Abstract
Introduction:
Oral contraceptives (OCs) are considered as one of the most common risk factor of venous thromboembolism (VTE) in childbearing age. Some of the recent researches indicate that the odds of VTE may be even higher with newer generations of OCs. The present meta-analysis was designed to evaluate the effect of different generation of OCs on the occurrence of VTE.
Methods:
Two researchers independently ran a thorough search in Pubmed, ISI Web of Science, EMBASE, CINAHL and Scopus databases regarding study keywords including thromboembolic event, thromboembolism, embolism, thromboembolic, thrombotic and thrombosis, combined with oral contraceptive. The outcomes were the incidence of diagnosed thromboembolism, such as deep vein thrombosis, pulmonary embolism and cerebral venous thrombosis. Based on the heterogeneity of the studies, random effect model was used and pooled odds ratio was reported.
Results:
Three cohort and 17 case-control studies with 13,265,228 subjects were entered into meta-analysis. Analysis showed that the odds of VTE in women taking OCs are more than three-fold (OR=3.13; 95% CI: 2.61-3.65). The risk of VTE in women taking first-, second- and third-generation OCs are 3.5 fold (OR=3.48; 95% CI: 2.01-4.94), 3 fold (OR=3.08; 95% CI: 2.43-3.74) and 4.3 fold (OR=4.35; CI: 3.69‒5.01), respectively.
Conclusion:
It seems that the risk of VTE is not same between different generations of OCs, so that third-generation has highest risk. Taking second and third-generation OCs increases the risk of VTE up to 3 and 4.3 fold, respectively. The researchers of the present study suggest that more trials be designed in relation to the effect of newer generations of OCs in different communities.
Key Words: Oral contraceptives, venous thromboembolism, intracranial thrombosis, pulmonary embolism, meta-analysis
Introduction
Thromboembolic events are multifactorial phenomena, involving both genetic and acquired factors (1). Some of the genetic factors include defects in and mutations of the genes of prothrombin and factor V Leiden (2-4), whereas acquired factors include pregnancy, the postpartum period, obesity, lack of activity, and aging (5-8). At present, oral contraceptives (OCs) are considered one of the most common risk factors of venous thromboembolism (VTE) in women at childbearing age. OCs are among the most commonly used methods to prevent pregnancy. The official reports of 2012 show that OCs have been used by 11 million women (17%) in the United States and 100 million women worldwide (9, 10). These pills may have life-threatening side effects, including myocardial infarction, strokes, and VTE (11), and the odds of such incidents in women taking OCs are three times higher than those in nonusers (12-14). In recent decades, the chemical composition of OCs has undergone changes. Compounds containing estrogen are known as important risk factors for this medical condition in postmenopausal women (15, 16). In this context, the estrogen content of OCs was decreased and new progestins were incorporated. Despite these changes, the incidence of vascular complications resulting from the use of these pills is still high (17, 18). A meta-analysis of observational studies and clinical trials on postmenopausal women showed that the use of OCs containing estrogen increased the risk of VTE by up to three fold, which significantly increased in the first year of the drug use and when combined with other risk factors (15). Furthermore, the newer generation OCs presented higher odds of a VTE risk, when compared with the older generation OCs (2, 12, 19). Therefore, the present meta-analysis was designed to evaluate the effects of different generations of OCs on the incidence of VTE.
Methods
This study was designed based on the instructions for conducting Meta-analysis of Observational Studies in Epidemiology Statement (20).
Search Strategy
Two independent reviewers conducted an extensive search in various databases. All the articles indexed in the electronic databases of PubMed, ISI Web of Sciences, EMBASE, CINAHL, and Scopus from 2000 to 2012 were evaluated. The keywords were determined by using the Medical Subject Headings (MeSH) of PubMed, which consisted of words related to “thrombosis,” including thromboembolic event, thromboembolism, embolism, thromboembolic, thrombotic, and thrombosis, combined with oral contraceptive. Only articles in English were evaluated. To evaluate additional articles with unpublished data, hand search was carried out in the list of “relevant studies.”
Selection Criteria
The cohort and case-control studies conducted on 15–50-year-old female subjects, who took oral contraceptives, were included. The inclusion criteria were as follows: 1) study population consisting of subjects taking oral contraceptives; 2) studies in which the clinical outcomes, including deep vein thrombosis (DVT), pulmonary embolism (PE), and cerebral venous thrombosis (CVT) had been evaluated; 3) studies in which diagnosis of thromboembolism had been carried out by using standard and well-validated diagnostic criteria; and 4) studies with a nonuser control group. Studies that were conducted before 2000, related to special populations such as postpartum, and of editorial, review, and letter to the editor types were excluded.
Quality assessment and data extraction
The summaries of the studies were independently evaluated and recorded in data sheets by the two reviewers. The data were collected in a blind manner in relation to the authors, journal, and organization or institution. The reason for exclusion was recorded and disagreement was resolved by a third reviewer. The number of subjects, adjusted odds ratio (OR), relative risk (rr), and rate ratio with 95% confidence interval (CI) were extracted from the relevant studies. If it was not possible to extract data from a study, the corresponding author was asked to provide the necessary data. The data and results in relation to the generation of OCs were recorded separately. Finally, the findings were incorporated into a flowchart designed based on Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement Guidelines (21).
