Abstract
Non-verbal communication is the basis of animal interactions. In dyadic leader–follower interactions, leaders master the ability to carve their motor behaviour in order to ‘signal’ their future actions and internal plans while these signals influence the behaviour of follower partners, who automatically tend to imitate the leader even in complementary interactions. Despite their usefulness, signalling and imitation have a biomechanical cost, and it is unclear how this cost–benefits trade-off is managed during repetitive dyadic interactions that present learnable regularities. We studied signalling and imitation dynamics (indexed by movement kinematics) in pairs of leaders and followers during a repetitive, rule-based, joint action. Trial-by-trial Bayesian model comparison was used to evaluate the relation between signalling, imitation and pair performance. The different models incorporate different hypotheses concerning the factors (past interactions versus online movements) influencing the leader's signalling (or follower's imitation) kinematics. This approach showed that (i) leaders' signalling strategy improves future couple performance, (ii) leaders used the history of past interactions to shape their signalling, (iii) followers' imitative behaviour is more strongly affected by the online movement of the leader. This study elucidates the ways online sensorimotor communication help individuals align their task representations and ultimately improves joint action performance.
Keywords: motor interactions, model comparison, sensorimotor communication
1. Introduction
The ability to coordinate in real time and to interact with our conspecifics is key for a number of social practices that range from team games to daily cooperative work. Interpersonal interactions may manifest in different forms ranging from automatic entrainment (i.e. synchronization) to intentional coordination and joint actions where individuals share a representation of the joint goal to be achieved [1]. Ideomotor theories [2–4] propose the idea that (predictive) simulative-like sensorimotor mechanisms such as internal forward models [5–7] support action perception. By extension, the motor coding of observed actions plays a major role in our ability to interact with others and to obtain efficient joint coordination [8]. The proposal is that we are able to predict, monitor and adapt to the behaviour of others by simulating their actions in our sensorimotor system. Experimental evidence has shown that we use the sensorimotor simulation of the movements we observe in others as much as they belong to our motor repertoire [9,10] and that this simulation allows us to code the correctness [11] and predict the fate of others' movement [12]. At a behavioural level, however, these simulations may have detrimental effects on one's own motor execution as these may induce visuomotor interference effects [13,14]. This is particularly true when two partners are engaged in an interaction that requires them to perform two complementary movements (e.g. reaching to the top or to the bottom of an object). Thus, sensorimotor simulation might be necessary to support prediction about the partner's action [15] yet it might result in involuntary imitative behaviours during the interaction.
Similarly to what happens in a conversation, partners engaged in joint actions may resort to a vocabulary of ‘sensorimotor signals’. The use of these signals, however, does not need to be static (as in the case of ritualized gestures) but must be constantly negotiated during the interaction as the individuals build and align their behaviours to a common representation of the task [16]. In this perspective, the asymmetric allocation of information between two partners (e.g. leader–follower) is known to shape the kinematics of their movements [14,17–21]. Role assignment gives the agents the possibility to support coordination by implementing so-called signalling strategies [22,23], for example by systematically making the trajectory of their movements less variable and hence more predictable [14,24,25]. In other words, individuals can signal their future behaviour to their partner by modulating the way they move (e.g. their movement trajectory or speed). At the same time, signalling strategies have costs (e.g. the biomechanical cost required to ‘distort’ the movement kinematics) that have to be compared against their benefits for the interaction (i.e. an increased interaction success). As an example, one may increase the height of his wrist trajectory (individual cost) while reaching the upper part of an object to let the partner understand, and thus adapt to it (thus providing a benefit for the couple), that his movement is not aimed at the lower part of the object. Learning how to minimize these costs makes us able to interact with a tight-knit partner, and these changes should be reflected in modulations of movement features (e.g. systematic changes in signalling and imitation dynamics) as the interaction proceeds based on ‘direct’ implicit sensorimotor learning or on higher-order predictions such as those based on ‘sequence-rule’ learning. This hypothesis implies that the signalling-and-decoding strategies adopted by dyads should change when the interaction is repeated in time and task regularities can be learned.
To test this hypothesis, we studied and modelled the kinematics of leaders' signalling and followers' imitative behaviour during repetitive, rule-based, complementary motor interactions. Pairs of leaders and followers, facing each other, were asked to reach and grasp as synchronously as possible two objects resembling the shape of a bottle by performing complementary movements (i.e. leader to the top of the bottle, follower to its bottom; figure 1). Given the geometrical structure of the present experimental set-up, leaders' signalling is indexed as an increase of their wrist maximum height during the reaching phase to the upper part of the bottle or its reduction when reaching the lower part (i.e. an increase of trajectory curvature) during interactive sessions with respect to baseline behaviour (i.e. when acting alone). In other words, leaders might emphasize the curvature of their reaching trajectory to provide the followers with additional cues and thus enable followers to predict where they would grasp the bottle. This implies leaders have to modify their reaching pattern which thus represent a ‘cost’ for them (i.e. they have to modify their movement planning and execution) but might benefit the pair performance. Conversely, given the assignment to perform opposite movements with respect to those of their partners, followers' automatic tendency to imitate the leaders is indexed by the tendency to increase their wrist maximum height during the reaching phase to the lower part of the bottle or its reduction when reaching the upper part during interactive sessions with respect to when acting alone: indeed, followers might involuntarily imitate the leader's trajectory and follow, for instance, a higher trajectory while grasping the lower part of the bottle, because they are observing the leader grasping the higher part of the bottle (i.e. they might be influenced by the leader's complementary movement kinematics). First, we show and analyse performance and kinematics indexes associated with signalling and imitation (behavioural and kinematics results). Second, we show a trial-by-trial, Bayesian model-based analysis that compares alternative hypotheses on what factors modulate the relation between signalling, imitative behaviour and pairs' performance during the task. We compare six alternative models (M1–M6) that describe signalling and imitative dynamics as being uniform (M1), modulated by task history and previous performance (M2–M4), task structure (M5) or online information and movement kinematics (M6). The results of the Bayesian model comparison are intended to shed light on the nature of signalling and imitative strategies of leaders and followers, respectively, and their relation to pair performance in order to assess whether they are essentially fixed (in the sense that they are not modulated by the task, i.e. M1), strategic (in the sense that participants proactively modulate them based on their knowledge of what happened in previous trials, i.e. M2–M5), or reactive (in the sense that participants adapt them based on the online information they receive in the current trial, i.e. M6).
Figure 1.
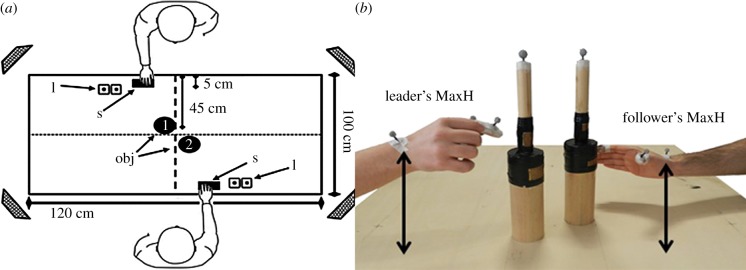
Experimental set-up (a). The letter ‘l’ indicates the LEDs and the letter ‘s’ indicates the starting position. (b) An example trial. (Online version in colour.)
2. Methods
2.1. Participants
Thirty right-handed participants [26] (two males, age 23.5 ± 2.45) took part in the experiment and were randomly assigned to 15 same-gender pairs. All participants reported normal or corrected-to-normal vision and were naive as to the purpose of the experiment. The experimental protocol was approved by the ethics committee of the Fondazione Santa Lucia and was carried out in accordance with the ethical standards of the 1964 Declaration of Helsinki. Participants gave their written informed consent to take part in the study, received reimbursement for their participation and were debriefed on the purpose of the experiment at the end of the experimental procedure.
2.2. Experimental set-up
Paired participants were seated opposite to each other in front of the work surface. Each participant had to reach and grasp the bottle-shaped object composed of two superimposed cylinders of different diameters (small, 2.5 cm; large, 7.0 cm) placed 45 cm in front of him and 5 cm to the right of the midline (figure 1). Auditory instructions concerning the movement to be performed were given simultaneously to both participants via headphones. The possible instructions were three sounds with the same intensity (4.5 dB) and duration (200 ms), but different frequencies: (i) ‘high-pitch’, (ii) ‘low-pitch’, (iii) ‘whistle’. In order to record the participants' touch-time on the bottle, two pairs of touch-sensitive copper plates were placed at 15 and 23 cm along the vertical length of both objects measured from the base. The arm and hand kinematics of each participant were recorded using a SMART-D motion capture system (Bioengineering Technology & System, BTS). Four infrared cameras, with wide-angle lenses (sampling rate 100 Hz), placed about 100 cm away from each of the four corners of the table, captured the movement of the markers in three-dimensional space. The standard deviation of the reconstruction of error was always lower than 0.5 mm for the three axes. Three infrared reflective markers (5 mm) were attached to the participants' right upper limb at the following points: (i) wrist, dorsodistal aspect of the radial styloid process, (ii) thumb, ulnar side of the nail, (iii) index finger, radial side of the nail.
