Abstract
The human SMN2 transgenic mice are well-established models of spinal muscular atrophy (SMA). While the severe type I mouse model has a rapidly progressive condition mimicking type I SMA in humans, the mild type III mice do not faithfully recapitulate chronic SMA variants affecting children. A SMA mouse model that clinically mimics the features of type II and III SMA in human is therefore needed. In this study, we generated intermediately affected SMA mice by delivering low-dose morpholino oligomer (PMO25) into the existing severe SMA mice. We show that a single low-dose administration of PMO25 moderately extended the survival of severe type I SMA mice. The neuromuscular pathology is also modestly but significantly improved in these mice. A second administration of PMO25 at postnatal day 5 (PND5) demonstrated an additive effect on survival. Additional systemic administration of low-dose PMO25 at 2-week intervals suppressed the occurrence of distal necrosis beyond postnatal day 100, and induced more complete phenotypic rescue than a single bolus high-dose injection at PND0. Our study demonstrates that survival of motor neuron (SMN) is required early at a critical threshold to prevent symptoms and suggests that subsequent systemic administration of low-dose PMO25 in SMA mice can provide therapeutic benefit and phenotypic rescue, presumably via peripheral SMN restoration. Our work also provides additional insight into the time window of response to administration of antisense oligonucleotides to SMA mice with an intermediate phenotype. This information is crucial at a time when a number of therapeutic interventions are in clinical trials in SMA patients.
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive disease which is characterized by progressive trunk and limb muscle paralysis as a result of degeneration of motor neurons in the spinal cord (1). It is the most common genetic cause of infant mortality and affects approximately 1 in 6000 to 1 in 10 000 live births, with a carrier frequency of 1 in 35–50 in the general population (2).
The genetic defect in SMA patients is the loss of survival of motor neuron (SMN) protein, caused in 95% of cases by homozygous deletions of the Survival of Motor Neuron 1 (SMN1) gene (3). The SMN locus is part of a genomic inverted duplication region on human chromosome 5, which contains a paralogue gene, SMN2. SMN2 is intact in all SMA patients. Furthermore SMN2 copy number varies in the general population and in SMA there is an inverse correlation between the number of copies of SMN2 and the severity of the disease (4,5). The SMN1 and SMN2 genes differ by several exonic and intronic single nucleotide polymorphisms, although no amino acid substitution is induced by these changes. Among them, a single-nucleotide cytosine to thymidine transition in SMN2 at position 6 of exon 7, that either disrupts an exonic splicing enhancer (6), or creates an exonic splicing silencer (6,7), causes predominant (∼90%) exon 7 skipping which results in an unstable truncated protein (6,8,9). The remaining 10% transcripts can correctly splice exon 7, but this low level fails to fully compensate for the loss of the SMN1 gene.
Significant progress in the development of experimental therapies in SMA has been achieved in recent years. These include viral vector-mediated SMN1 gene therapy (10–13), exon 7 splicing-switching antisense oligonucleotides (AOs) (14–17) and small-molecule drug therapy (18–22). Early phase clinical trials of AO therapy have been well tolerated and preliminary data are encouraging (23). Other strategies, such as small molecules and adenovirus-mediated SMN1 gene therapy, have very recently also entered clinical trials (www.clinicaltrials.org, ID: NCT02240355 and NCT02122952).
The clinical subtypes of SMA range from the most severe type I to intermediate type II, milder type III and adult-onset (type IV) SMA. Transgenic mouse models of SMA carrying the human SMN2 gene have contributed significantly to our understanding of SMA pathogenesis and more importantly in evaluating experimental therapies, such as AOs that augment the splicing of SMN2 gene (14,17,24) or small molecule therapy that targets the splicing or transcript regulation of the SMN2 gene (25). There are several commonly used human SMN2 transgenic SMA mouse models, including a model with the genotype of SMN2+/+; SMNΔ7; Smn−/−, referred to as SMNΔ7 SMA mice (26), and the model with the genotype of (SMN2)2+/−; Smn−/−, referred to as SMN2 mice or Taiwanese SMA mice (27). These mouse models show severe phenotypes with multiple organs involved and a short lifespan of approximately 15 or 10 days, respectively. Previous studies also show the existence of an extremely narrow therapeutic time window in the severe transgenic SMA mouse models, with maximal therapeutic benefit achievable only when treatment is received pre-symptomatically (28).
