Abstract
Purpose
Use of paracetamol during pregnancy may increase the risk of asthma in offspring. The association between prenatal exposure to maternal use of paracetamol and risk of asthma was investigated.
Methods
A cohort study of 197,060 singletons born in northern Denmark in 1996–2008 was conducted, with follow-up until the end of 2009. Maternal paracetamol use during pregnancy was defined as a redeemed prescription. Asthma in offspring was defined as at least two prescriptions of both a β-agonist and an inhaled glucocorticoid and/or a hospital diagnosis of asthma during follow-up. Absolute risk of asthma in offspring was estimated using the Kaplan–Meier method and incidence rate ratios adjusted for known risk factors were estimated using Cox proportional-hazards regression.
Results
Overall, 976 (0.5%) children were exposed prenatally to maternal use of prescription paracetamol. During follow-up, 24,506 (12.4%) children developed asthma. Absolute risk of asthma was 7.5% after 2 years and 14.4% after 10 years among the unexposed children. Corresponding risks were 12.7% and 21.6% among the exposed children. The adjusted incidence rate ratio was 1.35 (95% confidence interval: 1.17–1.57) for exposure in any trimester of pregnancy. A similar association was present for paracetamol exposure in each of the trimesters and for maternal use of prescription nonsteroidal anti-inflammatory drugs. Furthermore, maternal prescription use in the year following the relevant delivery also showed similar associations.
Conclusion
A robust association was found between prenatal exposure to maternal use of prescription paracetamol and the risk of asthma; however, noncausal explanations could not be ruled out for such association.
Keywords: acetaminophen, asthma, children, paracetamol, pregnancy
Introduction
Asthma has become one of the most common diseases in children, with increasing prevalence worldwide, ranging from less than 2% in developing countries to more than 20% in industrialized countries.1–3 Although much is still unknown about the etiology of asthma, epidemiological evidence suggests a concerning association with paracetamol use in both children and adults.4–6 Paracetamol, also referred to as acetaminophen, is an analgesic commonly used by pregnant women.7,8 Based on self-reported use, prevalence of paracetamol use in pregnancy was 54% in Denmark and 69% in the US.7,9 Studies that investigated the relation between prenatal paracetamol exposure and the risk of childhood asthma and wheezing7,9–15 yielded estimates of association consistent with no or protective effect,7 and with as much as a 110% increase in risk.13 These studies, mainly based on self-reported information, were, however, likely to be limited by recall and selection bias. Therefore, a cohort study was conducted using large population-based registries, including routine electronic prescription dispensation records, to investigate the association between maternal use of prescription paracetamol and the risk of childhood asthma. Using these registries the risk of selection bias is nearly nonexistent and the information has been collected with independent recording of exposure and outcome data, thus reducing the risk of recall bias.
Methods
Study population and design
This registry-based cohort study was conducted in northern Denmark, a region which comprises 1.8 million inhabitants, representing approximately 33% of the Danish population. The study population was identified through the Danish Medical Birth Registry (MBR), which has recorded all births in Denmark since 1973.16,17 All singletons born alive in northern Denmark from January 1, 1996 to December 31, 2008 were included; in this period a complete prescription history can be established for all mothers and their children. Multiple births were not included since twinning is a risk factor for perinatal morbidity and obstetric complications.18 The children were followed from date of birth until the date of asthma diagnosis, death, emigration, or the end of follow-up on December 31, 2009 – whichever came first.
Data on paracetamol and nonsteroidal anti-inflammatory drug (NSAID) exposure
The Danish National Health Service provides universal tax-supported health coverage and refunds part of the costs of most prescribed drugs.19 Reimbursement ensues through a computerized accounting system maintained at Danish pharmacies.19 In Denmark, paracetamol is available over the counter (OTC), but can be given by prescription for chronic use, allowing for reimbursement.20 Data on maternal by-prescription paracetamol was extracted from the Aarhus University Prescription Database (AUPD), which includes data on all reimbursed medicines dispensed at community pharmacies in northern Denmark.21 AUPD records type of drug prescribed according to the Anatomical Therapeutic Chemical classification system, and the date of the dispensation. Prenatal exposure to maternal use of prescription paracetamol was defined as at least one paracetamol dispensation during pregnancy. To evaluate potential confounding by indication, data on maternal use of prescription NSAIDs were also extracted.
