Abstract
The colourful wing patterns of butterflies play an important role for enhancing fitness; for instance, by providing camouflage, for interspecific mate recognition, or for aposematic display. Closely related butterfly species can have dramatically different wing patterns. The phenomenon is assumed to be caused by ecological processes with changing conditions, e.g. in the environment, and also by sexual selection. Here, we investigate the birdwing butterflies, Ornithoptera, the largest butterflies of the world, together forming a small genus in the butterfly family Papilionidae. The wings of these butterflies are marked by strongly coloured patches. The colours are caused by specially structured wing scales, which act as a chirped multilayer reflector, but the scales also contain papiliochrome pigments, which act as a spectral filter. The combined structural and pigmentary effects tune the coloration of the wing scales. The tuned colours are presumably important for mate recognition and signalling. By applying electron microscopy, (micro-)spectrophotometry and scatterometry we found that the various mechanisms of scale coloration of the different birdwing species strongly correlate with the taxonomical distribution of Ornithoptera species.
Keywords: biophotonic structures, reflectance, iridescence, pigmentation, filtering, biogeography
1. Background
The beauty and brilliancy of this insect are indescribable.
— Alfred Russel Wallace on capturing a birdwing butterfly in Indonesia in 1859, as described in The Malay Archipelago, 1869
The coloured wing patterns of butterflies have attracted the intense interest of scientists, artists and collectors alike for more than a century [1]. Butterfly wings are covered by a lattice of wing scales, which have colours generated by two different mechanisms, structural (physical) and pigmentary (chemical) [2]. A structural colour results when a tissue is made up of materials with different refractive indices arranged in periodic structures with distances of the order of the wavelengths of the incident light, and a pigmentary colour occurs when irregularly structured matter contains pigment absorbing in a restricted wavelength range [1–3]. Structural colours are usually connected to directionally reflected light and are called iridescent when they depend on the direction of illumination and viewing [1–5]. Pigmentary colours are generally less brilliant than structural colours, due to diffuse scattering by the irregularly arranged material components. Often the two coloration mechanisms are combined, i.e. many structural coloured butterfly wing scales contain pigment that tunes the coloration [4].
Birdwing butterflies, next to Morpho butterflies, are probably the most amazingly coloured living butterflies and thus are often seen as synonymous for beauty. The Australasian genus Ornithoptera, which includes all birdwings, belongs to the subfamily Troidinae of the swallowtails (Papilionidae [6–8]), that evolved from the ancestral troidines [9,10]. The genus contains 10 species which are known for their extremely large body size (wing span of up to 28 cm), angular wings and bird-like flight. The Ornithoptera genus includes three distinct subgenera: Schoenbergia, Aetheoptera and Ornithoptera. Each of these subgenera differs in its coloration and the ecosystem they inhabit. The striking wing colorations can range over the whole visible spectrum: from blue-green in the Cape York Birdwing, O. urvillianus, to orange-red in Wallace's Golden Birdwing, O. croesus.
Previous studies have demonstrated that the wing scales of papilionid butterflies harbour a unique class of pigments, the papiliochromes. Notably, the yellow-cream wing colour of the Japanese yellow swallowtail, Papilio xuthus, was shown to be caused by papiliochrome II. This pigment has a narrow-band, violet-peaking absorption spectrum and displays a marked green fluorescence when excited by violet-blue light [11–14]. In the birdwing butterflies, anatomical studies of the wing scales suggested, however, that their beautiful colours are created by photonic structures [2,15–17]. In which way and to what extent the morphology of the birdwing scales determines the spectral and spatial reflection characteristics, or whether pigments play a principal role, has so far remained unresolved. To clarify this, we investigated seven representative examples of Ornithoptera by applying morphological as well as various optical methods. We thus found that the coloration of this special group of butterflies is due to a unique combination of structural and pigmentary effects, together causing a diffuse optical signal. We discuss the results in light of the evolution and geographical distribution of the butterflies and we also consider the advantages of the unique structural architecture of the wing scales for coloration and display.
2. Material and methods
2.1. Butterflies
Mounted specimens of the birdwing butterflies Ornithoptera priamus (Linnaeus, 1758), O. urvillianus (Boisduval, 1832), O. croesus (Wallace, 1859) of the subgenus Ornithoptera (Boisduval 1832), O. tithonus (de Haan, 1840), O. goliath (Oberthür, 1888), O. rothschildi (Kenrick, 1911) of the subgenus Schoenbergia (Pagenstecher, 1893), and O. victoriae (Gray, 1850) of the subgenus Aethoptera (Rippon, 1890) (figure 1) were purchased from Worldwide Butterflies (Dorset, UK; www.wwb.co.uk). O victoriae features two different coloured spots on the forewings, one cyan and one green, which are named as two subspecies in the tables and figures.
Figure 1.
