Key Clinical Message
It is well-known that the major organisms for mycotic aneurysms are Staphylococcus aureus, Salmonella spp but is extremely rare in Streptococcus pneumoniae in postantibiotic era. We demonstrated the first case with multiple mycotic aneurysms simultaneously generated in the splenic and hepatic arteries in a patient with pneumococcal pneumonia.
Keywords: Hepatic artery aneurysm, mycotic aneurysm, splenic artery aneurysm, Streptococcus pneumoniae
Case Presentation
A 74-year-old woman was referred to our emergency department because of pyrexia and productive cough for a few days. She underwent replacements of three valves for mitral, tricuspid, and aortic regurgitation at 15, 10, and 2 years earlier. She had a pacemaker implanted for sick sinus syndrome 5 years previously. She never smoked and occasionally consumed alcohol. There was no relevant family or travel history. She denied contact with any sick persons and had no significant animal contact. Vital signs showed blood pressure of 110/60 mmHg, temperature of 37°C, respiratory rate of 24 breaths/min, heart rate of 84 beats/min, and oxygen saturation of 88% on ambient air. Physical examination demonstrated coarse crackles in bilateral middle-to-lower lung fields, and a Levine III/IV systolic murmur at the right 2nd intercostal space. Chest X-ray on admission showed consolidation in bilateral middle to lower lung fields (Fig.1A), cardiomegaly with a permanent pacemaker, and three postoperative valves, which were confirmed by thoracic computed tomography (CT) (Fig.1B). Although two sets of blood cultures were negative, sputum Gram stain revealed engulfment of Gram-positive diplococci within neutrophils, suggesting Streptococcus pneumoniae pneumonia. Based on the diagnosis of pneumococcal pneumonia, she was admitted to our hospital (day 1) and treated with imipenem/cilastatin (0.5 g q6 h). Thereafter, S. pneumoniae was isolated from the sputum, which was susceptible to all antibiotics, suggesting penicillin-susceptible S. pneumoniae (PSSP).
Figure 1.
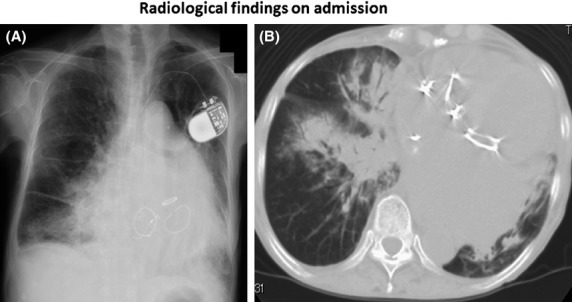
Chest X-ray (A) on admission shows consolidation in bilateral middle to lower lung fields and cardiomegaly. A pacemaker and three replaced valves are also noted. Thoracic computed tomography (B) on the same day demonstrates consolidation with an air bronchogram in the right middle lobe and bilateral lower lobes, as well as dilatation of the left atrium and left ventricle.
After the start of treatment, her general condition improved, with defervescence and no requirement for oxygen within a few days, but on day 9, her temperature increased to 38.4°C with sudden left upper quadrant abdominal pain. Non-enhanced thoraco-abdominal CT (Fig.2A) taken on day 9 showed faint low density areas (LDAs) in the upper portion of the spleen, whereas enhanced CT on the same day (Fig.2B) showed LDA involving two-thirds of the spleen and a faint LDA located in the right hepatic lobe, suggesting hepatic and splenic infarctions of undetermined cause. Blood cultures taken on day 9 were positive for Candida albicans, along with elevation of serum (1→3)-β-d-glucan to 42.2 pg/mL (Wako Inc, Osaka, Japan). Therefore, she was immediately treated with intravenous infusion of micafungin (150 mg/day) on day 10, and the subsequent blood cultures were negative.
Figure 2.