Data on asthma
Asthma among children was defined using an algorithm that combines use of anti-asthma medications and a hospital diagnosis of asthma.22 Hence, the asthma outcome was defined as a hospitalization, outpatient visit, or an emergency room visit with a diagnosis of asthma or a dispensation record for anti-asthma medication. For the medication-based criterion, prescriptions of both a β-agonist and an inhaled glucocorticoid were required. In patients aged 5–45 years, this algorithm has a positive predictive value of 100% for “any asthma” (including definitive asthma, wheezing, chronic obstructive pulmonary disease, or allergy) and a positive predictive value of 80% for “definitive asthma.”16,22 To ensure ongoing use of anti-asthma medication and to avert possible misclassification as asthmatics of small children with wheezing only, it was additionally required that the medication regimen be dispensed twice in order for a child to be counted as having asthma.
Diagnoses of asthma were ascertained from the Danish National Patient Registry (DNPR). The diagnoses were coded using the World Health Organization’s International Classification of Diseases, eighth revision before 1994 and International Classification of Diseases, tenth revision thereafter. The DNPR has tracked all discharges from nonpsychiatric acute care hospitals since 1977; reporting of emergency room and outpatient clinic contacts started in 1995. Prescription dispensations were ascertained from the AUPD.
Data on risk factors for asthma
From the DNPR, MBR, and the AUPD, information was collected on a priori selected risk factors for asthma in children: year of birth, county of residence, child’s sex, gestational age, birth order, delivery mode, mother’s age at delivery, maternal smoking during pregnancy, maternal use of systemic antibiotics during pregnancy, and maternal asthma, identified using the same algorithm as that for children.16,23–26 Maternal prepregnancy body mass index (BMI) was also included as this has been associated with asthma in children.27,28 BMI has been reportable to the MBR since 2004. All relevant codes are listed in the Appendix.
The Civil Personal Registration number, a personal identifier assigned to all Danish residents at birth or immigration, was used to unambiguously link records from different data sources. The Civil Personal Registration number is used in all prescription and hospital records, and a child’s birth record contains a maternal Civil Personal Registration number.29 The Danish Data Protection Agency approved the study.
Statistical analysis
Prenatal exposure was defined as at least one maternal paracetamol prescription from 30 days before the first day of the last menstrual period and until delivery. Overall exposure during gestation and trimester-specific exposure were examined: the first trimester was defined as the first 12 weeks of pregnancy counted from the first day of last menstrual period; the second and third trimesters (examined together) were defined as the remainder of the pregnancy. According to this definition, children exposed during the first trimester could also be exposed later in pregnancy, but not vice versa. The reference group was defined as children unexposed to maternal use of prescription paracetamol at any time during gestation. Last menstrual period date was estimated from the gestational age at birth, recorded in the MBR.
The Kaplan–Meier method was applied to estimate 2-year and 10-year risk of asthma according to paracetamol exposure and Cox proportional-hazards regression to estimate crude and adjusted incidence rate ratios (IRR and aIRR, respectively) with 95% confidence intervals (95% CI). The assumption of proportional hazards was assessed graphically and found appropriate.
To examine whether the association varied by severity of asthma, an analysis restricted to children with any hospital diagnosis of asthma (excluding prescriptions) was performed. Because asthma cannot be diagnosed with certainty before age 5–6 years,1,30 an analysis restricted to children with asthma diagnosed after age 5 years was also performed.
Additionally, the following analyses were conducted: computation of adjusted estimates including maternal prepregnancy BMI for the subset of children born in 2004 and onwards; a sensitivity analysis examining the impact of counting maternal paracetamol prescriptions 0 days and 60 days before last menstrual period; an analysis of an association between prenatal exposure to NSAIDs and asthma; and analyses of the association between maternal use of paracetamol in the year after birth but not during pregnancy. The two latter analyses were conducted to address confounding by indication.
Data were analyzed using SAS® (v 9.2; SAS Institute, Inc, Cary, NC).
Results
The study population consisted of a total of 197,060 children (51% boys). A total of 976 (0.5%) children were exposed prenatally to maternal use of prescription paracetamol, and 566 of these (58%) were exposed during the first trimester. Maternal use of antibiotics during pregnancy was more frequent among paracetamol-exposed children than among the unexposed children. So were maternal smoking, maternal asthma, and high maternal prepregnancy BMI (Table 1).
Table 1.