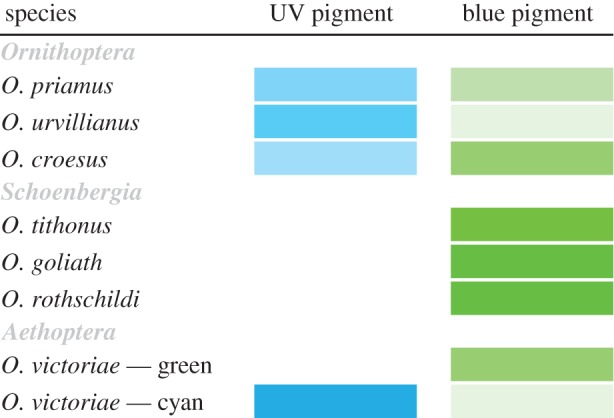
Investigated ornithopterans and pigment distribution in the different coloured wing scales. The saturation of the colour corresponds to the (relative) amount of pigment scaled to the maximum absorbance observed in all investigated scales (see also figure 4a).
2.2. Photography
The purchased butterflies were photographed with a Canon EOS 550D digital camera using a ring flash. Some butterflies were photographed in the collection of the Western Australian Museum (Perth, Australia) using a Canon EOS 7D with a Passport II macro photography system (Dun Inc., Palmyra, VA, USA). For UV-photographs (electronic supplementary material, figure S2), the specimens were illuminated with a Wood's lamp and photographed with a Nikon D70 digital camera fitted with a UV-transmission filter (combined Schott glasses UG3 and BG17). Details of the scale lattice on the wing surface were photographed with a Zeiss Axioscope-A1 Pol microscope, applying white-light epi-illumination and using a Point Grey Grasshopper 3 GS3-U3-50S5C-C or a Mueller DCM310 digital camera. For fluorescence pictures, we used the Zeiss Axioscope with 450–490 nm excitation light and a greater than 520 nm barrier filter.
2.3. Spectrophotometry
The absorbance spectra of the wing scales' pigments were measured on single wing scales immersed in a fluid with refractive index 1.55 (Series A; Cargille Labs, Cedar Grove, NJ, USA) with a microspectrophotometer [4]. Reflectance spectra of the intact wing were measured with an integrating sphere connected to an AvaSpec-2048-2 photodiode spectrometer. The light source was a deuterium–halogen lamp (Avantes D(H)-S). A white standard (Avantes WS-2) served as the reference.
2.4. Scanning electron microscopy
The ultrastructure of the wing scales was investigated using a Philips XL30-ESEM or a Tescan Mira 3 LM scanning electron microscope (SEM). Samples were sputtered with palladium or gold to prevent charging effects prior to imaging.
2.5. Transmission electron microscopy
For transmission electron microscopy (TEM) of the scales, wing parts were prefixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 mol l−1 sodium cacodylate buffer (CB, pH 7.3) for approximately 45 min. After dehydrating with a graded series of ethanol and infiltration with propylene oxide, the tissues were embedded in Spurr's resin. The tissues were cut into 50 nm ultrathin sections, double-stained with uranyl acetate and lead citrate and observed using a Hitachi H7650 (Tokyo, Japan) TEM (as outlined in [18]).
2.6. Imaging scatterometry
The hemispherical far-field light scattering pattern of single scales was visualized with an imaging scatterometer [18–20]. The scatterometer is built around an ellipsoidal mirror, which collects light from a full hemisphere around its first focal point, where the sample is positioned. A small piece of magnesium oxide served as a white diffuse reference object. Images were acquired with an Olympus DP-70 camera and were subsequently corrected for geometrical distortions using a MATLAB routine.
3. Results
3.1. Wing coloration and morphology
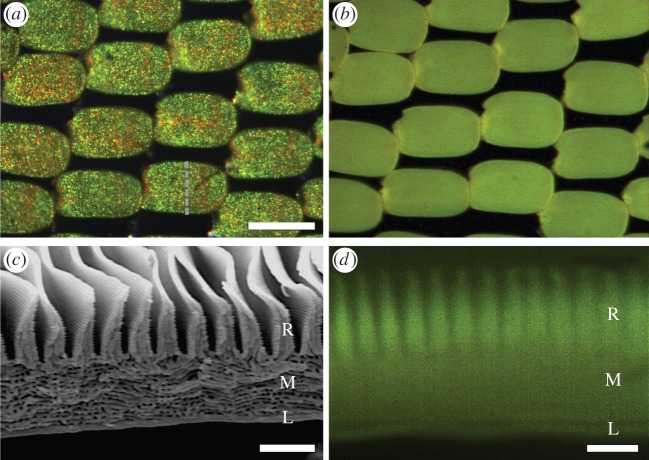
The dorsal wing surfaces of male birdwing butterflies feature brightly coloured patches on both wings, marked within a jet-black framing that can be superposed with yellow patches on the hindwing. Figure 2 (column I) shows the wing patterns of a few typical ornithopterans, where the colour range varies from blue in O. urvillianus, via green-yellow in O. priamus and O. tithonus to orange-red in O. croesus. All animals have strongly coloured cover scales overlapping a dense lattice of jet-black ground scales (figure 2, column II; electronic supplementary material, figure S1). To investigate whether the coloration of the scales is due to structural effects, we used TEM. The cover scales of the different species all appeared to have a very similar architecture. The scales have a unique layer of pointed ridges covering a lumen existing of an extensive multilayer (figure 2, column III). We derived the average layer distance by performing a fast-Fourier transform (FFT) of the anatomical figures, yielding for instance for the blue and orange wing scales thicknesses of approximately 170 nm and approximately 215 nm, respectively (table 1). The difference in layer spacing between the differently coloured scales suggests that the scale-specific multilayer properties principally determine the scales' colour. We note here that the multilayers are rather disordered and chirped (i.e. the thickness of adjacent layers varies significantly from layer to layer) when compared with the lumen multilayers of lycaenid butterflies, for instance [19,21].
