Abstract
Cyclin B3 is a relatively new member of the cyclin family whose functions are little known. We found that depletion of cyclin B3 inhibited metaphase-anaphase transition as indicated by a well-sustained MI spindle and cyclin B1 expression in meiotic oocytes after extended culture. This effect was independent of spindle assembly checkpoint activity, since both Bub3 and BubR1 signals were not observed at kinetochores in MI-arrested cells. The metaphase I arrest was not rescued by either Mad2 knockdown or cdc20 overexpression, but it was rescued by securin RNAi. We conclude that cyclin B3 controls the metaphase-anaphase transition by activating APC/Ccdc20 in meiotic oocytes, a process that does not rely on SAC activity.
Key words: Cyclin B3, oocyte, meiosis, SAC, anaphase initiation
Introduction
The proliferation of all eukaryotic cells depends on the E3 ubiqutin ligase activity of the anaphase promoting complex/cyclosome (APC/C), as without it cells would not enter anaphase and sister chromatids would not separate. The main co-factors of APC/C are Cdh1 and Cdc20; they are the direct activators of APC/C, and they share the same sequence: C-box1 and IR-tail 2,3, both mediating their binding to APC/C, and WD40 domain helping to recognize the D-box4 and KEN-box5 in the substrates of APC/C.6 APC/C is a very large complex of 1.5 MDa and it has over 10 subunits; the core subunits have cullin and Ring-finger domains, which are the markers of E3 ubiquitin ligase.7-9 The Apc2's cullin domain associates with Apc11's Ring-finger domain3,10,11 and interacts with E2 ligase.11,12 Without considering the complexity of APC/C, Apc11 itself can perform E3 ligase activity in vitro, indicating that other subunits may not be essential for E3 function.2,10,12
In both mitosis and meiosis, there are several checkpoints that control cell cycle progression. One of them, the spindle assembly checkpoint (SAC) senses the existence of kinetochores not being attached to spindle microtubules or not being under tension; if the checkpoint is activated, cells are not able to continue mitosis or meiosis. Through this mechanism a cell ensures accurate chromosome segregation to maintain genomic stability, thus avoiding aneuploidy.13-17 The key target of SAC is Cdc20, the activator of APC/C, and if Cdc20 is inhibited the APC/C will not be able to perform ubiquitin ligase activity thus cyclin B1 and securin remain stable, thereby delaying the anaphase onset and mitotic exit.18,19
SAC proteins recruited onto kinetochores and other multiple components form a large complex between kinetochores and microtubules. The components of SAC are conserved from yeast to humans, indicating that this pathway has been consistent throughout evolution. Aurora B kinase and Mps1 are at the top of this pathway and they are thought to regulate each other,20-23 the downstream components Bub1, BubR1, Bub3, Mad1 and Mad2 form 3 complexes: Bub1-Bub3, BubR1-Bub3 and Mad1-Mad2.24 They are recruited to kinetochores in a KMN-dependent manner,25-27 and when the kinetochore is unattached, the checkpoint is activated.
The function of SAC that inhibits APC/C is mainly related to the activity of the mitotic checkpoint complex (MCC), consisting of BubR1-Bub3, Cdc20 and Mad2.24 When Cdc20 forms the MCC, Mad2 and BubR1 change Cdc20 binding to APC/C, and Cdc20 loses its function as an activator. Cdc20 is the activator of APC/C in anaphase when it is released from the complex. On the other hand, knockdown of Mad2 causes precocious chromosome separation and cells divide ahead of schedule.28
Cyclin B3 is a newly discovered cyclin, and its functions are little known. One study reports that its activity regulates leptotene and zygotene events such as recombination and synapsis in meiotic cells,29 while another report shows that its accurate expression is required for mitotic anaphase onset in C. elegans.30 Cyclin B3-depleted C. elegans embryos have defects in mitotic entry and their SAC proteins and dynein accumulate on chromosomes. But aside from these few findings our understanding of cyclin B3s function to date is very limited.
To reveal the functions of cyclin B3 in meiotic cells, we conducted a series of experiments in mouse oocytes. When cyclin B3 was knocked down, oocytes did not undergo anaphase onset during first meiosis. Unlike in C. elegans, cyclin B3-depleted mouse oocytes showed SAC inactivation, while cyclin B1 remained un-degraded; so we conclude that cyclin B3 affects APC/C activity.
