Abstract
Purpose:
To evaluate the efficacy of subconjunctival bevacizumab (ScB) as adjuvant therapy to primary trabeculectomy with mitomycin C (MMC) in primary open-angle glaucoma.
Materials and Methods:
Forty-six eyes of primary open-angle glaucoma patients were randomized to receive ScB (1.25 mg/0.05 mL) injections (the MMC+ScB group) at the end of the operations, or sham-treated controls (the MMC group). Intraocular pressure (IOP) was the primary outcome and secondary outcomes included bleb appearance, visual acuity, number of medications, complications, and proportion of eyes achieving successful outcomes at the 12-month follow-up.
Results:
Of 39 eyes, 20 eyes from the MMC+ScB group, and 19 eyes from the MMC group completed the follow-up. The mean postoperative IOP was 15.5±4.1 mm Hg in the MMC+ScB group (P<0.01; 40% reduction), and 14.7±4.3 mm Hg in the MMC group (P<0.01; 44% reduction). The differences in IOPs, at all follow-up visits, were not significant (P>0.05). The mean bleb vascularity score, at 1 month, in the MMC+ScB group was lower than the MMC group (1.55±0.51 vs. 2.26±0.6, respectively, P=0.01), but was not retained at follow-ups. The success rates at 12 months after surgery were 85% in the MMC+ScB group and 89.5% in the MMC group (P=0.53). The cumulative probabilities of surgical success were 80% and 73.7% in the MMC+ScB and in the MMC group, respectively (P=0.52).
Conclusion:
Single adjunctive ScB injection did not appear to have an additive benefit on outcomes of MMC trabeculectomy, in terms of IOPs and success rates.
Key Words: trabeculectomy, anti-VEGFs, bevacizumab, bleb morphology, mitomycin C
Wound healing modulation is crucial to ensure long-term surgical success of trabeculectomy.1,2 Mitomycin C (MMC) and 5-fluorouracil have been widely used to reduce the wound healing response.2 Unfortunately, surgery still fails in some cases, and has serious adverse effects. Thus, efforts have focused on agents that are more efficacious, safer, or have synergistic effects with MMC or 5-fluorouracil as alternative agents to optimize the healing response.2
Bevacizumab (Avastin; Genentech Inc., San Francisco, CA) is a humanized nonselective monoclonal antibody against vascular endothelial growth factor (VEGF). Bevacizumab may also have utility in filtering surgery, owing to its inhibitory effect on vascular and fibroblast proliferation, resulting in decreased scarring.3,4 Recent studies investigating the utility of subconjunctival bevacizumab (ScB) injection, for wound modulation at time of trabeculectomy, reported promising results in terms of intraocular pressure (IOP) control and bleb appearance.5–11 However, it seems unlikely that the ScB alone will be as effective as MMC.7,8 The use of bevacizumab in glaucoma is currently off-label, and evidence for its efficacy in trabeculectomy, as an adjunctive with MMC, is limited.
The present study sought to compare the efficacy of a single, adjunctive, intraoperative ScB injection, or a placebo injection on the outcomes of primary trabeculectomy with MMC in primary open-angle glaucoma (POAG) patients.
MATERIALS AND METHODS
Study Design
A single-center, 12-month, masked, randomized, placebo-controlled study was carried out at the Department of Ophthalmology, Prince of Songkla University, October 2010 to December 2013. The study adhered to the tenets of the Declaration of Helsinki and was performed according to the principles of Good Clinical Practice, the guidelines on design and reporting of glaucoma surgical trials, and the Consolidated Standards of Reporting Trials (CONSORT) statement.12 The study protocol was approved by the Institutional Review Board of Prince of Songkla University. All patients provided written informed consent before participation in the study. The trial was registered at the Clinical Trials Registry (NCT01263834).
