Abstract
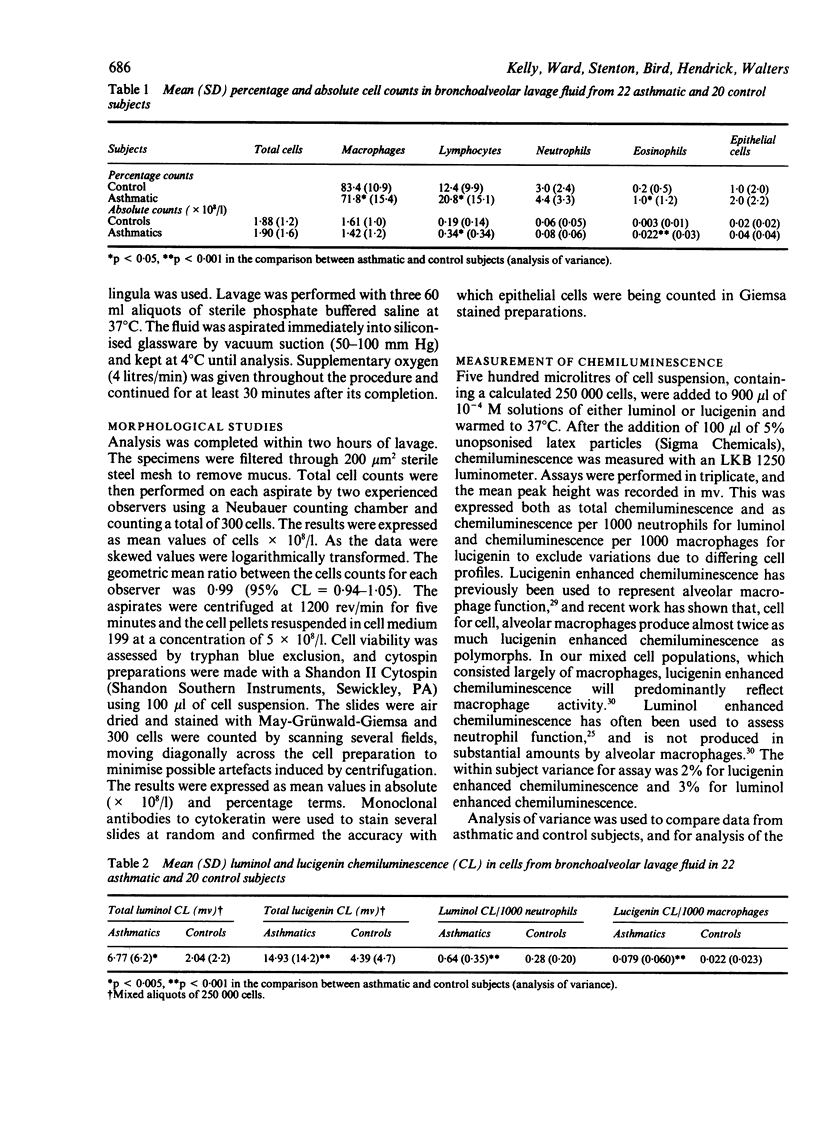
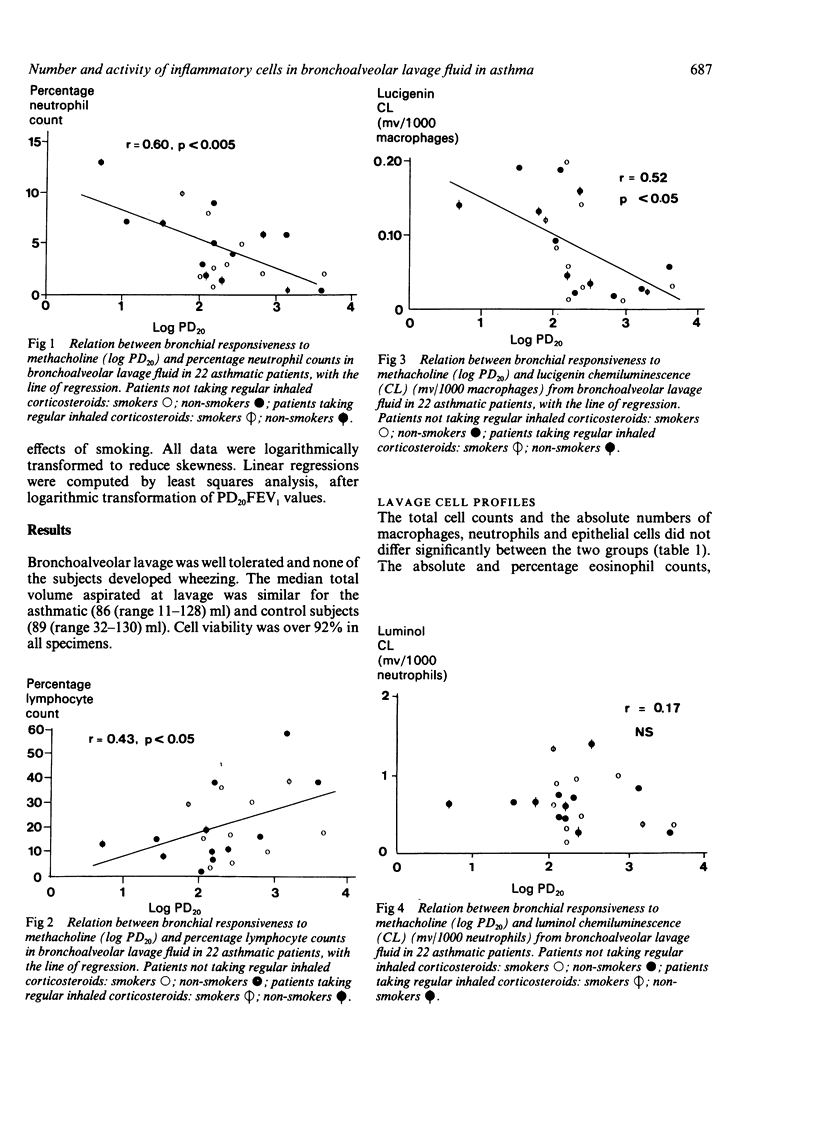
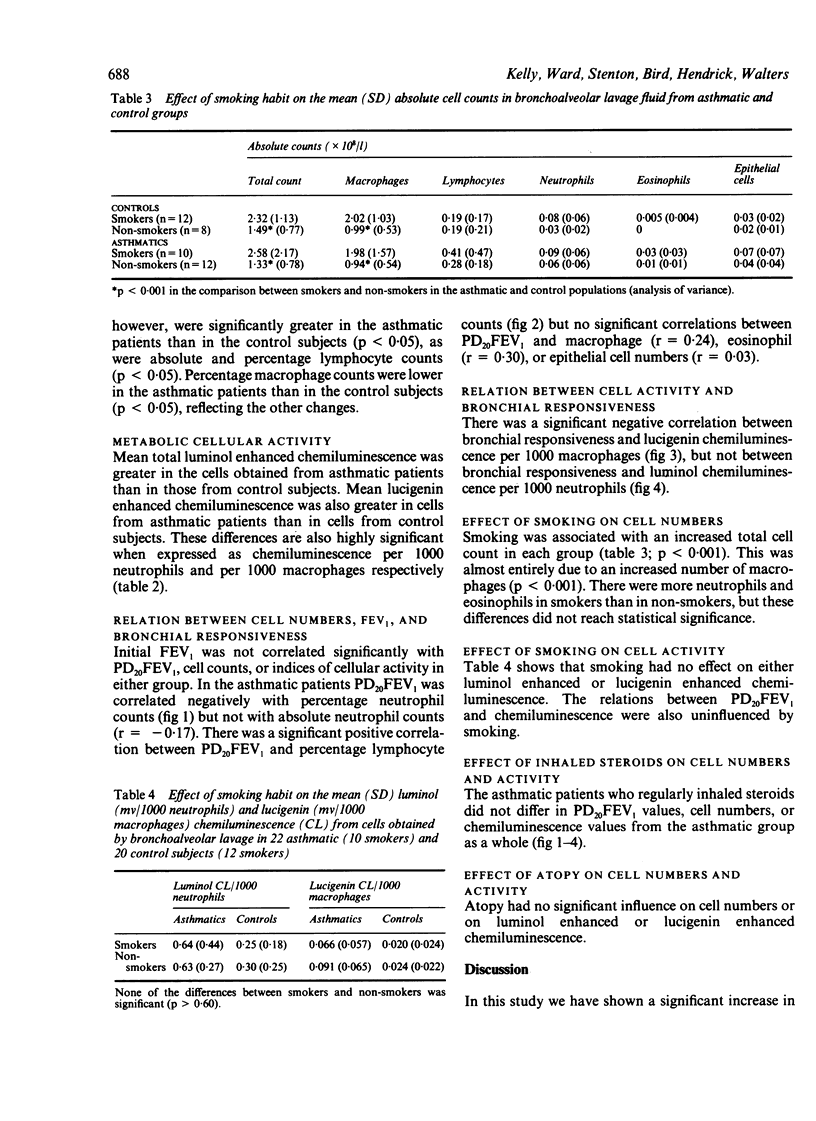
Bronchial responsiveness to inhaled methacholine was measured four to six days before fibreoptic bronchoscopy in 22 asthmatic patients (10 smokers) and 20 control subjects (12 smokers). The asthmatic patients had a baseline FEV1 greater than 60% predicted and a PD20FEV1 (provocative dose of methacholine causing a 20% fall in FEV1) of 0.006-3.7 mg. The 20 control subjects had normal pulmonary function and a PD20FEV1 above the maximum cumulative dose of methacholine of 6.4 mg. Bronchoalveolar lavage of a middle lobe segment (lingula in four subjects) was performed with three sequential 60 ml aliquots of sterile saline. Cellular metabolic activity was stimulated with latex in aliquots of resuspended cells, and measured by means of luminol enhanced chemiluminescence to assess neutrophil activity and lucigenin enhanced chemiluminescence to assess macrophage activity. Mean absolute total cell counts were similar in the asthmatic and control groups but there were differences in differential cell counts, with a significant increase in eosinophil (p less than 0.05) and lymphocyte (p less than 0.005) counts in asthma. PD20FEV1 was negatively correlated with percentage neutrophil counts (p less than 0.005). Luminol enhanced chemiluminescence/1000 neutrophils was increased about twofold in asthmatic subjects (p less than 0.001), but was not correlated with PD20FEV1 Lucigenin enhanced chemiluminescence/1000 macrophages was increased nearly fourfold in asthmatic patients (p less than 0.001) and showed a negative correlation with PD20FEV1 (p less than 0.01). The macrophage count was increased twofold in current smokers in both groups, but other cell numbers were not altered significantly. Smoking did not affect cellular metabolic activity in either group. This study supports the idea that an inflammatory process is present in the airways of those with asthma, and suggests a relation between bronchial responsiveness and both neutrophil numbers and macrophage activity.
Full text