Results
Cylin B3 depletion does not affect GVBD, but arrests oocytes at the MI stage in the presence of normal spindle and chromosome alignment
To clarify the expression of cyclin B3, we collected oocytes at the germinal vesicle (GV), germinal vesicle break down (GVBD), metaphase I (MI), anaphase I, and metaphase II stages. Because a working antibody is not available, we used real-time PCR (RT-PCR) to measure cyclin B3 mRNA expression. We found that in mouse oocytes cyclin B3 expression increased about 3 to fold4- in metaphase compared with the GV stage (Fig. 1A), indicating that cyclin B3 may have functions after GVBD.
Figure 1.

Cyclin B3 regulates meiotic anaphase onset. (A) Oocytes in 4 different maturation stages were collected and used for RT-PCR, each sample contained 100 oocytes. The polyline showed cyclin B3 mRNA changing tendency. (B) Oocytes were injected with mixed cyclin B3 siRNAs (50 µM), incubated in M2 medium containing Milrinone for 24 h, then oocytes were washed with Milrinone-free M2 medium to resume meiosis. Knockdown efficiency was measured through RT-PCR. (C) In cyclin B3-depleted oocytes, polar body extrusion rate was observed after 14h of maturation; the RNAi group contained 236 oocytes and the control group contained 241 oocytes. (D) Cyclin B3 RNAi oocytes cultured for 14h were fixed and used for confocal imaging to show the spindles and chromosomes. Bar = 20 µM.
SiRNAs (50 μM) were injected into GV oocytes to investigate the effects of cyclin B3 depletion. To verify the knockdown efficiency, we mixed 3 siRNAs for injection, and 65.3 ± 1.7% of cyclin B3 mRNA was depleted (p < 0.05) (Fig. 1B). RNAi oocytes went through the GVBD stages, but they hardly could complete first meiosis, and the polar body extrusion (PBE) rate was only 5.8 ± 3.08 % (Fig. 1C). In contrast, control oocytes injected with ddH2O displayed high rates of GVBD and PBE, and the difference was significant (p < 0.05). This phenotype shows that cyclin B3 is a critical regulator of oocyte maturation.
Immunofluorescence staining showed that the spindles of RNAi-injected oocytes were still arrested at metaphase I (Fig. 1D) at 14 hour of culture, which is the time when normal oocytes had extruded their first polar body. Both chromosomes and microtubules were the same as in MI oocytes, and this phenotype remained until 24 hour of culture. Overall, our results show that cyclin B3 depletion does not destroy spindle structure, but blocks oocytes in metaphase.
Cyclin B3 knockdown causes metaphase-anaphase transition failure in a SAC-independent pathway
As cyclin B3 knockdown blocked oocytes in metaphase I, we wondered if cyclin B3 is essential for inactivation of SAC function. In mouse oocytes, SAC contains Bub1, BubR1, Bub3 and Mad2, and they assemble on kinetochores as dot signals when analyzed with immunofluorescence microscopy. To analyze SAC in clear detail we applied chromosome spread staining and Bub3 antibody staining. At 6 hour of culture, both cyclin B3 RNAi oocytes and control oocytes were observed in typical pro-metaphase and Bub3 was located on kinetochores (Fig. 2A). Unexpectedly, both groups lost Bub3 signal on kinetochores at 9 hour of culture, indicating that SAC was inactivated in cyclin B3 RNAi oocytes (Fig. 2A). To confirm the phenotype we incubated the oocytes with BubR1 antibody, and obtained the same result (Fig. 2B). Through these experiments we conclude that cyclin B3 regulates metaphase-anaphase transition through a SAC-independent pathway.
Figure 2.

SAC activity in cyclin B3 RNAi ooctyes. (A) Cyclin B3 RNAi oocytes and control oocytes were used for chromosome spread staining at 6h and 9h of culture. Bub3 (green fluorescence) was used as marker of SAC; the red crosses are metaphase chromosomes stained by PI. (B) BubR1 (green fluorescence) staining was used to confirm the phenotype.
Knockdown of Mad2 does not rescue meiotic defects caused by RNAi cyclin B3 depletion
Because we found that SAC inactivation was not affected by cyclin B3 depletion, the mechanism(s) underlying metaphase arrest might be related to later events. Next to the SAC checkpoint, it is the APC/C that controls the metaphase-anaphase progression for which the prerequisites are degradation of cyclin B1 and securin, and inactivation of MPF. Western blotting showed that both cyclin B1 expression and p-CDK1 in control oocytes had already been degraded while in cyclin B3 RNAi oocytes they remained un-degraded (Fig. 3A) at 9.5 hour of culture, providing direct evidence for the correlation between APC/C activation and cyclin B3 function.
