Key Clinical Message
Prompt serum copper and zinc in addition to vitamin B12 levels should be measured in patients suffering from refractory anemia with neurological symptoms. A timely copper supplementation can help revert the hematological and possibly the neurological manifestations.
Keywords: Anemia, copper deficiency, myelodysplastic syndrome, neuropathy
Copper is an important trace element with an integral role in metabolism due to its requirement in the function of many critical enzymes involved in production of hemoglobin, myelin, melanin, and regulation of the thyroid gland function 1. Hematological manifestations of acquired copper deficiency include anemia and neutropenia. Classical bone marrow findings comprise of prominent vacuoles in erythroid and myeloid precursors along with iron-containing plasma cells and ring sideroblasts 2. The neurological manifestations of copper deficiency very closely resemble the clinical findings of subacute combined degeneration of the spinal cord (SCD) which is similar to the presentation in vitamin B12 deficiency 3. We report a case of macrocytic anemia with SCD-type neuropathy due to copper deficiency in a patient with supra normal serum zinc concentration. Copper deficiency is now a better known masquerader of myelodysplastic syndrome. Our case emphasizes the need for prompt copper and zinc level measurements in addition to vitamin B12 levels in patients suffering from refractory anemia with neurological symptoms.
Case Presentation
A 45-year-old white female was admitted to the hospital in May 2012 with complaints of progressively increasing fatigue and shortness of breath on exertion for 4 months. She had no previous history of any chronic medical conditions and had normal blood counts 1 year ago. About 3 weeks before presenting to the hospital, the patient developed paresthesia of her toes and subsequently the numbness extended up to her mid-shin bilaterally. She also complained of numbness in her fingertips. There were no motor deficits. She denied any history of fever, night sweats, and anorexia or weight loss at that time and or any symptoms of easy bruising and bleeding. There was no history of gastric surgery, chronic diarrhea, or urinary complaints. She was a social drinker. Patient declined any recreational drugs and was a lifelong nonsmoker. On physical examination the patient was comfortable at rest. Her vital signs were stable. Head, ears, nose, and throat examination was normal. No lymphadenopathy or hepatosplenomegaly was noted and there were no abnormal skin findings. Her central nervous system examination was only remarkable for absent deep tendon reflexes without any objective sensory loss. Babinski reflex was negative.
Routine laboratory investigations revealed macrocytic anemia with a hemoglobin of 8.0 g/dL and a mean corpuscular volume of 104 femtoliter. The total leukocyte count was noted to be 1.7 × 109/L with a differential count of Polymorphs – 34%, Lymphocytes – 53%, and Monocytes – 10%. Her platelet count was normal at 2.48 × 109/L and so was the reticulocyte count – 1.3% (absolute reticulocyte count 0.04 × 106/μL). The iron panel demonstrated normal parameters; serum iron – 30 μg/dL, TIBC of 474 μg/dL and a ferritin level of 192 ng/mL. Her serum vitamin B12 and methylmalonate levels as well as her thyroid functions were normal. The peripheral smear revealed slight anisopoikilocytosis with macrocytes and also demonstrated leukopenia but without any cell line dysplasia or blasts. Hemolytic work-up was negative for Coombs test with acceptable lactate dehydrogenase and haptoglobin levels. Chemistries revealed optimal liver and renal function.
The day after admission her hemoglobin declined to 7.6 g/dL and she required transfusion of two units of packed red blood cells with an appropriate posttransfusion response. No autoantibodies were found on screening. A bone marrow biopsy was recommended and performed as an outpatient.
Bone marrow biopsy finding demonstrated a hypercellular bone marrow with marked erythroid hyperplasia (Fig.1). Prominent cytoplasmic vacuolization of immature erythroid and granulocytic precursors with ring sideroblast was also noted on the bone marrow aspirate (Figs2 and 3) These peculiar changes were suggestive of acquired copper deficiency and serum copper and zinc levels were measured. Her copper levels were markedly low at 5 μg/L (normal range 70–155 μg/L) and zinc levels were noted to be elevated at 213 μg/L (normal 70–150 μg/L). Cytogenetic and flow cytometry testing revealed a normal female karyotype with no genetic aberrations associated with MDS, thus confirming the diagnosis of copper deficiency.
