Key Clinical Message
We report a rare case of recurrent trisomy 21 caused by an isochromosome 21q and what is very likely to be maternal germ-line cell mosaicism. Over 90% of cases of rob(21;21) reported in the literature are due to an isochromosome 21q, with a risk of recurrence of more than 10%.
Keywords: Down syndrome; homologous Robertsonian translocation; isochromosome 21; rea(21,21)
Introduction
Trisomy 21 (also referred to as Down syndrome) is the most frequent chromosomal abnormality at birth. It affects approximately one in every 800 newborns 1. In France, prenatal diagnosis is always suggested when indicated by ultrasound findings and maternal serum marker assays. About 92% of cases of Down syndrome are due to the presence of an extra, free chromosome 21 in all cells, whereas 2–3% are due to mosaicism and 5–6% are due to unbalanced heterologous or homologous acrocentric rearrangements (of which rob(14;21) and rob(21;21) are the most common). Around 70% of rob(14;21) rearrangements are de novo, and this value is over 95% for rob(21;21) 2,3. Prenatal diagnosis of recurrent Down syndrome due to unbalanced, de novo homologous acrocentric rearrangement is rare. Here, we report on an exceptionally rare case: a recurrent rea(21;21) Down syndrome that was probably caused by maternal germ-line cell mosaicism of the rearrangement.
A 27-year-old woman (gravida 2 para 1) requested a prenatal diagnosis after the detection of increased nuchal translucency in the fetus. The (unrelated) parents were healthy and had unremarkable medical histories. Their first pregnancy had been uneventful and the infant (a girl) was healthy. Chorionic villus sampling at 13 weeks of gestation (w.g.) revealed an abnormal karyotype, with a rearrangement between two chromosomes 21 (compatible with a rob(21;21) event) on the 16 metaphases analyzed. Karyotyping enabled us to diagnose Down syndrome in a female fetus (46,XX,+21,rob(21;21)(q10;q10)). Following a request by the couple, the pregnancy was terminated. Given that (1) the parents' first child was healthy, (2) the parental karyotypes (in blood lymphocytes) were normal, and (3) the vast majority of rob(21;21) are de novo, the subsequent genetic counseling was reassuring with regard to future pregnancies. The woman then experienced four consecutive miscarriages after conceiving with the same partner. No cytogenetic analyses had been performed. During the seventh pregnancy, the patient requested a prenatal diagnosis because of increased nuchal translucency and distended jugular lymphatic sacs. Chorionic villus sampling at 13 w.g. revealed rob(21;21) Down syndrome, with a 46,XY,+21,rob(21;21)(q10;q10) karyotype on the 15 metaphases analyzed. The pregnancy was subsequently terminated.
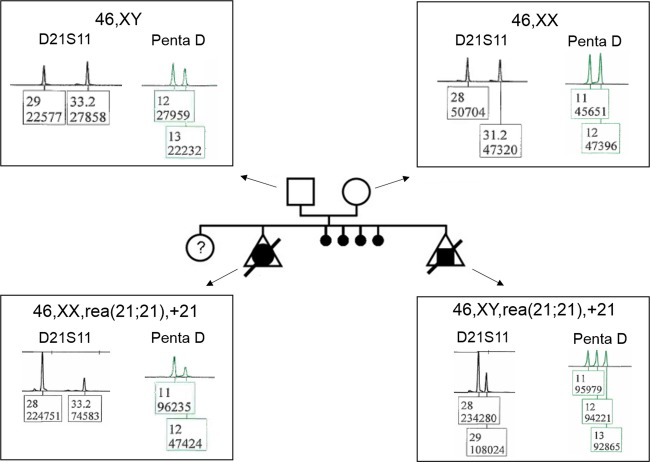
In order to determine the parental origin of this apparently recurrent rob(21;21), we performed a microsatellite marker analysis (focusing on two shorts tandem repeats: the D21S11 and Penta D loci on chromosome 21) in the fetus and parents. This showed that the rearrangement involving chromosomes 21 was an isochromosome 21 of maternal origin, rather than a rob(21;21) (Fig.1). With a view to detecting potential low-grade mosaicism in peripheral blood cells from the mother, we performed FISH analysis (focusing on DSCR1 locus on 21q22) of 1000 interphase nuclei; all were normal.
Figure 1.