We have previously reported the efficacy of the 25-mer morpholino antisense oligomer PMO25, targeting the intronic splicing silencer (−10, −34) in intron 7 of SMN2 gene, which significantly rescued the severe type I SMA mice when administered at postnatal day 0 (PND0) by a single bolus injection at 40 µg/g. Both intracerebral ventricular (ICV) injection and subcutaneous (SC) injection in neonatal mice increased the life span of the severe SMA mice dramatically, up to 30-fold. This SMN2 transgenic mouse model, which carries only two copies of the human SMN2 gene ((SMN2)2+/−; Smn−/−), resembles the severe phenotype observed in patients with type I SMA. However, the milder type III mouse model, which carries four copies of the human SMN2 gene ((SMN2)2+/+; Smn−/−), does not show features in line with patients with type II and type III SMA. These mice have essentially normal motor function and no muscle pathology well into adulthood (>9 months) but develop necrosis in the ears and tail at an early adult stage (29). Therefore, there is a need for an intermediate SMA mouse model in this mouse strain in order to mimic the less severe type of SMA, e.g. type II and type III SMA, which composes the majority of surviving SMA children. Lorson's group has recently created an intermediate mouse model of SMA by introducing a slightly more functional SMNΔ7 read-through transgene (SMNRT) onto the background of a severe SMA mouse model (SMN2+/+; Smn−/−) (30), and subsequently showed the value of this model in evaluating a morpholino antisense oligomer targeting the intronic repressor element 1 in intron 6 of the SMN2 gene (31). An SMN2 mis-splicing AO induced adult-onset SMA model was also reported recently by Krainer's group (32). These studies highlight the importance and requirement of the alternative SMA mouse models in this field.
In this study, we have generated SMA mice with an intermediate level of clinical severity by using low-dose morpholino antisense oligomer PMO25, administered to neonatal severe SMA mice of the Taiwanese model. Mice receiving a single low-dose PMO25 exhibited a moderately extended lifespan with improved but still defective neuromuscular pathology, with intermediate phenotypes between the severe type I and mild type III SMA mice. By using a combination of different doses and frequency of PMO25 administration we showed that long-term therapeutic benefit was achieved in mice receiving regular systemic administration of low-dose PMO25. These studies provide further insight into the therapeutic regimens of AOs in less severe SMA and will benefit the design of future clinical trials.
Results
Effect of morpholino oligomer PMO25 on the survival of severe SMA mice is dose-dependent
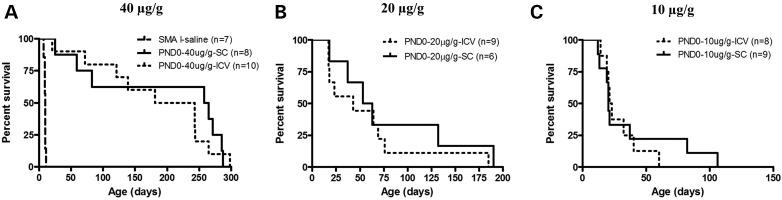
We have previously reported the efficient rescue of severe type I SMA mice by PMO25 (17). To further investigate the efficacy of PMO25 in SMA mice, we compared different delivery routes and different dosages on the survival of the severe SMA mice. A single dose of 40 µg/g (high-dose) administered at PND0 prolonged median survival to 212 days if given by a single ICV injection and to 261 days by a single SC delivery, compared to the 9.5 days survival of saline-treated SMA mice (Fig. 1A). A single injection of 20 µg/g at PND0 increased median survival to 43 days by ICV and 58 days by SC delivery (Fig. 1B). The efficacy was dramatically reduced when the dosage was decreased to 10 µg/g (low-dose), and the median survival was increased to only 22 days by either ICV or SC delivery at PND0 (Fig. 1C)
Figure 1.