Characteristics of the study population according to prenatal exposure to maternal use of prescription paracetamol in northern Denmark 1996–2008 (N = 197,060)
| Characteristics | Exposed during gestation (N = 976)
|
Unexposed during gestation N = (196,084)
|
||
|---|---|---|---|---|
| n | % | n | % | |
| Sex of child | ||||
| Girl | 473 | 48.5 | 95,518 | 48.7 |
| Boy | 503 | 51.5 | 100,566 | 51.3 |
| Mother’s age at time of birth | ||||
| <25 | 102 | 10.5 | 24,446 | 12.5 |
| 25–29 | 299 | 30.6 | 71,578 | 36.5 |
| 30–34 | 351 | 36.0 | 69,572 | 35.5 |
| ≥35 | 224 | 23.0 | 30,488 | 15.5 |
| Prenatal exposure to antibiotics | ||||
| No | 490 | 50.2 | 133,363 | 68.0 |
| Yes | 486 | 49.8 | 62,721 | 32.0 |
| Maternal smoking during pregnancy | ||||
| Missing | 22 | 2.3 | 4086 | 2.1 |
| No | 685 | 70.1 | 154,596 | 78.8 |
| ≤10 cigarettes | 163 | 16.7 | 28,532 | 14.6 |
| >10 cigarettes | 106 | 10.9 | 8870 | 4.5 |
| Gestational age (weeks) | ||||
| <37 | 68 | 7.0 | 9714 | 5.0 |
| 37–41 | 847 | 86.8 | 171,822 | 87.6 |
| ≥42 | 61 | 6.3 | 14,548 | 7.4 |
| Birth order | ||||
| 1 | 359 | 36.8 | 80,611 | 41.1 |
| 2 | 335 | 34.3 | 74,135 | 37.8 |
| ≥3 | 282 | 28.9 | 41,338 | 21.1 |
| Maternal asthma | ||||
| No | 875 | 89.7 | 187,671 | 95.7 |
| Yes | 101 | 10.3 | 8413 | 4.3 |
| Mode of delivery | ||||
| Missing | 8 | 0.8 | 3827 | 2.0 |
| Cesarean | 263 | 27.0 | 31,945 | 16.3 |
| Vaginal | 705 | 72.2 | 160,312 | 81.8 |
| Maternal prepregnancy body mass index* | (N = 447) | (N = 78,224) | ||
| 15–19.9 | 45 | 10.1 | 10,480 | 13.4 |
| 20–24.9 | 173 | 38.7 | 40,268 | 51.5 |
| 25–29.9 | 115 | 25.7 | 17,501 | 22.4 |
| ≥30 | 114 | 25.5 | 9975 | 12.8 |
Note:
N = 78,671; data on maternal body mass index were available from 2004 onwards.
During follow-up, 24,506 (12.4%) children with asthma were identified (median follow-up time 6.8 years). The median age at asthma diagnosis was 1.6 years for unexposed children and 1.5 years for children exposed any time during gestation. Among the unexposed children, the absolute risk of asthma was 7.5% after 2 years as compared with 12.7% among the exposed children. After 10 years of follow-up, the absolute risk was 14.4% in the unexposed and 21.6% in the exposed children (Figure 1).
Figure 1.
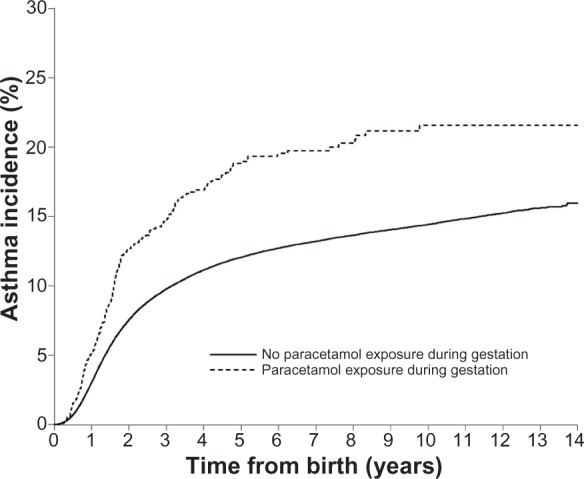
Cumulative incidence of asthma in children according to prenatal exposure to prescription paracetamol.
The risk of asthma was 35% higher (aIRR = 1.35, 95% CI: 1.17–1.57) for children prenatally exposed to maternal use of prescription paracetamol any time during gestation as compared to unexposed children after adjusting for the covariates (Table 2). The aIRR was 1.40 (95% CI: 1.16–1.71) for paracetamol exposure in the first trimester and 1.28 (95% CI: 1.02–1.62) for exposure in second/third trimester. Results did not differ substantially from the main analyses when restricted to 10,632 children with an asthma hospital contact or to 4966 children with asthma diagnoses after age 5 years (Table 2).
Table 2.