2.3. Procedure
The overall structure of the experiment was as follows: baseline1 (24 trials), test1 (72 trials), learning 1, 2, 3, 4, 5 (72 trials for each session = 360 trials), test2 (72 trials identical to test1), baseline2 (24 trials), leading to a total of 552 trials (2 h experiment; figure 2). During the whole experiment, participants were asked not to verbally communicate and to grasp their bottle-shaped object as synchronously as possible with their partner according to specific instructions that are described below.
Figure 2.
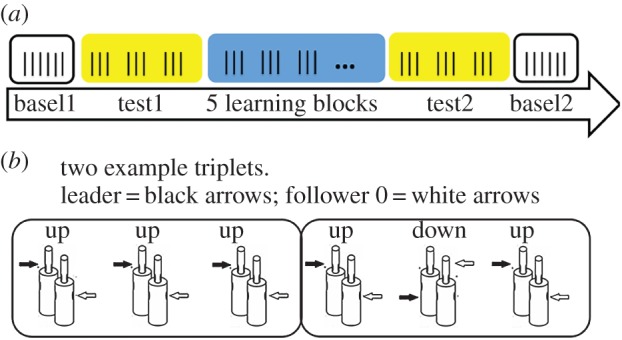
Experimental structure (a), and two example triplets (b). The left rectangle in b shows a triplet where the leader (black arrow) aimed three times in a row at the higher part of the bottle while the follower (white arrow) aimed at the lower part of the bottle; the right rectangle shows a triplet where the leader (black arrow) aimed at the top, bottom and top of the bottle while the follower (white arrow) executed down, up, down movements. (Online version in colour.)
2.3.1. Baseline1 and 2
The first baseline session (baseline1) was repeated at the very end of the experiment (baseline2) in order to investigate overall improvement in synchrony unrelated to the learning of the rules of the interaction. Participants received opposite instructions, implying the execution of complementary movements (i.e. opposite movement with respect to the partner's). The instructions could either be (i) a high-pitch sound, meaning ‘grasp the upper part of the object’ (up), or (ii) a low-pitch sound, meaning ‘grasp the lower part of the object’ (down). Presentation and randomization of instructions was controlled by E-Prime v. 2 software (Psychology Software Tools, Inc., Pittsburgh, PA, USA).
2.3.2. Test1 and 2
After the first baseline session, leader–follower roles were randomly assigned to the two participants. The sequence of instructions (triplets) provided to participants was identical in test1 and test2, allowing to compare pairs' performance in these two sessions and to test the presence of implicit sequence learning achieved during the learning sessions (see below).
Leader instructions specified that in the subsequent blocks, the trials would be grouped in sequences of three movements (triplets) to be executed according to two possible rules (i.e. four different sequences): (i) three identical movements (e.g. up–up–up) or (ii) three alternate movements (e.g. up–down–up; figure 2). Leaders were told that the follower would be unaware of these rules. The rationale behind the rules is that, based on the first two trials of the triplet, the follower might become able to predict where the leader will grasp the object in the third movement.
Follower instructions specified that in the subsequent blocks the participant would hear only a GO signal (whistle), and that he/she was required to coordinate with the partner by performing complementary movements.
2.3.3. Learning sessions 1, 2, 3, 4, 5
These sessions were performed between test1 and test2. Each of the learning sessions comprised 72 trials grouped in randomized triplets (in a different order from that of test1 and 2 which were instead identical between them so as to be comparable) according to the same procedure of the test1/2.
2.3.4. Visual analogue scale judgements
At the end of sessions test1/2, leaders were asked to judge how well the follower seemed to understand the sequences of movements, and followers were asked to judge how predictable were the movements of their leader, through a visual analogue scale (VAS, vertical 10 cm line, 0 = ‘lowest’, 100 = ‘highest’).
2.4. Data processing
Only correct trials were analysed (i.e. when both participants followed their individual instructions). All trials in which participants started their movement before hearing the auditory instruction (false start) or did not execute the instruction correctly were excluded as incorrect trials (mean number of excluded trials per couple = 8; number of trials excluded in total, 6%).
For each trial, we considered two parameters:
(1) Pairs' performance behavioural index: grasping asynchrony (ms): (i.e. the absolute value of the time delay between subjects' index–thumb contact times on their bottle [abs (sbjA's contact time on the bottle–sbjB's contact time on the bottle)] where the contact time is defined as the time from the auditory instruction to the instant of each participant's index–thumb contact on the bottle) (RTs, starting asynchrony and their standard deviations in electronic supplementary material, S1 text).
(2) Kinematic index: maximum wrist height (MaxH) (mm): (max index–finger aperture in electronic supplementary material, S1 text).
MaxH describes the wrist trajectory of participants (reaching component of the movement) and is indexed by the maximum peak of wrist height on the vertical plane from the level of the table (figure 3).
Figure 3.
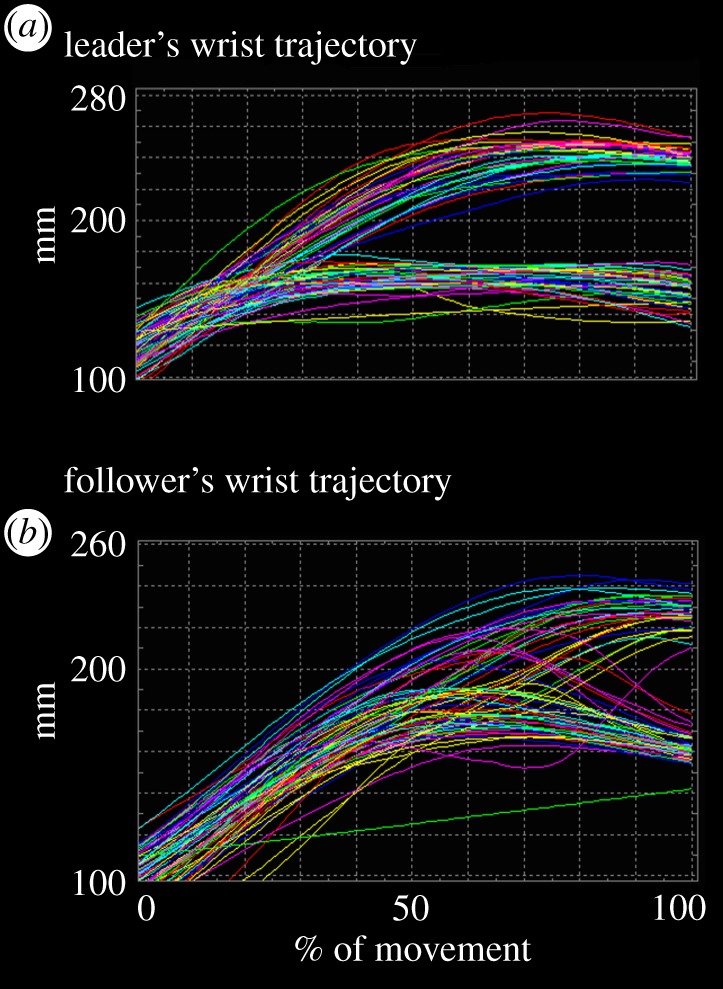
Wrist trajectory of an example leader (a) and follower (b). Millimetres on the ordinate, and percentage of movement duration on the abscissa. (Online version in colour.)
Each behavioural and kinematic value that fell 2.5 standard deviations (s.d.) above or below each individual mean for each experimental condition was excluded as an outlier value (mean number of outliers for subject = 2.26; 2% of the total). No participant exhibited behavioural or kinematics values 2.5 s.d. above or below the group mean.
2.5. Data analysis
2.5.1. Baseline analyses
In order to test whether individuals improved their ability to interact (indexed by pairs' grasping asynchrony), regardless the presence of any rule, baseline analyses were performed by means of repeated measure ANOVAs on grasping asynchrony with session (base1/base2), and by means of repeated measure ANOVAs with session (base1/base2) × movement type (up/down grasping) as within subjects factors for the kinematic index (MaxH).