Study Population
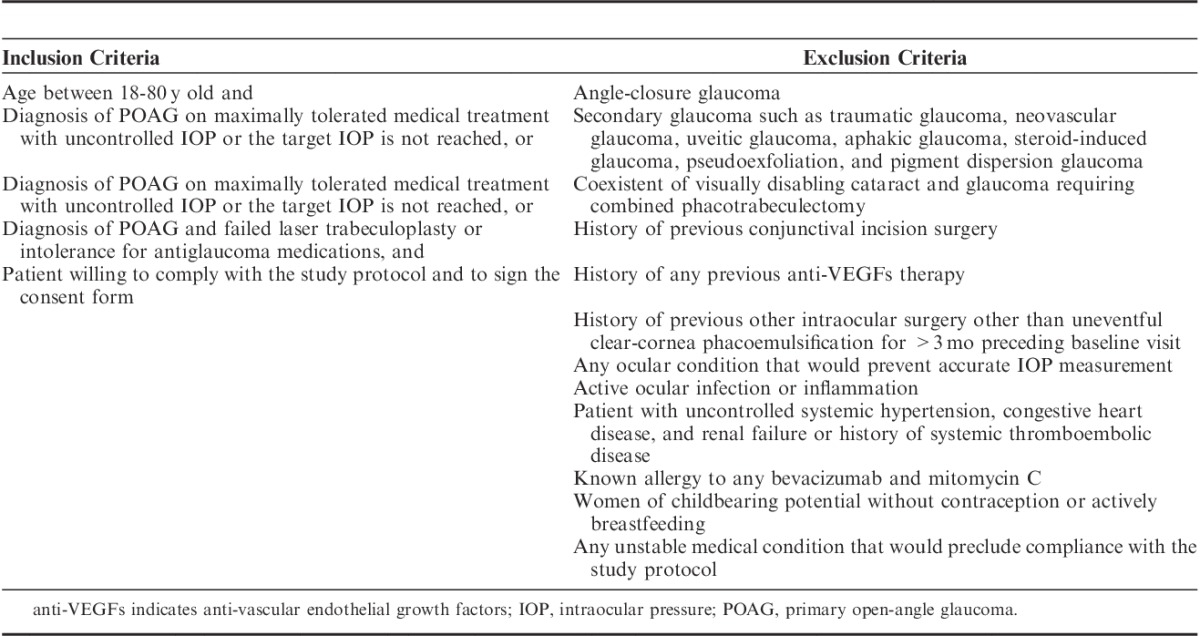
Fifty patients were assessed for eligibility, of which 46 patients satisfied the inclusion and exclusion criteria that are summarized in Table 1. Only 1 eye, if both eyes were eligible, was randomly included in the trial. Recruited patients underwent baseline assessment of best-corrected visual acuity (BCVA), IOP measured by calibrated Goldmann applanation tonometer, gonioscopy, dilated fundoscopy with a 78-D lens, and Humphrey 24-2 perimetry. The number and duration of topical antiglaucoma medications used were recorded. Fixed combinations were noted according to the active medications.
TABLE 1.
Summary of Inclusion and Exclusion Criteria for Determining Patient Eligibility
Randomization and Masking
Randomization was performed using a computer-generated randomization list. Patients were randomized, 1:1, into one of 2 study groups: those treated with trabeculectomy with MMC (0.04%) and a single-dose ScB (1.25 mg/0.05 mL) injection (the MMC+ScB group) at the end of the operation; and those treated with trabeculectomy with MMC and receiving placebo, balanced salt solution (BSS) subconjunctival injections (the MMC group). The allocation was performed using a sealed envelope system. Patients, operating physician (W.K.), and outcome assessors (L.O., K.K.) were masked to the treatment allocation during the operation, the follow-up assessment, and the bleb appearance analyses (by W.K.).
Surgical Technique
All surgeries were performed under local anesthesia, using the same technique, by a single surgeon (W.K.). An 8-0 nylon corneal traction suture was used. A fornix-based conjunctiva was dissected at the superonasal quadrant after injection of 1 mL of 2% lidocaine with epinephrine. Hemostasis was done meticulously, using a 15-degree knife to delineate and a crescent knife to dissect and create a half-thickness, 4×5 mm, rectangular-shaped scleral flap. In all eyes, 3 pieces of MMC 0.04% (0.4 mg/mL) (Kyowa Hokko Kirin Co. Ltd., Tokyo, Japan) soaked Weck-Cel sponges (Inami Co. Ltd., Tokyo, Japan) were applied in the Tenon’s capsule pocket, and under the sclera flap, for 3 minutes. The sponges were then removed and the surgical area was rinsed with 30 mL BSS. Two diagonal scleral flap sutures were preplaced, using 10-0 nylon. A corneal paracentesis was made using the 15-degree knife. No viscoelastic was injected into the anterior chamber. Sclerectomy was performed with a Kelly-Descemet’s punch, and peripheral iridectomy was performed with a Vannas scissors (Katena Products Inc., NJ). The scleral flap was approximated with 3 interrupted 10-0 nylon sutures. The conjunctiva was closed with 2 interrupted 10-0 nylon wing sutures.
The trabeculectomy with MMC was performed in the same manner in both the groups. In the MMC+ScB group, at the end of the operation, 1.25 mg/0.05 mL of bevacizumab was injected subconjunctivally, over the scleral flap area, with a 30-G needle. The needle entrance was at least 8 mm away from the bleb to prevent any needle track leakage. In the MMC group, 0.05 mL of BSS was injected, using the same procedure. Postoperative treatment included topical antibiotic eyedrops (4 times daily, for 1 wk), topical 1% prednisolone acetate (every 2 h, for 2 wk, then decreased, over 6 to 8 wk). Antiglaucoma medications were discontinued on the day of surgery. After surgery, ocular massage, laser suture lysis (LSL), antiglaucoma medications, and postoperative procedures such as needling were permitted if necessary, depending on the target IOP, and based on the physician’s discretion.