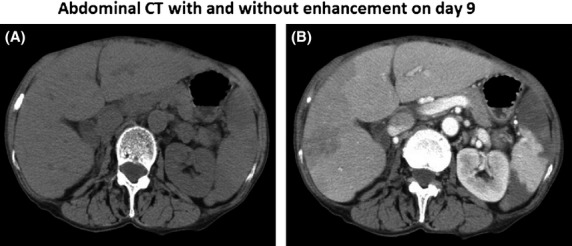
Nonenhanced abdominal CT (A) taken on day 9 shows faint low density areas (LDAs) in the upper portion of the spleen, whereas enhanced CT on the same day (B) shows that an LDA occupies two-thirds of the spleen and a faint LDA is located in the right hepatic lobe, suggesting hepatic and splenic infarction of unknown cause.
However, on day 26, a new, sudden, right upper quadrant pain emerged, and enhanced abdominal CT demonstrated saccular enhanced lesions measuring up to 35 mm (Fig.3A, arrow head), surrounded by an LDA of the right hepatic lobe together with a huge LDA in the spleen. Two approximately 10-mm enhanced nodules were noted (Fig.3B, arrows) surrounded by an LDA at the upper portion of the spleen together with an LDA in lower portion of the spleen. The LDA measuring 5 cm in size in the right hepatic lobe had a dense component (Fig.3B, arrow head). On day 26, urgent abdominal angiography was performed, which confirmed that the above-mentioned enhanced lesions on abdominal CT corresponded to two mycotic hepatic artery aneurysms (Fig.3C, arrow heads) and two splenic artery aneurysms (Fig.3D, arrows).
Figure 3.
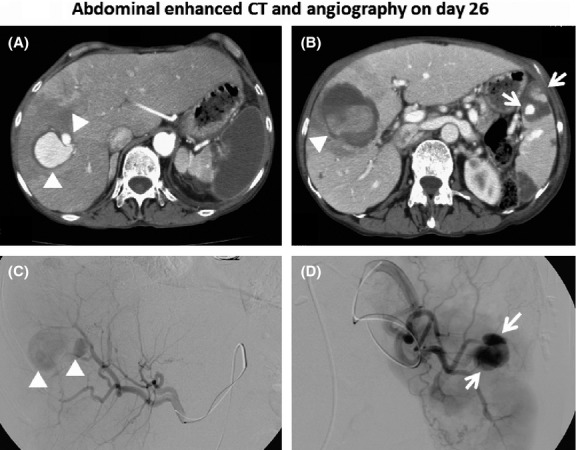
Enhanced abdominal CT taken on day 26 demonstrates a saccular enhanced lesion measuring 35 mm (A, arrow head) with an LDA in the right hepatic lobe and a huge LDA in the spleen. Two 10-mm enhanced nodules (B, arrows) were noted surrounded by an LDA at the upper portion of the spleen together with an LDA in the lower portion of the spleen. The LDA measuring 5 cm in size in the right hepatic lobe has a dense component (B, arrow head). Abdominal angiography on the same day clearly demonstrates the two mycotic hepatic artery aneurysms (C, arrow heads) and two splenic artery aneurysms (D, arrows).
To examine the source of the infection, whole CT and echocardiography (two times transthoracic and three times transesophageal) were performed in due course (Fig.4), but no apparent vegetation or abscesses were noted. Furthermore, after the start of treatment with intravenous micafungin, 10 sets of repeated blood cultures in the subsequent days were all negative, with normalization of the value of serum (1→3)-β-d-glucan (Fig.4).
Figure 4.