Figure 3.
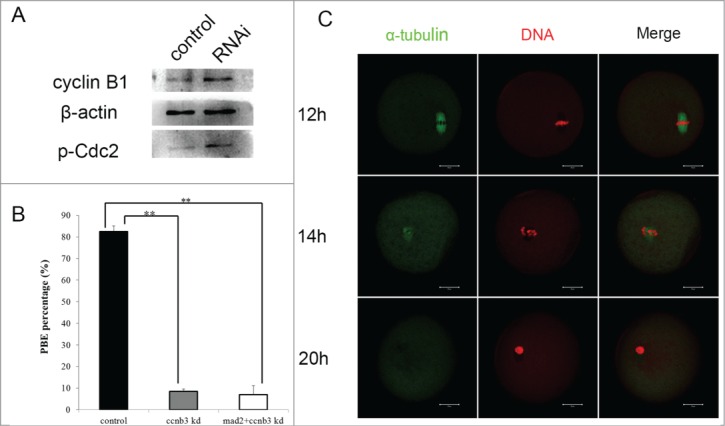
(A) Cyclin B3 RNAi oocytes and normal oocytes at 9h of culture were collected and used for WB. Cyclin B1 and p-CDK1 first antibodies were incubated to show the MPF activity and β-actin was the reference protein. (B) Cyclin B3 and Mad2 siRNAs were mixed and injected into GV oocytes at a concentration of 50 µM, and oocytes were incubated in M2 medium containing Milrinone for 24 h, then they were washed in Milrinone-free M2 medium to resume meiosis. After 14 h the PBE was analyzed. The RNAi group contained 217 oocytes and the control group contained 194 oocytes. (C) Cylin B3 RNAi oocytes were collected at different times of culture, 12 h, 14 h and 20 h, then used for confocal imaging; α-tubulin was incubated with antibody displaying green fluorescence and DNA was detected with PtdIns staining. Bar = 20 µM
In general, APC/C is activated by the regulatory subunit Cdc20 and inhibited by Emi2. Cdc20 forms MCC with SAC components like Mad2 and inhibits APC/C in normal cells; we therefore wondered if cyclin B3 might promote MCC degradation. So we knocked down Mad2 and released Cdc20 from MCC, considering that it might activate APC/C. We mixed cyclin B3 and Mad2 siRNAs (50 μM) and injected the mixture into GV oocytes. After more than 14 hours of culture we analyzed the maturation rates. The oocyte maturation rate in the co-RNAi group (6.9 ± 4.2%) showed no difference to that of the cyclin B3 knockdown group (8.5 ± 1.0%), but was significantly lower than that of the control group (82.5 ± 2.7%) (Fig. 3B). Though oocytes did not recover to progress through maturation, knockdown of Mad2 caused apoptosis as indicated by chromatin condensation (Fig. 3C). These results further confirm that cyclin B3 activates APC/C downstream of the SAC checkpoint system.
Cdc20 overexpression cannot rescue cyclin B3 knockdown phenotype but securin RNAi can rescue the phenotype
Our experiments revealed the function of cyclin B3 in the meiotic cell cycle and we tried to explain the mechanism, but we could only tell that cyclin B3 did not interact with SAC to control the activation of APC/C. If we could activate APC/C directly and rescue the phenotype of metaphase I arrest, we would be able to narrow down and detail the scope of the investigation, so we applied Cdc20 overexpression in cyclin B3 RNAi oocytes. Because cyclin B3 siRNA requires an incubation time for about 24 hours, but mRNA usually only requires 2–4 hours to take effect, we tested if the cdc20-myc mRNA injection time would make a difference. We showed that a reaction for 24 hours showed the same effect as a reaction for 4 hours, so we mixed cyclin B3 siRNA and cdc20-myc mRNA for injection and blocked oocytes at prophase for 24 hour, and we then released the inhibition. Unexpectedly, Cdc20 overexpression could not increase the oocyte maturation rate, and there was no difference between cyclin B3 knockdown oocytes (4.4 ± 1.2%) and cyclin B3 knockdown plus Cdc20 overexpression oocytes (3.6 ± 2.7%) (Fig. 4A). IF staining was used to show the expression and location of Cdc20-myc protein (Fig. 4B). We found that Cdc20-myc (green fluorescence) was expressed significantly, and it was mainly located on the spindle. To test APC/C activity we performed another western blotting experiment and analyzed the cyclin B1 level at 9 hour of culture. We could observe an obvious signal of Cdc20-myc in Cdc20 overexpression plus cyclin B3 RNAi oocytes and cyclin B1 was not degraded, while it was degraded in the control group (Fig. 4C). The results indicate that in cyclin B3 knockdown oocytes, APC/C could not be activated only by Cdc20 overexpression.
