(00)03114-0) [DOI] [PubMed] [Google Scholar]
- 63.Falkenberg G, Fleissner GE, Fleissner GUE, Schuchardt K, Kühbacher M, Chalmin E, Janssens K. 2009. High resolution micro-XRF maps of iron oxides inside sensory dendrites of putative avian magnetoreceptors. J. Phys. Conf. Ser. 186, 012084 ( 10.1088/1742-6596/186/1/012084) [DOI] [Google Scholar]
- 64.Hanzlik M, Heunemann C, Holtkamp-Rötzler E, Winklhofer M, Petersen N, Fleissner G. 2000. Superparamagnetic magnetite in the upper beak tissue of homing pigeons. Biometals 13, 325–331. ( 10.1023/A:1009214526685) [DOI] [PubMed] [Google Scholar]
- 65.Wajnberg E, Acosta-Avalos D, El-Jaick LJ, Abraçado L, Coelho JLA, Bakuzis AF, Morals PC, Esquivel DMS. 2000. Electron paramagnetic resonance study of the migratory ant Pachycondyla marginata abdomens. Biophys. J. 78, 1018–1023. ( 10.1016/S0006-3495(00)76660-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esquivel DMS, Wajnberg E, Cernicchiaro GR, Alves OC. 2004. Comparative magnetic measurements of migratory ant and its only termite prey. J. Magn. Magn. Mater. 278, 117–121. ( 10.1016/j.jmmm.2003.12.327) [DOI] [Google Scholar]
- 67.Wajnberg E, Cernicchiaro G, Motta De Souza Esquivel D. 2004. Antennae: the strongest magnetic part of the migratory ant. Biometals 17, 467–470. ( 10.1023/B:BIOM.0000029443.93732.62) [DOI] [PubMed] [Google Scholar]
- 68.Abracado LG, Esquivel DM, Alves OC, Wajnberg E. 2005. Magnetic material in head, thorax, and abdomen of Solenopsis substituta ants: a ferromagnetic resonance study. J. Magn. Reson. 175, 309–316. ( 10.1016/j.jmr.2005.05.006) [DOI] [PubMed] [Google Scholar]
- 69.Giraldo D, Hernandez C, Molina J. 2013. In search of magnetosensitivity and ferromagnetic particles in Rhodnius prolixus: behavioral studies and vibrating sample magnetometry. J. Insect Physiol. 59, 345–350. ( 10.1016/j.jinsphys.2012.12.005) [DOI] [PubMed] [Google Scholar]
- 70.Alves OC, Wajnberg E, De Oliveira JF, Esquivel DM. 2004. Magnetic material arrangement in oriented termites: a magnetic resonance study. J. Magn. Reson. 168, 246–251. ( 10.1016/j.jmr.2004.03.010) [DOI] [PubMed] [Google Scholar]
- 71.Gould JL. 1978. Bees have magnetic remanence. Science 201, 1026–1028. ( 10.1126/science.201.4360.1026) [DOI] [PubMed] [Google Scholar]
- 72.El-Jaick LJ, Acosta-Avalos D, De Souza Esquivel DM, Wajnberg E, Linhares MP. 2001. Electron paramagnetic resonance study of honeybee Apis mellifera abdomens. Eur. Biophys. J. 29, 579–586. ( 10.1007/s002490000115) [DOI] [PubMed] [Google Scholar]
- 73.Desoil M, Gillis P, Gossuin Y, Pankhurst QA, Hautot D. 2005. Definitive identification of magnetite nanoparticles in the abdomen of the honeybee Apis mellifera. J. Phys. Conf. Ser. 17, 45–49. ( 10.1088/1742-6596/17/1/007) [DOI] [Google Scholar]
- 74.Chambarelli LL, Pinho MA, Abraçado LG, Esquivel DMS, Wajnberg E. 2008. Temporal and preparation effects in the magnetic nanoparticles of Apis mellifera body parts. J. Magn. Magn. Mater. 320, e207–e210. ( 10.1016/j.jmmm.2008.02.049) [DOI] [Google Scholar]
- 75.Lucano MJ, Cernicchiaro G, Wajnberg E, Esquivel DM. 2006. Stingless bee antennae: a magnetic sensory organ? Biometals 19, 295–300. ( 10.1007/s10534-005-0520-4) [DOI] [PubMed] [Google Scholar]
- 76.Walker MM, Quinn TP, Kirschvink JL, Groot C. 1988. Production of single-domain magnetite throughout life by sockeye salmon, Oncorhynchus nerka. J. Exp. Biol. 140, 51–63. [DOI] [PubMed] [Google Scholar]