Incidence rate ratios for asthma in northern Denmark (1996–2008) for all children and children with a hospital diagnosis of asthma and children aged 5+ years according to prenatal exposure to maternal use of prescription paracetamol
| Number of children | Children with asthma n (%) | IR (per 1000 PY) | IRR (95% CI) | aIRR* (95% CI) | |
|---|---|---|---|---|---|
| All children | |||||
| No paracetamol exposure during gestation | 196,084 | 24,330 (12.4) | 19.5 | 1.00 | 1.00 |
| Paracetamol exposure during gestation | 976 | 176 (18.0) | 32.5 | 1.57 (1.35–1.82) | 1.35 (1.17–1.57) |
| Paracetamol exposure | |||||
| First trimester | 566 | 104 (18.4) | 33.6 | 1.62 (1.33–1.96) | 1.40 (1.16–1.71) |
| Second/third trimester | 410 | 72 (17.6) | 31.0 | 1.52 (1.20–1.91) | 1.28 (1.02–1.62) |
| Children with an asthma diagnosis | |||||
| No paracetamol exposure during gestation | 196,084 | 10,542 (5.4) | 8.1 | 1.00 | 1.00 |
| Paracetamol exposure during gestation | 976 | 90 (9.2) | 15.5 | 1.81 (1.47–2.23) | 1.52 (1.24–1.88) |
| Paracetamol exposure | |||||
| First trimester | 566 | 55 (9.7) | 16.6 | 1.92 (1.47–2.50) | 1.64 (1.26–2.15) |
| Second/third trimester | 410 | 35 (8.5) | 14.0 | 1.66 (1.19–2.31) | 1.37 (0.98–1.91) |
| Children aged 5+ years | |||||
| No paracetamol exposure during gestation | 129,304 | 4930 (3.8) | 9.9 | 1.00 | 1.00 |
| Paracetamol exposure during gestation | 584 | 36 (6.2) | 17.1 | 1.71 (1.23–2.37) | 1.54 (1.11–2.14) |
| Paracetamol exposure | |||||
| First trimester | 326 | 24 (7.4) | 20.0 | 2.01 (1.35–3.01) | 1.80 (1.20–2.69) |
| Second/third trimester | 258 | 12 (4.7) | 13.2 | 1.31 (0.74–2.31) | 1.20 (0.68–2.12) |
Note:
Adjustment was made for year of birth, county, sex of child, gestational age, birth order, mode of delivery, mother’s age, maternal use of antibiotics during pregnancy, maternal smoking during pregnancy, and maternal asthma.
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio; PY, person-years.
The analysis among children born in 2004 and onwards adjusting for BMI included 447 exposed and 78,224 unexposed children. A crude IRR of 1.34 (95% CI: 1.05–1.71) and an aIRR of 1.13 (95% CI: 0.88–1.44) were found for exposure any time during gestation. When examined according to trimester of exposure an aIRR of 1.20 (95% CI: 0.88–1.64) was found when exposed during first trimester and when exposed in second and/or third trimester the aIRR was 1.03 (95% CI: 0.68–1.53).
The same overall pattern was seen when redefining first-trimester exposure from 30 days before the first day of last menstrual period to 0 days and 60 days, respectively (results not shown). The results for the comparison group of children exposed prenatally to maternal use of prescription NSAIDs showed results similar to those for paracetamol (Table 3).
Table 3.
Incidence of asthma in all children born in northern Denmark 1996–2008 according to prenatal exposure to maternal use of prescription nonsteroidal anti-inflammatory drugs
| N = 197,060 | Children with asthma N = 24,506 n (%) | IR (per 1000 PY) | IRR (95% CI) | aIRR* (95% CI) | |
|---|---|---|---|---|---|
| No NSAID exposure during gestation | 187,811 | 22,958 (12.2) | 19.2 | 1.00 | 1.00 |
| NSAID exposure during gestation | 9249 | 1548 (16.7) | 28.8 | 1.43 (1.36–1.51) | 1.26 (1.20–1.33) |
| NSAID exposure | |||||
| First trimester | 7466 | 1244 (16.7) | 28.7 | 1.43 (1.35–1.51) | 1.26 (1.19–1.33) |
| Second/third trimester | 1783 | 304 (17.0) | 29.0 | 1.45 (1.30–1.63) | 1.27 (1.14–1.43) |
Note:
Adjustment was made for year of birth, county, sex of child, gestational age, birth order, mode of delivery, mother’s age, maternal use of antibiotics during pregnancy, maternal smoking during pregnancy, and maternal asthma.
Abbreviations: aIRR, adjusted incidence rate ratio; CI, confidence interval; IR, incidence rate; IRR, incidence rate ratio; NSAID, nonsteroidal anti-inflammatory drug; PY, person-years.