Figure 4.

The rescue of cyclin B3 RNAi phenotype. (A) GV oocytes were injected with cyclin B3 siRNA (50 µM) either alone or with cdc20 mRNA (5 mg/ml), incubated in M2 medium containing Milrinone for 24 h, then oocytes were washed in Milrinone-free M2 medium to resume meiosis. PBE rates were analyzed at 14 h of culture. The cyclin B3 group contained 224 oocytes, the mixed group contained 189 oocytes and the control group contained 207 oocytes. (B) Oocytes injected with cdc20 mRNA were incubated with FITC-myc antibody; IF was used to show Cdc20 expression and location. (C) Cyclin B3 siRNA plus cdc20 mRNA injected oocytes and control oocytes were collected at 9 h of culture and protein gel blotting was performed. Cyclin B1 showed the MPF activity, myc antibody showed expression of injected cdc20 mRNA and β-actin was stained as reference protein. (D) Cyclin B3 siRNA and securin siRNA were mixed to a concentration of 50 µM and injected into GV oocytes; cyclin B3 alone injected oocytes and normal oocytes were used as control. Oocytes were incubated in M2 medium containing Milrinone for 24 h, then they were washed in Milrinone-free M2 medium to resume meiosis. PBE rates were analyzed at 14 h of culture. The cyclin B3 group contained 211 oocytes, the mixed group contained 208 oocytes and the control group contained 227 oocytes. Bar = 20 µM.
To further clarify the mechanisms for the metaphase I arrest in cyclin B3 depleted oocytes, we set out to rescue the phenotype by artificially depleting the substrates of APC/C. As the substrates of APC/C were mainly cyclin B1 and securin, and cyclin B1 was essential for GVBD, we only knocked down securin. SiRNAs were mixed to a concentration of 50 μM, and after injection the oocytes were blocked for 24 hours at the GV stage. After release and culture for 12 hours we calculated the PBE rate of each group, and obtained an interesting result: 62.8 ± 6.8% of the oocytes in which both cyclin B3 and securin were knocked down completed maturation (Fig. 4D), thus the phenotype caused by cyclin B3 depletion was rescued. These results confirmed that in cyclin B3 knockdown oocytes, the metaphase I arrest was caused by APC/C inactivation, and that cyclin B3 was an activator of APC/C, but the detailed mechanisms of its function still remain to be clarified.
Discussion
It is generally accepted that the anaphase onset is strictly controlled by SAC both in mitosis and meiosis. When chromosomes are not properly attached to kinetochores the checkpoint system is activated, which inhibits APC/C from degrading cyclin B1 and securin, thus delaying metaphase-to-anaphase transition. Once SAC is satisfied Cdc20 is released from MCC and APC/C is activated to ubiquinate cyclin B1 and securin for degradation, and thus separase is activated to cleave cohesin, followed by anaphase initiation. Here, we found a new molecular mechanism controlling the metaphase-to-anaphase transition in meiosis: cyclin B3 is also a strict controlling component of the anaphase onset, a process that is independent of SAC. Depletion of cyclin B3 results in oocyte arrest at metaphase I and this arrest is not controlled by SAC but by directAPC/C inactivation.