Figure 2.
Characteristic birdwing butterflies. (a) O. croesus; (b) O. tithonus; (c) O. priamus; (d) O. urvillianus. Column I: picture of the animal; II: scale lattice of the colour patch on the dorsal wing surface; III: TEM cross-section images of structural coloured wing scales; IV: scatterograms of single wing scales. Scale bars for photos in columns I–III are given in row d; column I, 1 cm; II, 100 µm; III, 2 µm. Column IV: red circles indicate angles 5°, 30°, 60° and 90°.
Table 1.
Structural parameters of the wing scales extracted from TEM images. The average layer distance has been determined by FFT of the TEM images of figure 1 and thus shows the ensemble average over the full-scale picture.
| species | no. layers | average distance (nm) | colour |
|---|---|---|---|
| O. croesus | 11–13 | 214 ± 5 | orange-red |
| O. tithonus | 12–14 | 191 ± 6 | green-yellow |
| O. priamus | 10–12 | 194 ± 5 | green |
| O. urvillianus | 9–11 | 168 ± 6 | blue |
A classical multilayer reflects light very directionally, and to test if this was also the case for the birdwing scales we measured the spatial distribution of the light scattered by single wing scales with an imaging scatterometer. The obtained scatterograms (figure 2, column IV) demonstrated that the wing scales of the ornithopterans reflect incident light somewhat diffusely, arguing against structural coloration and rather favouring pigmentary coloration. We, therefore, decided to explore the possibility that the scales were coloured by wavelength-selective absorbing pigments.
3.2. Pigmentary coloration
The pigments of papilionids belong to the class of papiliochromes, which can be distinctly fluorescent [11–13]. To investigate whether the scales contained papiliochrome pigments, we therefore applied fluorescence microscopy. Figure 3 shows results obtained from the wing scales of the Tithonus Birdwing, O. tithonus. Using blue excitation light, the green-yellow coloured wing scales (figure 3a) appeared to display a distinct, green emission, strongly suggesting that the scales contained papiliochrome pigment (figure 3b). To uncover the pigment's localization, we observed cross-sectioned single wing scales with the fluorescence microscope. The whole wing scale body appeared to be fluorescent (figure 3b), but the fluorescence emerged predominantly from the upper structured layer with structured ridges (R, figure 3c,d), which is a location well suited for a spectral filter.
Figure 3.
Fluorescence of the wing scales of the Tithonus Birdwing, O. tithonus. (a) Epi-illumination light micrograph of the scale lattice on the dorsal side of the forewing. (b) Blue-induced fluorescence (excitation 450–490 nm, emission > 520 nm) of the scale pattern shown in (a). (c) Scanning electron micrograph of a cross-sectioned yellow-green wing scale (cross-section indicated by the dashed line in (a)). The scale structure exists of three parts: a ridge array of tall, peaked ridges (R) on top of a disordered multilayer in the scale lumen (M), with below a flat lower lamina (L). (d) Blue-induced green fluorescence of a cross-section of a single scale. All three parts of the scale contain the pigment. Scale bars: (a,b) 100 µm, (c,d) 2 µm.
To identify the pigments of the scales of the various birdwing species and to determine their spectral characteristics, we immersed single scales in refractive index matching fluid (n = 1.55) and measured absorbance spectra with a microspectrophotometer (MSP, figure 4a). The scales of the studied birdwing butterflies yielded two classes of absorbance spectra. The scales of O. urvillianus contained a predominantly UV-absorbing pigment, with peak absorbance at approximately 375 nm, whereas the scales of O. tithonus contained a blue-absorbing pigment, absorbing maximally at approximately 460 nm. Some scales, like those of O. croesus contained a mixture of both pigments (figures 1, 4a).
Figure 4.
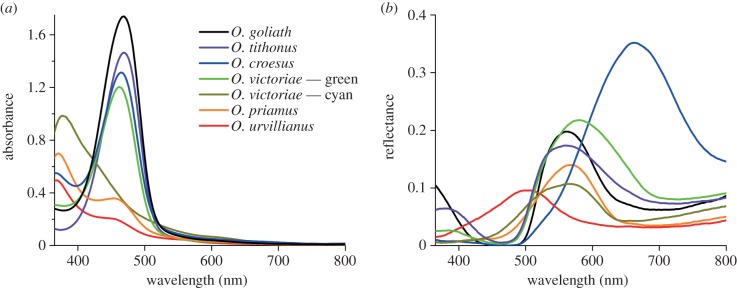
Spectral tuning of the scale reflectance. (a) Absorbance spectra of single wing scales immersed in a refractive index liquid. The wing scales contain different compositions of two different absorbing pigments (see also table 1). (b) Reflectance spectra of the wings measured with an integrating sphere. Most birdwings are green, with maximal reflectance at approximately 550 nm, but the blue-coloured O. urvillianus has a reflectance peaking at approximately 490 nm and the orange O. croesus reflects maximally at approximately 660 nm.