PDF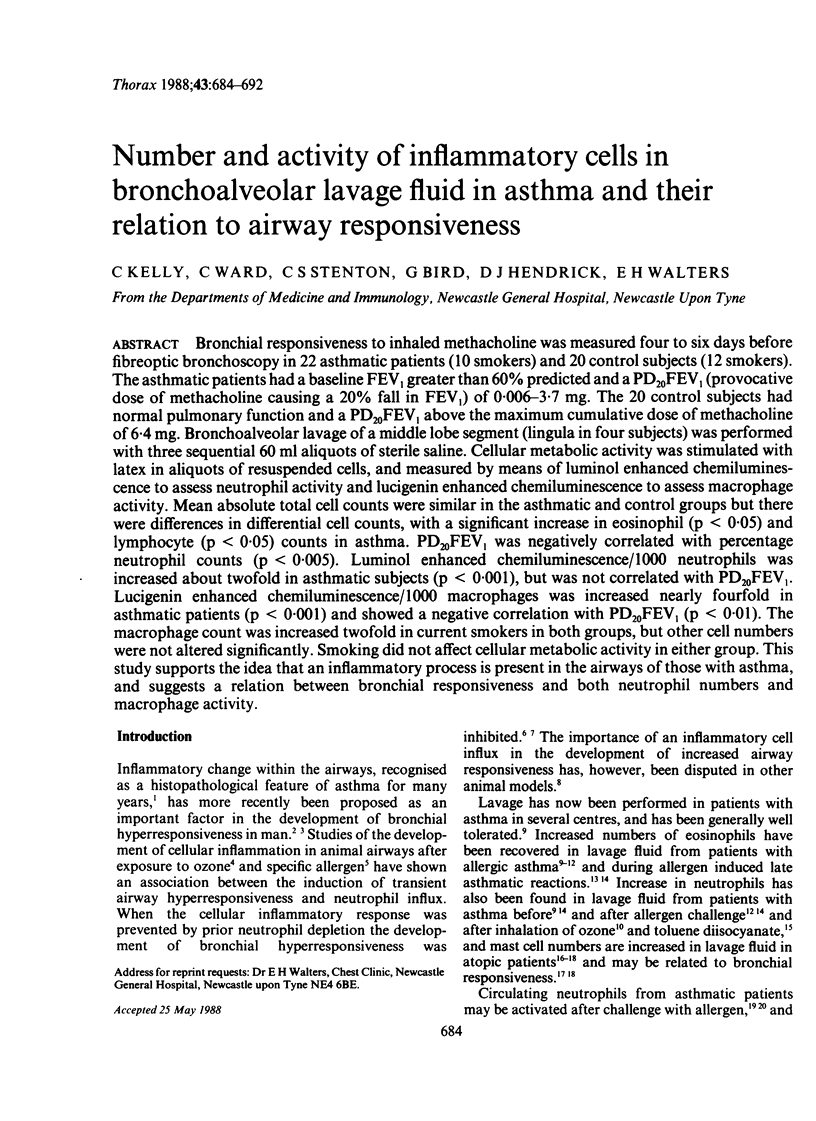





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam R., Kuna P., Rozniecki J., Kuzminska B. The magnitude of the spontaneous production of histamine-releasing factor (HRF) by lymphocytes in vitro correlates with the state of bronchial hyperreactivity in patients with asthma. J Allergy Clin Immunol. 1987 Jan;79(1):103–108. doi: 10.1016/s0091-6749(87)80023-4. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel P. L., de Monchy J. G., Verhagen J., Kauffman H. F. The eosinophilic granulocyte an active participant in the late phase asthmatic reaction? Bull Eur Physiopathol Respir. 1986;22 (Suppl 7):54–61. [PubMed] [Google Scholar]
- Carroll M. P., Durham S. R., Walsh G., Kay A. B. Activation of neutrophils and monocytes after allergen- and histamine-induced bronchoconstriction. J Allergy Clin Immunol. 1985 Feb;75(2):290–296. doi: 10.1016/0091-6749(85)90060-0. [DOI] [PubMed] [Google Scholar]
- Chung K. F. Role of inflammation in the hyperreactivity of the airways in asthma. Thorax. 1986 Sep;41(9):657–662. doi: 10.1136/thx.41.9.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluzel M., Damon M., Chanez P., Bousquet J., Crastes de Paulet A., Michel F. B., Godard P. Enhanced alveolar cell luminol-dependent chemiluminescence in asthma. J Allergy Clin Immunol. 1987 Aug;80(2):195–201. doi: 10.1016/0091-6749(87)90129-1. [DOI] [PubMed] [Google Scholar]
- Connolly M. J., Avery A. J., Walters E. H., Hendrick D. J. The relationship between bronchial responsiveness to methacholine and bronchial responsiveness to histamine in asthmatic subjects. Pulm Pharmacol. 1988;1(1):53–58. doi: 10.1016/0952-0600(88)90011-7. [DOI] [PubMed] [Google Scholar]
- DUNNILL M. S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960 Jan;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Monchy J. G., Kauffman H. F., Venge P., Koëter G. H., Jansen H. M., Sluiter H. J., De Vries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am Rev Respir Dis. 1985 Mar;131(3):373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Durham S. R., Carroll M., Walsh G. M., Kay A. B. Leukocyte activation in allergen-induced late-phase asthmatic reactions. N Engl J Med. 1984 Nov 29;311(22):1398–1402. doi: 10.1056/NEJM198411293112202. [DOI] [PubMed] [Google Scholar]