Follow-up Evaluations and Outcome Measures
Follow-up visits were scheduled on day 1; at weeks 1 and 2; and at months 1, 3, 6, and 12 postoperatively. The primary outcome measure was the IOP. Secondary outcome measures included BCVA, bleb appearance, numbers of antiglaucoma medications used, postoperative interventions and complications, and proportion of eyes achieving successful outcomes at the 12-month follow-up. Two measurements of IOP were taken and averaged to determine the mean IOP. Bleb photographs were obtained at months 1, 3, 6, and 12. The bleb appearances were graded by a masked investigator (W.K.), as per the Indiana Bleb Appearance System (IBAS), which is based on bleb height, extent, vascularity, and Seidel test results (H0-3, E0-3, V0-4, S0-2).13
A treatment success criterion was defined as the following: (1) an IOP≤target IOP (21 or 14 mm Hg) if preoperative IOP>21 mm Hg; or (2) IOP≥20% reduction from baseline if preoperative IOP≤21 mm Hg. Success was defined as “complete” when these criteria were achieved without any glaucoma medications and as “qualified” with or without medication. Failure of the treatment was defined as IOP<6 mm Hg or IOP>target IOP despite medication, or needing MMC, or further glaucoma surgery, or as a loss of light perception, or development of adverse events that needed treatment in the operating room, such as suturing bleb leakage and anterior chamber reformation. Ocular massage, LSL, and needling without antifibrotic agent injection, were not considered as failures.
Statistical Analysis
A sample size of at least 18 patients in each arm was estimated to give an 80% power of detecting at least a difference of 3 mm Hg between groups with the SD of 1 mm Hg as significant at the 2-sided 5% level. Adjusting for a possible dropout rate of 20%, we planned to recruit 22 eyes per group. Descriptive statistics were used to summarize patient demographics and baseline ocular characteristics. Categorical variables were analyzed using Pearson χ2 test. Fisher exact test was used for comparison of proportions. Paired and independent t tests were used to evaluate within-group and between-group mean differences, respectively. Repeated measures of 1-way analysis of variance were used for IOP comparisons. Nonparametric data were analyzed using the Mann-Whitney test. The cumulative probability of success was assessed with the Kaplan-Meier survival analysis with log-rank test. The analyses were performed with Stata software (version 12; StataCorp, College Station, TX) and MedCalc statistical software (version 13.0.2; MedCalc Software, Ostend, Belgium). P values <0.05 were considered statistically significant.
RESULTS
Figure 1 lists the flow of patients through the study. Of the 39 eyes completing the 12-month follow-up, 20 eyes were in the MMC+ScB group and 19 eyes were in the MMC group. Demographic and baseline characteristics are summarized in Table 2. No statistically significant differences were found between the groups, except the duration of antiglaucoma medication use before surgery. The durations of the medical treatments in the MMC+ScB group and the MMC group were 20.5±8.9 months [95% confidence interval (CI), 16.3-24.7] and 14.8±5.7 months (95% CI, 12.1-17.6), respectively (P=0.04).
FIGURE 1.
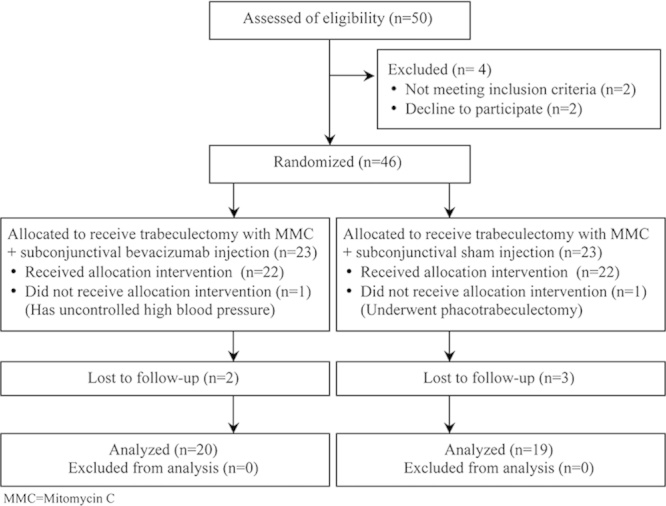
Flow diagram according to the Consolidated Standards of Reporting Trials (CONSORT) statement, showing recruitment, randomization, and patient flow in this study.
TABLE 2.