2.5.2. Learning analysis
In order to test whether leader–follower pairs learned to improve their pair performance during the learning sessions (i.e. reduce their grasping asynchrony), we performed an ANOVA with factors session (test1/L1/L2/L3/L4/L5/test2) × trial (1/2/3) on the grasping asynchrony. All data were normalized (divided) on the mean of the two baselines so to get an index of pair performance with respect to baseline behaviour.
2.5.3. Test analyses
In order to test whether the presence of signalling and interference was modulated owing to the individuals' learning, kinematic data of the test1/2 sessions were normalized (divided) on the mean of the two baselines and analyses were run only on the test1/2 sessions: mixed-model ANOVA with session (test1/test2) × trial (1/2/3) × movement type (up/down grasping) as within subjects factors and group (leader/follower) as between subjects factor. All tests of significance were based upon an α level of 0.05. Significant interactions and main effects were further analysed performing post hoc tests using the Newman–Keuls method.
Importantly, here we interpret increases in the curvature of the reaching trajectory as an index of signalling in leaders, i.e. a higher MaxH when grasping the upper part of the bottle and a lower MaxH when grasping the lower part of the bottle, when compared with kinematics in the baseline condition: indeed, this would indicate leaders emphasize the curvature of their reaching trajectory to provide the followers with additional cues about their goal and thus enable them to adapt and program where to grasp their own bottle. Similarly, we interpreted as an index of automatic imitation in followers any change in their wrist trajectory on the vertical plane (when compared with baseline condition) which indicates the followers' wrist trajectory is influenced by the leaders' one, i.e. for instance, a higher MaxH when grasping the lower part of the bottle when interacting with a leader who was grasping the upper part of the bottle: indeed, this might indicate followers have been attracted by the leader's complementary movement kinematics. Crucially, this rationale guided the interpretation of both kinematic data analyses and model analyses.
2.5.4. Model analyses
We considered six models (M1–M6) for a model-based trial-by-trial statistical analysis [27]. With MaxHt, we denote the max height of wrist of the subject during the trial t (the same parameter was used for leaders' signalling kinematics and followers' imitative kinematics, respectively). With CoactMaxHt we denote the max height of the co-actor's wrist at trial t (this measure is only used for M6). Prior to the Bayesian model comparison, we executed a logistic regression of the parameters for models M2–M6. For the leaders' signalling, we first distinguished data from the baseline sessions and all the experimental sessions by labelling them using the labels 0 or 1, respectively. The assumption that signalling is present only in the experimental sessions is in keeping with the results reported in section ‘Leader–follower effects’. Analogously, for the followers' imitation, we first distinguished data into baseline sessions and experimental trials 3 (with label 0) and experimental trials 1 and 2 (with label 1).
We thus regressed the distribution (equation (2.1)):
| 2.1 |
where P(Lt) denotes the probability of signalling (in the case of leaders) or imitation (in the case of followers); c0 and c1 are the parameters of the regression curves, different for each subject (leader in the case of signalling, follower in the case of imitation).
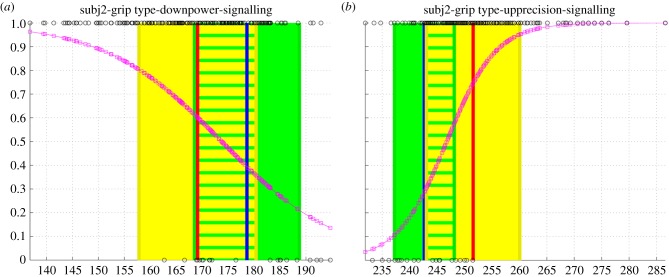
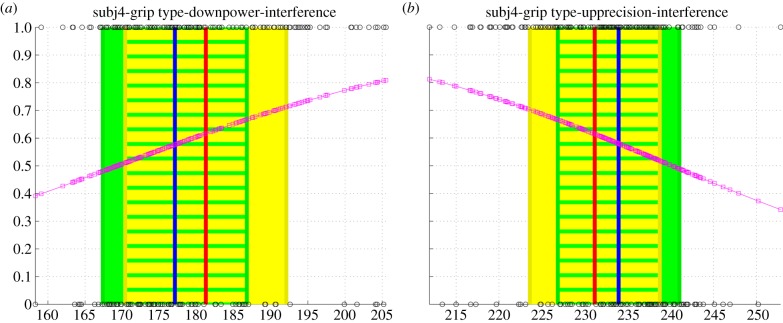
Figures 4 and 5 show two examples of the resulting distributions: the signalling distribution of the leader of pair 2 and the imitation distribution of the follower of pair 4, respectively. Electronic supplementary material, S1 text shows the distributions for all pairs.
Figure 4.
(a,b) Probability of signalling P(Lt|MaxHt) of the leader of couple 2 given his max height of the wrist, MaxHt. Probabilities are on the ordinate, values of MaxHt are on the abscissa. (Online version in colour.)
Figure 5.
(a,b) Probability of imitation P(Lt|MaxHt) of the follower of couple 4 given his max height of the wrist, MaxHt. Probabilities are on the ordinate, values of MaxHt are on the abscissa. (Online version in colour.)
Figure 4 shows the probability P(Lt|MaxHt) of signalling (Lt) of an example subject (the leader of the couple 2) given his max height of the wrist, MaxHt. Figure 4a shows the results for power (down) grasps, whereas figure 4b shows the results for precision (up) grasps of each trial of the same subject. The procedure for calculating the probability P(Lt|MaxHt) is as follows. First, the MaxHt measured in each trial is assigned a value of 0 on the ordinate axis if the trial belongs to the baseline (label = 0) or a value of 1 if it belongs to an experimental trial (label = 1). These label values are shown as circles in figure 4. Second, a logistic regression is performed on those label values in order to capture the best parameters of P(Lt|MaxHt), following equation (2.1). The resulting probability distribution P(Lt|MaxHt) is shown as the curve passing through values denoted with squares (each square corresponds to the value of P(Lt|MaxHt) for each trial, given its corresponding MaxHt). As explained later, this probability distribution is used by the models M2–M6, but not the model M1. Figure 4 shows also the mean value of MaxHt in the baseline (right vertical line in panel (a) and left vertical line in panel (b)) and the mean value of the MaxHt in the experimental trials (left vertical line in panel (a) and right vertical line in panel (b)), together with their variance.
Figure 5 shows the probability of imitation P(Lt|MaxHt) of an example subject (the follower of the couple 4) given his max height of the wrist, MaxHt, using the same notation as for figure 4. The left panel shows the results for power grasps, whereas the right panel shows the results for precision grasps of each trial of the same subject. Here, different to figure 4, the MaxHt of each trial is assigned a label of 0 if the trial belongs to the baseline trial or to experimental trials 3, or a label of 1 if it belongs to experimental trials 1 or 2. Similar to figure 4, a logistic regression is then performed on these labels, whose resulting values are shown in figure 5 (squares). Figure 5 also shows the mean value of MaxHt in the baseline trials and on experimental trials 3 (left vertical line in panel (a) and right vertical line in panel (b)) and the mean value of MaxHt in the experimental trials 1 and 2, (right vertical line in panel (a) and left vertical line in panel (b) together with their variance.
We next evaluated the resulting models by computing the model evidence, i.e. probability of reconstructing dataset D given the ith model Mi, P(D|Mi). We use Bayesian information criterion (BIC) to approximate the evidence [28] (equation (2.2)):
| 2.2 |
where n denotes the number of elements in the dataset D, m the number of model parameters, and θMi the parameters that are optimized to maximize the likelihood P(Mi|D,θMi) (table 1).
Table 1.