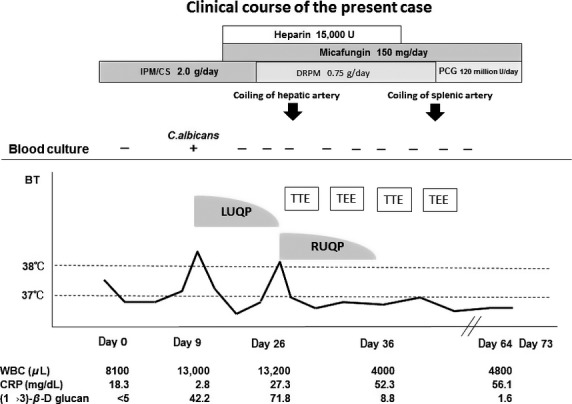
Clinical course of the present case. BT, body temperature; CRP, C-reactive protein; DRPM, doripenem; IMP/CS, imipenem/cilastatin; LUQP, left upper quadrant pain; RUQP, right upper quadrant pain; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; UCG, ultrasound cardiography; WBC, white blood cell count.
The patient was thus diagnosed with multiple mycotic aneurysms of undetermined infectious cause generated sequentially in the hepatic and splenic arteries in association with pneumococcal pneumonia. She was successfully treated with transcatheter coil embolization for the hepatic (day 26) and splenic aneurysms (day 40). After completion of 6 weeks of intravenous treatments with doripenem (0.75 g/day) followed by penicillin G (120 million U/day) for PSSP and 6 weeks treatment with micafungin for C. albicans, she was discharged uneventfully on day 73.
Discussion
Infected aortic aneurysms were first described by Sir William Osler in 1885 1. Mycotic aneurysms comprise 2.5% of all vascular aneurysms 2. It is well known that the major organisms for mycotic aneurysms are Staphylococcus aureus and Salmonella spp 3, but S. pneumoniae is rare in the post-antibiotic era 4. Furthermore, only 2% of mycotic aneurysms have true fungal infections 5, to which immunocompromised patients, such as those with acquired immunodeficiency syndrome, chronic disease like diabetes mellitus, malignant neoplasm, prolonged use of chemotherapy or broad-spectrum antibiotics, invasive medical care for transplantation, or indwelling catheters are vulnerable 6.
In this respect, the present case is an immunocompetent patient except for the medical histories of repeated cardiac operations and the presence of a pacemaker and its lead. These facts led us to the hypothesis that both of the pathogens (C. albicans or S. pneumoniae) could have been associated with the mycotic aneurysms. Mycotic aneurysms caused by C. albicans or S. pneumoniae are extremely rare. Thus, to compare and characterize them, all cases from PubMed were retrieved using the terms “mycotic aneurysm”, “S. pneumoniae”, and “C. albicans” during the period from 1987 to 2014.
This intensive review gathered a total of 63 cases, which consisted of 34 cases with S. pneumoniae (Table S1) and 29 cases with C. albicans (Table S2). The age of the S. pneumoniae group (64.0 ± 10.9 years) was significantly higher than that of C. albicans group (49.3 ± 17.3 years), mean ± SD), and the male to female ratio was not significantly different (20/14 vs. 23/6). However, the frequency of underlying disease was significantly higher in the C. albicans group (100%) than in the S. pneumoniae group (52.9%, excluding the eight cases whose data were not available, P < 0.001) (Tables S1 and S2). Especially in the C. albicans group, the proportion of immunocompromised patients was high, as reported in previous cases 6. The average number (1.2 ± 0.5 vs. 1.1 ± 0.3) and size (48.2 ± 18.8 vs. 47.8 ± 21.8 mm) of the mycotic aneurysms in each group (S. pneumoniae vs. C. albicans) were equal (Table1). In this respect, the present case simultaneously had a large number of mycotic aneurysms (n = 4) with typical saccular form (Fig.3), as previously reported 7.
Table 1.