Patient Demographics and Baseline Ocular Characteristics of the Study Eyes in the Groups Receiving Subconjunctival Bevacizumab Injection Versus Placebo Adjunctive to MMC Trabeculectomy
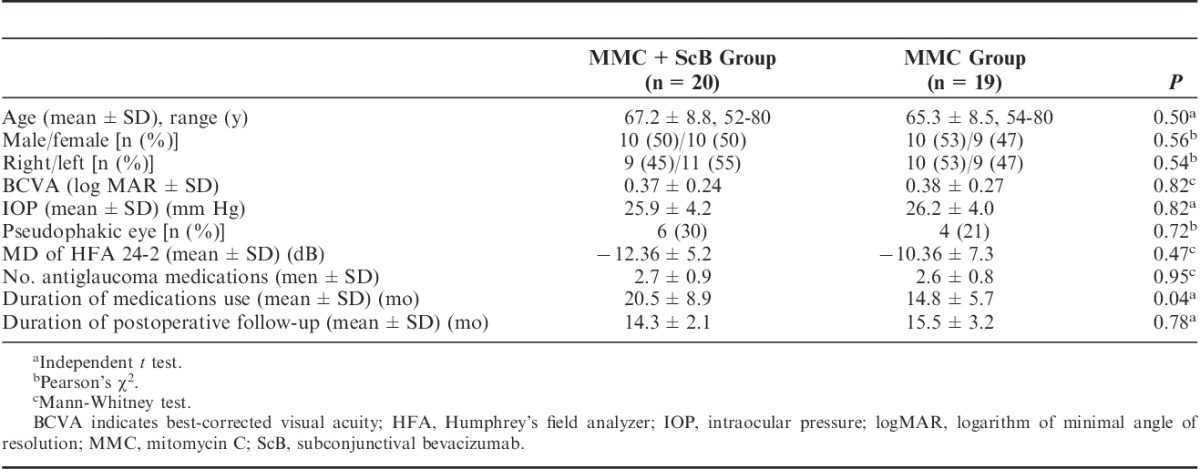
Figure 2 shows the mean IOP measurements and P values determined at each visit, in both groups. Mean preoperative IOP levels were comparable in both groups (MMC+ScB group, 25.9±4.2 mm Hg; MMC group, 26.2±4.0 mm Hg; P=0.82). One year after surgery, the mean IOP improved significantly to 15.5±4.1 mm Hg in the MMC+ScB group (P<0.01, 40% reduction) and to 14.7±4.3 mm Hg (P<0.01, 44% reduction) in the MMC group. The difference between the IOPs in both groups, during the follow-ups, including the last visit, was not statistically significant (P>0.05).
FIGURE 2.
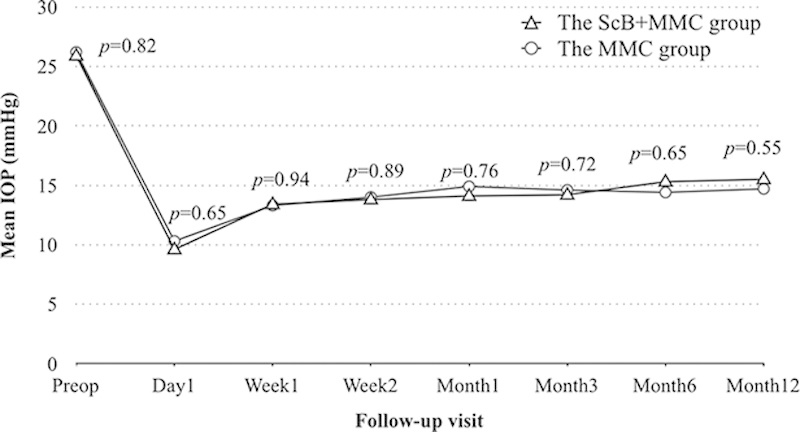
Mean intraocular pressure (IOP) and P values throughout the follow-up visits, in both groups.MMC indicates mitomycin C; ScB, subconjunctival bevacizumab.
After the surgery, no significant difference was detected in BCVA between the groups at the 12-month follow-up (P=0.90, Table 3). The number of antiglaucoma medications dropped from 2.7±0.9 before surgery, to 0.6±0.7, at month 12 (P<0.001), in the MMC+ScB group, and from 2.6±0.8 to 0.5±0.7, at month 12 (P<0.001), in the MMC group. No statistically significant differences were found between the groups in number of medications at 12 months (P=0.92).
TABLE 3.