To clarify the spectral consequences of the absorbing pigments, we measured the reflectance spectra of the wings of the various birdwing butterflies with an integrating sphere (figure 4b). All reflectance spectra featured pronounced bands in the blue to green wavelength ranges, with a peak value between approximately 0.1 (O. urvillianus) and approximately 0.35 (O. croesus). In addition, the reflectance spectra had characteristic troughs in the shorter wavelength range (figure 4b), strikingly corresponding to the pigments' absorption wavelength range (figure 4a). We conclude, therefore, that the pigments act as spectral filters, suppressing the reflectance in the restricted wavelength range of the pigment absorption bands.
4. Discussion
4.1. The colours of birdwing scales
The scales of birdwings contain distinctly fluorescing pigments, which most likely are intimately related to the papiliochrome pigments of other papilionid butterflies. The violet-absorbing pigment papiliochrome II is evidently amply present in the scales of O. priamus and O. urvillianus (see also electronic supplementary material, figure S3 for a comparison with Troides ssp.), but another, blue-absorbing pigment exists most prominently in the scales of O. goliath and O. tithonus, for example.
The wing scales of the related P. xuthus, representing the tribe Papilionini, contain the same or very similar blue-absorbing pigments, but their morphological structure is rather irregular [11,12]. The scales hence act more or less as wavelength-independent, diffuse scatterers, and because the scales' pigment suppresses the reflectance only in the short-wavelength range an overall cream-yellow colour remains. The coloration of P. xuthus wings hence is fully pigmentary.
Quite differently, the reflectance spectra of the birdwing scales feature clear bands, with at long wavelengths a low reflectance. Therefore, the birdwings are principally structural coloured, due to the multilayers in the scale lumen reflecting only in a restricted wavelength range. Furthermore, the structural coloured cover scales are backed by strongly absorbing, melanin-containing ground scales (figure 2; electronic supplementary material, figure S1). This organization evidently serves to optimize colour contrast, e.g. by the ground scales absorbing straylight [22–24].
4.2. Tuning of wing coloration
The multilayers of the brightly reflecting wing scales of the birdwing butterflies are covered by tapered ridges. This organization resembles that of the wing scales of the Emerald-patched Cattleheart, Parides sesostris, also a member of the tribe Troidini, which consist of a highly reflective photonic crystal covered by a ‘honeycomb’ layer acting as both a spectral filter and a diffuser/scrambler [4]. Although the exact anatomy differs, the upper layer of the ornithopteran scales clearly also acts as a spectral filter as well as a strongly scattering medium that causes the broad-angled reflections shown in the scatterograms of figure 2, column IV.
The anatomy of the birdwing scales is very different from that in most other butterfly species, especially concerning its thickness of approximately 10 µm (see also [15,25,26]). Most butterflies, which are usually considerably smaller than the birdwings, feature much thinner scales, hinting at an optimization of the weight of the scales with respect to their colour signalling function. Small butterflies often achieve a strong reflection signal by stacking similar coloured scales, e.g. the Cabbage White, Pieris rapae [27]. The high reflectance achieved by the single scales of the birdwings is presumably sufficient to avoid scale stacking.
The combination of pigmentary and structural coloration to achieve unique optical effects is encountered in the scales of several other butterfly species, and also in bird feathers, e.g. in budgerigars and parrots [28,29]. For instance, the wing scales of the nireus group papilionids (Papilio: Papilionini) have inconspicuous ridges above a pigmented layer of irregular cylinders, separated from a flat lower lamina, which acts as a thin film reflector [12]. This principle of coloration is also practiced in the scales of other butterfly families [30–32]. The scales of birdwings, on the other hand, have very pronounced ridges above a disordered multilayer in the lumen, consisting of up to 14 chitin layers (figure 2, table 1). The large stack of somewhat irregularly tilted layers in the scale lumen together with the highly pointed ridge layer causes light reflection into a wide angular space, which further causes significant shape anisotropy causing a slight polarization-dependent structural colour [17]. The additional filtering pigment enhances the strongly chromatic colour. Preliminary finite-difference time-domain (FDTD) simulations using the TEM ultrastructures of figure 2 (results not shown) confirm that the chirped multilayers create broadband, violet-to-green-peaking reflectance spectra and that the papiliochrome pigments effectively suppress the reflectance in the short-wavelength range.
4.3. Colour–phylogeny correlation
The three subgenera of the birdwings show different methods of coloration: (i) the true Ornithoptera all feature similar wing patterns, which however, strongly vary in colour; (ii) the Schoenbergia are monocoloured with strongly coloured yellow-green scales and angular (i.e. asymmetric-sized) wing features that further only contain the blue-absorbing papiliochrome pigment leading to strongly chromatic signals (figure 4; electronic supplementary material, figure S2); (iii) the Aethoptera, though a small clade, feature different structural-coloured spots with species-dependent pigment expressions (figure 5; [7,8]).