Studies of cyclin B3 have been scarse and sometimes results are inconsistent and confusing. Through our study we show that cyclin B3 has multiple functions because it is the activator of APC/C and APC/C plays roles in both prophase and anaphase. The expression pattern of cyclin B3 in oocytes exhibited a typical feature of a protein being activated in metaphase or anaphase, and cyclin B3 RNAi caused a phenotype of metaphase I arrest. Metaphase arrest is typically caused by unattached chromosomes, which activates SAC to delay the anaphase onset; this at first prompted us to hypothesize that cyclin B3 might be the inhibitor of SAC, either related to its transport or to its disassembly (inactivation). However, further experiments showed that this was not the case because neither SAC transport nor disassembly had been affected by cyclin B3 depletion. SAC disappeared from kinetochores at the correct time after reaching metaphase I, and Mad2 RNAi could not rescue the phenotype of metaphase I arrest. So we doubt the idea that cyclin B3 interacts with SAC protein and dynein, as reported in a previous study.30
Here we propose that cyclin B3 activates APC/C directly after metaphase, then Cdc20 can combine with APC/C, forming the functional APC/Ccdc20 to promote cell cycle progression. APC/C is composed of 12 subunits in humans, and its structure is so complicated that we know little about it except for its 3D image. There are many phosphorylation sites on these subunits that either positively or negatively control the complex. We think that cyclin B3 may take part in some of the phosphorylation reactions. Apc2 and Apc 11 together are the catalytic domain3,31 and they can perform functions like E3 ligase in vitro, but in vivo their activity is obviously controlled by multiple mechanisms, the classical Cdh1/Cdc20 activation mode and our proposed cyclin B3 control mode. Like other cyclins, cyclin B3 must have its partner. Molecules that interact with cyclins are mainly CDKs; the typical Cdks are Cdk1 and Cdk2. Cdk1 is known to combine with cyclin B1 to form MPF, while Cdk2 interacts with cyclin A. Cyclin B3 has both cyclin A and B domains so it is possible for both CDKs to be its partner. Because MPF is not inactivated in our research, it indicates that cyclin B3 acts earlier than Cdk1 releases from MPF. When we knocked down securin the oocytes completed maturation. There are reports in buddying yeast that Cdk1 phosphorylates securin and protects it from being degraded by APC/C.32-34 However, in human cells Cdk1 is less important in the metaphase-anaphase transition, and the main mechanism is that securin and cyclin B1 inhibit separase activity to protect cohesion so that chromosomes are not able to separate.34,35 Considering others' findings and our results we conclude that securin is the main molecule that protects cohesion.
In summary, our study provides evidence showing that cyclin B3 is a critical regulator of APC/C activity and anaphase onset in oocyte meiosis, and its effect is independent of SAC activity.
Materials and methods
Ethics statement
Mouse handling was conducted following policies promulgated by the Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences. ICR mice were housed in the animal core facility which holds a license from the experimental animal committee of the city of Beijing.
Mouse oocyte collection and in vitro maturation
We isolated GV stage oocytes from minced ovaries from 8 to 10 week-old ICR female mice. Oocytes were cultured in M2 medium for at least 12 hours. The culture was conducted in an incubator under environmental conditions of 5% CO2, 37°C, and saturated humidity.
RNA interference
The GV oocytes were microinjected with 5-10 pl of siRNA in M2 medium containing 2.5 uM Milrinone, cyclin B3-1 siRNA GCAGCAGGCUAUUACUAAAtt, cyclin B3-2 siRNA GGCCCUCUAUAUGAGGAAUtt, cyclin B3-3 siRNA GAUCAGUGUUUGAAGAUGUtt, Mad2l1 siRNA GGACUCACCUUGCUUACAAtt, securin siRNA GAUGAUGCCUACCCAGAAAtt, control siRNA UUCUCCGAACGUGUCACGUtt, the concentration of siRNAs was 50 µM. Injected oocytes were incubated in M2 medium containing Milrinone for 24h, then oocytes were washed in Milrinone-free M2 medium and allowed to resume meiosis and further maturation.
Real-time PCR
Total RNA was extracted from 100 oocytes using RNeasy micro purification kit (Qiagen). Single-strand cDNA generated with the cDNA synthesis kit (Takara), using polyT primers. The cDNAs were used as templates to amplify cyclin B3 and Ppia using the following primers cyclin B3: 5′-GAAGCAACCCATACAAAGAAGCC-3′ (forward) and 5′-TTGTCTGGCAGTACAGATGGC-3′ (reverse). Real-time PCR was performed using SYBR Premix (Kangwei) in Roche Light Cycler 480. Analysis of relative gene expression was measured by real-time quantitative PCR and the 2(−Delta DeltaC(T)) method.
Plasmid construction and Cdc20-myc overexpression
We used oocyte total RNA to conduct reverse transcription and generate cDNA as above, cdc20 DNA was amplified using nest PCR. The outer primers were 5′-TGTGTTCGGGAGAGCTGAGTACG-3′ (forward) and 5′-GGAAGATACGAACTTTATTAGA-3′ (reverse), the primers with restriction enzyme site were 5′-CGAATTCGCGCTTGGTCGCCTTTCGC-3′ (forward with EcoRI site) and 5′-CCGGCGCGCCGGAAGATACGAACTTTATTAGA-3′ (reverse with AscI site). The 1592bp fragment was then recombined into EcoR1 and Asc1 cutted pCS2+-myc plasmid, transfected into Trans10 competent cells (TransGene). Plasmids were extracted from bacteria using TIANprep Mini Plasmid Kit (TIANGEN) and linearized by SalI then purified by Gel and PCR Clean-Up System (Promega). We used Sp6 MmessagemMACHINE kit (Qiagen) to produce capped mRNA and we used RNeasy cleanup kit (Qiagen) to purify it. mRNA was dissolved in nuclease-free water to a concentration of 5.0 mg/ml before injection into oocytes. Injected oocytes were incubated in M2 medium containing Milrinone for 2h (changes in different conditions), then oocytes were washed in Milrinone-free M2 medium and allowed to resume meiosis and maturation.