- Fabbri L. M., Boschetto P., Zocca E., Milani G., Pivirotto F., Plebani M., Burlina A., Licata B., Mapp C. E. Bronchoalveolar neutrophilia during late asthmatic reactions induced by toluene diisocyanate. Am Rev Respir Dis. 1987 Jul;136(1):36–42. doi: 10.1164/ajrccm/136.1.36. [DOI] [PubMed] [Google Scholar]
- Flint K. C., Leung K. B., Hudspith B. N., Brostoff J., Pearce F. L., Johnson N. M. Bronchoalveolar mast cells in extrinsic asthma: a mechanism for the initiation of antigen specific bronchoconstriction. Br Med J (Clin Res Ed) 1985 Oct 5;291(6500):923–926. doi: 10.1136/bmj.291.6500.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godard P., Aubas P., Calvayrac P., Taib J., Michel F. B. Endoscopie et lavage bronchiolo-alvéolaire chez l'asthmatique allergique. Nouv Presse Med. 1981 Oct 24;10(38):3141–3148. [PubMed] [Google Scholar]
- Gosset P., Tonnel A. B., Joseph M., Prin L., Mallart A., Charon J., Capron A. Secretion of a chemotactic factor for neutrophils and eosinophils by alveolar macrophages from asthmatic patients. J Allergy Clin Immunol. 1984 Dec;74(6):827–834. doi: 10.1016/0091-6749(84)90186-6. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Olsen G. N., Castle J. R., Maloney A. S. Comparison of proteolytic enzyme activity in pulmonary alveolar macrophages and blood leukocytes in smokers and nonsmokers. Am Rev Respir Dis. 1975 May;111(5):579–586. doi: 10.1164/arrd.1975.111.5.579. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S. T., Hardy C., Howarth P. H., Robinson C., Church M. K., Agius R. M. Bronchial mucosal mast cells and their implications in the pathogenesis of asthma. Bull Eur Physiopathol Respir. 1986;22 (Suppl 7):39–47. [PubMed] [Google Scholar]
- Holt P. G. Immune and inflammatory function in cigarette smokers. Thorax. 1987 Apr;42(4):241–249. doi: 10.1136/thx.42.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman M. J., Fabbri L. M., O'Byrne P. M., Gold B. D., Aizawa H., Walters E. H., Alpert S. E., Nadel J. A. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis. 1983 Jun;127(6):686–690. doi: 10.1164/arrd.1983.127.6.686. [DOI] [PubMed] [Google Scholar]