Figure 5.
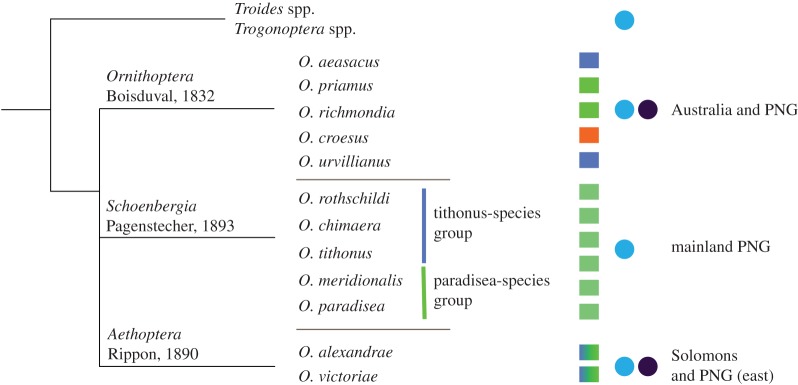
Birdwing phylogeny and geographical distribution. The different coloured squares indicate the colour of the wing patches, similar colours indicate a roughly identical photonic structure, and the blue and black dots the appearance of blue-absorbing and UV-absorbing papiliochromes in the wing scales, respectively. PNG, Papua New Guinea.
The different characteristics are also recognizable in the distribution of the butterflies in the Australo-Indomalayan region. Weber's line acts as a divider for species having evolved in Asia from those in Wallacea (the Australian genera) [33,34]. Also the Ornithoptera and Schoenbergia appear to belong to the Australian genera, whereas Aethoptera is restricted to small areas to the west of Weber's line. The origin of this diversification is unknown, but interestingly the colour mechanisms are distinct between different groups (figure 5).
4.4. Biological importance and butterfly vision
Larval stages of Ornithoptera are monophagous on the poisonous Aristolochiaceae, the main foodplants [9]. Both male and female adults have yellow coloured wings, presumably for aposematic warning to predators, e.g. spiders or birds.
As butterfly colour vision extends from the UV into the red [35], the reflection of UV- and yellow-green light by the birdwing wings into a wide angular space creates a powerful visual signal: a broad-angled, bright ‘butterfly purple’. This is quite in contrast with what is generally assumed to hold for structural coloration, namely that the associated iridescence functions in angle-dependent, directional signalling [5]. In the well-known Neotropical Morpho butterflies (Morphinae: Nymphalidae), multilayer-reflectors in the folded scale ridges create a directional blue-coloured iridescence, which is presumably tuned to the blue-receptors of conspecifics [18].
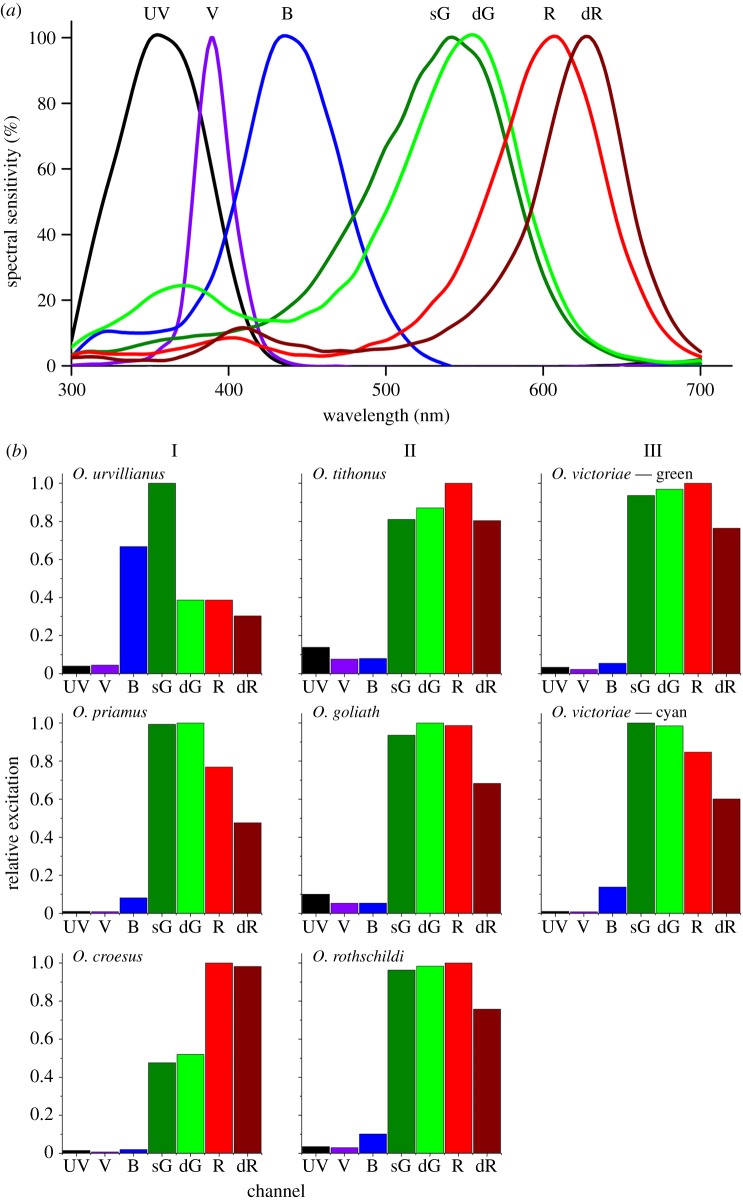
Sexual dimorphism is strong in Ornithoptera species, where males are black combined with bright green, blue, orange or yellow markings (figure 2), while the larger and less colourful females are overall black or dark brownish with white, pale-brown or yellow markings [33,34]. The sexual dichromatism functions in mate recognition by using the set of spectral photoreceptors [5]. Most likely the Troides and Ornithoptera, which are in the same tribe (Troidini) in the family of Papilionidae, have similar sets of spectral receptors. Comparing the photoreceptor spectral sensitivities of the Golden Birdwing butterfly, Troides aeacus formosanus, determined by intracellular recordings [36], with the measured wing reflectance spectra suggests that the high chromatic contrast created by the pigmentary tuning is well discriminated by the set of different photoreceptors, and thus will facilitate mate recognition in a highly complex visual environment (figure 6a).