Immunofluorescence (IF) labeling
Oocytes were first fixed in 4% paraformaldehyde at room temperature for 30 min, and then permeabilized in PBS containing 0.5% Triton X-100 for 20 min. Next, oocytes were blocked in PBS with 1% BSA for 1h, and then incubated with first antibody at 4°C overnight. After washing 3 times, they were incubated with second antibody at room temperature for 1 h. DNA was stained with PI. The samples were then mounted on slides. The antibodies used included FITC-α-tubulin (F2618, Sigma), FITC-myc (R953–25, Invitrogen) and Bub 3(sc-28258, Santa Cruz). The oocytes were observed under a laser-scanning confocal microscope (Zeiss LSM 710, Germany). At least 40 oocytes were examined in each treatment, and each treatment was repeated 3 times.
Western blotting analysis
First we gathered at least 150 oocytes and boiled for 5 min with loading buffer; then the proteins were separated by 10% SDS-PAGE and electrically transferred to polyvinylidene fluoride membranes. Following this the membranes were blocked in 5% BSA dissolved in TBST for 2 h at room temperature, then incubated with first antibodies overnight at 4°C. After washing 3 times in TBST, 10 min each time, the membranes were incubated for 1 h at 37°C with secondary antibodies. Finally the membranes were imaged using the enhanced chemiluminescence detection system.
Chromosome spread staining
The chromosome spread was performed as described previously (Hodges and Hunt, 2002). Briefly, the oocytes were exposed to acid Tyrode's solution (Sigma) to remove the zona pellucida; the whole process was monitored under the microscope to avoid over-digestion. After a brief recovery in M2 medium, the oocytes were transferred onto glass slides and fixed in a solution of 1% paraformaldehyde in distilled H2O (pH 9.2) containing 0.15% Triton X-100 and 3 mM dithiothreitol. The slides were allowed to dry slowly in a humidified chamber for several hours, and then blocked with 1% BSA in PBS for 1h at room temperature or overnight at 4°C and incubated with primary antibodies of anti-Bub3 or anti-BubR1 (1:50), overnight at 4°C. After brief washes with washing buffer, the slides were then incubated with corresponding secondary antibodies for 2h at room temperature. DNA on the slides was stained with PtdIns for 10 min and slides were mounted for immunofluorescence microscopy observation.
Data analysis
All experiments were repeated at least 3 times. Statistical analysis was performed using Student's T test and shown as mean ± SEM.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Shi-Wen Li and Hua Qin for their technical help with confocal laser microscopy. We also thank the other members in Dr. Sun's laboratory for their kind discussions and help.
Note
While we were preparing this manuscript, a paper was published to show that cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition (Yuan K, O'Farrell PH. Cyclin B3 is a mitotic cyclin that promotes the metaphase-anaphase transition. Curr Biol 2015; 25(6):811–6). Our conclusion in meiosis is similar to that in mitosis.
Funding
This study was supported by the National Basic Research Program of China (No 2012CB944404, 2011CB944501) and the National Natural Science Foundation of China (No.31371451).