- Joseph M., Tonnel A. B., Capron A., Voisin C. Enzyme release and superoxide anion production by human alveolar macrophages stimulated with immunoglobulin E. Clin Exp Immunol. 1980 May;40(2):416–422. [PMC free article] [PubMed] [Google Scholar]
- Juniper E. F., Frith P. A., Hargreave F. E. Airway responsiveness to histamine and methacholine: relationship to minimum treatment to control symptoms of asthma. Thorax. 1981 Aug;36(8):575–579. doi: 10.1136/thx.36.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J. G., Hargreave F. E., Gleich G. J., O'Byrne P. M. Bronchoalveolar cell profiles of asthmatic and nonasthmatic subjects. Am Rev Respir Dis. 1987 Aug;136(2):379–383. doi: 10.1164/ajrccm/136.2.379. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Gallin J. I. Inhibition of neutrophil Fc receptor function by cotricosteroids. Clin Exp Immunol. 1978 Oct;34(1):137–142. [PMC free article] [PubMed] [Google Scholar]
- Luelmo F. BCG vaccination. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):70–72. doi: 10.1164/arrd.1982.125.3P2.70. [DOI] [PubMed] [Google Scholar]
- Mann J. S., Cushley M. J., Holgate S. T. Adenosine-induced bronchoconstriction in asthma. Role of parasympathetic stimulation and adrenergic inhibition. Am Rev Respir Dis. 1985 Jul;132(1):1–6. doi: 10.1164/arrd.1985.132.1.1. [DOI] [PubMed] [Google Scholar]
- Martin R. R. Altered morphology and increased acid hydrolase content of pulmonary macrophages from cigarette smokers. Am Rev Respir Dis. 1973 Apr;107(4):596–601. doi: 10.1164/arrd.1973.107.4.596. [DOI] [PubMed] [Google Scholar]
- Martin T. R., Raghu G., Maunder R. J., Springmeyer S. C. The effects of chronic bronchitis and chronic air-flow obstruction on lung cell populations recovered by bronchoalveolar lavage. Am Rev Respir Dis. 1985 Aug;132(2):254–260. doi: 10.1164/arrd.1985.132.2.254. [DOI] [PubMed] [Google Scholar]
- McLeod R., Mack D. G., McLeod E. G., Campbell E. J., Estes R. G. Alveolar macrophage function and inflammatory stimuli in smokers with and without obstructive lung disease. Am Rev Respir Dis. 1985 Mar;131(3):377–384. doi: 10.1164/arrd.1985.131.3.377. [DOI] [PubMed] [Google Scholar]