Finally, the analysis for maternal prescription paracetamol use in the year after delivery included 194,959 unexposed and 1125 exposed women and also showed an increased risk of asthma; crude IRR was 1.56 (95% CI: 1.36–1.79) and aIRR was 1.44 (95% CI: 1.25–1.65).
Discussion
It was found that prenatal exposure to maternal use of prescription paracetamol was associated with an increased risk of asthma in offspring. However, no clear evidence was found that this association was restricted to a specific trimester of exposure, or to prenatal exposure, as the risk of asthma was also increased in children whose mothers used paracetamol in the year after giving birth but not during pregnancy. Hence, confounding by indication could not be ruled out.
The results are similar to the findings of several published studies of maternal self-reported paracetamol use and childhood asthma and/or wheezing.5,6,9–14,31–33 The largest of them, based on the Danish National Birth Cohort, included 90,549 women of whom 66,445 participated when their child was 18 months and 12,733 when their child was 7 years old. In the 18-months-old population, 54.7% women used paracetamol during pregnancy and the risk of physician diagnosed asthma/bronchitis in the children increased by 18% as compared to children with no prenatal paracetamol exposure. In the 7-years-old population, 53.0% women used paracetamol during pregnancy and the risk of asthma in their children increased by 15%.9
Similarly, a study with nearly 14,000 women in Great Britain found that prenatal exposure to paracetamol before week 20 was associated with a 25% increased risk of childhood asthma at age 7 years and exposure later than week 20 with a 29% increase.14 Another study found no increased risk of asthma after prenatal exposure to paracetamol.7
The timing of paracetamol use during pregnancy may be relevant in regard to the possible biological mechanisms underlying asthma. However, the results for timing in the current study and other studies are conflicting, and the reason for this remains unclear. Glucuronidation, which is the main pathway for paracetamol metabolism in adults, is distinctly reduced in the fetus in first trimester. Glutathione S transferase, which detoxifies the oxidative paracetamol metabolites, is gradually reduced after week 15 of gestation.6,9,34,35 If the balance between these pathways is affected, it may predispose the offspring to increased oxidant-induced airway inflammation due to an accumulation of toxic oxidative metabolites in the fetus.36,37
Furthermore, paracetamol may affect the immune system and the development of asthma by altering antigen recognition towards favoring T helper 2 (Th2) over Th1 cytokines.38 This mechanism could be due to endocrine disrupting compounds that may be harmful to the developing immune system.39 Recently these compounds have been suggested to include nonselective cyclooxygenase inhibitors such as paracetamol, ibuprofen, and acetylsalicylic acid.40–42 Cyclooxygenase inhibitors have a structure nearly similar to phthalates,42 which in mice have been found to facilitate Th2 differentiation, production of Th2 cytokines, and enhanced levels of Th2-promoted immunoglobulins.39,43,44 This endocrine disruption hypothesis could explain the current findings of an association of both paracetamol and NSAIDs with an increased risk of asthma. The development of the fetal immune system begins in early gestation and continues after birth, which could potentially explain the increased risk observed for both first trimester and second/third trimester exposure if the observed association was indicative of a true association.45
The strengths of the current study are its large size, long and complete follow-up, and the use of data from population-based medical databases in a setting of universal health care, reducing the risk of recall bias and selection bias. The study also had limitations of which misclassification is pivotal. Without doubt, the unexposed group included women with OTC use of paracetamol. However, the authors believe this misclassification would be unrelated to the development of asthma in offspring, ie, nondifferential, and therefore the observed association is expected to represent diluted, not inflated, IRRs. It is also possible that some women, especially those with only one prescription for paracetamol, did not use all the pills. This misclassification is likely to be nondifferential as well and consequently leading to diluted IRRs. In addition, although a validated algorithm was used for identifying children with asthma, the misclassification of asthma status could still occur, for example, since anti-asthma medications are used by children with wheezing without asthma.1,16,46 If this were true it would cause an overestimation of the absolute risk of asthma. Nevertheless, restriction to children with a hospital diagnosis of asthma and children diagnosed with asthma after 5 years of age produced estimates similar to those in the main analysis.