Comparison of Postoperative Outcome Measures in the Groups Receiving Subconjunctival Bevacizumab Injection versus Placebo Adjunctive to MMC Trabeculectomy
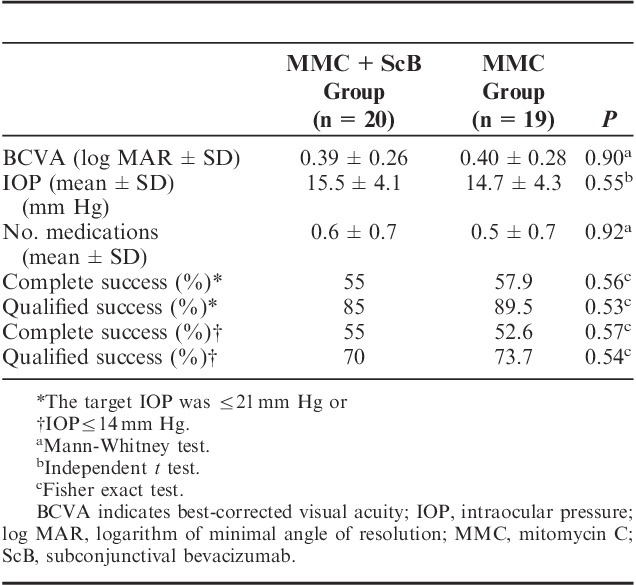
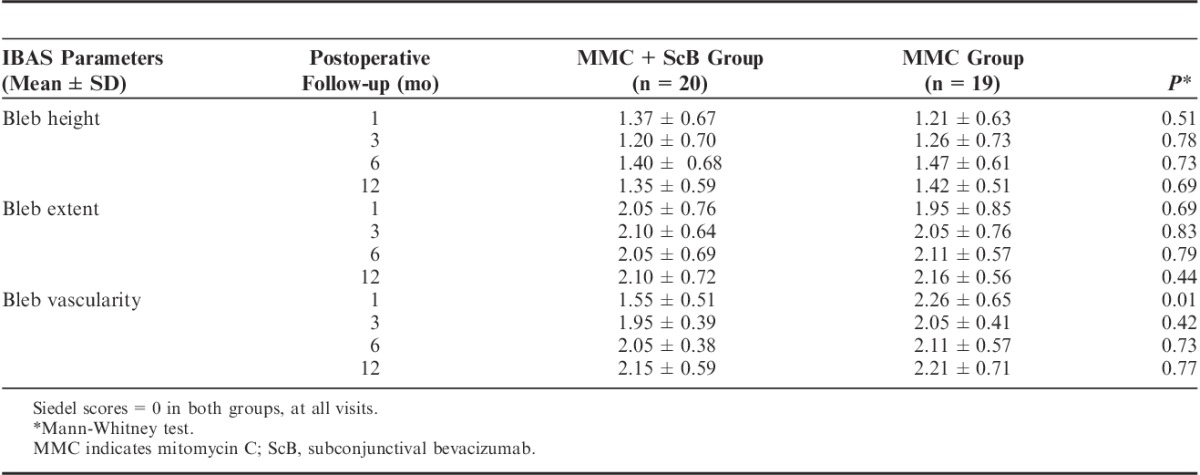
Table 4 demonstrates the bleb appearances according to the IBAS at 1, 3, 6, and 12 months, postoperatively. There were no statistically significant differences in bleb height, bleb extent, and leakage scores between the 2 groups. However, the vascularity scores of the MMC+ScB group were significantly lower when compared with the MMC group scores at the 1-month follow-up (1.55±0.51 vs. 2.26±0.65, P=0.01). This was not observed at 3-, 6-, and 12-month follow-ups.
TABLE 4.
Bleb Morphologic Scores at 1, 3, 6, and 12-mo Follow-up in Groups, According to the Standard Indiana Bleb Appearance System (IBAS)
The proportions of eyes achieving successful outcomes at 12 months, in both groups, are listed in Table 3. When using the criteria that was defined as an IOP≤21 mm Hg (if preoperative IOP>21 mm Hg), or IOP reduction was ≥20% from baseline (if preoperative IOP≤21 mm Hg) as a success, complete success was obtained in 55% and 57.9% of the MMC+ScB group and the MMC groups, respectively (P=0.56). A qualified success was obtained in 85% and 89.5% of the MMC+ScB group and the MMC group, respectively (P=0.53). The cumulative probabilities of surgical success at 12 months were 80% and 73.7% in the MMC+ScB group and in the MMC group, respectively (P=0.52, log-rank test; Fig. 3). Failures at the 12-month follow-up were observed in 3 and 2 eyes in the MMC+ScB group and the MMC group, respectively, and were due to bleb failures that required further glaucoma surgery.
FIGURE 3.