Data Synthesis
The outcomes consisted of DVT, PE, and CVT. Three cohort (22-24) and 17 case-control studies (2-4, 14, 19, 25-36) met the inclusion criteria. One study had calculated the rate ratios (23), one had determined the rr (24) and the others had evaluated the OR (2-4, 14, 19, 22, 25-36).
As the type of the study had no effect on the results, based on a logistic regression model, all the 20 articles were included into one meta-analysis. In the sensitivity analysis, only studies with quality rates of good and fair were included. The funnel plot was used to evaluate selection bias (37) and “trim and fill” technique was used to identify publication bias (38, 39). The Methods Guide for Effectiveness and Comparative Effectiveness Reviews of the Agency for Healthcare Research and Quality was used to evaluate the quality of the studies (40). The reviewers evaluated each study in relation to its design, presence of bias in the selection of samples, and performance and reporting of outcomes. Each study was given a general score of good, fair, or poor. Studies with the least bias were given a score of “good;” studies in which there was a possibility of bias, but their results had not been influenced, were given a score of “fair;” and studies with obvious indications of bias and elimination of large amounts of data or great discrepancies in reporting the outcomes were given a general score of “poor.” The inter-rater reliability of the two reviewers was 83%.
Chi-squared and I2 tests were used to evaluate the heterogeneity among the studies, and statistical significance was defined at p < 0.1. If the studies were homogeneous, then the fixed effect model was used; otherwise, meta-analysis was conducted based on the random effect model. The results of the studies were pooled and an overall OR was calculated, which indicated the odds of affliction with thromboembolism in women taking OCs, when compared with that in nonusers. The calculated OR was also presented separately in relation to the generation of OCs. As meta-analyses were performed in at least three studies in which OR had been presented, it was not possible to report the OR for the fourth-generation OCs. Statistical analyses were carried out by STATA 11.0 (Stata Corporation, College Station, TX).
Results
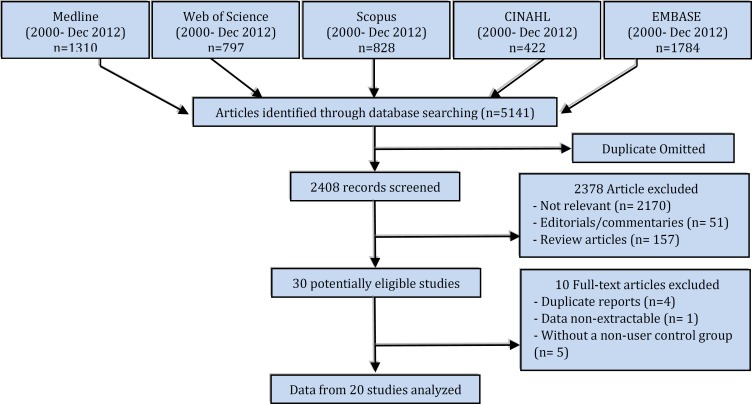
After elimination of duplicate reports, 162 potentially relevant articles were identified (Figure 1). A total of 20 articles (13,265,228 subjects) were included in the meta-analysis, consisting of three cohort (22-24) and 17 case-control studies (2-4, 14, 19, 25-36) (Table 1 and Figure 1). Five studies had evaluated the relationship between first-generation OCs and VTE (4, 14, 24, 30, 32); eight studies had examined the second-generation OCs (4, 14, 19, 23, 24, 29-32) and seven studies had investigated the third-generation OCs (14, 19, 23, 24, 29, 30, 32). The endpoint of all these studies was the occurrence of DVT, pulmonary embolism, and cerebral embolism. The diagnostic tests used were Doppler ultrasound for DVT and computed tomography angiography for pulmonary and cerebral embolism. Logistic regression analysis showed the feasibility of pooling the studies (p = 0.12).
Figure 1.
the flowchart of the study
Table 1.
Studies of oral contraceptive use, thrombosis and Thromboembolism event
| Authors, year and location of study | No. of cases/controls | Results | Adjustments | Weaknesses | Quality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case-Control studies | ||||||||||||
|
Austin et al.,
2009 ( 25 ) USA |
46 cases and 170 controls | The risk of VTE: OR=2.6 |
Age and household income | Analysis was not adjusted for other Potential confounders Small sample size. The participation rate of cases and controls was low Selection bias: The use of clinic controls may result in an overestimate of OCs. |
Fair | |||||||
|
Aznar et al.,
2000 ( 26 ) Spain |
84 cases and 89 controls | The risk of VTE: Healthy users: OR= 3.5 Suspected thrombophilia: OR= 14.3 DVT patients: OR=6.9 |
None | Unadjusted analysis for potential confounders. The participation rate of cases and controls was low. |
Fair | |||||||
|
Barsoum et al.,
2010 ( 34 ) USA |
125 cases and 143 controls | The risk of VTE: Total: OR=3.0 Estrogen alone: OR=1.81 Progestin alone: OR=2.53 (NS) Non- estrogen OCs plus progestin: OR=2.53 |