Figure 6.
Photoreceptor spectral sensitivities and coloration of birdwing butterflies. (a) Normalized spectral sensitivities of the most common photoreceptors of the Golden Birdwing butterfly, Troides aeacus formosanus [36]. (b) Bar graphs of the relative excitation of the set of photoreceptors for the different coloured wing areas of the butterflies of the subgenera Ornithoptera (I), Schoenbergia (II) and Aethoptera (III). The reflectance spectra of figure 4b were convoluted with a CIE D65 standard illuminant spectrum and each of the photoreceptor spectral sensitivities. The obtained values were subsequently normalized to the highest excitation per species. Each bar represents the result of such a calculation for an individual photoreceptor. Note the strong trend within Ornithoptera and Aethoptera species, whereas all excitation profiles are basically identical for the different Schoenbergia species.
To show this more directly, we calculated the relative excitation of the different photoreceptors by the wing reflectances of the different butterflies. We, therefore, convoluted the spectral reflectance of the various wing areas with the common daylight spectrum and the photoreceptor spectral sensitivities, using those of T. aeacus as the closest approximation. Comparing the different bar graphs (figure 6b) shows that the different wing colours are well discriminable by the colour vision system of the birdwings and that especially the butterflies of the subgenus Ornithoptera excite strongly different photoreceptors, as expected from the different colours. Interestingly, species whose photoreceptor spectral sensitivities are sex-dependent, like pierid butterflies [37], tend to have wings of very different colours between sexes. The wings of papilionid species generally do not feature clear sexual dimorphism, and neither the eyes of papilionid butterflies [35,36]. In the sister genus of the ornithopterans, Troides, there is weak sexual dimorphism as the two sexes have similarly coloured wings, whereas the wing colours of ornithopteran males and females are quite different (see above). It would, therefore, be interesting to study whether and how photoreceptor spectral sensitivities are sexually dimorphic in ornithopterans. However, the protected status of these species will hamper the necessary experimental studies.
5. Conclusion
The showy coloration of birdwings is controlled by two mechanisms: diffuse reflection of incident light by chirped multilayers and spectral filtering by the embedded pigments. Changes in the multilayer dimensions and the pigment absorption spectrum will modify the colour. This is especially interesting as the different birdwing species cover the full range of visible colours, ranging from deep-blue (O. urvillianus) to orange-red (O. croesus) [10]. The characterization of the novel combination of structural and pigmentary coloration in birdwings expands our insight into biophotonic coloration, especially in insects. This may provide biomimetic inspiration, e.g. for adjusting the colour of photonic materials or for improvement of the viewing angle of displays [1,38,39].
Supplementary Material
Acknowledgements
We thank H. L. Leertouwer for expert technical assistance and B. Hamich for access to additional butterflies and help with taking macrophotographs.
Authors' contributions
B.D.W. and D.G.S. designed the study, all authors performed experiments, collected and analysed data, B.D.W. and D.G.S. wrote the paper with input from all authors.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the AFOSR/EOARD (grant no. FA8655-08-1-3012 to D.G.S.) and by the JSPS KAKENHI (grant no. 26251036 to K.A.).