Figure 1.
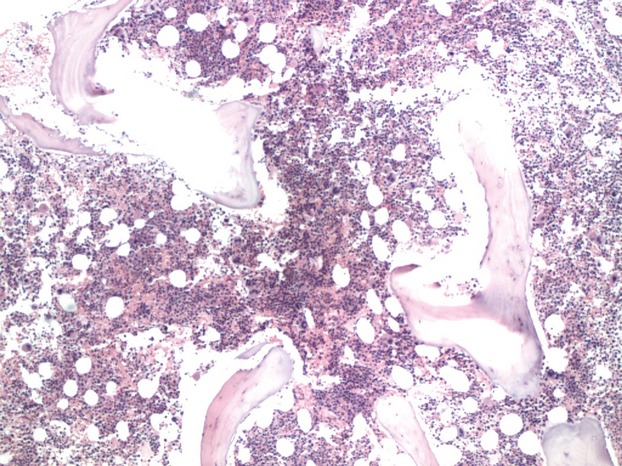
Bone marrow biopsy- Hypercellular bone marrow with marked erythroid hyperplasia.
Figure 2.
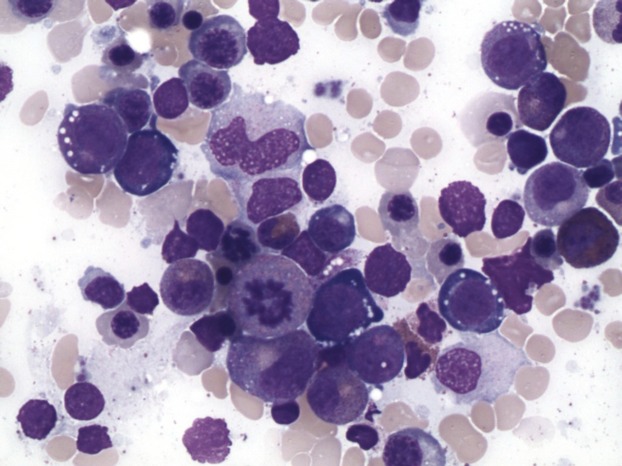
Bone marrow aspirate- Prominent cytoplasmic vacuolization of immature erythroid and granulocytic precursors.
Figure 3.
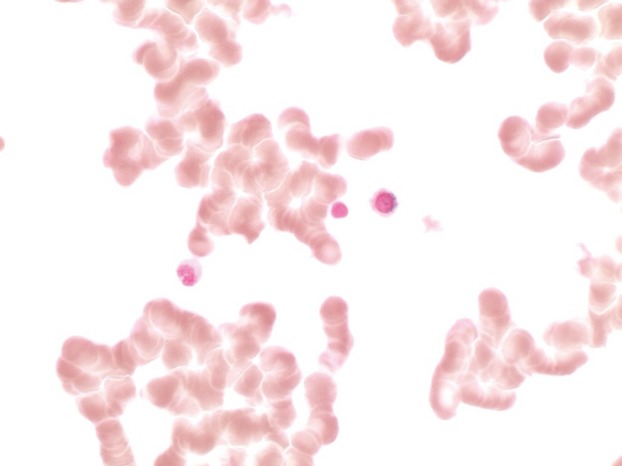
Bone marrow aspirate: Ring sideroblast.
Patient denied any history of excessive zinc intake except occasional over the counter multivitamin tablets. She was started on daily copper sulfate supplementation at 2 mg orally three times a day for 6 months. Her condition improved rapidly with resolution of anemia and leukopenia within 1 month, though neuropathy persisted. Repeat serum copper and zinc levels 6 months later were normal at 158 and 73 μg/L, respectively. Patient remains symptom free 2 years later with normal blood counts; hemoglobin (15.6 g/dL), MCV (101.6 femtoliter) and WBC count (8.5 × 109/L). Her neurological symptoms have also improved substantially with slight residual numbness in lower extremities.