Age, BMI and all previously identified VTE risk factors | The participation rate of cases and controls was low. Findings may not be generalizable to other races or ethnicities. Sample size was too small to test the effect of different estrogen and progestin combinations, doses. |
Good | |||||||
|
Bergendal et al., 2012 (
33
)
Sweden |
766 cases and 674 controls | Use of CHC was associated with an eight-fold increased risk of VTE: OR=8.45 No risk increase associated with use of POC: OR=0.98 Use of MHT increased risk for VTE: OR=3.73 |
Age, BMI, smoking, use of hormones, bed rest/minor trauma, surgery, cast, surgery and cast, the prothrombin mutation and/or factor V Leiden | The participation rate of cases and controls was low. Retrospective design. Higher non-participant rate among the controls. Possible recall-bias. No specified diagnostic criteria for assessment of menopausal status. |
Fair | |||||||
|
Bloemenkamp et al., 2000 (
27
)
Netherlands |
155 cases and 169 controls | The risk of DVT in healthy user: First six month of use: OR=3.0 First years of use: OR=2.0 The risk of development of DVT in combined with thrombophilia: First six month of use: OR=18.5 First years of use: OR=11.0 |
Age, family history of venous thrombosis, history of pregnancy | The most important genetic risk factors for venous thrombosis were not discovered. Large confidence intervals. The participation rate of cases and controls was low. |
Good | |||||||
|
Dinger et al.,
2010 ( 28 ) Germany |
680 cases and 2,720 controls | The risk of VTE associated with current COC use: OR=2.3 DNG/EE vs. any other low-dose COCs: OR=0.9 DNG/EE vs. low-dose LNG/EE: OR=1.1 DRSP/EE vs. low-dose LNG/EE: OR=1.0 |
Personal history of VTE, family history of VTE, body mass index, duration of combined oral contraceptive use, parity, educational level, chronic disease, concomitant medication and smoking | Recruit only survivors of VTE. Possible recall bias. |
Good | |||||||
|
Heinemann et al., 2002 (
30
)
Germany |
605 cases and 2,941 controls | The risk of VTE: For all cases: OR=3.4 For hospital cases: OR=3.7 The risk of Idiopathic VTE: For all cases: OR=5.4 For hospital cases:OR=9.1 |
Age, BMI, parity, ever-use of OCs | Did not assess of other potential confounder. | Good | |||||||
|
Heinemann et al., 2010 (
29
)
Austria |
362 cases and 1,505 controls | The risk of VTE: OCs containing gestodene: OR=3.39 OCs containing progestin: OR=3.14 |
Age, BMI, parity and ever-use of hormonal contraceptives | Limit the generalizability of results to other regions and/or other racial and ethnic groups. Mild or atypical VTE cases were under-reported in this study. |
Good | |||||||
|
Legnani et al.,
2002 ( 2 ) Italy |
301 cases and 650 controls | The risk of DVT: In the absence of both mutations was : OR=2·4 In the presence of R506Q mutation: OR=41·0, In the presence of G20210A mutation: OR=58·6 Both mutations: OR=86·5. |
Age and presence of other thrombophilic defects | Lack of reporting of other potential confounders. | Good | |||||||
|
Lidegaard et al., 2002 (
14
)
Denmark |
654 cases and 1,921 controls | The risk of VTE: Second generation OCs: OR=2.9 Third generation OCs: OR=4.0 |
Age, year, family history of VTE, BMI, years of schooling, smoking, diabetes, coagulation disturbances, and previous delivery | Analysis was not adjusted for duration of use and other potential confounders. | Fair | |||||||
|
Pomp et al.,
2008 ( 31 ) Netherlands |
362 cases and 357 controls | The Risk of VTE: Current smokers and OCs users: OR= 8.8 Non-smoker and OCs user: OR=3.9 |
Age, sex, BMI, parity and fibrinogen levels | The participation rate of cases and controls was low. | Fair | |||||||
|
Santamaria et al., 2001 (
3
)
Spain |
100 cases and 273 controls | The Risk of VTE: Without defect: OR=1.3 (NS) PT20210A mutation+ OCs use: OR=2.9 Factor V Leiden carriers + OCs: OR=1.2 (NS) |
Age, including the PT20210-A and the FVL mutations | Sample size was too small Self-report of OCs use. Unadjusted analysis for other potential confounders. |
Good | |||||||
|
Santamaria et al., 2001 (
3
)
Spain |
100 cases and 273 controls | The Risk of VTE: Without defect: OR=1.3 (NS) PT20210A mutation+ OCs use: OR=2.9 Factor V Leiden carriers + OCs: OR=1.2 (NS) |
Age, including the PT20210-A and the FVL mutations | Sample size was too small Self-report of OCs use. Unadjusted analysis for other potential confounders. |
Good | |||||||
|
Sidney et al.,
2004 ( 4 ) USA |
196 cases and 746 controls | The Risk of DVT: OCs user: OR=4.07 Factor V Leiden mutation: OR=7.1 Prothrombin mutation: OR=2.83 MTHFR C677T mutation: OR=0.26 |