- 77.Beason RC, Brennan WJ. 1986. Natural and induced magnetization in the bobolink, Dolichonyx oryzivorus (Aves: Icteridae). J. Exp. Biol. 125, 49–56. [Google Scholar]
- 78.Tian L, Lin W, Zhang S, Pan Y. 2010. Bat head contains soft magnetic particles: evidence from magnetism. Bioelectromagnetics 31, 499–503. ( 10.1002/bem.20590) [DOI] [PubMed] [Google Scholar]
- 79.Wajnberg E, Acosta-Avalos D, Alves OC, de Oliveira JF, Srygley RB, Esquivel DM. 2010. Magnetoreception in eusocial insects: an update. J. R. Soc. 7(Suppl 2), S207–S225. ( 10.1098/rsif.2009.0526.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jandacka P, Kasparova B, Jiraskova Y, Dedkova K, Mamulova-Kutlakova K, Kukutschova J. 2015. Iron-based granules in body of bumblebees. Biometals 28, 89–99. ( 10.1007/s10534-014-9805-9) [DOI] [PubMed] [Google Scholar]
- 81.de Oliveira JF, Alves OC, Esquivel DM, Wajnberg E. 2008. Ingested and biomineralized magnetic material in the prey Neocapritermes opacus termite: FMR characterization. J. Magn. Reson. 191, 112–119. ( 10.1016/j.jmr.2007.12.006) [DOI] [PubMed] [Google Scholar]
- 82.Maher BA. 1998. Magnetite biomineralization in termites. Proc. R. Soc. Lond. B 265, 733–737. ( 10.1098/rspb.1998.0354) [DOI] [Google Scholar]
- 83.Heyen U, Schuler D. 2003. Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl. Microbiol. Biotechnol. 61, 536–544. ( 10.1007/s00253-002-1219-x) [DOI] [PubMed] [Google Scholar]
- 84.Kuterbach DA, Walcott B, Reeder RJ, Frankel RB. 1982. Iron-containing cells in the honey bee (Apis mellifera). Science 218, 695–697. ( 10.1126/science.218.4573.695) [DOI] [PubMed] [Google Scholar]
- 85.Hsu CY, Ko FY, Li CW, Fann K, Lue JT. 2007. Magnetoreception system in honeybees (Apis mellifera). PLoS ONE 2, e395 ( 10.1371/journal.pone.0000395) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Walker MM, Kirschvink JL, Chang SBR, Dizon AE. 1984. A candidate magnetic sense organ in the yellowfin tuna, Thunnus albacares. Science 224, 751–753. ( 10.1126/science.224.4650.751) [DOI] [PubMed] [Google Scholar]
- 87.Walker MM, Kirschvink JL, Dizon AE. 1985. Magnetoreception and biomineralization of magnetite Fish. In Magnetite biomineralization and magnetoreception in organisms (eds Kirschvink JL, Jones DS, MacFadden BJ), pp. 417–437. New York, NY: Plenum Press. [Google Scholar]
- 88.Kirschvink JL, Walker MM, Chang SB, Dizon AE, Peterson KA. 1985. Chains of single-domain magnetite particles in chinook salmon, Oncorhynchus tshawytscha. J. Comp. Physiol. A 157, 375–381. ( 10.1007/BF00618127) [DOI] [Google Scholar]
- 89.Moore A, Freake SM, Thomas IM. 1990. Magnetic particles in the lateral line of the Atlantic salmon (Salmo salar L.). Phil. Trans. R. Soc. B 329, 11–15. ( 10.2307/76891) [DOI] [Google Scholar]
- 90.Moore A, Riley WD. 2009. Magnetic particles associated with the lateral line of the European eel Anguilla anguilla. J. Fish. Biol. 74, 1629–1634. ( 10.1111/j.1095-8649.2009.02197.x) [DOI] [PubMed] [Google Scholar]
- 91.Walcott C, Gould JL, Kirschvink JL. 1979. Pigeons have magnets. Science 205, 1027–1029. ( 10.1126/science.472725) [DOI] [PubMed] [Google Scholar]
- 92.Winklhofer M, Holtkamp-Rötzler E, Hanzlik M, Fleissner G, Petersen N. 2001. Clusters of superparamagnetic magnetite particles in the upper-beak skin of homing pigeons: evidence of a magnetoreceptor? Eur. J. Min. 13, 659–669. ( 10.1127/0935-1221/2001/0013-0659) [DOI] [Google Scholar]