References
- 1.Kinoshita S. 2008. Structural colors in the realm of nature. Singapore: World Scientific. [Google Scholar]
- 2.Srinivasarao M. 1999. Nano-optics in the biological world: beetles, butterflies, birds, and moths. Chem. Rev. 99, 1935–1962. ( 10.1021/cr970080y) [DOI] [PubMed] [Google Scholar]
- 3.Wilts BD, Michielsen K, De Raedt H, Stavenga DG. 2014. Sparkling feather reflections of a bird-of-paradise explained by finite-difference time-domain modeling. Proc. Natl Acad. Sci. USA 111, 4363–4368. ( 10.1073/pnas.1323611111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilts BD, Michielsen K, De Raedt H, Stavenga DG. 2012. Iridescence and spectral filtering of the gyroid-type photonic crystals in Parides sesostris wing scales. Interface Focus 2, 681–687. ( 10.1098/rsfs.2011.0082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doucet SM, Meadows MG. 2009. Iridescence: a functional perspective. J. R. Soc. Interface 6, S115–S132. ( 10.1098/rsif.2008.0395.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonsen TJ, Zakharov EV, Djernaes M, Cotton AM, Vane-Wright RI, Sperling FAH. 2011. Phylogenetics and divergence times of Papilioninae (Lepidoptera) with special reference to the enigmatic genera Teinopalpus and Meandrusa. Cladistics 27, 113–137. ( 10.1111/j.1096-0031.2010.00326.x) [DOI] [PubMed] [Google Scholar]
- 7.d'Abrera B. 1975. Birdwing butterflies of the world. London, UK: Lansdowne Press. [Google Scholar]
- 8.Deslisle G. 2004. A taxonomic revision of the birdwing butterflies of paradise, genus Ornithoptera, based on the adult morphology. Lambillionea 104, 3–93. [Google Scholar]
- 9.Condamine FL, Sperling FAH, Wahlberg N, Rasplus J, Kergoat GJ. 2012. What causes latitudinal gradients in species diversity? Evolutionary processes and ecological constraints on swallowtail biodiversity. Ecol. Lett. 15, 267–277. ( 10.1111/j.1461-0248.2011.01737.x) [DOI] [PubMed] [Google Scholar]
- 10.Parsons M. 1996. A phylogenetic reappraisal of the birdwing genus Ornithoptera (Lepidoptera: Papilionidae: Troidini) and a new theory of its evolution in relation to Gondwanan vicariance biogeography. J. Nat. Hist. 30, 1707–1736. ( 10.1080/00222939600771001) [DOI] [Google Scholar]
- 11.Stavenga DG, Matsushita A, Arikawa K. 2015. Combined pigmentary and structural effects tune wing scale coloration to color vision in the swallowtail butterfly Papilio xuthus. Zool. Lett. 1, 14 ( 10.1186/s40851-015-0015-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilts BD, Trzeciak TM, Vukusic P, Stavenga DG. 2012. Papiliochrome II pigment reduces the angle-dependency of structural wing colouration in nireus group papilionids. J. Exp. Biol. 215, 796–805. ( 10.1242/jeb.060103) [DOI] [PubMed] [Google Scholar]
- 13.Umebachi Y. 1985. Papiliochrome, a new pigment group of butterfly. Zool. Sci. 2, 163–174. [Google Scholar]
- 14.Umebachi Y. 1975. Yellow pigments in the wings of Papilio xuthus (papilionid butterfly). Acta Vitaminol. Enzymol. 29, 219–222. [PubMed] [Google Scholar]
- 15.Prum RO, Quinn T, Torres RH. 2006. Anatomically diverse butterfly scales all produce structural colours by coherent scattering. J. Exp. Biol. 209, 748–765. ( 10.1242/jeb.02051) [DOI] [PubMed] [Google Scholar]
- 16.Ingram AL, Parker AR. 2008. A review of the diversity and evolution of photonic structures in butterflies, incorporating the work of John Huxley (The Natural History Museum, London from 1961 to 1990). Phil. Trans. R. Soc. B 363, 2465–2480. ( 10.1098/rstb.2007.2258) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang K, Zhou S, Tang Y, Wang G, Zhou H, Fan T, Zhang D. 2014. Polarization-sensitive color in iridescent scales of butterfly Ornithoptera. RSC Adv. 4, 51 865–51 871. ( 10.1039/C4RA07988D) [DOI] [Google Scholar]
- 18.Stavenga DG, Leertouwer HL, Pirih P, Wehling MF. 2009. Imaging scatterometry of butterfly wing scales. Opt. Express 17, 193–202. ( 10.1364/OE.17.000193) [DOI] [PubMed] [Google Scholar]
- 19.Wilts BD, Leertouwer HL, Stavenga DG. 2009. Imaging scatterometry and microspectrophotometry of lycaenid butterfly wing scales with perforated multilayers. J. R. Soc. Interface 6, S185–S192. ( 10.1098/rsif.2008.0299.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vukusic P, Stavenga DG. 2009. Physical methods for investigating structural colours in biological systems. J. R. Soc. Interface 6, S133–S148. ( 10.1098/rsif.2008.0386.focus) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bálint Z, Kertész K, Piszter G, Vértesy Z, Biró LP. 2012. The well-tuned blues: the role of structural colours as optical signals in the species recognition of a local butterfly fauna (Lepidoptera: Lycaenidae: Polyommatinae). J. R. Soc. Interface 9, 1745–1756. ( 10.1098/rsif.2011.0854) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason CW. 1926. Structural colors in insects. I . J. Phys. Chem. 30, 383–395. ( 10.1021/j150261a009) [DOI] [Google Scholar]