Description of the models, which includes the model name, the mathematical formulation of the conditional probability for the different models, and the model parameters. It is possible to note that the models have a different number of parameters; the BIC method allows them to be compared on equal grounds [28].
| model name | probability formulation | parameters |
|---|---|---|
| M1 | 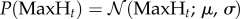 |
μ, σ |
| M2 | 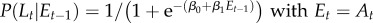 |
β0, β1 |
| M3 | 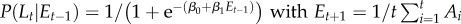 |
β0, β1 |
| M4 | 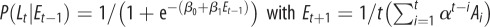 |
α, β0, β1 |
| M5 | 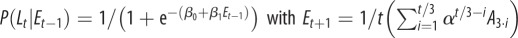 |
α, β0, β1 |
| M6 | 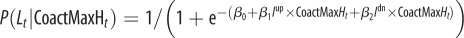 |
β0, β1, β2 |
| M6 | 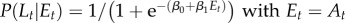 |
β0, β1 |
Model M1 assumes that the probability of signalling (or imitation) Lt does not change during the trials, but is sampled from a normal distribution (mean and variance are computed separated on trials with different labels and different grasp types). In the models M2–M5, the probability of signalling (or imitation) Lt depends probabilistically on an ‘error’ term Et. In turn, this error term depends on a variable At (asynchrony) that is calculated differently for each model, reflecting their underlying hypothesis on what causes signalling (or imitation). In M2, the error term depends on the asynchrony in the previous trial (more formally, Et −1 corresponds to the value At −1, thus this model takes into account only the variable At −1 at trial t−1). In M3, the error term includes the asynchrony values of all the previous trials. In M4, the variable Et considers a fading window of previous At values, with the amplitude of the window regulated by a decay parameter α. M5 is analogous to M4, but the fading window takes into account only the third trials of each triplet of the experimental sessions. In M6, the conditioning variable is not an error term but instead CoactMaxHt, and in the definition of the conditional probability depends on an extra index I that encodes movements directed to the top Iup or bottom Idn. The impact of this index is treated separately for precision grip trials (where Iup = 1 and Idn = 0) and power grasp trials (where Iup = 0 and Idn = 1) reflecting the fact that precision grips are aimed at the top, whereas power grasps are aimed to the bottom. While we used M6 for the analysis of signalling and imitation performance, we used instead a separate model M6 for the analysis of how signalling affects the pairs' performance. This model M6 takes into account the value of the variable At at the same trial t of Lt (table 2).
Table 2.
Meaning of the variables in the different analyses. In the analysis of leaders' signalling behaviour the variable Lt represents the signalling of the leaders and the variable At represents the grasping asynchrony. In the analysis of followers' imitation behaviour the variable Lt represents the imitation of the followers and the variable At represents the grasping asynchrony. In the analysis of pairs' performance the variable Lt represents the performance (grasping asynchrony thresholded and rescaled between 0 and 1) and the variable At represents the signalling of the Leaders.
| analysis name | leaders' signalling behaviour | followers' interference behaviour | pairs' performance behaviour |
|---|---|---|---|
| variable Lt | signalling | interference | performance |
| variable At | grasping asynchrony | grasping asynchrony | signalling |
| variable MaxHt | max height of the leaders' wrist | max height of the followers' wrist | max height of the leaders' wrist |
| variable CoactMaxHt | max height of the followers' wrist | max height of the leaders' wrist | max height of the followers' wrist |
3. Results
3.1. Behavioural results: grasping asynchrony
3.1.1. Baselines (no leader and follower role)
Results showed no significant main effect of baseline session (baseline1 versus baseline2) [F(1,14) = 1.130, p = 0.306], indicating participants achieved the same level of performance in baseline1 and baseline2. Thus, we averaged the two sessions and used their mean to normalize (i.e. divide) the data from test and learning sessions. By doing so, we are able to test whether pairs increased their ability to synchronize owing to the implicit learning of the rules.
3.1.2. Learning to interact
The session (test1/L1/L2/L3/L4/L5/test2) × trial (1/2/3) ANOVA on grasping asynchrony (note that the factor group (leader/follower) is missing in this analysis as grasping asynchrony represents a couple index) failed to show a significant main effect of trial [F(2,28) = 2.399, p = 0.109] while it highlighted a significant main effect of session [F(6,84) = 3.78, p = 0.002], suggesting the presence of a learning effect throughout the experiment. Post hoc test showed grasping asynchrony in test1 was significantly higher (i.e. pairs were less synchronous, indicating poorer performance) when compared with the other sessions (all ps < 0.03; figure 6). This effect was not modulated by trial (session × trial interaction, p = 0.717).
Figure 6.
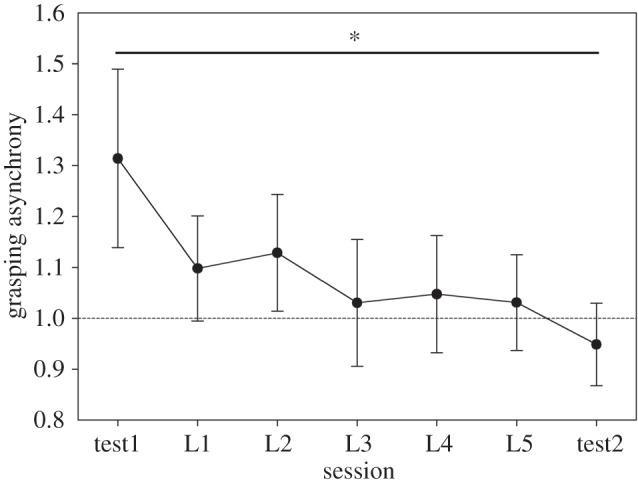
Results of the grasping asynchrony. The graph shows the reduction of pairs' grasping asynchrony (i.e. their increase in the ability to be synchronous) along the different sessions of the experiment (baseline = value 1). Normalized means ± s.e. mean.
3.1.3. Test1/2
In order to directly compare the pair performance at the beginning of the interaction and after having interacted in the learning sessions, we performed a test session (test1/test2) × trial ANOVA on grasping asynchrony. This analysis showed a main effect of test session [F(1,14) = 5.898, p = 0.029] indicating that the pair performance improved overall in the second test session. The main effect of trial did not reach statistical significance [F(2,28) = 1.541, p = 0.232]. The test session × trial interaction was not significant (p = 0.287) showing that the reduction of grasping asynchrony generalized to all trials of the triplets to the same extent.
Given our specific hypotheses of signalling and imitation regarded the comparison between test1 and test2, the kinematic analyses were performed only on these two sessions.
3.2. Kinematics results
3.2.1. Baseline (no leader and follower role)
The baseline session (base1/base2) × movement type (up/down grip) ANOVA on MaxH failed to show a significant main effect of baseline session [F(1,29) = 2.098, p = 0.158]. Thus, as for the grasping asynchrony, data from the two baselines were pooled together in order to normalize (i.e. divide) the data from the test sessions and be able to compare the kinematics of learned interactions with those of a baseline condition controlling for the peculiar grasping kinematics of each participants measured at baseline (when no imitation or signalling is expected).
3.2.2. Normalized tests
We first describe the kinematic results that do not show a group effect, and then we describe results involving the interactional role.
3.2.3. Motor effects
The test session (test1/test2) × movement type (up/down grip) × trial (1/2/3) × group (leader/follower) mixed-model ANOVA on MaxH showed a significant main effect of trial [F(2,56) = 4.72, p = 0.012], indicating that only during the third trial of the triplets individuals showed a smaller wrist height with respect to the first trial (p = 0.009) but not with respect to the second trial (all ps > 0.079). The main effects of group, test session and movement type were not significant (all ps > 0.074). This analysis highlighted also a significant trial × movement type interaction [F(2,56) = 9.28, p < 0.001], where the first trial of the sequence, during down grips, was significantly higher than in all the other trials (all ps < 0.041). This effect was further specified by the third-order interaction with the factor group (see below).
3.2.4. Leader–follower effects
MaxH showed a significant movement type × group interaction [F(1,28) = 40.197, p < 0.001], indicating leaders emphasized their movements by increasing their MaxH when compared with followers when grasping the upper part of the bottle (p = 0.003) and by decreasing it when grasping the lower part of the bottle (p < 0.001). This shows leaders implemented a signalling strategy.
The significant trial × movement type × group interaction [F(2,56) = 8.51, p < 0.001] further specified the second-order interaction showing a reduction of followers' MaxH during down grips in the second and third trial when compared with the first one (p = 0.011 and p < 0.001, respectively) and third trial when compared with the second (p = 0.008). The interaction shows that the imitation effect was higher in the first trials of the triplets while it started to decrease in the second trial and was most reduced in the third trial probably based on the fact followers became able to predict where the leader would grasp the object and were thus less prone to visuomotor interference (figure 7).
Figure 7.
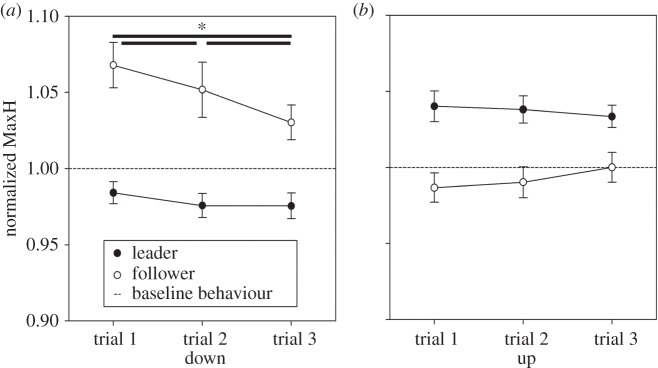
Results of the MaxH. The graph shows the modulation amplitude of wrist MaxH with respect to the baseline (value 1) for leaders and followers in the three trials of the triplets in up and down grips of test1/2. Normalized means ± s.e. mean.