- 93.Tian L, Xiao B, Lin W, Zhang S, Zhu R, Pan Y. 2007. Testing for the presence of magnetite in the upper-beak skin of homing pigeons. Biometals 20, 197–203. ( 10.1007/s10534-006-9027-x) [DOI] [PubMed] [Google Scholar]
- 94.Beason RC, Nichols JE. 1984. Magnetic orientation and magnetically sensitive material in a transequatorial migratory bird. Nature 309, 151–153. ( 10.1038/309151a0) [DOI] [Google Scholar]
- 95.Kirschvink JL, Kobayashi-Kirschvink A, Woodford BJ. 1992. Magnetite biomineralization in the human brain. Proc. Natl Acad. Sci. USA 89, 7683–7687. ( 10.1073/pnas.89.16.7683) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cranfield CG, Dawe A, Karloukovski V, Dunin-Borkowski RE, de Pomerai D, Dobson J. 2004. Biogenic magnetite in the nematode Caenorhabditis elegans. Biol. Lett. 271(Suppl 6), S436–S439. ( 10.1098/rsbl.2004.0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walker MM, Bitterman ME. 1985. Conditioned responding to magnetic fields by honeybees. J . Comp. Physiol. 157, 67–71. ( 10.1007/BF00611096) [DOI] [Google Scholar]
- 98.Gould JL, Kirschvink JL, Deffeyes KS, Brines ML. 1980. Orientation of demagnetized bees. J. Exp. Biol. 86, 1–8. [Google Scholar]
- 99.Kirschvink JL, Diaz Ricci J, Nesson MH, Kirschvink SJ. 1993. Magnetite-based magnetoreceptors in animals: structural, behavioral, and biophysical studies, 42 Palo Alto, CA: Electric Power Research Institute. [Google Scholar]
- 100.Walker MM, Bitterman ME. 1989. Attached magnets impair magnetic field discrimination by honeybees. J. Exp. Biol. 141, 447–451. [Google Scholar]
- 101.Chobotow J, Strachecka A. 2013. Morphology and function of insect fat bodies taking into account Apis mellifera L. Honey bees. Medycyna Weterynaryjna 69, 712–715. [Google Scholar]
- 102.Locke M, Nichol H. 1992. Iron economy in insects: transport, metabolism, and storage. Annu. Rev. Entomol. 37, 195–215. ( 10.1146/annurev.en.37.010192.001211) [DOI] [Google Scholar]
- 103.Nichol H, Locke M, Kirschvink JL, Walker MM, Nesson MH, Hsu CY, Li CW. 1995. Honeybees and magnetoreception. Science 269, 1888–1890. ( 10.1126/science.269.5232.1888) [DOI] [PubMed] [Google Scholar]
- 104.Fleissner G, Fleissner G, Stahl B, Falkenberg G. 2007. Iron-mineral-based magnetoreception in birds: the stimulus conducting system. J. Ornithol. 148, 643–648. ( 10.1007/s10336-007-0229-y) [DOI] [Google Scholar]
- 105.Lefeldt N, Heyers D, Schneider NL, Engels S, Elbers D, Mouritsen H. 2014. Magnetic field-driven induction of ZENK in the trigeminal system of pigeons (Columba livia). J. R. Soc. Interface 11, 20140777 ( 10.1098/rsif.2014.0777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Michel FM, et al. 2010. Ordered ferrimagnetic form of ferrihydrite reveals links among structure, composition, and magnetism. Proc. Natl Acad. Sci. USA 107, 2787–2792. ( 10.1073/pnas.0910170107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jandacka P, Burda H, Pistora J. 2014. Magnetically induced behaviour of ferritin corpuscles in avian ears: can cuticulosomes function as magnetosomes? J. R. Soc. Interface 12, 20141087 ( 10.1098/rsif.2014.1087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gagliardo A, Ioalè P, Savini M, Wild JM. 2006. Having the nerve to home: trigeminal magnetoreceptor versus olfactory mediation of homing in pigeons. J. Exp. Biol. 209, 2888–2892. ( 10.1242/jeb.02313) [DOI] [PubMed] [Google Scholar]