No information was available on the children’s use of paracetamol in early life, which also has been associated with childhood asthma.5 Such use is mainly likely to be OTC use which is not obtainable from registries. Furthermore, it is expected that prescription paracetamol use during pregnancy is associated with other potential risk factors for asthma such as maternal use of alcohol during pregnancy, reduced duration of breastfeeding, and socioeconomic status, which were unable to be adjusted for in the current analysis (ie, unmeasured confounding). Accordingly, residual confounding may also have affected the results since the adjustment for some of the included covariates may have been incomplete. In addition, no direct information was available on the underlying condition or disease leading to prescription paracetamol use, which may reflect complicated conditions predisposing the children to asthma.47 Therefore, the increased risk of asthma being caused by such conditions rather than paracetamol itself cannot be ruled out. Corresponding risks of asthma among children exposed prenatally to maternal use of prescription NSAIDs were observed, indicating either a common pathway or that the association may be related to the underlying condition more than an effect of paracetamol itself. Risk of asthma was also found to be increased among children whose mothers used paracetamol after giving birth, also indicating that the results may be affected by confounding by indication. However, it is likely that some of the women without prescription use during pregnancy used OTC paracetamol during pregnancy. Moreover, the possibility that the association observed for postpartum use of paracetamol could be caused by paracetamol exposure through breastfeeding, which could not be measured, cannot be disregarded.
In conclusion, the results suggest that prenatal exposure to paracetamol increases the risk of asthma. Although a nontrivial association was found, noncausal explanations for such association cannot be ruled out.
Acknowledgments
/disclosure
The work was supported by the Clinical Epidemiology Research Foundation, Aarhus University Hospital, Denmark. The authors report no conflicts of interest in this work. The Department of Clinical Epidemiology, Aarhus University Hospital receives funding for other studies from companies in the form of research grants to (and administered by) Aarhus University. None of these studies have any relation to the present study. All authors had access to the data and played a significant role in writing the manuscript.
Appendix tables
Table A1.
Included Anatomical Therapeutic Chemical codes
| Drug | ATC-code |
|---|---|
| Paracetamol | N02BE01 |
| NSAIDs | M01A |
| β-agonist | R03AC |
| Inhaled glucocorticoid | R03BA |
| Systemic antibiotics | J01 |
Abbreviations: ATC, Anatomical Therapeutic Chemical; NSAIDs, nonsteroidal anti-inflammatory drugs.
Table A2.
Included International Classification of Diseases codes
| Diagnoses | ICD classification |
|---|---|
| Asthma | ICD-8: 493 |
| Asthma | ICD-10: J45, J46 |
Abbreviations: ICD, International Classification of Diseases; ICD-8, eighth revision; ICD-10, tenth revision.
References
- 1.Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5–34. doi: 10.1111/j.1398-9995.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355(21):2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Asher MI, Montefort S, Bjorksten B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet. 2006;368(9537):733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 4.Barr RG, Wentowski CC, Curhan GC, et al. Prospective study of acetaminophen use and newly diagnosed asthma among women. Am J Respir Crit Care Med. 2004;169(7):836–841. doi: 10.1164/rccm.200304-596OC. [DOI] [PubMed] [Google Scholar]
- 5.Etminan M, Sadatsafavi M, Jafari S, Doyle-Waters M, Aminzadeh K, Fitzgerald JM. Acetaminophen use and the risk of asthma in children and adults: a systematic review and metaanalysis. Chest. 2009;136(5):1316–1323. doi: 10.1378/chest.09-0865. [DOI] [PubMed] [Google Scholar]
- 6.Eyers S, Weatherall M, Jefferies S, Beasley R. Paracetamol in pregnancy and the risk of wheezing in offspring: a systematic review and meta-analysis. Clin Exp Allergy. 2011;41(4):482–489. doi: 10.1111/j.1365-2222.2010.03691.x. [DOI] [PubMed] [Google Scholar]