Age, race/ethnicity, income and BMI | Possible recall bias and diagnostic bias. Unadjusted analysis for potential confounders. |
Fair | |||||||
|
Smith et al.,
2004 ( 35 ) USA |
493 cases and 1728 controls | The Risk of VTE: Total: OR= 1.75 Estrogen only: OR: 0.92 (NS) CEE: OR: 1.65 CEE + Progestin:OR: 2.17 |
Age, hypertension, calendar year, race, and cancer history | Use of hormone therapy was not randomly assigned. Findings are generalizable only to similar populations. |
Good | |||||||
|
Suissa et al.,
2000 ( 19 ) Germany and UK |
128 cases and 650 controls | The risk of VTE: Second generation OCs: RR=4.7 Third generation OCs: RR=2.9 |
Age, country, BMI, alcohol, smoking and duration of use | Defect in randomization. | Fair | |||||||
|
Van Hylckama et l., 2009 (
32
)
Netherlands |
1,524 cases and 1,760 controls | The risk of DVT: Total:OR= 5.0 OCs containing levonorgestrel: OR=3.6 OCs containing gestodene: OR=5.6 OCs containing desogestrel: OR=7.3 OCs containing Cyproterone acetate: OR=6.8 OCs containing drospirenone: OR=6.3 |
Age and period of inclusion | Unadjusted analysis for potential confounders. The participation rate of cases and controls was low. Potential recall bias. |
Good | |||||||
| Cohort studies | ||||||||||||
|
Huerta et al.,
2007 ( 22 ) UK |
5,866 OCs Users and 9,326 Non-user |
The risk of VTE in OCs users and hormone therapy group was same: RR=1.9 | Sex, age, calendar year, BMI, smoking, cancer, fractures in the last month, surgery in the last 6 months, use of warfarin sodium, | The authors did not calculate the incidence of VTE by weighting the number of newly diagnosed VTE cases identified by the confirmation rate obtained in the validation study. | Good | |||||||
|
Lidegaard et al., 2009 (
23
)
Denmark |
2,302,045 OCs Users and 4,802,168 Non-user |
The Risk of VTE: <1 year: RR=4.17 1-4 years: RR= 2.98 >4 years: RR= 2.76 |
Current use of oral contraceptives, calendar year, and educational level | Family predisposition and body mass index were not adjusted. Validity of each included diagnosis of VTE was not checked. |
Good | |||||||
|
Lidegaard et al., 2011 (
24
)
Denmark |
231,675 OCs Users and 5,892,182 Non-user |
The risk of VTE compared with users of combined oral contraceptives containing levonorgestrel: Transdermal patches: RR=2.3 The vaginal ring: RR=1.9 |
Age, calendar year, and education |
Analysis could not control for family disposition or for BMI. | Good | |||||||
VTE: Venous thromboembolism; DVT: Deep vein thrombosis; VT: Venous thrombosis; CHC: Combined hormonal contraceptives; POC: Progestogen-only contraception; MHT: Multi hormone therapy; NS: Non-significant; CEE: Conjugated equine estrogen; BMI: Body mass index
Heterogeneity and publication bias
The studies included in the meta-analysis were not sufficiently homogeneous to allow conducting meta-analysis based on the fixed effect model. Therefore, in all the analyses, random effect model was used. There were no selection and publication biases in three of the four meta-analyses (one meta-analysis of the first-generation OCs had publication bias). The limited number of studies (five studies) did not allow the exclusion of outlier studies to eliminate bias. The results of these four meta-analyses were as follows.
Meta-analysis
A) Effect of OCs on incidence of VTE (without generation consideration)
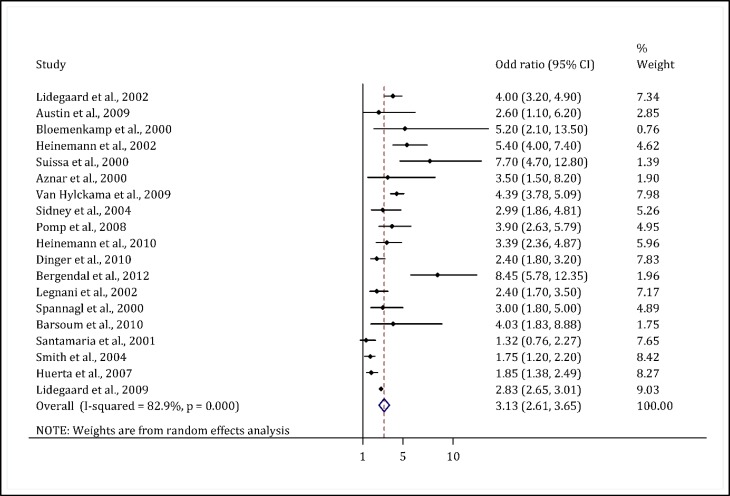
Systematic review of 19 studies showed an OR/rr range of 1.32–8.45, which was statistically significant (2-4, 14, 19, 22-36). Only one study did not indicate an increase in the OR (3). The meta-analysis showed that the odds of VTE in women taking OCs was threefold higher than that in nonusers (OR = 3.13; 95% CI: 2.61–3.65) (Figure 2).
Figure 2.
Odds ratio of the incidence of venous thromboembolism due to the use of OCP compared to non-users
B) Effect of first-generation OCs on incidence of VTE
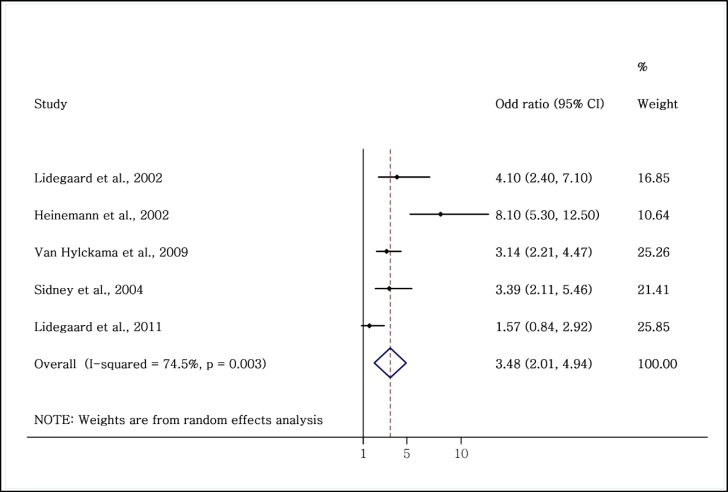
All the five studies on the relationship between the incidence of VTE and first-generation OCs showed a high OR (26,327 users and 5,909,630 nonusers). Of these five studies, four were case-control (4, 14, 30, 32) and one was cohort (24). This increased risk was significant in four studies (4, 14, 30, 32). The adjusted OR range in the studies was 1.57–8.1. The meta-analysis showed that the odds of VTE in women taking first-generation OCs was 3.5-fold higher than that in nonusers (OR = 3.48; 95% CI: 2.01–4.94) (Figure 3).