We also examined whether the mean value of MaxH was significantly different from baseline behaviour by means of single-sample t-tests against 1. Leaders' MaxH was significantly higher than baseline (significant p value corrected for 12 comparisons is p = 0.004) during all three trials in up grips (all ps < 0.001), while it did not differ from 1 during down grips (all ps > 0.009). Followers' MaxH in the three trials was significantly higher than baseline only during the first trial of the triplets in down grips (p < 0.001, all other ps > 0.013; corrected significance level p = 0.004).
These results suggest that leaders increased their signalling strategy by modulating their MaxH during all three trials of the triplets, especially when performing precision grips, whereas followers tended to imitate leaders' complementary movements during the first trial of the triples in power grips.
3.2.5. Implicit sequence learning
Leaders evaluated that their follower learned the rule of the triplets at the same level after test1 and test2 (T(14) = 0.154, p = 0.879) thus indicating that they were unaware of the actual comprehension of the rules by the followers. Followers declared they did not note whether there was any rule at the base of the movements of the leader after test2 with respect to test1 (T(14) = 0.1816, p = 0.859).
3.3. Models
We performed a model-based trial-by-trial statistical analysis [27] to compare different explicatory hypotheses on leaders' signalling kinematics and followers' imitation. We designed six models that incorporate different hypotheses on which variables modulate the leaders' and followers' kinematics (generating signalling and imitation, respectively) and used BIC to compare them [28]. The models are designed to study which features of the interaction (past pair performance or online motor behaviour) explain the probability of leaders to signal and followers to imitate the leaders' movements. In this analysis, we considered MaxHt (the max height of wrist of the subjects at trial t) as the kinematic index for signalling and imitation (see Methods section).
Figure 8 shows the model comparison results for leaders' signalling behaviour. Figure 8a shows the BIC score of each hypothesis (M1–M6) for each of the 15 leaders in the experiment. Figure 8b shows the aggregate results, calculated by averaging the performance of the hypotheses (M1–M6) shown in figure 8a. In these two panels, for better readability, results are shown in comparison with M1, which is considered to be the worse one thus representing the value of 100%. Here, smaller values indicate a better BIC score and thus better performance. Figure 8c, instead, shows the best explanatory hypothesis, calculated by first counting how many times a given hypothesis (M1–M6) is the best explanation of the behaviour of each of the leaders, and then normalizing to 100% the number of leaders whose behaviour is best explained by each model. In this case, thus, values are a percentage, and so higher values indicate better performance.
Figure 8.
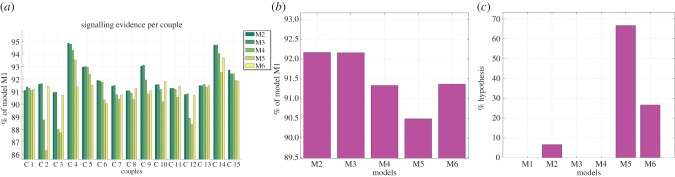
(a–c) Model-based analysis of signalling. See main text for explanation. (Online version in colour.)
Despite the variability in the subjects' performance (a), a quite clear pattern emerges in the analysis of leaders' signalling strategies. The model incorporating the task structure (M5) is the best explanation for most subjects. Models M4–M5, incorporating the history of past trials, are the best explanation on average; by comparing figure 8b,c, it is possible to note that M4 is never the best explanation, still its average score is good (ranking second in most comparisons). This suggests that leaders use information of past trials (and particularly of the most informative ones: the third trials of each triplet) to modulate strategically their signalling kinematics, and specifically to reduce the amount of signalling as the grasping asynchrony in those trials decreases, which is coherent with the notion that signalling has a motor cost [22]. The fact that models M4–M5 (which consider in various ways the history of past trials) explain the data better than M6 (which uses an information available during the online interaction) suggests a strategic modulation of the leaders' kinematics that depends on the performance of the couple over the experiment rather than only on the current trial.
Figure 9 shows the model comparison results for followers' imitative behaviour; the panels are the same as for figure 8. Here, the best strategy on average is M6, but M4 and M5 are also good explanations in many cases. The fact that here (differently from the case of signalling) M6 is a good explanation suggests that followers consistently use the cues offered by the leader during the online interaction, rather than (only) the history of previous trials. This result thus supports the idea that followers' behaviour is more reactive (or less strategic) compared with leaders'.
Figure 9.
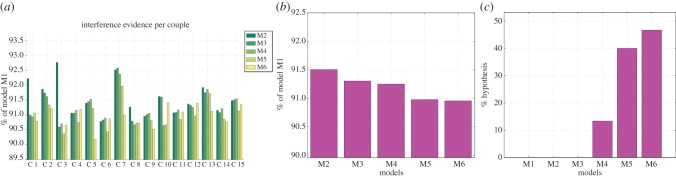
(a–c) Model-based analysis of imitation. See main text for explanation. (Online version in colour.)
The first important result that emerges from our model-based analysis is that neither for signalling nor for imitation is M1 a good explanation. This implies that signalling and imitation dynamics are modulated by contextual factors rather than being randomly expressed during the experiment. The second important result is that the structure of the task and the history of previous interactions are the best predictors of leaders' behaviour while information that is available online is the best predictor of followers' behaviour. This pattern of results indicates that leaders' behaviour is significantly more strategic than followers', pointing to an error-correction mechanism that considers task- and history-related information.
The model-based analyses performed so far show that the leaders' signalling kinematics are well explained by the pairs' performance in the previous trials. Our next analysis tests the hypothesis that the reverse relation holds too, and that the leaders' signalling strategy has (positive) effects on pairs' performance. We designed six models that are analogous to the previous ones except that they test the (probabilistic) relation between signalling and pairs' performance rather than vice versa as done before. Specifically, M1 tests the hypothesis that pairs' performance in a given trial is not related to the leaders' signalling strategy and is instead better modelled as a normal distribution. All the remaining models incorporate the hypothesis that signalling increases pairs' performance but differ in what signalling strategy they hypothesize to be more efficacious. M2–M5 test the hypotheses that pairs' performance in a given trial depends probabilistically on leaders' signalling behaviour in the other experimental trials (only one trial for M2; all the experimental trials for M3; a window of k previous trials for M4 and the third trials of each triplet for M5), whereas M6 tests the hypothesis that pairs' performance in a given trial depends probabilistically on leaders' signalling behaviour in the same trial. In this analysis, pairs' performance is measured in terms of grasping asynchrony, and leaders' signalling is measured in terms of MaxHt.
Figure 10 shows the results of the model-based comparison; the panels are the same as for figure 6. The first result of this analysis is that, for all leaders, signalling increases pair performance (for one couple, C9, the increase is reduced). The second result of this analysis is that various signalling strategies perform similarly, but M5 is the best predictor of pairs' performance. Interestingly, our previous analyses (figure 8) have shown that most leaders use a signalling strategy that is well captured by M5, suggesting that they implicitly select an adaptive (if not optimal) way to convey information to their co-actors.
Figure 10.
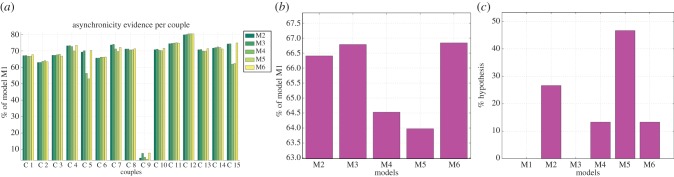
(a–c) Model-based analysis of grasping asynchrony. See main text for explanation. (Online version in colour.)
4. Discussion
We studied how pairs of leaders and followers shape the kinematic features of their movements when engaged in repetitive joint actions that are based on sequence rules. On the one hand, we found that followers' implicit learning of the rules reduces their tendency to imitate their leader (reduction of visuomotor interference). These results suggest that when followers have low uncertainty on their own motor plan and need not to infer it from leaders' behaviour, interferential visuomotor imitation [13] does not emerge [14]. On the other hand, we show that leaders tend to signal their motor intentions via their kinematics adopting a signalling strategy. Trial-by-trial mathematical models specify that leaders' signalling dynamics flexibly change according to pair performance history. This result is compatible with the hypothesis that leaders maintain an estimate of the pair's performance and use cost–benefit criteria to decide when to use signalling to improve performance [22,23]. Rather, the same trial-by-trial analysis shows that followers mainly rely on online movement cues offered by leaders, at least in this experimental setting where task rules were not explicit. The benefit of this dyadic strategy can be appreciated by finding that leaders' signalling behaviour boosts couple performance in a positive way: the more they signal, the more the couple achieves synchrony. Taken together, these results show that repetitive joint actions with asymmetric information induce sophisticated forms of ‘sensorimotor communication’ and suggest that leaders and followers tend to use past interactions in different ways in order to control their behaviour, which in turn results in a difference between a more ‘strategic’ behaviour of leaders and a more ‘reactive’ behaviour of followers. Below, we discuss these differences—and their putative neuronal underpinnings—in more detail.