- 7.Kang EM, Lundsberg LS, Illuzzi JL, Bracken MB. Prenatal exposure to acetaminophen and asthma in children. Obstet Gynecol. 2009;114(6):1295–1306. doi: 10.1097/AOG.0b013e3181c225c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Headley J, Northstone K, Simmons H, Golding J, ALSPAC Study Team Medication use during pregnancy: data from the Avon Longitudinal Study of Parents and Children. Eur J Clin Pharmacol. 2004;60(5):355–361. doi: 10.1007/s00228-004-0775-7. [DOI] [PubMed] [Google Scholar]
- 9.Rebordosa C, Kogevinas M, Sorensen HT, Olsen J. Pre-natal exposure to paracetamol and risk of wheezing and asthma in children: a birth cohort study. Int J Epidemiol. 2008;37(3):583–590. doi: 10.1093/ije/dyn070. [DOI] [PubMed] [Google Scholar]
- 10.Shaheen SO, Newson RB, Henderson AJ, et al. Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35(1):18–25. doi: 10.1111/j.1365-2222.2005.02151.x. [DOI] [PubMed] [Google Scholar]
- 11.Koniman R, Chan YH, Tan TN, Van Bever HP. A matched patient-sibling study on the usage of paracetamol and the subsequent development of allergy and asthma. Pediatr Allergy Immunol. 2007;18(2):128–134. doi: 10.1111/j.1399-3038.2006.00484.x. [DOI] [PubMed] [Google Scholar]
- 12.Perzanowski MS, Miller RL, Tang D, et al. Prenatal acetaminophen exposure and risk of wheeze at age 5 years in an urban low-income cohort. Thorax. 2010;65(2):118–123. doi: 10.1136/thx.2009.121459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaheen SO, Newson RB, Sherriff A, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57(11):958–963. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen SO, Newson RB, Ring SM, Rose-Zerilli MJ, Holloway JW, Henderson AJ. Prenatal and infant acetaminophen exposure, antioxidant gene polymorphisms, and childhood asthma. J Allergy Clin Immunol. 2010;126(6):1141–1148.e7. doi: 10.1016/j.jaci.2010.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bakkeheim E, Mowinckel P, Carlsen KH, Haland G, Carlsen KC. Paracetamol in early infancy; the risk of childhood allergy and asthma. Acta Paediatr. 2011;100(1):90–96. doi: 10.1111/j.1651-2227.2010.01942.x. [DOI] [PubMed] [Google Scholar]
- 16.Yuan W, Fonager K, Olsen J, Sorensen HT. Prenatal factors and use of anti-asthma medications in early childhood: a population-based Danish birth cohort study. Eur J Epidemiol. 2003;18(8):763–768. doi: 10.1023/a:1025390420122. [DOI] [PubMed] [Google Scholar]
- 17.Blenstrup LT, Knudsen LB. Danish registers on aspects of reproduction. Scand J Public Health. 2011;39(7 Suppl):79–82. doi: 10.1177/1403494811399957. [DOI] [PubMed] [Google Scholar]
- 18.Obiechina N, Okolie V, Eleje G, Okechukwu Z, Anemeje O. Twin versus singleton pregnancies: the incidence, pregnancy complications, and obstetric outcomes in a Nigerian tertiary hospital. Int J Womens Health. 2011;3:227–230. doi: 10.2147/IJWH.S22059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 20.Lipworth L, Friis S, Mellemkjaer L, et al. A population-based cohort study of mortality among adults prescribed paracetamol in Denmark. J Clin Epidemiol. 2003;56(8):796–801. doi: 10.1016/s0895-4356(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 21.Ehrenstein V, Antonsen S, Pedersen L. Existing data sources for clinical epidemiology: Aarhus University Prescription Database. Clin Epidemiol. 2010;2:273–279. doi: 10.2147/CLEP.S13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osborne ML, Vollmer WM, Johnson RE, Buist AS. Use of an automated prescription database to identify individuals with asthma. J Clin Epidemiol. 1995;48(11):1393–1397. doi: 10.1016/0895-4356(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 23.Dehlink E, Yen E, Leichtner AM, Hait EJ, Fiebiger E. First evidence of a possible association between gastric acid suppression during pregnancy and childhood asthma: a population-based register study. Clin Exp Allergy. 2009;39(2):246–253. doi: 10.1111/j.1365-2222.2008.03125.x. [DOI] [PubMed] [Google Scholar]
- 24.Davidson R, Roberts SE, Wotton CJ, Goldacre MJ. Influence of maternal and perinatal factors on subsequent hospitalisation for asthma in children: evidence from the Oxford record linkage study. BMC Pulm Med. 2010;10:14. doi: 10.1186/1471-2466-10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thavagnanam S, Fleming J, Bromley A, Shields MD, Cardwell CR. A meta-analysis of the association between Caesarean section and childhood asthma. Clin Exp Allergy. 2008;38(4):629–633. doi: 10.1111/j.1365-2222.2007.02780.x. [DOI] [PubMed] [Google Scholar]
- 26.Renz-Polster H, David MR, Buist AS, et al. Caesarean section delivery and the risk of allergic disorders in childhood. Clin Exp Allergy. 2005;35(11):1466–1472. doi: 10.1111/j.1365-2222.2005.02356.x. [DOI] [PubMed] [Google Scholar]