Figure 3.
Evaluation of the OR of venous thromboembolism in women taking first-generation OCs compared to non-users.
C) Effect of second-generation OCs on incidence of VTE
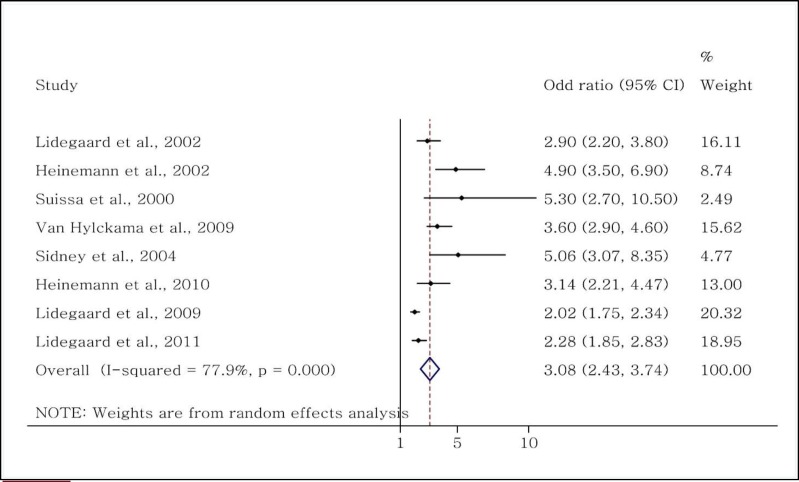
The eight relevant studies indicated a significant relationship between the second-generation OCs and VTE (4, 14, 19, 23, 24, 29, 30, 32). Among these studies (2,537,189 users and 10,703,873 nonusers), six were case-control (4, 14, 19, 29, 30, 32) and two were cohort (23, 24). Separate subgroup analysis of the study type showed that the overall OR reported in the case-control studies was 3.57 (95% CI: 2.92–4.2) and the rr reported in the two cohort studies was 2.09 (95% CI: 1.82–2.34). The overall OR for the incidence of VTE in the second-generation OC users was 3.08 (95% CI: 2.43–3.74) (Figure 4).
Figure 4.
Evaluation of the OR of venous thromboembolism in women taking second-generation OCs.
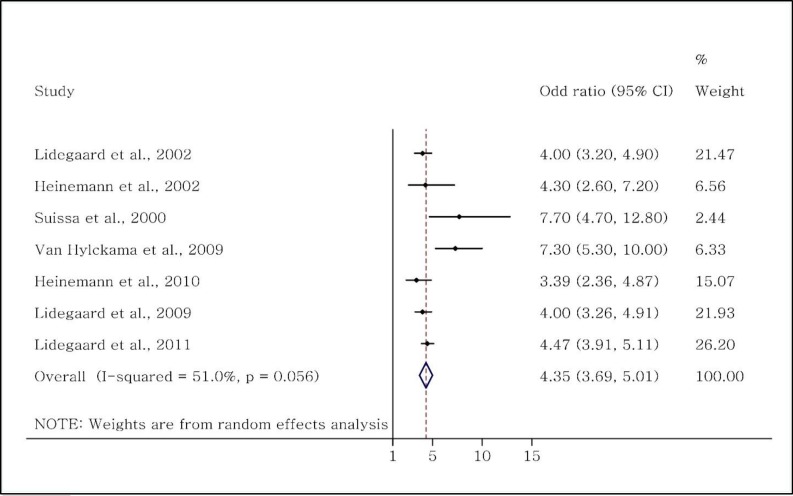
D) Effect of third-generation OCs on incidence of VTE
Seven studies (2,536,993 users and 10,703,127 nonusers) had evaluated the effects of third-generation OCs on the incidence of VTE (14, 19, 23, 24, 29, 30, 32). Among these, five were case-control (4, 14, 19, 29, 30, 32) and two were cohort (23, 24). Subgroup analysis based on the type of the study showed an OR range of 3.39–7.7 (overall OR = 4.74; CI: 3.42–6.08). The rr calculated from the two cohort studies were 4.0 and 4.47, respectively. The pooled analysis of all the seven studies showed an OR of 4.35 (CI: 3.69–5.01) (Figure 5).
Figure 5.
Evaluation of the OR of venous thromboembolism in women taking third-generation OCs.
Discussion
The results of this meta-analysis showed that OCs are important risk factors for VTE in women. The risk of such events was higher with the use of third-generation OCs, as evidenced by other meta-analyses (41-43).
A study performed in 2001 showed that the risk of VTE in women taking third-generation OCs was 1.7-fold higher than that in women taking second-generation OCs (42).
A similar result was also reported by Martinez et al., who demonstrated that the risk of VTE associated with third-generation OCs was greater than that associated with second-generation OCs (43).