4.1. Leaders' signalling—past interactions shape leaders' signalling
We unconsciously pre-shape our hands configuration when reaching objects of different sizes. Similarly, it has been shown that the mere presence of another (passive) individual changes basic features of our reaching movement [18]. These kinematic cues are crucial when we interact with others because they might provide our partner with information concerning our intentions and goals [29–31]. We already showed that the asymmetric allocation of information concerning an action's goal is reflected in the way we perform that action and thus communicate with our partners during motor interactions [14]. Thus, the kinematics of our movements are not resistant to higher-order plans such as the representation we hold of the overall goal of an action [32,33]. During motor interactions, we tend to build a representation of what we think our partner will do based on the information we have and those we are able to infer from his behaviour [34]. Along the interaction, in case information concerning the structure of the sequences of interactions is available to only one member of the pair, people may align their representation of the task and use this cognitive framework to strategically change their behaviour even when the representation is outside conscious control ([8,34–37]; see also [38]).
Here, we expand this notion by showing that the modulation of our kinematic features is influenced by what we expect the other knows based on the efficacy of past interactions we had with him and on his online behaviour. The results of the model comparison analysis are coherent with the idea that leaders maintain a representation of the shared task (and of the dyad performance) in order to change their signalling. Conversely, followers tend to base their imitative behaviour upon online cues of the leaders' movements.
4.2. Followers' implicit predictions shape their tendency to imitate the leader
Imitation of observed actions is evident under a number of conditions (automatic motor priming, [39]; visuomotor interference, [13,40]) and is thought to be mostly automatic [3,41]. In a recent experiment applying the same scenario we used here [14], it has been shown that this imitative interference might be captured also in interactive situations and that it has a detrimental effect on pairs' coordination. Even more striking is that individuals tend to imitate each other also during competitive interactions where imitating our opponent is completely against the achievement of the individual goal [42,43]. It is suggested that imitation might be supported by internal sensorimotor models [5,6,44] that might provide the observer with the ability to anticipate the others' movements and their outcomes [12,15]. Along these lines, it is possible to speculate that followers' imitative behaviour is a by-product of their attempts to estimate the leaders' actions using the same internal models as those involved in their own action execution. In our task, followers need to estimate leaders' actions to select their own actions. When they have sufficient information to act (e.g. in the third elements of triplets), they do not use their internal models to estimate the leaders' movements (or use them to a lesser extent), and this, in turn, would reduce automatic imitation. At a neural level, it has been proposed that action observation–execution effects might be based on the activity of cells in frontoparietal regions (mirror neurons, [45–48]) that seem to transform visual information about others' actions into a motor code. Seminal studies on the neural correlates of motor interactions suggested this frontoparietal system might be at play also in this condition [49]. More recently, it has been proposed that joint interactions are based on the activity of larger neural networks comprising frontoparietal regions within and outside the classical action observation system [50] under the idea that, for example, parietal regions may integrate one's own and others' motor goals in a stable joint goal [51–55]. This ‘integration’ in a joint goal might be crucial to interpret the pattern of kinematic results in this study: indeed, one might suggest that because leaders' signalling manifest as an emphasis in the curvature of movement trajectory, a ‘signalling’ movement is also more interfering because it implies more ‘extreme’ movement features. Instead, when the follower becomes able to ‘integrate’ the leader's movement into his/her own motor plan (e.g. because he/she has understood the rules behind movement sequence), the leader's movements become predictable and they are thus not interfering anymore.
4.3. The costs of non-verbal communication within social interactions
It is now well established that kinematic laws of motion exist (for example Fitts's law [56], two-third power law [57]) and it is thought that performance of different movements might be described at the muscular, kinematic and neural level as the combination of a limited number of motor primitives [58]. The laws governing these primitives are constrained by the structure of the muscular system, and the neural systems that control our movements where motor synergies seem to play a major role [59]. Several computational motor control theories incorporate a notion of motor cost in one way or another. For example, the objective of optimal control is providing control signals that prescribe trajectories that are optimal in relation to some cost function [60]. In active inference, costs (and value functions) are absorbed in the (Bayesian) priors or the desired states of the environment that an agent tries to achieve through action [61]. Importantly, these costs can be of various kinds, from movement error to motor effort (which often interact, [62]).
Here, in its most simplified form, we can define as ‘cost’ any violation of stereotypical movement patterns (e.g. a default grasping trajectory), which determines increased demands at the motor/biomechanical level (e.g. to execute a less optimal trajectory) as well as at the cognitive/planning level (e.g. to plan a novel trajectory). In an optimization perspective, the increased cost can be justified in terms of extra requirements, e.g. not only reaching and grasping an object, but also making one's own movement more communicative [22,63]. Once we recognize that our movements have a social dimension, it becomes clear that (virtually any) movement might achieve combined pragmatic and communicative functions, with different ‘costs’: in the present case, grasping an object and communicating our intention to a partner via the movement's kinematics.
Because the neural underpinnings of individual grasping control have been extensively studied [64,65], grasping movements have become an experimental test case for studying the influence of social factors on motor performance. These studies have shown that the kinematics of reaching–grasping–placing movements is modulated even by the mere presence of another person [29]. Notably, others are able to catch the distortions of the kinematics of observed movements possibly, because the visual perceptual system is tailored to the same laws governing action execution in the motor system [66–69]. For example, observational learning seems to be associated with the observation of ‘unnatural’ kinematics, i.e. we are able to learn how to perform a movement by observing somebody else's erroneous movement while he learns [70,71] thus, it appears that we are able to read the costs of observed movements for different purposes (e.g. learn, predict, interact).
Previous studies have shown that interactions impact on a number of higher-level perceptuocognitive representations as they change the way we co-represent the physical space [22], display our attention [54], perceive our partner [37,72–75] and build a common representation of the task at hand [51]. Here, we expand this knowledge by showing that repeatedly interacting with a partner shapes the way in which the pair communicate and organize their behaviour by aligning their representation of the knowledge shared between individuals. An interesting open objective for future research is to understand how these changes are incorporated in the neuronal (e.g. sensorimotor) representations that individuals use for performing joint actions.
Supplementary Material
Acknowledgements
We acknowledge Dr Enea Francesco Pavone (BrainTrends, Ltd, Applied Neuroscience, Rome, Italy) for experimental set-up development.
Data accessibility
Data are available from the Department of Psychology Institutional Data. Access for researchers who meet the criteria for access to confidential data.
Authors' contributions
M.C., A.C., L.M.S., F.D., G.P. designed the experiment; M.C., A.C., L.M.S. recorded and analysed the data; F.D., G.P. performed the model comparison analyses; M.C., A.C., L.M.S., F.D., G.P. wrote the paper.
Competing interests
We declare we have no competing interests.
Funding
M.C. was funded by University Sapienza, Rome (C26A13ZJN4) and by the EU Information and Communication Technologies Grant (VERE project, FP7-ICT-2009-5, protocol number 257695). G.P. and F.D. were funded by the European Community's Seventh Framework Programme under grant agreement no. FP7-270108 (Goal-Leaders).