Discussion
Copper is an ubiquitous trace element which is essential for numerous important metabolic pathways in the body. Several factors have been described which cause serum copper levels to fall below required concentration for normal cellular function. The most common cause for copper deficiency is malabsorption, especially due to dumping syndrome as a result of an increasing incidence of gastrointestinal bypass surgeries for the obesity epidemic 4. Another important factor is hyperzincemia due to nutritional supplementation. High zinc levels result in the increased production of metallothionein in intestinal cells. Copper binds to metallothionein displacing zinc, leading to accumulation of copper in the intestinal enterocytes. These enterocytes are ultimately shed and lost through the gastrointestinal tract, leading to copper elimination and deficiency 5. Interestingly, increased iron levels as seen in hemochromatosis have also been implicated to cause hyperzincemia and associated copper deficiency. Idiopathic cases with no apparent cause have also been described 1.
The classic hematological findings include bicytopenia generally affecting the myeloid and erythroid precursors, though rare cases of thrombocytopenia have also been reported 6. Copper is a vital cofactor for various redox enzymes such as ceruloplasmin and cytochrome oxidase, and decreased activity of these enzymes have been postulated to be a potential cause of anemia. Mitochondria isolated from copper-deficient cells failed to synthesize heme from ferric iron and protoporphyrin 1. The anemia in copper deficiency can present in varied morphological forms and anisopoikilocytosis is frequently observed in the peripheral smear. Neutropenia is generally the most frequent laboratory abnormality noted 7,8. The etiology has been unclear though arrested maturation of granulocyte precursors has been demonstrated in in vivo studies of copper-deficient mouse cells 9. This presentation generally mimic myelodysplastic syndrome. The bone marrow findings, though not pathognomonic, are very characteristic of copper deficiency and most cases are suspected due to the findings of increased vacuolization in the erythroid and myeloid precursors, as well as presence of ring sideroblasts and iron deposition in plasma cells 2,10,11. Such was the case in our patient who presented with symptoms of fatigue, exertional dyspnea, and neuropathy. With the absence of increased myeloblasts along with normal cytogenetic and no immunophenotypic abnormalities of MDS, copper deficiency became highly likely. Importantly, these hematological abnormalities are completely reversible with adequate copper supplementation.
Neurological manifestations of acquired copper deficiency include presentations of myelopathy and peripheral neuropathy which can closely mimic the symptoms and clinical signs of SCD, most commonly caused due to vitamin B12 deficiency. Spinal and peripheral nerve injury can also occur and patients can have presentations varying from indolent gait disorders to sensory ataxia with or without spasticity 3,12–14. Magnetic resonance imaging may show subcortical white matter changes, cerebellar atrophy, and signal changes in the dorsal columns in either the cervical or thoracic cord 12. More recent published reports have also described the causal relationship between copper deficiency and optic neuropathy (acute, subacute, chronic) presenting several years after gastric bypass surgery 15,16. Dake et al. 17 had previously demonstrated in mouse studies that copper depletion could lead to demyelination of optic nerve, thus probably explaining the irreversible neurological deficiency if not treated promptly.
The diagnosis of acquired copper deficiency requires a high clinical suspicion, especially in patients with combined hematological and neurological deficiency. Although hematologic manifestations of copper deficiency are reversible with early supplementation, many patients experience partial relief of their neurological symptoms and have irreversible neurological debilitation as also shown in other published reports 16,18. Such easily treatable causes should be thus promptly diagnosed and treated for a better clinical outcome. Our case emphasizes the need for an early evaluation of serum copper and zinc levels along with vitamin B12 levels in a patient suffering from refractory macrocytic anemia with myelopathy or neuropathy. A timely copper supplementation can help revert the hematological and possibly the neurological manifestations.
Conflict of Interest
None declared.