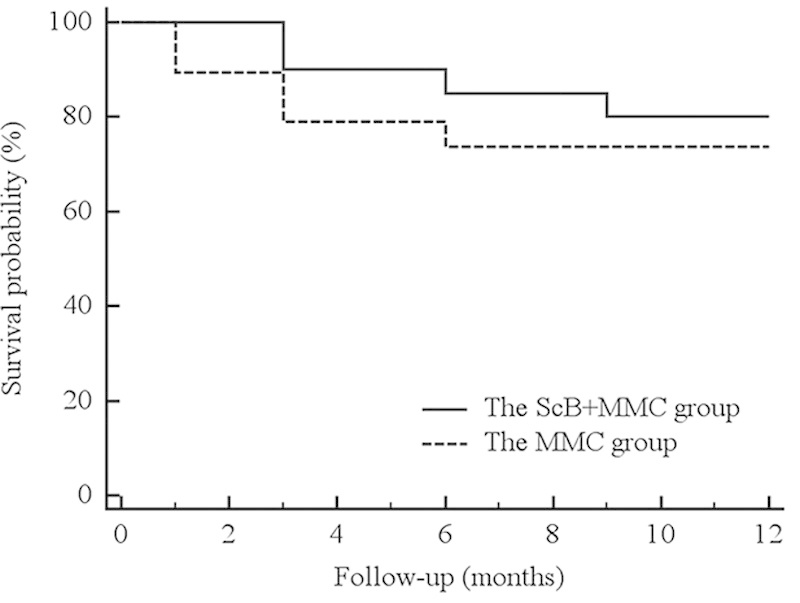
Kaplan-Meier survival curve analyses showing the cumulative probabilities of success in both treatment groups during the 12-month follow-up period. Log-rank test identified nonsignificant differences between the 2 groups (P=0.52). MMC indicates mitomycin C; ScB, subconjunctival bevacizumab.
None of the patients had intraoperative or systemic complications. Five eyes (25%) and 4 eyes (21%) in the MMC+ScB group and the MMC group, respectively, required LSL, all occurring 1 to 2 weeks after surgery. An encapsulated bleb was seen in 4 eyes (20%) and in 3 eyes (15.8%) in the MMC+ScB group and the MMC group, respectively (P=0.34). In the MMC+ScB group, treatment with MMC (0.02%) was performed in all cases, 3 months after surgery. Of 4 eyes, 1 eye had a central cystic avascular bleb without any leakage, 3 months after needling. In the MMC group, MMC needling was performed in all cases at 1 to 3 months postoperatively. One of these cases developed hypotony (IOP of 4 mm Hg) without choroidal effusion, and improved gradually in 1 month without any further intervention. No flat anterior chamber, aqueous misdirection, bleb leakage, and bleb-related infection were encountered in either group.
DISCUSSION
VEGF played a key role in the proliferative phase of wound healing, and VEGF levels were also found to be elevated in aqueous from POAG patients and posttrabeculectomy.2,14 After application of anti-VEGF antibody, the concentration of VEGF was significantly reduced.4,15 Therefore, VEGF antibody, may be beneficial in POAG patients treated by trabeculectomy. Thus far, no comparative study has investigated the synergistic effects of intraoperative ScB injection, in conjunction with MMC, in trabeculectomy. We prospectively studied 39 POAG patients who underwent primary trabeculectomy with MMC, with or without intraoperative ScB injection, during a 12-month follow-up period. We observed an approximately 40% reduction of IOPs in both groups. Nevertheless, the cumulative probabilities of the surgical success at 12 months was higher in the ScB+MMC group (80%), when compared with the MMC group (73.7%), but was not statistically significant (P=0.52).
The intraoperative use of bevacizumab for trabeculectomy in nonrefractory glaucoma focused on 3 routes of administration including soaked sponges,16 intracameral injection,6 and subconjunctival injection.5,7–9 The optimum route of administration remains unclear, however, the studies showed that intracameral and subconjunctival injection worked well for filtration surgery.9,16,17 Few studies have investigated the use of intraoperative ScB injection in primary trabeculectomy.5,7,8,16 Most studies focused on the use of ScB alone, compared with MMC. The common dosages used were 1.25 mg/0.05 mL5,9,16 and 2.5 mg/0.1 mL.7,8 Grewal et al5 reported results from a noncomparative interventional case series. Twelve glaucoma patients received a single-dose ScB injection (1.25 mg/0.05 mL), at the time of primary trabeculectomy, without using MMC. The study demonstrated the potential benefit of bevacizumab-augmented trabeculectomy. Nilforushan et al7 and Akkan and Cilsim8 have recently reported similar findings, in a prospective, randomized, comparative studies, that a single ScB injection (2.5 mg/0.1 mL), at time of trabeculectomy without using MMC, provided favorable IOP control, but it was less effective when compared with the group receiving MMC (0.02% for 3 min). Therefore, it seems unlikely that bevacizumab, as a stand-alone treatment, will be as effective as MMC, when used as an augmentative agent in trabeculectomy.
There was also evidence suggesting that anti-VEGF agents may have a synergistic effect with MMC.18,19 Biteli and Prata9 reported, in a small noncomparative case series, that 1.25 mg ScB injection, combined with MMC in trabeculectomy, was safe and effective.9 Similar to the present study, a 43% reduction of IOP at the last follow-up was observed. The qualified success rates at 12 months had a range of 84% to 96%. Their success rates were better than those of the ScB+MMC group in our study, and were possibly because of the higher dosage and the longer duration of MMC application. Our study used a fixed dose of 0.4 mg/mL MMC, applied for 3 minutes, for all cases, to reduce surgical technique variations. The study of Biteli et al9 was limited by the lack of a control group. In contrast, the present study was a direct comparison of MMC trabeculectomy, with or without additional ScB injections. An additional benefit of ScB injection on IOP reduction and success rate was not found.