- Metzger W. J., Richerson H. B., Worden K., Monick M., Hunninghake G. W. Bronchoalveolar lavage of allergic asthmatic patients following allergen bronchoprovocation. Chest. 1986 Apr;89(4):477–483. doi: 10.1378/chest.89.4.477. [DOI] [PubMed] [Google Scholar]
- Murlas C. G., Roum J. H. Sequence of pathologic changes in the airway mucosa of guinea pigs during ozone-induced bronchial hyperreactivity. Am Rev Respir Dis. 1985 Mar;131(3):314–320. doi: 10.1164/arrd.1985.131.3.314. [DOI] [PubMed] [Google Scholar]
- Murphy K. R., Wilson M. C., Irvin C. G., Glezen L. S., Marsh W. R., Haslett C., Henson P. M., Larsen G. L. The requirement for polymorphonuclear leukocytes in the late asthmatic response and heightened airways reactivity in an animal model. Am Rev Respir Dis. 1986 Jul;134(1):62–68. doi: 10.1164/arrd.1986.134.1.62. [DOI] [PubMed] [Google Scholar]
- O'Byrne P. M., Walters E. H., Gold B. D., Aizawa H. A., Fabbri L. M., Alpert S. E., Nadel J. A., Holtzman M. J. Neutrophil depletion inhibits airway hyperresponsiveness induced by ozone exposure. Am Rev Respir Dis. 1984 Aug;130(2):214–219. doi: 10.1164/arrd.1984.130.2.214. [DOI] [PubMed] [Google Scholar]
- Okuda S., Motomura K., Sanai T., Hirakata H., Nanishi F., Onoyama K., Fujishima M. Effect of different levels of protein intake on renal deterioration and nutritional state in experimental renal disease. Clin Sci (Lond) 1987 Jul;73(1):33–39. doi: 10.1042/cs0730033. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Inflammation and superoxide production by macrophages. Agents Actions Suppl. 1980;7:174–179. [PubMed] [Google Scholar]
- Podleski W. K., Grimes J. R. Circulating hyperreactive lymphocytes in bronchial asthma. Clin Immunol Immunopathol. 1978 Feb;9(2):236–239. doi: 10.1016/0090-1229(78)90075-2. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Newball H. H. Analysis of proteins and respiratory cells obtained from human lungs by bronchial lavage. J Lab Clin Med. 1974 Oct;84(4):559–573. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Chemiluminescence and superoxide production by myeloperoxidase-deficient leukocytes. J Clin Invest. 1976 Jul;58(1):50–60. doi: 10.1172/JCI108458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J., Bigby B. G., Stulbarg M., Holtzman M. J., Nadel J. A., Ueki I. F., Leikauf G. D., Goetzl E. J., Boushey H. A. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol (1985) 1986 Apr;60(4):1321–1326. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- Tomioka M., Ida S., Shindoh Y., Ishihara T., Takishima T. Mast cells in bronchoalveolar lumen of patients with bronchial asthma. Am Rev Respir Dis. 1984 Jun;129(6):1000–1005. doi: 10.1164/arrd.1984.129.6.1000. [DOI] [PubMed] [Google Scholar]
- Tonnel A. B., Joseph M., Gosset P., Fournier E., Capron A. Stimulation of alveolar macrophages in asthmatic patients after local provocation test. Lancet. 1983 Jun 25;1(8339):1406–1408. doi: 10.1016/s0140-6736(83)92356-5. [DOI] [PubMed] [Google Scholar]
- Wallaert B., Aerts C., Bart F., Hatron P. Y., Dracon M., Tonnel A. B., Voisin C. Alveolar macrophage dysfunction in systemic lupus erythematosus. Am Rev Respir Dis. 1987 Aug;136(2):293–297. doi: 10.1164/ajrccm/136.2.293. [DOI] [PubMed] [Google Scholar]
- Wallaert B., Bonniere P., Prin L., Cortot A., Tonnel A. B., Voisin C. Primary biliary cirrhosis. Subclinical inflammatory alveolitis in patients with normal chest roentgenograms. Chest. 1986 Dec;90(6):842–848. doi: 10.1378/chest.90.6.842. [DOI] [PubMed] [Google Scholar]
- Walters E. H., Parrish R. W., Bevan C., Smith A. P. Induction of bronchial hypersensitivity: evidence for a role for prostaglandins. Thorax. 1981 Aug;36(8):571–574. doi: 10.1136/thx.36.8.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Human bronchoalveolar lavage cells and luminol-dependent chemiluminescence. J Clin Pathol. 1981 Feb;34(2):167–171. doi: 10.1136/jcp.34.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. J., Cole P. J. Investigation of alveolar macrophage function using lucigenin-dependent chemiluminescence. Thorax. 1981 Nov;36(11):866–869. doi: 10.1136/thx.36.11.866. [DOI] [PMC free article] [PubMed] [Google Scholar]