A review of the literature and meta-analysis of 19 case-control and cohort studies showed that, in general, the use of OCs increased the odds of VTE by almost threefold, when compared with the nonusers (11). These OR values are consistent with the results obtained in the present study. Although the duration of drug use was different, in the present meta-analysis, VTE frequently occurred in the first year of use. Previous studies have demonstrated that the incidence of VTE was higher in women who recently started taking OCs (23, 44-46).
Quality issue
In the present study, the application of three techniques resulted in qualitative confirmation of meta-analysis. Initially, subgroup analysis was carried out separately for each generation of OCs. In this context, only confirmed and documented cases of thrombosis were included in the meta-analysis. In the second stage, to evaluate the implementation of appropriate adjustments for confounding factors, OR was separately calculated for approximate OR or rr and was adjusted. As the OR calculated from both the analyses were almost the same, the presence of a confounding factor was unlikely. Therefore, only adjusted OR or rr was reported. In addition, in the present meta-analysis, only women at childbearing age (age under 50 years) were included, which eliminated the effect of age. In the third stage, the quality assessment of exposure was evaluated, which was conducted by separating the different generations of OCs. Although the definitions of drug generations were not completely similar in the included studies, they did not affect the pooled OR, because of the much less weight of studies with incongruent definitions (4, 19, 30, 32) (Figure 2-4). However, the calculated pooled OR might have been underestimated; because (i) it was not possible to completely eliminate publication bias, particularly in the case of OR calculated for the first-generation OCs; (ii) some pharmaceutical companies might refrain from disclosing the results of studies to protect personal benefits, resulting in an increase in publication bias (47); and (iii) the use of rr calculated from the community data (especially from cohort studies) is generally less frequent, when compared with the results obtained from matched regression analyses.
Furthermore, the differences in the incidence rate of VTE with the use of first-, second-, and third- generation OCs might be attributed to the differences in the populations of women taking these drugs, because new users have a tendency to use new generations, but old users prefer to use the same drug that they had been using. Moreover, new users might consist of women who are genetically predisposed to VTE or have an acquired susceptibility to VTE. In this context, it has been shown that older users are resistant to the complications of the drug, which might result in erroneously reporting a higher risk of VTE in newer generations, when compared with older generations. However, as drug use duration had not been adjusted only in one study (14) the presence of confounding factors was very improbable.
Limitation
One of the most important limitations of the present study was the meta-analysis nature of the observational studies. The observational studies, with their inherent limitation for the evaluation of all the confounding factors, could not reliably establish the cause-and-effect relationships. Another limitation was the heterogeneity of the studies, resulting in designing of meta-analyses based on random effect model. Although an attempt was made to choose studies similar in methodology and to control the confounding factors, this aim could not be completely achieved even under ideal conditions. Therefore, in the present meta-analysis, the definitions, controls for confounding factors, and study populations in the studies included were different to some extent. For example, the methods used to diagnose and confirm thromboembolism in the selected studies were not similar. Although only the data of confirmed cases were included in the meta-analyses, as the diagnosis of thromboembolism was hampered by limitations, some patients might have been erroneously classified, thus influencing the results.
Advantages
In the present study, the databases were extensive searched and attempts were made to contact the corresponding authors for acquisition of data. More importantly, apart from evaluation of the effects of all the OCs, the results were reported separately for all the three generations of OCs. This analysis significantly contributed to decreasing bias, and among the four analyses, only one had selection and publication biases. Another advantage of the present study was the elimination of data of switchers (women who changed the drug that they used owing to pooled side effects) because changing of the drug has been reported to increase the risk of VTE (19).
Conclusion:
It seems that the risk of VTE was not the same between different generations of OCs, with third-generation OCs presenting the highest risk. The use of second- and third-generation OCs increased the risk of VTE by up to threefold and 4.3-fold, respectively. Nevertheless, further clinical trials in relation to the effect of newer generations of OCs on different communities are necessary.
Acknowledgments:
We thank Dr. Mahmoud Yousefifard who kindly helped us in revising this manuscript. This work is a part of the academic thesis of Dr Pauline Haroutunian in Shahid Beheshti University of Medical Sciences.
Conflict of interest:
All authors declare no conflict of interest.
Funding support:
None declared.
Authors’ contributions:
Alireza Baratloo and Pauline Haroutunian are the principle investigators; Farhad Rahmati, Maryam Motamedi, Mohammadmehdi Forouzanfar, and Behrooz Hashemi are the co-investigators who collected the basic data; Pauline Haroutunian wrote the first draft and collected data with Internet research; Saeed Safari and Alaleh Rouhipour commented on the final manuscript; and Alireza Baratloo provided critical revisions.