Survival curves of severe SMA mice treated with a single administration of different dosages of PMO25 at PND0. (A) Mice were given a single dose of 40 µg/g PMO25 at PND0 by ICV (n = 10) or SC (n = 8). (B) Mice were given a single dose of 20 µg/g PMO25 at PND0 by ICV (n = 9) or SC (n = 6). (C) Mice were given a single dose of 10 µg/g PMO25 at PND0 by ICV (n = 8) or SC (n = 9).
Low-dose treated SMA mice showed intermediate phenotypes between type I and type III SMA mice
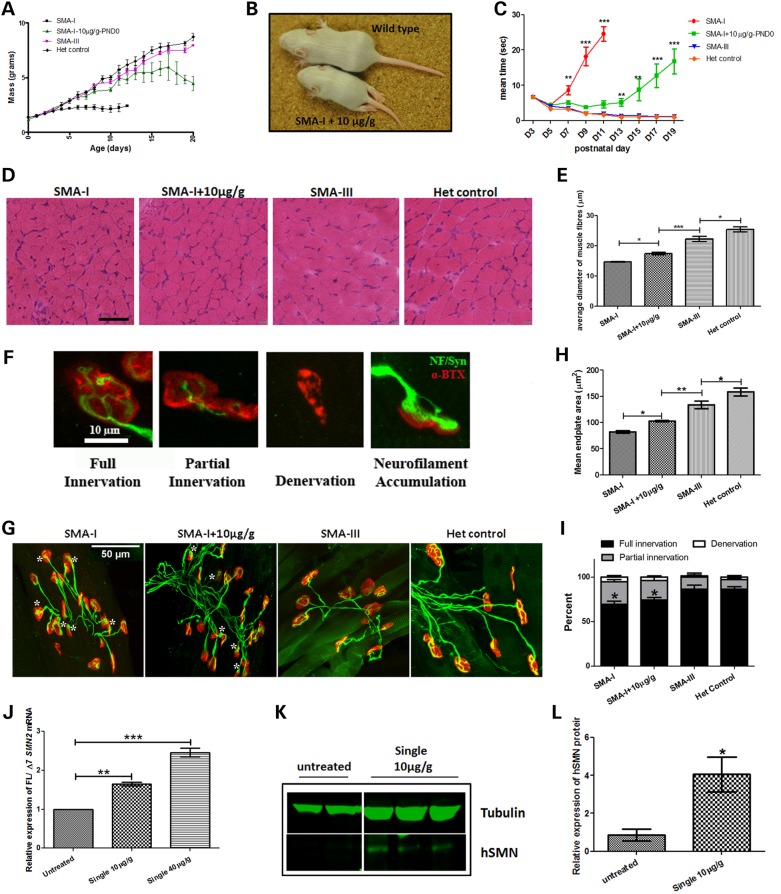
Mice treated with 10 µg/g of PMO25 at PND0 had a modest increase in lifespan in comparison to untreated type I SMA mice and they remained apparently healthy until PND 14-15 (Fig. 2A). However, the body weight declined significantly after PND14-PND17, which is consistent with the onset of the SMA-like symptom in these mice; indeed more than 60% of the low-dose treated SMA mice exhibited hindlimb paralysis starting as early as PND 12 (Fig. 2B). Additional features observed in these mice were necrotic lesions in the anal region and constipation, symptoms that were also observed in untreated severe mice.
Figure 2.