References
- 1.Knoblich G, Sebanz N. 2008. Evolving intentions for social interaction: from entrainment to joint action. Phil. Trans. R. Soc. B 363, 2021–2031. ( 10.1098/rstb.2008.0006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James W. 1890. The principles of psychology. New York, NY: Holt. [Google Scholar]
- 3.Prinz W. 1997. Perception and action planning. Eur. J. Cognit. Psychol. 9, 129–154. ( 10.1080/713752551) [DOI] [Google Scholar]
- 4.Jeannerod M. 1999. The 25th Bartlett lecture. To act or not to act: perspectives on the representation of actions. Q. J. Exp. Psychol. A 52, 1–29. ( 10.1080/713755803) [DOI] [PubMed] [Google Scholar]
- 5.Wolpert DM, Doya K, Kawato M. 2003. A unifying computational framework for motor control and social interaction. Phil. Trans. R. Soc. Lond. B 358, 593–602. ( 10.1098/rstb.2002.1238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilner JM, Friston KJ, Frith CD. 2007. Predictive coding: an account of the mirror neuron system. Cogn. Process. 8, 159–166. ( 10.1007/s10339-007-0170-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pezzulo G, Candidi M, Dindo H, Barca L. 2013. Action simulation in the human brain: twelve questions. New Ideas Psychol. 31, 270–290. ( 10.1016/j.newideapsych.2013.01.004) [DOI] [Google Scholar]
- 8.Vesper C, van der Wel RP, Knoblich G, Sebanz N. 2013. Are you ready to jump? Predictive mechanisms in interpersonal coordination. J. Exp. Psychol. Hum. Percept. Perform. 39, 48–61. ( 10.1037/a0028066) [DOI] [PubMed] [Google Scholar]
- 9.Buccino G, Binkofski F, Fink GR, Fadiga L, Fogassi L, Gallese V. 2001. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 13, 400–404. ( 10.1111/j.1460-9568.2001.01385.x) [DOI] [PubMed] [Google Scholar]
- 10.Calvo-Merino B, Glaser DE, Grezes J, Passingham RE, Haggard P. 2005. Action observation and acquired motor skills: an fMRI study with expert dancers. Cereb. Cortex 15, 1243–1249. ( 10.1093/cercor/bhi007) [DOI] [PubMed] [Google Scholar]
- 11.Candidi M, Sacheli ML, Mega I, Aglioti SM. 2014. Somatotopic mapping of piano fingering errors in sensorimotor experts: TMS studies in pianists and visually trained musically naives. Cereb. Cortex 24, 435–443. ( 10.1093/cercor/bhs325) [DOI] [PubMed] [Google Scholar]
- 12.Aglioti SM, Cesari P, Romani M, Urgesi C. 2008. Action anticipation and motor resonance in elite basketball players. Nat. Neurosci. 11, 1109–1116. ( 10.1038/nn.2182) [DOI] [PubMed] [Google Scholar]
- 13.Kilner JM, Paulignan Y, Blakemore SJ. 2003. An interference effect of observed biological movement on action. Curr. Biol. 13, 522–525. ( 10.1016/S0960-9822(03)00165-9) [DOI] [PubMed] [Google Scholar]
- 14.Sacheli LM, Tidoni E, Pavone EF, Aglioti SM, Candidi M. 2013. Kinematics fingerprints of leader and follower role-taking during cooperative joint actions. Exp. Brain Res. 226, 473–486. ( 10.1007/s00221-013-3459-7) [DOI] [PubMed] [Google Scholar]
- 15.Knoblich G, Jordan JS. 2003. Action coordination in groups and individuals: learning anticipatory control. J. Exp. Psychol. Learn. Mem. Cogn. 29, 1006–1016. ( 10.1037/0278-7393.29.5.1006) [DOI] [PubMed] [Google Scholar]
- 16.Pickering MJ, Garrod S. 2013. An integrated theory of language production and comprehension. Behav. Brain Sci. 36, 329–347. ( 10.1017/S0140525X12001495) [DOI] [PubMed] [Google Scholar]
- 17.Noy L, Dekel E, Alon U. 2011. The mirror game as a paradigm for studying the dynamics of two people improvising motion together. Proc. Natl Acad. Sci. USA 108, 20 947–20 952. ( 10.1073/pnas.1108155108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sartori L, Becchio C, Bara BG, Castiello U. 2009. Does the intention to communicate affect action kinematics? Conscious Cogn. 18, 766–772. ( 10.1016/j.concog.2009.06.004) [DOI] [PubMed] [Google Scholar]
- 19.Konvalinka I, Vuust P, Roepstorff A, Frith CD. 2010. Follow you, follow me: continuous mutual prediction and adaptation in joint tapping. Q. J. Exp. Psychol. (Hove) 63, 2220–2230. ( 10.1080/17470218.2010.497843) [DOI] [PubMed] [Google Scholar]
- 20.Hart Y, Noy L, Feniger-Schaal R, Mayo AE, Alon U. 2014. Individuality and togetherness in joint improvised motion. PLoS ONE 9, e87213 ( 10.1371/journal.pone.0087213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skewes J, Skewes L, Michael J, Kovalinka I. 2015. Synchronised and complementary coordination mechanisms in an asymmetric joint aiming task. Exp. Brain Res. 233, 551–565. ( 10.1007/s00221-014-4135-2) [DOI] [PubMed] [Google Scholar]
- 22.Pezzulo G, Donnarumma F, Dindo H. 2013. Human sensorimotor communication: a theory of signaling in online social interactions. PLoS ONE 8, e79876 ( 10.1371/journal.pone.0079876) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pezzulo G, Dindo H. 2011. What should I do next? Using shared representations to solve interaction problems. Exp. Brain Res. 211, 613–630. ( 10.1007/s00221-011-2712-1) [DOI] [PubMed] [Google Scholar]
- 24.Vesper C, van der Wel RP, Knoblich G, Sebanz N. 2011. Making oneself predictable: reduced temporal variability facilitates joint action coordination. Exp. Brain Res. 211, 517–530. ( 10.1007/s00221-011-2706-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vesper C, Richardson MJ. 2014. Strategic communication and behavioral coupling in asymmetric joint action. Exp. Brain Res. 232, 2945–2956. ( 10.1007/s00221-014-3982-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briggs GG, Nebes RD. 1975. Patterns of hand preference in a student population. Cortex 11, 230–238. ( 10.1016/S0010-9452(75)80005-0) [DOI] [PubMed] [Google Scholar]
- 27.Daw ND. 2011. Trial-by-trial data analysis using computational models. In Decision making, affect, and learning, pp. 3–38. Oxford, UK: Oxford University Press, Attention and Performance. [Google Scholar]
- 28.Schwarz G. 1978. Estimating the dimension of a model. Ann. Stat. 6, 461–464. ( 10.1214/aos/1176344136) [DOI] [Google Scholar]
- 29.Becchio C, Sartori L, Bulgheroni M, Castiello U. 2008. The case of Dr Jekyll and Mr hyde: a kinematic study on social intention. Conscious Cogn. 17, 557–564. ( 10.1016/j.concog.2007.03.003) [DOI] [PubMed] [Google Scholar]
- 30.Becchio C, Sartori L, Bulgheroni M, Castiello U. 2008. Both your intention and mine are reflected in the kinematics of my reach-to-grasp movement. Cognition 106, 894–912. ( 10.1016/j.cognition.2007.05.004) [DOI] [PubMed] [Google Scholar]
- 31.Ansuini C, Cavallo A, Bertone C, Becchio C. 2014. The visible face of intention: why kinematics matters. Front. Psychol. 5, 815 ( 10.3389/fpsyg.2014.00815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ansuini C, Giosa L, Turella L, Altoè G, Castiello U. 2008. An object for an action, the same object for other actions: effects on hand shaping. Exp. Brain Res. 185, 111–119. ( 10.1007/s00221-007-1136-4) [DOI] [PubMed] [Google Scholar]
- 33.Sartori L, Straulino E, Castiello U. 2011. How objects are grasped: the interplay between affordances and end-goals. PLoS ONE 6, e25203 ( 10.1371/journal.pone.0025203) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sebanz N, Rebbechi D, Knoblich G, Prinz W, Frith CD. 2007. Is it really my turn? An event-related fMRI study of task sharing. Soc. Neurosci. 2, 81–95. ( 10.1080/17470910701237989) [DOI] [PubMed] [Google Scholar]
- 35.Atmaca S, Sebanz N, Prinz W, Knoblich G. 2008. Action co-representation: the joint sNaRc effect. Soc. Neurosci. 3, 410–420. ( 10.1080/17470910801900908) [DOI] [PubMed] [Google Scholar]
- 36.Sebanz N, Knoblich G, Prinz W. 2003. Representing others’ actions: just like one's own? Cognition 88, 11–21. ( 10.1016/S0010-0277(03)00043-X) [DOI] [PubMed] [Google Scholar]
- 37.Inzlicht M, Gutsell JN, Legault L. 2012. Mimicry reduces racial prejudice. J. Exp. Soc. Psychol. 48, 361–365. ( 10.1016/j.jesp.2011.06.007) [DOI] [Google Scholar]