Representative electrophoretograms (using the microsatellite markers D21S11 and Penta D) for the couple and the aborted male and female fetuses, suggesting an i(21q) of maternal origin in the first (female) fetus and the second (male) fetus. Considering the area under the curve, the electrophoretograms with the D21S11 marker revealed (1) a maternal duplication of allele 28 and a paternal allele 33.2 in the female fetus, and (2) a maternal duplication of allele 28 and a paternal allele 29 in the male fetus. The area under curve for the Penta D marker shows (1) a maternal duplication of allele 11 and a paternal allele 12 in the female fetus, and (2) the presence of three alleles in the male fetus (a paternal allele 13, a maternal allele 11 and an allele 12 whose origin cannot be proven but which is very probably maternal, in view of the results for the D21S11 marker). The results of these microsatellite marker analyses suggest that the i(21q) is of maternal origin in both fetuses.
The karyotypes of the two aborted fetuses were thus determined to be 46,XX,i(21)(q10) and 46,XY,i(21)(q10), respectively.
Our results raise a number of questions. In view of the first normal pregnancy, what genetic counseling should have been given after the termination of pregnancy? What mechanism led to the maternally confined mosaicism? And what genetic counseling should be given to the couple's healthy daughter?
As mentioned in the Introduction, about 95% of rob(21;21) translocations arise de novo 4. An isochromosome 21 (i(21q)) is derived from replication of a single chromosome 21, whereas a homologous rob(21;21) involves translocation between two different homologous chromosomes 21. Conventional cytogenetic analysis is unable to distinguish between rob(21;21) and i(21q), and so the use of molecular assays of microsatellite markers is required.
In the literature, most of the initial reports of rob(21;21) Down syndrome feature an i(21q) 5. Shaffer et al. found that 17 of 19 cases were i(21q), with roughly equivalent maternal (n = 10) and paternal (n = 9) inheritance. When additionally considering four previous studies, 32 of 36 cases were found to be i(21q) 5–7. Again, the frequencies of maternally inheritance (n = 17) and paternal inheritance (15) were equivalent. The four true rob(21;21) were all maternally inherited.
Kovaleva and Shaffer 8 later analyzed published cases of rea(21;21) Down syndrome and found that the prevalence of mosaicism among the parents was unusually high. However, the recurrence rate for de novo rea(21;21) (rob(21;21) or i(21q)) Down syndrome is reportedly low. Steinberg et al. 9 studied 112 families in which the child's Down syndrome was caused by a de novo t(Gq;Gq) event. This event was a 21q21q translocation in 77 families, and none of the couples had a second pregnancy affected by Down syndrome. However, Steinberg et al. identified three couples (one mother and two fathers) with low-grade mosaicism for rea(21;21) translocation Down syndrome in blood samples and an additional couple in which the mother carried a pericentric inversion of chromosome 21. Two of the three mosaic couples had a healthy normal child. Given that four of the 112 couples (3.6%) had an abnormal karyotype, the researchers estimated the risk recurrence to be between 2% and 3.6%. Accordingly, Steinberg et al. advocated that “caution should be exercised when counseling that the recurrence risk of this event is remote”.
A few reports on couples experiencing recurrent de novo rea(21;21) Down syndrome have demonstrated that mosaicism for rea(21;21) in the skin or ovaries of one parent was combined with normal or low-level mosaicism in blood lymphocytes 9 (Table1).
Table 1.