Comparison of the patients with mycotic aneurysms between Streptococcus pneumoniae and Candida albicans
| Patient characteristics | S. pneumoniae | C. albicans | P value |
|---|---|---|---|
| Total number of patients | 34 | 29 | |
| Age (ave ± SD) | 64.0 ± 10.9 | 49.3 ± 17.3 | <0.001 |
| Male/Female | 20/14 | 23/6 | NS |
| Presence of pneumonia | 47% (n = 16) | 3.4% (n = 1) | <0.001 |
| Blood culture | 27 | 20 | |
| Positive/Negative/NA | 18/9/4 | 10/8/2 | NS |
| Local pain | |||
| YES/NO | 18/16 | 14/15 | NS |
| RTD | 5.8 ± 4.1 | 3.0 ± 2.3 | 0.021 |
| Duration of treatment (week) | 8.7 ± 10.8 | 8.9 ± 12.6 | NS |
| Lifetime treatment | 2.9% (n = 1) | 31.0% (n = 9) | 0.004 |
| Prognosis (Alive/Dead) | 27/7 | 21/8 | NS |
| Total number of aneurysm in all patients | 40 | 30 | |
| Number of aneurysm in each patient (ave ± SD) | 1.2 ± 0.5 | 1.1 ± 0.3 | NS |
| Size of aneurysm (ave ± SD) (mm) | 48.2 ± 18.8 | 47.8 ± 21.8 | NS |
| Portion | |||
| Aortic aneurysm | 76.4% (n = 26) | 41.3% (n = 12) | 0.009 |
| Total number of suprarenal aneurysm | N = 22 (55%) | N = 12 (40%) | NS |
| Cranial artery | 0 | 1 | |
| Pulmonary artery | 0 | 2 | |
| Ascending aorta | 0 | 3 | |
| Thoracic aorta | 11 | 0 | |
| Descending aorta | 2 | 4 | |
| Abdominal aorta | 4 | 0 | |
| Celiac trunk | 1 | 0 | |
| Inferior mesenteric | 1 | 0 | |
| Others | 3 | 2 | |
| Total number of Infrarenal aneurysm | N = 18 (45%) | N = 18 (60%) | NS |
| Renal artery | 2 | 4 | |
| Femoral artery | 2 | 2 | |
| Popliteal artery | 2 | 0 | |
| Crural artery | 0 | 1 | |
| Iliac artery | 2 | 6 | |
| Others | 10 | 5 | |
RTD, required time from initial onset to diagnosis; NA, not available; SD, standard deviation.
In general, mycotic aneurysm have a diverse distribution, while almost 90% of atherosclerotic aneurysms located are in the area of the infrarenal aorta 7, which was confirmed in the review both in the S. pneumoniae (supra vs. infrarenal: 22:18) and the C. albicans groups (12:18). Interestingly, the S. pneumoniae group (n = 26, 76.4%, P = 0.09) was more likely to have aortic involvement than the C. albicans group (n = 12, 41.3%). The frequency of clinical symptoms, such as chest, abdominal, or back pain, was similar in both groups (Table1). Those symptoms are usually considered associated with rupture of the aneurysm, hemorrhage, dissection, embolization, or infarction, but do not depend on the presence of the aneurysm itself 8. Therefore, abdominal pain in the present case was thought to be due to splenic or hepatic infarction, seen as LDAs in each organ on abdominal CT.
Of note, the proportion of patients with positive blood cultures was not significantly different between the groups (S. pneumonia vs. C. albicans: 66.7% vs. 50%), and the required time from initial onset to diagnosis (RTD) was significantly shorter in the C. albicans group (mean ± SD: 3.0 ± 2.3 weeks, P = 0.021) than in the S. pneumoniae group (mean ± SD: 5.8 ± 4.1 weeks). The RTD of the present case was approximately 2.4 weeks (17 days), which raised the suspicion that the C. albicans was likely the cause, while, in the S. pneumoniae group, most of the patients had only pneumonia (47%, n = 16) but not bacteremia (Table1), which was a diagnostic dilemma when considering the pathogens, as in the present case. The duration of treatment was similar in both groups, but lifetime treatment was given to a higher proportion of patients with C. albicans (31.0%, n = 9) than with S. pneumoniae (2.9%, n = 1), suggesting a high frequency of immunocompromised patients in the former group.