- 109.Gagliardo A, Loalè P, Savini M, Wild M. 2009. Navigational abilities of adult and experienced homing pigeons deprived of olfactory or trigeminally mediated magnetic information. J. Exp. Biol. 212, 3119–3124. ( 10.1242/jeb.031864) [DOI] [PubMed] [Google Scholar]
- 110.Holland R, Filannino C, Gagliardo A. 2013. A magnetic pulse does not affect homing pigeon navigation: a GPS tracking experiment. J. Exp. Biol. 216, 2192–2200. ( 10.1242/jeb.083543) [DOI] [PubMed] [Google Scholar]
- 111.Alerstam T. 2003. Animal behaviour: the lobster navigators. Nature 421, 27–28. ( 10.1038/421027a) [DOI] [PubMed] [Google Scholar]
- 112.Lohmann KJ, Lohmann CMF. 2006. Sea turtles, lobsters, and oceanic magnetic maps. Mar. Freshw. Behav. Physiol. 39, 49–64. ( 10.1080/10236240600563230) [DOI] [Google Scholar]
- 113.Phillips JB, Borland SC, Freake MJ, Brassart J, Kirschvink JL. 2002. ‘Fixed-axis’ magnetic orientation by an amphibian: non-shoreward-directed compass orientation, misdirected homing or positioning a magnetite-based map detector in a consistent alignment relative to the magnetic field? J. Exp. Biol. 205, 3903–3914. [DOI] [PubMed] [Google Scholar]
- 114.Vidal-Gadea A, et al. 2015. Magnetosensitive neurons mediate geomagnetic orientation in Caenorhabditis elegans. eLife, 4, e07493 ( 10.7554/eLife.07493) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walker MM, Kirschvink JL, Perry A, Dizon AE. 1985. Detection, extraction, and characterization of biogenic magnetite. In Magnetite biomineralization and magnetoreception in organisms (eds Kirschvink JL, Jones DS, MacFadden BJ), pp. 155–166. New York, NY: Plenum Press. [Google Scholar]
- 116.Lowenstam HA, Weiner S. 1989. On biomineralization, 324 p. New York, NY: Oxford University Press. [Google Scholar]
- 117.Teja AS, Koh P-Y. 2009. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Crystal Growth Charact. Mater. 55, 22–45. ( 10.1016/j.pcrysgrow.2008.08.003) [DOI] [Google Scholar]
- 118.Wahajuddin, Arora S. 2012. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 7, 3445–3471. ( 10.2147/IJN.S30320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karl S, Woodward RC, Davis TME, St. Pierre TG. 2010. Manufacture and testing of a high field gradient magnetic fractionation system for quantitative detection of Plasmodium falciparum gametocytes. In AIP Conf. Proc, 25–29 May, Rostock, Germany, pp. 135–140. College Park, MD: American Institute of Physics. [Google Scholar]
- 120.Becker K, Jährling N, Saghafi S, Dodt HU. 2013. Ultramicroscopy: light-sheet-based microscopy for imaging centimeter-sized objects with micrometer resolution. Cold Spring Harb. Protoc. 2013, 704–713. ( 10.1101/pdb.top076539) [DOI] [PubMed] [Google Scholar]
- 121.Vladimirov N, Mu Y, Kawashima T, Bennett DV, Yang CT, Looger LL, Keller PJ, Freeman J, Ahrens MB. 2014. Light-sheet functional imaging in fictively behaving zebrafish. Nat. Methods 11, 883–884. ( 10.1038/nmeth.3040) [DOI] [PubMed] [Google Scholar]
- 122.Taylor A, Herrmann A, Moss D, Sée V, Davies K, Williams SR, Murray P. 2014. Assessing the efficacy of nano- and micro-sized magnetic particles as contrast agents for MRI cell tracking. PLoS ONE 9, 883–884. ( 10.1371/journal.pone.0100259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peddie CJ, Collinson LM. 2014. Exploring the third dimension: volume electron microscopy comes of age. Micron 61, 9–19. ( 10.1016/j.micron.2014.01.009) [DOI] [PubMed] [Google Scholar]