- 27.Scholtens S, Wijga AH, Brunekreef B, et al. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes (Lond) 2010;34(4):606–613. doi: 10.1038/ijo.2009.194. [DOI] [PubMed] [Google Scholar]
- 28.Reichman NE, Nepomnyaschy L. Maternal pre-pregnancy obesity and diagnosis of asthma in offspring at age 3 years. Matern Child Health J. 2008;12(6):725–733. doi: 10.1007/s10995-007-0292-2. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(7 Suppl):22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann F, Glaeske G. Prescriptions as a proxy for asthma in children: a good choice? Eur J Clin Pharmacol. 2010;66(3):307–313. doi: 10.1007/s00228-009-0755-z. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Marcos L, Sanchez-Solis M, Perez-Fernandez V, Pastor-Vivero MD, Mondejar-Lopez P, Valverde-Molina J. Is the effect of prenatal paracetamol exposure on wheezing in preschool children modified by asthma in the mother? Int Arch Allergy Immunol. 2009;149(1):33–37. doi: 10.1159/000176304. [DOI] [PubMed] [Google Scholar]
- 32.Persky V, Piorkowski J, Hernandez E, et al. Prenatal exposure to acetaminophen and respiratory symptoms in the first year of life. Ann Allergy Asthma Immunol. 2008;101(3):271–278. doi: 10.1016/S1081-1206(10)60492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persky VW. Acetaminophen and asthma. Thorax. 2010;65(2):99–100. doi: 10.1136/thx.2009.127977. [DOI] [PubMed] [Google Scholar]
- 34.Pacifici GM. Sulfation of drugs and hormones in mid-gestation human fetus. Early Hum Dev. 2005;81(7):573–581. doi: 10.1016/j.earlhumdev.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Wilkes JM, Clark LE, Herrera JL. Acetaminophen overdose in pregnancy. South Med J. 2005;98(11):1118–1122. doi: 10.1097/01.smj.0000184792.15407.51. [DOI] [PubMed] [Google Scholar]
- 36.Chen TS, Richie JP, Jr, Lang CA. Life span profiles of glutathione and acetaminophen detoxification. Drug Metab Dispos. 1990;18(6):882–887. [PubMed] [Google Scholar]
- 37.Micheli L, Cerretani D, Fiaschi AI, Giorgi G, Romeo MR, Runci FM. Effect of acetaminophen on glutathione levels in rat testis and lung. Environ Health Perspect. 1994;102(Suppl 9):63–64. doi: 10.1289/ehp.94102s963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peterson JD, Herzenberg LA, Vasquez K, Waltenbaugh C. Glutathione levels in antigen-presenting cells modulate Th1 versus Th2 response patterns. Proc Natl Acad Sci U S A. 1998;95(6):3071–3076. doi: 10.1073/pnas.95.6.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larsson M, Hagerhed-Engman L, Kolarik B, et al. PVC – as flooring material – and its association with incident asthma in a Swedish child cohort study. Indoor Air. 2010;20(6):494–501. doi: 10.1111/j.1600-0668.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- 40.Jensen MS, Rebordosa C, Thulstrup AM, et al. Maternal use of acetaminophen, ibuprofen, and acetylsalicylic acid during pregnancy and risk of cryptorchidism. Epidemiology. 2010;21(6):779–785. doi: 10.1097/EDE.0b013e3181f20bed. [DOI] [PubMed] [Google Scholar]
- 41.Gravel A, Vijayan MM. Salicylate disrupts interrenal steroidogenesis and brain glucocorticoid receptor expression in rainbow trout. Toxicol Sci. 2006;93(1):41–49. doi: 10.1093/toxsci/kfj166. [DOI] [PubMed] [Google Scholar]
- 42.Kristensen DM, Hass U, Lesne L, et al. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod. 2011;26(1):235–244. doi: 10.1093/humrep/deq323. [DOI] [PubMed] [Google Scholar]
- 43.Jaakkola JJ, Knight TL. The role of exposure to phthalates from polyvinyl chloride products in the development of asthma and allergies: a systematic review and meta-analysis. Environ Health Perspect. 2008;116(7):845–853. doi: 10.1289/ehp.10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bornehag CG, Nanberg E. Phthalate exposure and asthma in children. Int J Androl. 2010;33(2):333–345. doi: 10.1111/j.1365-2605.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 45.Holt PG, Jones CA. The development of the immune system during pregnancy and early life. Allergy. 2000;55(8):688–697. doi: 10.1034/j.1398-9995.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 46.Hermann C, De Fine Olivarius N, Host A, Begtrup K, Hollnagel H. Prevalence, severity and determinants of asthma in Danish five-year-olds. Acta Paediatr. 2006;95(10):1182–1190. doi: 10.1080/08035250600582814. [DOI] [PubMed] [Google Scholar]
- 47.Goksor E, Thengilsdottir H, Alm B, Norvenius G, Wennergren G. Prenatal paracetamol exposure and risk of wheeze at preschool age. Acta Paediatr. 2011;100(12):1567–1571. doi: 10.1111/j.1651-2227.2011.02403.x. [DOI] [PubMed] [Google Scholar]