The duration of antiglaucoma medication in the present study may have adversely affected the outcome of treatment in the MMC+ScB group. The mean preoperative duration of antiglaucoma medication in the MMC+ScB group was higher than that of the MMC group (P=0.04). These differences were significant, however, given the overlap of the 95% CI (16.3-24.7 vs. 12.1-17.6, respectively). A recent study has not shown a relationship between duration of glaucoma treatment and surgical outcomes in first-time trabeculectomy.20
Regarding the effect of anti-VEGF on bleb appearances, several studies determined the impact of ScB injection on postoperative bleb vascularity.5,7–11,18,21 Li et al4 reported, in a rabbit model, that ScB (2.5 mg) had beneficial effects on bleb area, but not on IOP. Grewal et al5 reported bleb appearance, using the Moorfields Bleb Grading System (MBGS), in human eyes, after trabeculectomy with ScB injection. Bleb-related complications, such as thin cystic bleb leakage, and bleb-related infections were not observed, and the area of blebs decreased over time, but not the height or vascularity. A possible adverse effect of ScB on the trabeculectomy bleb was reported in the study of Akkan and Cilsim.8 They found that, within 1 month postoperatively, encapsulated bleb formation was not significant, and was observed in 29% of the bevacizumab group, and in 10% of the MMC group (P=0.23). However, at the end of the study, significant differences in bleb area, height, and vascularity were not observed. There is no study using a high dose of ScB (2.5 mg) plus MMC, in trabeculectomy. Biteli and Prata9 used a 1.25-mg ScB injection, combined with MMC, and reported that no avascular bleb was observed. As reported in a previous study,9 and because of safety concerns, we determined the synergistic effects of ScB plus MMC by using the lower dose (1.25 mg) of bevacizumab.
According to the parameters defined by IBAS, we did not find significant differences in bleb height, bleb extent, and leakage scores between the 2 groups. Interestingly, the mean vascularity score of the MMC+ScB group was significantly lower than the MMC group at the 1-month follow-up (1.55±0.51 vs. 2.26±0.65, P=0.01). The effect on bleb vascularity was not retained throughout the follow-up visits after a single injection of ScB. In contrast to the study by Akkan and Cilsim,8 we did not observe a high incidence of bleb encapsulation in the MMC+ScB group. The trend of bleb vascularity score in our study was similar to the study of Sengupta et al.16 They studied the use of ScB (1.25 mg) alone, compared with soaked-sponge bevacizumab, and with MMC alone, in phacotrabeculectomy. Bleb vascularity of the ScB group was significantly lower when compared with other groups, at 1 month after ScB injections (using the IBAS scoring system, P=0.05), and showed gradually increasing vascularization, thereafter. To maintain therapeutic concentrations, a total of 3 injections of ScB were given immediately before surgery, immediately after surgery, and on postoperative day 7.16 Li et al4 showed that bevacizumab reduced the number of both human and rabbit fibroblasts in a dose-dependent manner. We hypothesized that the use of a higher dose of ScB (2.5 mg), in association with MMC, would have better results on trabeculectomy and bleb appearance outcomes.
To the best of our knowledge, the present study is the first randomized placebo-controlled trial that investigated the synergistic effects of ScB as an adjunct to trabeculectomy with MMC. The major limitations of the study were the small sample size and the short duration of follow-up. With the criteria for inclusion and exclusion, the study findings may not be applicable for other types of glaucoma. On the basis of the results of the bleb vascularity grading, in the first postoperative month, in the MMC+ScB group, multiple injections of ScB in the early postoperative period may sustain bleb survival. Further studies are warranted to investigate the utility of postoperative multiple injections of ScB as an addition to its intraoperative use in MMC trabeculectomy. The optimum bevacizumab dosage and timing to repeated injections should be further evaluated.
In conclusion, a single subconjunctival injection of bevacizumab, when given intraoperatively, and adjunctive to trabeculectomy with MMC, was safe, but did not appear to have an additive benefit, when compared with MMC alone, in reducing IOPs and improving success rates. However, patients receiving bevacizumab-augmented MMC trabeculectomy may experience benefits on bleb vascularity, at least for 1 month postoperatively.
ACKNOWLEDGMENTS
The authors thank the Faculty of Medicine, Prince of Songkla University for funded support.
Footnotes
Supported by the Faculty of Medicine, Prince of Songkla University.