- 23.Mäthger LM, Bell GR, Kuzirian AM, Allen JJ, Hanlon RT. 2012. How does the blue-ringed octopus (Hapalochlaena lunulata) flash its blue rings? J. Exp. Biol. 215, 3752–3757. ( 10.1242/jeb.076869) [DOI] [PubMed] [Google Scholar]
- 24.Vignolini S, Rudall PJ, Rowland AV, Reed A, Moyroud E, Faden RB, Baumberg JJ, Glover BJ, Steiner U. 2012. Pointillist structural color in Pollia fruit. Proc. Natl Acad. Sci. USA 109, 15 712–15 715. ( 10.1073/pnas.1210105109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vigneron JP, Kertesz K, Vertesy Z, Rassart M, Lousse V, Balint Z, Biro LP. 2008. Correlated diffraction and fluorescence in the backscattering iridescence of the male butterfly Troides magellanus (Papilionidae). Phys. Rev. E 78, 021903 ( 10.1103/PhysRevE.78.021903) [DOI] [PubMed] [Google Scholar]
- 26.Lawrence C, Vukusic P, Sambles R. 2002. Grazing-incidence iridescence from a butterfly wing. Appl. Opt. 41, 437–441. ( 10.1364/AO.41.000437) [DOI] [PubMed] [Google Scholar]
- 27.Stavenga DG, Giraldo MA, Hoenders BJ. 2006. Reflectance and transmittance of light scattering scales stacked on the wings of pierid butterflies. Opt. Express 14, 4880–4890. ( 10.1364/OE.14.004880) [DOI] [PubMed] [Google Scholar]
- 28.D'Alba L, Kieffer L, Shawkey MD. 2012. Relative contributions of pigments and biophotonic nanostructures to natural color production: a case study in budgerigar (Melopsittacus undulatus) feathers. J. Exp. Biol. 215, 1272–1277. ( 10.1242/jeb.064907) [DOI] [PubMed] [Google Scholar]
- 29.Tinbergen J, Wilts BD, Stavenga DG. 2013. Spectral tuning of Amazon parrot feather coloration by psittacofulvin pigments and spongy structures. J. Exp. Biol. 216, 4358–4364. ( 10.1242/jeb.091561) [DOI] [PubMed] [Google Scholar]
- 30.Stavenga DG. 2014. Thin film and multilayer optics cause structural colors of many insects and birds. Mater. Today Proc. 1, 109–121. ( 10.1016/j.matpr.2014.09.007) [DOI] [Google Scholar]
- 31.Stavenga DG, Leertouwer HL, Wilts BD. 2014. Coloration principles of nymphaline butterflies—thin films, melanin, ommochromes and wing scale stacking. J. Exp. Biol. 217, 2171–2180. ( 10.1242/jeb.098673) [DOI] [PubMed] [Google Scholar]
- 32.Stavenga DG, Leertouwer HL, Wilts BD. 2014. The colouration toolkit of the Pipevine Swallowtail butterfly, Battus philenor: thin films, papiliochromes, and melanin. J. Comp. Phys. A 200, 547–561. ( 10.1007/s00359-014-0901-7) [DOI] [PubMed] [Google Scholar]
- 33.Kondo K, Shinkawa T, Matsuka H. 2003. Molecular systematics of birdwing butterflies (Papilionidae) inferred from mitochondrial ND5 gene. J. Lepid. Soc. 57, 17–24. [Google Scholar]
- 34.Morinaka S, Maeyama T, Maekawa K, Erniwati D, Prijono SN, Ginarsa IK, Nakazawa T, Hidaka T. 1999. Molecular phylogeny of birdwing butterflies based on the representatives in most genera of the tribe Troidini (Lepidoptera: Papilionidae). Entomol. Sci. 2, 347–358. [Google Scholar]
- 35.Koshitaka H, Kinoshita M, Vorobyev M, Arikawa K. 2008. Tetrachromacy in a butterfly that has eight varieties of spectral receptors. Proc. R. Soc. B 275, 947–954. ( 10.1098/rspb.2007.1614) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen P, Arikawa K, Yang E. 2013. Diversity of the photoreceptors and spectral opponency in the compound eye of the Golden Birdwing, Troides aeacus formosanus. PLoS ONE 8, e62240 ( 10.1371/journal.pone.0062240) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arikawa K, Wakakuwa M, Qiu X, Kurasawa M, Stavenga DG. 2005. Sexual dimorphism of short-wavelength photoreceptors in the small white butterfly, Pieris rapae crucivora. J. Neurosci. 25, 5935–5942. ( 10.1523/JNEUROSCI.1364-05.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee LP, Szema R. 2005. Inspirations from biological optics for advanced photonic systems. Science 310, 1148–1150. ( 10.1126/science.1115248) [DOI] [PubMed] [Google Scholar]
- 39.Parker AR, Townley HE. 2007. Biomimetics of photonic nanostructures. Nat. Nanotechnol. 2, 347–353. ( 10.1038/nnano.2007.152) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.