Reports of apparent de novo der(21;21), parental mosaicism and the presence or absence of recurrence
| Parental mosaicism | |||||||
|---|---|---|---|---|---|---|---|
| References | Number of families | Tissue | Karyotype | i(21q) | Offspring with trisomy | Normal children | Recurrence |
| 10 | 1 | Mother's skin Ovary | 46,XX[46]/46,XX,-G+?t(GqGq)[4] 46,XX[29]/46,XX,-G+t(GqGq)[1] | Not determined | 2 | 0 | 1 |
| 11 | 1 | Mother's skin | 46,XX[435]/ 45,XX,t(21q21q)[1] | Not determined | 2 | 1 | 1 |
| 12 | 1 | Not studied | Not determined | 2 | 1 | 1 | |
| 13 | 1 | Mother's skin | 46,XX[993]/46,XX,-21,+t(21q21q)[7] | Not determined | 3 | 1 | 2 |
| 14 | 2 | Not studied | Not determined | 3 | 1 | 2 | |
| Not studied | Not determined | 2 | 0 | 1 | |||
| 15 | 1 | Mother's blood | 46,XX[99]/46,XX,-21,+t(21q21q)[1] | Not determined | 2 | 0 | 1 |
| 16 | 2 | Mother's blood | 46,XX[6]/45,XX,-21[168]/46,-21,-21,+i(21q)[26] | Yes | 1 | 1 | 0 |
| Mother's blood | 46,XX[2]/45,XX,-21,+dup(21q)[73] | Yes | 2 | 2 | 1 | ||
| 17 | 1 | Mother's blood and skin | 46,XX[2648]/ 45,XX,-21,-21,+t(21q21q)[11] | Not determined | 1 | 0 | 0 |
| 18 | 3 | Parents' blood | No detailed analysis | Yes | 2 | 2 | 1 |
| Parents' blood | No detailed analysis | Yes | 2 | 0 | 1 | ||
| Parents' blood | No detailed analysis | Yes | 2 | 1 | 1 | ||
| This report | 1 | Mother's blood | 46,XX | Yes | 2 | 1 | 1 |
| Total (n) | 14 | 7 | 6 | 28 | 11 | 14 | |
With regard to our present case (with a healthy child born 2 years before the first i(21q) event, the recurrence of a maternally inherited i(21q), and normal peripheral lymphocyte karyotypes in both parents), what advice should be given? Since the occurrence of another fetus with the same karyotype cannot be ruled out, preimplantation genetic screening may provide this couple with the certitude that a baby will be free of Down syndrome. Oocyte donation and adoption are other options.
Even though we did not study the woman's ovarian tissue, the features of the present case suggest the existence of germ-line cell mosaicism with at least three different oocyte populations: those containing a chromosome 21 (giving rise to normal offspring), those containing an i(21q) (responsible for recurrent i(21q) Down syndrome), and those lacking a chromosome 21 (responsible for the four miscarriages due to monosomy 21). However, the couple's healthy daughter may have resulted from the rescue of an initial i(21q) Down syndrome zygote (and thus will require careful genetic counseling) or the rescue of a monosomy 21. Current French legislation prevents genetic analysis of the healthy child.
One possible explanation for the occurrence of the maternal i(21q) is the reduplication of one of the chromosomes 21, forming a second trisomic cell line with an isochromosome (46,XX,i(21q),+21). Subsequent rescue of the trisomy would have generated a third, balanced cell line with the isochromosome (45,XX,i(21q)).
In view of the present case and the literature data, the vast majority of apparently de novo cases of rea(21;21) feature an i(21q) with maternal or paternal inheritance. Recurrence of rea(21;21) Down syndrome does not therefore appear to be such a rare event, with at least 14 recurrences in 105 reported 9 and documented cases (Table1), that is to say an incidence of 13.3%. We consider that (1) the previously reported recurrence rate of 2% is a marked underestimation and (2) that clinical practice and genetic counseling should be revised accordingly. When parental mosaicism is evidenced by high-quality cytogenetic analyses, one can even question whether the first-reported cases of rea(21;21) in parents with an apparently normal karyotype were truly de novo.
In line with Kovaleva and Shaffer's report 8, we recommend performing a DNA microsatellite marker analysis as soon as rea(21;21) Down syndrome is detected; this should enable one to distinguish between a true rob(21;21) and an i(21q). When an i(21q) is detected, extensive cytogenetic analysis of at least 500 cells (from a variety of tissues, if possible) might enable the detection of low-grade parental mosaicism and thus the provision of appropriate genetic counseling for an inherited rearrangement.
Acknowledgments
The authors thank the couple for their cooperation.
Conflicts of Interest
None declared.
References
- Collins VR, Muggli EE, Riley M, Palma S. Halliday JL. Is Down syndrome a disappearing birth defect? J. Pediatr. 2008;152:20–24. doi: 10.1016/j.jpeds.2007.07.045. , and, 24 e1. [DOI] [PubMed] [Google Scholar]
- Shaffer L. 1990. Molecular and cytogenetic characterization of de novo acrocentric rearrangements in human. Doctoral thesis, Medical College of Virginia, Richmond.