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Azuara-Blanco A, Katz LJ. Dysfunctional filtering blebs. Surv Ophthalmol. 1998;43:93–126. [DOI] [PubMed] [Google Scholar]
- 2.Seibold LK, Sherwood MB, Kahook MY. Wound modulation after filtration surgery. Surv Ophthalmol. 2012;57:530–550. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 4.Li Z, Van Bergen T, Van de Veire S, et al. Inhibition of vascular endothelial growth factor reduces scar formation after glaucoma filtration surgery. Invest Ophthalmol Vis Sci. 2009;50:5217–5225. [DOI] [PubMed] [Google Scholar]
- 5.Grewal DS, Jain R, Kumar H, et al. Evaluation of subconjunctival bevacizumab as an adjunct to trabeculectomy a pilot study. Ophthalmology. 2008;115:2141–2145. [DOI] [PubMed] [Google Scholar]
- 6.Vandewalle E, Abegão Pinto L, Van Bergen T, et al. Intracameral bevacizumab as an adjunct to trabeculectomy: a 1-year prospective, randomized study. Br J Ophthalmol. 2014;98:73–78. [DOI] [PubMed] [Google Scholar]
- 7.Nilforushan N, Yadgari M, Kish SK, et al. Subconjunctival bevacizumab versus mitomycin C adjunctive to trabeculectomy. Am J Ophthalmol. 2012;153:352–357. [DOI] [PubMed] [Google Scholar]
- 8.Akkan JU, Cilsim S. Role of subconjunctival bevacizumab as an adjuvant to primary trabeculectomy: a prospective randomized comparative 1-year follow-up study. J Glaucoma. 2014[Epub ahead of print]. doi: 10.1097/IJG.0b013e318287abf3. http://journals.lww.com/glaucomajournal/Abstract/publishahead/Role_of_Subconjunctival_Bevacizumab_as_an_Adjuvant.99418.aspx. [DOI] [PubMed] [Google Scholar]
- 9.Biteli LG, Prata TS. Subconjunctival bevacizumab as an adjuvant in first-time filtration surgery for patients with primary glaucoma. Int Ophthalmol. 2013;33:741–746. [DOI] [PubMed] [Google Scholar]
- 10.Coote MA, Ruddle JB, Qin Q, et al. Vascular changes after intra-bleb injection of bevacizumab. J Glaucoma. 2008;17:517–518. [DOI] [PubMed] [Google Scholar]
- 11.Freiberg FJ, Matlach J, Grehn F, et al. Postoperative subconjunctival bevacizumab injection as an adjunct to 5-fluorouracil in the management of scarring after trabeculectomy. Clin Ophthalmol. 2013;7:1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomized trials. J Clin Epidemiol. 2010;63:e1–e37. [DOI] [PubMed] [Google Scholar]
- 13.Cantor LB, Mantravadi A, WuDunn D, et al. Morphologic classification of filtering blebs after glaucoma filtration surgery: the Indiana Bleb Appearance Grading Scale. J Glaucoma. 2003;12:266–271. [DOI] [PubMed] [Google Scholar]
- 14.Skuta GL, Parrish RK., II Wound healing in glaucoma filtering surgery. Surv Ophthalmol. 1987;32:149–170. [DOI] [PubMed] [Google Scholar]
- 15.Hu D-N, Ritch R, Liebmann J, et al. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J Glaucoma. 2002;11:406–410. [DOI] [PubMed] [Google Scholar]
- 16.Sengupta S, Venkatesh R, Ravindran RD. Safety and efficacy of using off-label bevacizumab versus mitomycin C to prevent bleb failure in a single-site phacotrabeculectomy by a randomized controlled clinical trial. J Glaucoma. 2012;21:450–459. [DOI] [PubMed] [Google Scholar]
- 17.Nomoto H, Shiraga F, Kuno N, et al. Pharmacokinetics of bevacizumab after topical, subconjunctival, and intravitreal administration in rabbits. Invest Ophthalmol Vis Sci. 2009;50:4807–4813. [DOI] [PubMed] [Google Scholar]
- 18.Kahook MY. Bleb morphology and vascularity after trabeculectomy with intravitreal ranibizumab: a pilot study. Am J Ophthalmol. 2010;150:399–403e1. [DOI] [PubMed] [Google Scholar]
- 19.Jue A. Angiogenesis: trabeculectomy and bevacizumab. Semin Ophthalmol. 2009;24:122–125. [DOI] [PubMed] [Google Scholar]
- 20.Edmunds B, Bunce CV, Thompson JR, et al. Factors associated with success in first-time trabeculectomy for patients at low risk of failure with chronic open-angle glaucoma. Ophthalmology. 2004;111:97–103. [DOI] [PubMed] [Google Scholar]
- 21.Chua BE, Nguyen DQ, Qin Q, et al. Bleb vascularity following post-trabeculectomy subconjunctival bevacizumab: a pilot study. Clin Experiment Ophthalmol. 2012;40:773–779. [DOI] [PubMed] [Google Scholar]