References
- 1.Rosendaal FR. Risk factors for venous thrombosis: prevalence, risk, and interaction. Semin Hematol. 1997;34(3):171–6. [PubMed] [Google Scholar]
- 2.Legnani C, Palareti G, Guazzaloca G, et al. Venous thromboembolism in young women. Role of thrombophilic mutations and oral contraceptive use. Eur Heart J. 2002;23(12):984–90. doi: 10.1053/euhj.2001.3082. [DOI] [PubMed] [Google Scholar]
- 3.Santamaría A, Mateo J, Oliver A, et al. Risk of thrombosis associated with oral contraceptives of women from 97 families with inherited thrombophilia: high risk of thrombosis in carriers of the G20210A mutation of the prothrombin gene. Haematologica. 2001;86(9):965–71. [PubMed] [Google Scholar]
- 4.Sidney S, Petitti DB, Soff GA, Cundiff DL, Tolan KK, Quesenberry Jr CP. Venous thromboembolic disease in users of low-estrogen combined estrogen-progestin oral contracep-tives. Contraception. 2004;70(1):3–10. doi: 10.1016/j.contraception.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Rott H. Thrombotic risks of oral contraceptives. Curr Opin Obstet Gynecol. 2012;24(4):235–40. doi: 10.1097/GCO.0b013e328355871d. [DOI] [PubMed] [Google Scholar]
- 6.Szarewski A. Combined oral contraceptive pill and venous thromboembolism. Expert Rev Obstet Gynecol. 2011;6(5):473–6. [Google Scholar]
- 7.Hosseini M, Taslimi S, Yousefifard M, et al. Serum Cholesterol Level Nomograms for Iranian Population; Suggestion for National Cut-Offs. Iran J public health. 2013;42(2):164–71. [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseini M, Yousefifard M, Taslimi S, et al. Trend of blood cholesterol level in iran: results of four national surveys during 1991-2008. Acta Med Iran. 2013;51(9):642–51. [PubMed] [Google Scholar]
- 9.Mosher WD, Jones J. Use of contraception in the United States: 1982-2008. Vital health stat. 2010;23(29):1–44. [PubMed] [Google Scholar]
- 10.Disease WSGoC, Contraception SH. Cardiovascular Disease and Steroid Hormone Contraception: Report of a WHO Scientific Group. World Health Organization; 1998. [PubMed] [Google Scholar]
- 11.Urrutia RP, Coeytaux RR, McBroom AJ, et al. Risk of Acute Thromboembolic Events With Oral Contraceptive Use: A Systematic Review and Meta-analysis. Obstet Gynecol. 2013;122(2):380–9. doi: 10.1097/AOG.0b013e3182994c43. [DOI] [PubMed] [Google Scholar]
- 12.Roach R, Lijfering W, Helmerhorst F, Cannegieter S, Rosendaal F, van Hylckama Vlieg A. The risk of venous thrombosis in women over 50 years old using oral contracept-ion or postmenopausal hormone therapy. J Thromb Haemost. 2013;11(1):124–31. doi: 10.1111/jth.12060. [DOI] [PubMed] [Google Scholar]
- 13.Wu O, Robertson L, Langhorne P, et al. Oral contraceptives, hormone replacement therapy, thrombophilias and risk of venous thromboembolism: a systematic review The Thrombosis: Risk and Economic Assessment of Thromboph-ilia Screening (TREATS) Study. Thromb Haemost. 2005;94(1):17–25. doi: 10.1160/TH04-11-0759. [DOI] [PubMed] [Google Scholar]
- 14.Lidegaard Ø, Edström B, Kreiner S. Oral contraceptives and venous thromboembolism: a five-year national case-control study. Contraception. 2002;65(3):187–96. doi: 10.1016/s0010-7824(01)00307-9. [DOI] [PubMed] [Google Scholar]
- 15.Canonico M, Plu-Bureau G, Lowe G, Scarabin P-Y. Hormone replacement therapy and risk of venous thromboembolism in postmenopausal women: systematic review and meta-analysis. BMJ. 2008;336(7655):1227–31. doi: 10.1136/bmj.39555.441944.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J, Chan BK, Nelson HD. Postmenopausal Estrogen Replacement and Risk for Venous ThromboembolismA Systematic Review and Meta-Analysis for the US Preventive Services Task Force. Ann Intern Med. 2002;136(9):680–90. doi: 10.7326/0003-4819-136-9-200205070-00011. [DOI] [PubMed] [Google Scholar]
- 17.Vercellini P, Eskenazi B, Consonni D, et al. Oral contrace-ptives and risk of endometriosis: a systematic review and meta-analysis. Hum Reprod Update. 2011;17(2):159–70. doi: 10.1093/humupd/dmq042. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. WHO Medical Eligibility Criteria for Contraceptive Use. World Health Organization; 2010. [PubMed] [Google Scholar]
- 19.Suissa S, Spitzer W, Rainville B, Cusson J, Lewis M, Heinemann L. Recurrent use of newer oral contraceptives and the risk of venous thromboembolism. Hum Reprod. 2000;15(4):817–21. doi: 10.1093/humrep/15.4.817. [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemi-ology (MOOSE) group. JAMA. 2000;283(15):2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Huerta C, Johansson S, Wallander M-A, Garcia Rodriguez LA. Risk factors and short-term mortality of venous thromb-oembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med. 2007;167(9):935–44. doi: 10.1001/archinte.167.9.935. [DOI] [PubMed] [Google Scholar]
- 23.Lidegaard Ø, Løkkegaard E, Svendsen AL, Agger C. Hormonal contraception and risk of venous thromboem-bolism: national follow-up study. BMJ: British Medical Journal. 2009;339:b2890. doi: 10.1136/bmj.b2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lidegaard Ø, Nielsen LH, Skovlund CW, Skjeldestad FE, Løkkegaard E. Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and oestrogen doses: Danish cohort study, 2001-9. BMJ. 2011;343:d6423. doi: 10.1136/bmj.d6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin H, Lally C, Benson JM, Whitsett C, Hooper WC, Key NS. Hormonal contraception, sickle cell trait, and risk for venous thromboembolism among African American women. Am J Obstet Gynecol. 2009;200(6):620.e1–e3. doi: 10.1016/j.ajog.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 26.Aznar J, Vayá A, Estellés A, et al. Risk of venous thrombosis in carriers of the prothrombin G20210A variant and factor V Leiden and their interaction with oral contraceptives. Haema-tologica. 2000;85(12):1271–6. [PubMed] [Google Scholar]