References
- 1.Schwab M, Neutzner M, Mocker D, Seufert W. Yeast Hct1 recognizes the mitotic cyclin Clb2 and other substrates of the ubiquitin ligase APC. The EMBO J 2001; 20:5165-75; PMID:11566880; http://dx.doi.org/ 10.1093/emboj/20.18.5165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passmore LA, McCormack EA, Au SW, Paul A, Willison KR, Harper JW, Barford D. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J 2003; 22:786-96; PMID:12574115; http://dx.doi.org/ 10.1093/emboj/cdg084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vodermaier HC, Gieffers C, Maurer-Stroh S, Eisenhaber F, Peters JM. TPR subunits of the anaphase-promoting complex mediate binding to the activator protein CDH1. Curr biol 2003; 13:1459-68; PMID:12956947; http://dx.doi.org/ 10.1016/S0960-9822(03)00581-5 [DOI] [PubMed] [Google Scholar]
- 4.Glotzer M, Murray AW, Kirschner MW. Cyclin is degraded by the ubiquitin pathway. Nature 1991; 349:132-8; PMID:1846030; http://dx.doi.org/ 10.1038/349132a0 [DOI] [PubMed] [Google Scholar]
- 5.Pfleger CM, Kirschner MW. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev 2000; 14:655-65; PMID:10733526 [PMC free article] [PubMed] [Google Scholar]
- 6.Kraft C, Vodermaier HC, Maurer-Stroh S, Eisenhaber F, Peters JM. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol Cell 2005; 18:543-53; PMID:15916961; http://dx.doi.org/ 10.1016/j.molcel.2005.04.023 [DOI] [PubMed] [Google Scholar]
- 7.Kramer KM, Fesquet D, Johnson AL, Johnston LH. Budding yeast RSI1/APC2, a novel gene necessary for initiation of anaphase, encodes an APC subunit. EMBO J 1998; 17:498-506; PMID:9430641; http://dx.doi.org/ 10.1093/emboj/17.2.498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu H, Peters JM, King RW, Page AM, Hieter P, Kirschner MW. Identification of a cullin homology region in a subunit of the anaphase-promoting complex. Science 1998; 279:1219-22; PMID:9469815; http://dx.doi.org/ 10.1126/science.279.5354.1219 [DOI] [PubMed] [Google Scholar]
- 9.Zachariae W, Shevchenko A, Andrews PD, Ciosk R, Galova M, Stark MJ, Mann M, Nasmyth K. Mass spectrometric analysis of the anaphase-promoting complex from yeast: identification of a subunit related to cullins. Science 1998; 279:1216-9; PMID:9469814; http://dx.doi.org/ 10.1126/science.279.5354.1216 [DOI] [PubMed] [Google Scholar]
- 10.Tang Z, Li B, Bharadwaj R, Zhu H, Ozkan E, Hakala K, Deisenhofer J, Yu H. APC2 Cullin protein and APC11 RING protein comprise the minimal ubiquitin ligase module of the anaphase-promoting complex. Mol Biol Cell 2001; 12:3839-51; PMID:11739784; http://dx.doi.org/ 10.1091/mbc.12.12.3839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gmachl M, Gieffers C, Podtelejnikov AV, Mann M, Peters JM. The RING-H2 finger protein APC11 and the E2 enzyme UBC4 are sufficient to ubiquitinate substrates of the anaphase-promoting complex. Proc Natl Acad Sci U S A 2000; 97:8973-8; PMID:10922056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leverson JD, Joazeiro CA, Page AM, Huang H, Hieter P, Hunter T. The APC11 RING-H2 finger mediates E2-dependent ubiquitination. Mol Biol Cell 2000; 11:2315-25; PMID:10888670; http://dx.doi.org/ 10.1091/mbc.11.7.2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bharadwaj R, Yu H. The spindle checkpoint, aneuploidy, and cancer. Oncogene 2004; 23:2016-27; PMID:15021889; http://dx.doi.org/ 10.1038/sj.onc.1207374 [DOI] [PubMed] [Google Scholar]
- 14.Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat Rev Mol Cell Biol 2007; 8:379-93; PMID:17426725; http://dx.doi.org/ 10.1038/nrm2163 [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Yu H. Mutual regulation between the spindle checkpoint and APC/C. Semin Cell Dev Biol 2011; 22:551-8; PMID:21439394; http://dx.doi.org/ 10.1016/j.semcdb.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lara-Gonzalez P, Westhorpe FG, Taylor SS. The spindle assembly checkpoint. Curr Biol 2012; 22:R966-80; PMID:23174302; http://dx.doi.org/ 10.1016/j.cub.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 17.Foley EA, Kapoor TM. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nat Rev Mol Cell biol 2013; 14:25-37; PMID:23258294; http://dx.doi.org/ 10.1038/nrm3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell biol 2006; 7:644-56; PMID:16896351 [DOI] [PubMed] [Google Scholar]