Thus, regarding the present case, one episode of transient candidemia with elevation of serum levels of (1→3)-β-d-glucan with advent of multiple aneurysms in a relatively short period (2.4 weeks) favors the diagnosis of C. albicans-related mycotic aneurysms, but the possibility of aneurysms related to S. pneumoniae can be still high, because (1) the antecedent episode of S. pneumoniae pneumonia; (2) the patient was relatively older and immunocompetent, unlike the usual case with C. albicans-associated mycotic aneurysms; and (3) anecdotal reports suggest that S. pneumoniae can cause vasculitis or infarction 9. From this literature review, we conclude that the present case is a first case with multiple hepatic and splenic mycotic aneurysms possibly caused by S. pneumoniae in a patient with pneumococcal pneumonia.
Conclusions
The first case with multiple mycotic aneurysms simultaneously generated in the splenic and hepatic arteries in a patient with pneumococcal pneumonia was reported, and the clinical and radiological features of mycotic aneurysms due to S. pneumoniae or C. albicans were examined in a literature review. Determining the responsible pathogen of mycotic aneurysms needs careful multidisciplinary assessment.
Consent
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
None.
Conflict of Interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Characteristics of patients with mycotic aneurysms infected with Streptococcus pneumoniae.
Table S2. Characteristics of patients with mycotic aneurysms infected with Candida albicans.
References
- Osler W. The Gulstonian lectures, on malignant endocarditis. Br. Med. J. 1885;1:467–470. doi: 10.1136/bmj.1.1262.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst GF. Dekcer JP. Bacterial aortitis and mycotic aneurysm of the aorta; a report of twelve cases. Am. J. Pathol. 1955;31:821–835. [PMC free article] [PubMed] [Google Scholar]
- Cordero JA, Jr, Darling RC, 3rd, Chang BB, Shah DM, Paty PS. Leather RP. In situ prosthetic graft replacement for mycotic thoracoabdominal aneurysms. Am. Surg. 1996;62:35–39. [PubMed] [Google Scholar]
- Cartery C, Astudillo L, Deelchand A, Moskovitch G, Sailler L, Bossavy JP, et al. Abdominal infectious aortitis caused by Streptococcus pneumoniae: a case report and literature review. Ann. Vasc. Surg. 2011;25:266. doi: 10.1016/j.avsg.2010.07.014. e9–16. [DOI] [PubMed] [Google Scholar]
- Brown SL, Busuttil RW, Baker JD, Machleder HI, Moore WS. Barker WF. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J. Vasc. Surg. 1984;1:541–547. [PubMed] [Google Scholar]
- Albes J, Haverich A, Freihorst J, von der Hardt H. Manthey-Stiers F. Management of mycotic rupture of the ascending aorta after heart-lung transplantation. Ann. Thorac. Surg. 1990;50:982–983. doi: 10.1016/0003-4975(90)91139-3. [DOI] [PubMed] [Google Scholar]
- Macedo TA, Stanson AW, Oderich GS, Johnson CM, Panneton JM. Tie ML. Infected aortic aneurysms: imaging findings. Radiology. 2004;231:250–257. doi: 10.1148/radiol.2311021700. [DOI] [PubMed] [Google Scholar]
- SIlen W. Cope's early diagnosis of the acute abdomen. 22nd ed. Oxford Univ. Press; 2010. Acute abdominal lesions arising in the left hyocondrium; pp. 141–144. [Google Scholar]
- Takada K, Kojima S, Hayashi T, Fujii T, Shirotani T, Kohyama S, et al. Adult bacterial meningitis complicated by cerebral infarction: report of three cases. No To Shinkei. 2006;58:329–334. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of patients with mycotic aneurysms infected with Streptococcus pneumoniae.
Table S2. Characteristics of patients with mycotic aneurysms infected with Candida albicans.