- 27.Bloemenkamp KW, Rosendaal FR, Helmerhorst FM, Vandenbroucke JP. Higher risk of venous thrombosis during early use of oral contraceptives in women with inherited clotting defects. Arch Intern Med. 2000;160(1):49–58. doi: 10.1001/archinte.160.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Dinger J, Bardenheuer K, Moehner S. The risk of venous thromboembolism in users of a drospirenone-containing oral contraceptive with a 24-day regimen – results from the INAS-OC Study. Fertil Steril. 2010;94(4, Supplement):S3. [Google Scholar]
- 29.Heinemann LAJ, Dinger JC, Assmann A, Minh TD. Use of oral contraceptives containing gestodene and risk of venous thromboembolism: outlook 10 years after the third-generation “pill scare”. Contraception. 2010;81(5):401–7. doi: 10.1016/j.contraception.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Heinemann LAJ, Lewis MA, Assmann A, Thiel C. Case-control studies on venous thromboembolism: bias due to design? A methodological study on venous thromboembolism and steroid hormone use. Contraception. 2002;65(3):207–14. doi: 10.1016/s0010-7824(01)00309-2. [DOI] [PubMed] [Google Scholar]
- 31.Pomp ER, Rosendaal FR, Doggen CJ. Smoking increases the risk of venous thrombosis and acts synergistically with oral contraceptive use. Am J Hematol. 2008;83(2):97–102. doi: 10.1002/ajh.21059. [DOI] [PubMed] [Google Scholar]
- 32.van Hylckama Vlieg A, Helmerhorst F, Vandenbroucke J, Doggen C, Rosendaal F. The venous thrombotic risk of oral contraceptives, effects of oestrogen dose and progestogen type: results of the MEGA case-control study. BMJ. 2009;339 doi: 10.1136/bmj.b2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergendal A, Bremme K, Hedenmalm K, et al. Risk factors for venous thromboembolism in pre-and postmenopausal women. Thromb Res. 2012;130(4):596–601. doi: 10.1016/j.thromres.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Barsoum MK, Heit JA, Ashrani AA, Leibson CL, Petterson TM, Bailey KR. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb Res. 2010;126(5):373–8. doi: 10.1016/j.thromres.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith N, Heckbert S, Lemaitre R, et al. sterified Estrogens and Conjugated Equine Estrogens and the Risk of Venous Thrombosis. JAMA. 2004;292(13):1581–7. doi: 10.1001/jama.292.13.1581. [DOI] [PubMed] [Google Scholar]
- 36.Spannagl M, Heinemann L, Schramm W. Are factor V Leiden carriers who use oral contraceptives at extreme risk for venous thromboembolism? Eur J of Contracep Reprod Healthcare. 2000;5(2):105–12. doi: 10.1080/13625180008500383. [DOI] [PubMed] [Google Scholar]
- 37.Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duval S, Tweedie R. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95(449):89–98. [Google Scholar]
- 39.Sutton AJ, Duval S, Tweedie R, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320(7249):1574–7. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agency for Healthcare Research and Quality. Agency for Healthcare Research and Quality; 2012. [cited 2012 September 12]. Methods guide for effectiveness and comparative effectiveness reviews. Available from: www.effectivehealthcare.ahrq.gov. [PubMed] [Google Scholar]
- 41.Wu C, Grandi S, Filion K, Abenhaim H, Joseph L, Eisenberg M. Drospirenone‐containing oral contraceptive pills and the risk of venous and arterial thrombosis: a systematic review. Int J Obstet Gynaecol. 2013;120(7):801–11. doi: 10.1111/1471-0528.12210. [DOI] [PubMed] [Google Scholar]
- 42.Kemmeren JM, Algra A, Grobbee DE. Third generation oral contraceptives and risk of venous thrombosis: meta-analysis. BMJ. 2001;323(7305):131–9. doi: 10.1136/bmj.323.7305.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martínez F, Ramírez I, Pérez-Campos E, Latorre K, Lete I. Venous and pulmonary thromboembolism and combined hormonal contraceptives Systematic review and meta-analysis. Eur J of Contracep Reprod Healthcare. 2012;17(1):7–29. doi: 10.3109/13625187.2011.643836. [DOI] [PubMed] [Google Scholar]
- 44.Dinger JC, Heinemann LAJ, Kühl-Habich D. The safety of a drospirenone-containing oral contraceptive: final results from the European Active Surveillance study on Oral Contraceptiv-es based on 142,475 women-years of observation. Contracep-tion. 2007;75(5):344–54. doi: 10.1016/j.contraception.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 45.Jick SS, Hernandez RK. Risk of non-fatal venous throm-boembolism in women using oral contraceptives containing drospirenone compared with women using oral contracept-ives containing levonorgestrel: case-control study using Unit-ed States claims data. BMJ. 2011;342:d2151. doi: 10.1136/bmj.d2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parkin L, Sharples K, Hernandez RK, Jick SS. Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: nested case-control study based on UK General Practice Research Database. BMJ. 2011;342:d2139. doi: 10.1136/bmj.d2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber W. maastricht Study on risks of third Wim Weber generation pill “kept secret by industry”. The Lancet. 2001;357(9258):779–85. [Google Scholar]