- Bandyopadhyay R, McCaskill C, Knox-Du Bois C, Zhou Y, Berend SA, Bijlsma E, et al. Mosaicism in a patient with Down syndrome reveals post-fertilization formation of a Robertsonian translocation and isochromosome. Am. J. Med. Genet. A. 2003;116A:159–163. doi: 10.1002/ajmg.a.10113. [DOI] [PubMed] [Google Scholar]
- Shaffer LG, McCaskill C, Haller V, Brown JA. Jackson-Cook CK. Further characterization of 19 cases of rea(21q21q) and delineation as isochromosomes or Robertsonian translocations in Down syndrome. Am. J. Med. Genet. 1993;47:1218–1222. doi: 10.1002/ajmg.1320470818. [DOI] [PubMed] [Google Scholar]
- Grasso M, Giovannucci Uzielli ML, Pierluigi M, Tavellini F, Perroni L. Dagna Bricarelli F. Isochromosome not translocation in trisomy 21q21q. Hum. Genet. 1989;84:63–65. doi: 10.1007/BF00210673. [DOI] [PubMed] [Google Scholar]
- Antonarakis SE, Adelsberger PA, Petersen MB, Binkert F. Schinzel AA. Analysis of DNA polymorphisms suggests that most de novo dup(21q) chromosomes in patients with Down syndrome are isochromosomes and not translocations. Am. J. Hum. Genet. 1990;47:968–972. [PMC free article] [PubMed] [Google Scholar]
- Brahe C, Tassone F, Moscetti A, Millington-Ward A, Bova R. Serra A. Molecular study of parental origin of extra chromosome 21 in regular and de novo translocation trisomies. Am. J. Med. Genet. Suppl. 1990;7:125–128. doi: 10.1002/ajmg.1320370725. [DOI] [PubMed] [Google Scholar]
- Kovaleva NV. Shaffer LG. Under-ascertainment of mosaic carriers of balanced homologous acrocentric translocations and isochromosomes. Am. J. Med. Genet. A. 2003;121A:180–187. doi: 10.1002/ajmg.a.20156. [DOI] [PubMed] [Google Scholar]
- Steinberg C, Zackai EH, Eunpu DL, Mennuti MT. Emanuel BS. Recurrence rate for de novo 21q21q translocation Down syndrome: a study of 112 families. Am. J. Med. Genet. 1984;17:523–530. doi: 10.1002/ajmg.1320170214. [DOI] [PubMed] [Google Scholar]
- Mark HF, Mendoza T, Abuelo D, Beauregard LJ, May JB. LaMarche PH. Reproduction in a woman with low percentage t(21q21q) mosaicism. J. Med. Genet. 1977;14:221–223. doi: 10.1136/jmg.14.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilroy RS, Jr, Summitt RL. Martens P. Reproduction in a woman with mosaicism. J. Med. Genet. 1978;15:406–407. doi: 10.1136/jmg.15.5.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R. Nitowsky HM. Recurrence of apparent de novo 21/21 translocation trisomy in a sibship. J. Pediatr. 1977;90:841–842. doi: 10.1016/s0022-3476(77)81269-9. [DOI] [PubMed] [Google Scholar]
- Jacobs PA, Mayer M. Rudak E. Structural chromosome abnormalities in Down syndrome: a study of two families. Cytogenet. Cell Genet. 1978;20:185–193. doi: 10.1159/000130850. [DOI] [PubMed] [Google Scholar]
- Garver KL, Marchese SG, Steele MW. Ketterer DM. Recurrence risk in 21q/21q translocation of Down syndrome. J. Pediatr. 1982;100:243–245. doi: 10.1016/s0022-3476(82)80646-x. [DOI] [PubMed] [Google Scholar]
- Hall BD. Recurrence risk in de novo 21q21q translocation Down syndrome. Am. J. Med. Genet. 1985;22:417–418. doi: 10.1002/ajmg.1320220228. [DOI] [PubMed] [Google Scholar]
- Priest JH, Blackston RD, Pearse LA. Warren ST. Molecular evidence for true isochromosome 21q. Hum. Genet. 1988;81:1–3. doi: 10.1007/BF00283718. [DOI] [PubMed] [Google Scholar]
- Croci G. Franchi F. Parental mosaicism in de novo translocation (21q21q) Down's syndrome. J. Med. Genet. 1991;28:502. doi: 10.1136/jmg.28.7.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CP, Chern SR, Tsai FJ, Wu PC, Chiang SS, Lee CC, et al. Down syndrome due to unbalanced homologous acrocentric rearrangements and its recurrence in subsequent pregnancies: prenatal diagnosis by amniocentesis. Taiwan J. Obstet. Gynecol. 2009;48:403–407. doi: 10.1016/S1028-4559(09)60331-4. [DOI] [PubMed] [Google Scholar]