References
- Gregg XT, Reddy V. Prchal JT. Copper deficiency masquerading as myelodysplastic syndrome. Blood. 2002;100:1493–1495. doi: 10.1182/blood-2002-01-0256. [DOI] [PubMed] [Google Scholar]
- Halfdanarson TR, Kumar N, Li CY, et al. Hematological manifestations of copper deficiency: a retrospective review. Eur. J. Haematol. 2008;80:523–531. doi: 10.1111/j.1600-0609.2008.01050.x. [DOI] [PubMed] [Google Scholar]
- Jaiser SR. Winston GP. Copper deficiency myelopathy. J. Neurol. 2010;257:869–881. doi: 10.1007/s00415-010-5511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Ahlskog JE, Gross JB., Jr Acquired hypocupremia after gastric surgery. Clin. Gastroenterol. Hepatol. 2004;2:1074–1079. doi: 10.1016/s1542-3565(04)00546-4. [DOI] [PubMed] [Google Scholar]
- Sutton L, Vusirikala M. Chen W. Hematogone hyperplasia in copper deficiency. Am. J. Clin. Pathol. 2009;132:191–199. doi: 10.1309/AJCPS3ENUQ5LKBSB. , and; quiz 307. [DOI] [PubMed] [Google Scholar]
- Lazarchick J. Update on anemia and neutropenia in copper deficiency. Curr. Opin. Hematol. 2012;19:58–60. doi: 10.1097/MOH.0b013e32834da9d2. [DOI] [PubMed] [Google Scholar]
- Gabreyes AA, Abbasi HN, Forbes KP, et al. Hypocupremia associated cytopenia and myelopathy: a national retrospective review. Eur. J. Haematol. 2013;90:1–9. doi: 10.1111/ejh.12020. [DOI] [PubMed] [Google Scholar]
- Robinson SD, Cooper B. Leday TV. Copper deficiency (hypocupremia) and pancytopenia late after gastric bypass surgery. Proc. Bayl. Univ. Med. Cent. 2013;26:382–386. doi: 10.1080/08998280.2013.11929011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimbakas J, Langkamp-Henken B. Percival SS. Arrested maturation of granulocytes in copper deficient mice. J. Nutr. 1998;128:1855–1860. doi: 10.1093/jn/128.11.1855. [DOI] [PubMed] [Google Scholar]
- Hoffman HN, II, Phyliky RL. Fleming CR. Zinc-induced copper deficiency. Gastroenterology. 1988;94:508–512. doi: 10.1016/0016-5085(88)90445-3. [DOI] [PubMed] [Google Scholar]
- Hedera P, Fink JK, Bockenstedt PL, et al. Myelopolyneuropathy and pancytopenia due to copper deficiency and high zinc levels of unknown origin: further support for existence of a new zinc overload syndrome. Arch. Neurol. 2003;60:1303–1306. doi: 10.1001/archneur.60.9.1303. [DOI] [PubMed] [Google Scholar]
- Kumar N, Gross JB., Jr Ahlskog JE. Copper deficiency myelopathy produces a clinical picture like subacute combined degeneration. Neurology. 2004;63:33–39. doi: 10.1212/01.wnl.0000132644.52613.fa. [DOI] [PubMed] [Google Scholar]
- Schleper B. Stuerenburg HJ. Copper deficiency-associated myelopathy in a 46-year-old woman. J. Neurol. 2001;248:705–706. doi: 10.1007/s004150170118. [DOI] [PubMed] [Google Scholar]
- Prodan CI, Holland NR, Wisdom PJ, et al. CNS demyelination associated with copper deficiency and hyperzincemia. Neurology. 2002;59:1453–1456. doi: 10.1212/01.wnl.0000032497.30439.f6. [DOI] [PubMed] [Google Scholar]
- Naismith RT, Shepherd JB, Weihl CC, et al. Acute and bilateral blindness due to optic neuropathy associated with copper deficiency. Arch. Neurol. 2009;66:1025–1027. doi: 10.1001/archneurol.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarandi SS, Griffith DP, Sharma R, et al. Optic neuropathy, myelopathy, anemia, and neutropenia caused by acquired copper deficiency after gastric bypass surgery. J. Clin. Gastroenterol. 2014;48:862–865. doi: 10.1097/MCG.0000000000000092. [DOI] [PubMed] [Google Scholar]
- Dake Y. Amemiya T. Electron microscopic study of the optic nerve in copper deficient rats. Exp. Eye Res. 1991;52:277–281. doi: 10.1016/0014-4835(91)90091-r. [DOI] [PubMed] [Google Scholar]
- Huff JD, Keung YK, Thakuri M, et al. Copper deficiency causes reversible myelodysplasia. Am. J. Hematol. 2007;82:625–630. doi: 10.1002/ajh.20864. [DOI] [PubMed] [Google Scholar]