- 38.Wenke D, Atmaca S, Hollander A, Liepelt R, Baess P, Prinz W. 2011. What is shared in joint action? Issues of co-representation, response conflict, and agent identification. Rev. Phil. Psychol. 2, 147–172. ( 10.1007/s13164-011-0057-0) [DOI] [Google Scholar]
- 39.Brass M, Bekkering H, Wohlschläger A, Prinz W. 2000. Compatibility between observed and executed finger movements: comparing symbolic, spatial, and imitative cues. Brain Cogn. 44, 124–143. ( 10.1006/brcg.2000.1225) [DOI] [PubMed] [Google Scholar]
- 40.Brass M, Bekkering H, Prinz W. 2001. Movement observation affects movement execution in a simple response task. Acta Psychol. (Amst). 106, 3–22. ( 10.1016/S0001-6918(00)00024-X) [DOI] [PubMed] [Google Scholar]
- 41.Prinz W. 2006. What re-enactment earns us. Cortex 42, 515–517. ( 10.1016/S0010-9452(08)70389-7) [DOI] [PubMed] [Google Scholar]
- 42.Naber M, Vaziri Pashkam M, Nakayama K. 2013. Unintended imitation affects success in a competitive game. Proc. Natl Acad. Sci. USA 110, 20 046–20 050. ( 10.1073/pnas.1305996110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cook R, Bird G, Lünser G, Huck S, Heyes C. 2012. Automatic imitation in a strategic context: players of rock–paper–scissors imitate opponents’ gestures. Proc. R. Soc. B 279, 780–786. ( 10.1098/rspb.2011.1024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dindo H, Zambuto D, Pezzulo G. 2011. Motor simulation via coupled internal models using sequential Monte Carlo. In Proc. IJCAI, 16-22 July, Barcelona, Spain, pp. 2113–2119. Palo Alto, CA: AAAI Press. [Google Scholar]
- 45.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. 1992. Understanding motor events: a neurophysiological study. Exp. Brain Res. 91, 176–180. ( 10.1007/BF00230027) [DOI] [PubMed] [Google Scholar]
- 46.Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. 2005. Parietal lobe: from action organization to intention understanding. Science 308, 662–667. ( 10.1126/science.1106138) [DOI] [PubMed] [Google Scholar]
- 47.Chong TT, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. 2008. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr. Biol. 18, 1576–1580. ( 10.1016/j.cub.2008.08.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kilner JM, Neal A, Weiskopf N, Friston KJ, Frith CD. 2009. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci. 29, 10 153–10 159. ( 10.1523/JNEUROSCI.2668-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman-Norlund RD, van Schie HT, van Zuijlen AM, Bekkering H. 2007. The mirror neuron system is more active during complementary compared with imitative action. Nat. Neurosci. 10, 817–818. ( 10.1038/nn1911) [DOI] [PubMed] [Google Scholar]
- 50.Kokal I, Gazzola V, Keysers C. 2009. Acting together in and beyond the mirror neuron system. Neuroimage 47, 2046–2056. ( 10.1016/j.neuroimage.2009.06.010) [DOI] [PubMed] [Google Scholar]
- 51.Sebanz N, Bekkering H, Knoblich G. 2006. Joint action: bodies and minds moving together. Trends Cogn. Sci. 10, 70–76. ( 10.1016/j.tics.2005.12.009) [DOI] [PubMed] [Google Scholar]
- 52.Ondobaka S, de Lange FP, Newman-Norlund RD, Wiemers M, Bekkering H. 2012. Interplay between action and movement intentions during social interaction. Psychol. Sci. 23 30–35. ( 10.1177/0956797611424163) [DOI] [PubMed] [Google Scholar]
- 53.Tunik E, Rice NJ, Hamilton A, Grafton ST. 2007. Beyond grasping: representation of action in human anterior intraparietal sulcus. Neuroimage 36(Suppl 2), T77–T86. ( 10.1016/j.neuroimage.2007.03.026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sebanz N, Knoblich G, Prinz W. 2005. How two share a task: corepresenting stimulus–response mappings. J. Exp. Psychol. Hum. Percept. Perform. 31, 1234–1246. ( 10.1037/0096-1523.31.6.1234) [DOI] [PubMed] [Google Scholar]
- 55.Sacheli LM, Candidi M, Era V, Aglioti SM. 2015. Causative role of left aIPS in coding shared goals during human–avatar complementary joint actions. Nat. Commun. 6, 7544 ( 10.1038/ncomms8544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fitts PM. 1954. The information capacity of the human motor system in controlling the amplitude of movement. J. Exp. Psychol. 47, 381–391. ( 10.1037/h0055392) [DOI] [PubMed] [Google Scholar]
- 57.Viviani P, Terzuolo C. 1982. Trajectory determines movement dynamics. Neuroscience 7, 431–437. ( 10.1016/0306-4522(82)90277-9) [DOI] [PubMed] [Google Scholar]
- 58.Flash T, Hochner B. 2005. Motor primitives in vertebrates and invertebrates. Curr. Opin. Neurobiol. 15, 660–666. ( 10.1016/j.conb.2005.10.011) [DOI] [PubMed] [Google Scholar]
- 59.Overduin SA, d'Avella A, Carmena JM, Bizzi E. 2012. Microstimulation activates a handful of muscle synergies. Neuron 76, 1071–1077. ( 10.1016/j.neuron.2012.10.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Todorov E. 2004. Optimality principles in sensorimotor control. Nat. Neurosci. 7, 907–915. ( 10.1038/nn1309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Friston K. 2011. What is optimal about motor control? Neuron 72, 488–498. ( 10.1016/j.neuron.2011.10.018) [DOI] [PubMed] [Google Scholar]
- 62.Lepora NF, Pezzulo G. 2015. Embodied choice: how action influences perceptual decision making. PLoS Comput. Biol. 11, e1004110 ( 10.1371/journal.pcbi.1004110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pezzulo G. 2013. Studying mirror mechanisms within generative and predictive architectures for joint action. Cortex 49, 2968–2969. ( 10.1016/j.cortex.2013.06.008) [DOI] [PubMed] [Google Scholar]
- 64.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. 1995. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 18, 314–320. ( 10.1016/0166-2236(95)93921-J) [DOI] [PubMed] [Google Scholar]
- 65.Castiello U. 2005. The neuroscience of grasping. Nat. Rev. Neurosci. 6, 726–736. ( 10.1038/nrn1744) [DOI] [PubMed] [Google Scholar]
- 66.Viviani P, Stucchi N. 1992. Biological movements look uniform: evidence of motor–perceptual interactions. J. Exp. Psychol. Hum. Percept. Perform. 18, 603–623. ( 10.1037/0096-1523.18.3.603) [DOI] [PubMed] [Google Scholar]
- 67.Flach R, Knoblich G, Prinz W. 2004. The two-third power law in motion perception. Visual Cogn. 11, 461–481. ( 10.1080/13506280344000392) [DOI] [Google Scholar]
- 68.Grosjean M, Shiffrar M, Knoblich G. 2007. Fitts's law holds for action perception. Psychol. Sci. 18, 95–99. ( 10.1111/j.1467-9280.2007.01854.x) [DOI] [PubMed] [Google Scholar]
- 69.Friston K, Mattout J, Kilner J. 2011. Action understanding and active inference. Biol. Cybern. 104, 137–160. ( 10.1007/s00422-011-0424-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattar AA, Gribble PL. 2005. Motor learning by observing. Neuron 46, 153–160. ( 10.1016/j.neuron.2005.02.009) [DOI] [PubMed] [Google Scholar]
- 71.Brown LE, Wilson ET, Gribble PL. 2009. Repetitive transcranial magnetic stimulation to the primary motor cortex interferes with motor learning by observing. J. Cogn. Neurosci. 21, 1013–1022. ( 10.1162/jocn.2009.21079) [DOI] [PubMed] [Google Scholar]
- 72.Hommel B, Colzato LS, van den Wildenberg WP. 2009. How social are task representations? Psychol. Sci. 20, 794–798. ( 10.1111/j.1467-9280.2009.02367.x) [DOI] [PubMed] [Google Scholar]
- 73.Valdesolo P, DeSteno D. 2011. Synchrony and the social tuning of compassion. Emotion 11, 262–266. ( 10.1037/a0021302) [DOI] [PubMed] [Google Scholar]
- 74.Sacheli LM, Candidi M, Pavone EF, Tidoni E, Aglioti SM. 2012. And yet they act together: interpersonal perception modulates predictive simulation and mutual adjustments during a joint-grasping task. PLoS ONE 7, e50223 ( 10.1371/journal.pone.0050223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sacheli LM, Christensen A, Giese MA, Taubert N, Pavone EF, Aglioti SM, Candidi M. 2015. Prejudiced interactions: implicit racial bias reduces predictive simulation during joint action with an out-group avatar. Sci. Rep. 5, 8507 ( 10.1038/srep08507) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Department of Psychology Institutional Data. Access for researchers who meet the criteria for access to confidential data.