- 19.Yu H. Cdc20: a WD40 activator for a cell cycle degradation machine. Mol Cell 2007; 27:3-16; PMID:17612486; http://dx.doi.org/ 10.1016/j.molcel.2007.06.009 [DOI] [PubMed] [Google Scholar]
- 20.Sun SC, Lee SE, Xu YN, Kim NH. Perturbation of Spc25 expression affects meiotic spindle organization, chromosome alignment and spindle assembly checkpoint in mouse oocytes. Cell Cycle 2010; 9:4552-9; PMID:21084868 [DOI] [PubMed] [Google Scholar]
- 21.Saurin AT, van der Waal MS, Medema RH, Lens SM, Kops GJ. Aurora B potentiates Mps1 activation to ensure rapid checkpoint establishment at the onset of mitosis. Nat Commun 2011; 2:316; PMID:21587233; http://dx.doi.org/ 10.1038/ncomms1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van der Waal MS, Saurin AT, Vromans MJ, Vleugel M, Wurzenberger C, Gerlich DW, Medema RH, Kops GJ, Lens SM. Mps1 promotes rapid centromere accumulation of Aurora B. EMBO Rep 2012; 13:847-54; PMID:22732840; http://dx.doi.org/ 10.1038/embor.2012.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heinrich S, Windecker H, Hustedt N, Hauf S. Mph1 kinetochore localization is crucial and upstream in the hierarchy of spindle assembly checkpoint protein recruitment to kinetochores. J Cell Sci 2012; 125:4720-7; PMID:22825872; http://dx.doi.org/ 10.1242/jcs.110387 [DOI] [PubMed] [Google Scholar]
- 24.Jia L, Kim S, Yu H. Tracking spindle checkpoint signals from kinetochores to APC/C. Trends Biochem Sci 2013; 38:302-11; PMID:23598156; http://dx.doi.org/ 10.1016/j.tibs.2013.03.004 [DOI] [PubMed] [Google Scholar]
- 25.Liu D, Vader G, Vromans MJ, Lampson MA, Lens SM. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science 2009; 323:1350-3; PMID:19150808; http://dx.doi.org/ 10.1126/science.1167000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang E, Ballister ER, Lampson MA. Aurora B dynamics at centromeres create a diffusion-based phosphorylation gradient. Journal Cell Biol 2011; 194:539-49; PMID:21844210; http://dx.doi.org/ 10.1083/jcb.201103044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Zhang B, Su H, Birchler JA, Han F. Molecular mechanisms of homologous chromosome pairing and segregation in plants. J Genetics Genomics = Yi chuan xue bao 2014; 41:117-23; PMID:24656232 [DOI] [PubMed] [Google Scholar]
- 28.Tsuchiya D, Gonzalez C, Lacefield S. The spindle checkpoint protein Mad2 regulates APC/C activity during prometaphase and metaphase of meiosis I in Saccharomyces cerevisiae. Mol Biol Cell 2011; 22:2848-61; PMID:21697504; http://dx.doi.org/ 10.1091/mbc.E11-04-0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Refik-Rogers J, Manova K, Koff A. Misexpression of cyclin B3 leads to aberrant spermatogenesis. Cell Cycle 2006; 5:1966-73; PMID:16929180; http://dx.doi.org/ 10.4161/cc.5.17.3137 [DOI] [PubMed] [Google Scholar]
- 30.Deyter GM, Furuta T, Kurasawa Y, Schumacher JM. Caenorhabditis elegans cyclin B3 is required for multiple mitotic processes including alleviation of a spindle checkpoint-dependent block in anaphase chromosome segregation. PLoS Genet 2010; 6:e1001218; PMID:21124864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thornton BR, Ng TM, Matyskiela ME, Carroll CW, Morgan DO, Toczyski DP. An architectural map of the anaphase-promoting complex. Genes Dev 2006; 20:449-60; PMID:16481473; http://dx.doi.org/ 10.1101/gad.1396906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilioti Z, Chung YS, Mochizuki Y, Hardy CF, Cohen-Fix O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr Biol 2001; 11:1347-52; PMID:11553328; http://dx.doi.org/ 10.1016/S0960-9822(01)00399-2 [DOI] [PubMed] [Google Scholar]
- 33.Holt LJ, Krutchinsky AN, Morgan DO. Positive feedback sharpens the anaphase switch. Nature 2008; 454:353-7; PMID:18552837; http://dx.doi.org/ 10.1038/nature07050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shindo N, Kumada K, Hirota T. Separase sensor reveals dual roles for separase coordinating cohesin cleavage and cdk1 inhibition. Dev Cell 2012; 23:112-23; PMID:22814604; http://dx.doi.org/ 10.1016/j.devcel.2012.06.015 [DOI] [PubMed] [Google Scholar]
- 35.Meadows JC, Millar JB. Sharpening the anaphase switch. Biochem Soc Trans 2015; 43:19-22; PMID:25619242; http://dx.doi.org/ 10.1042/BST20140250 [DOI] [PubMed] [Google Scholar]