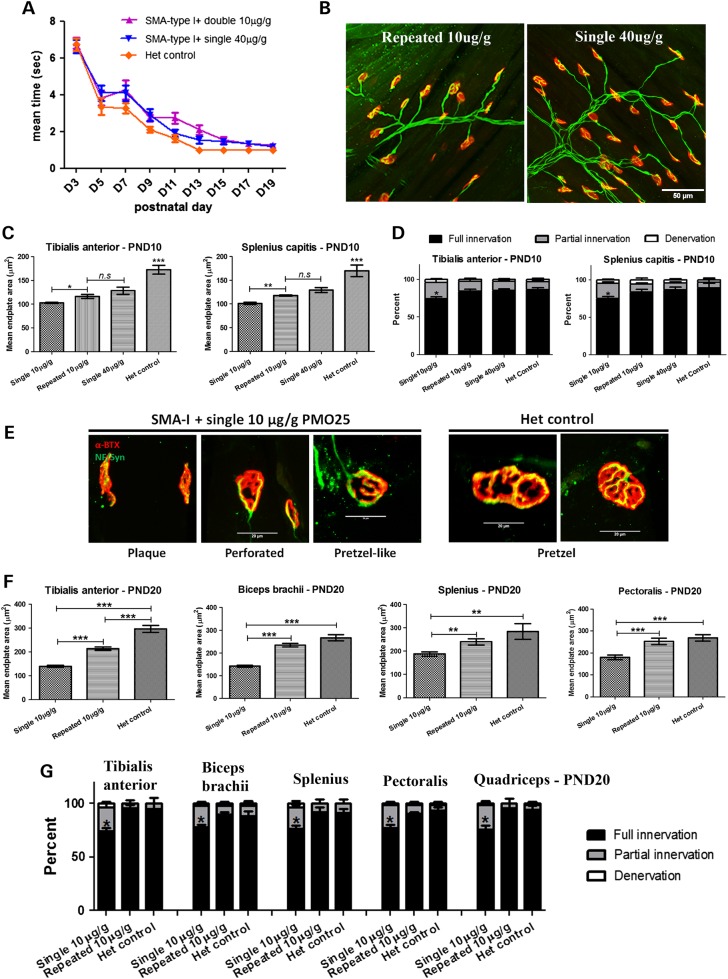
The phenotype of SMA mice that received single 10 µg/g low-dose PMO25. (A) The body weight gain (mass) in untreated severe type I SMA mice (SMA-I), type I mice received a single 10 µg/g PMO25 at PND0 (SMA-I + 10 µg/g-PND0), type III SMA mice (SMA-III) and unaffected heterozygous controls mice (Het control). (B) A representative picture of SMA-I mouse that had received a single 10 µg/g PMO25 by SC injection at PND0 (SMA-I + 10 µg/g) presenting hindlimb paralysis at 12 days old. (C) The ability of righting reflex in SMA mice. (D) Skeletal muscle histopathology by H&E staining of transverse sections from TA muscle of 10-day-old SMA mice. Scale bar = 50 µm. (E) Mean diameters of myofibres of SMA-I (n = 5), SMA-I + 10 µg/g-PND0 (n = 5), SMA-III (n = 5) and Het control (n = 5) mice at 10 days old. ***P < 0.001. (F) Representative images of NMJs that have full innervation, partial innervation, full denervation, or neurofilaments accumulation. The endplates were stained by α-bungarotoxin (α-BTX; red) and the nerves were stained by neurofilament antibody and synaptophysin antibody (green). (G) Representative images of NMJs in TA muscles from 10-day-old mice from different groups. Asterisk indicates endplates with neurofilament accumulations. (H) Histograms showing the quantification of the mean endplate areas in TA from SMA-I (n = 5), SMA-I + 10 µg/g-PND0 (n = 5), SMA-III (n = 5) and Het control (n = 5). (I) Quantification of fully innervated, partially innervated and fully denervated endplates in TA muscle at PND 10. *P < 0.05. (J) Real-time PCR showed the effects of different dosages on exon 7 inclusion at PND10 in spinal cord samples of SMA mice. The mice received either a single 10 μg/g (n = 3) or a single 40 μg/g injection (n = 3) at PND0. (K) The effect of a single 10 μg/g PMO25 injection on SMN protein restoration in spinal cord in SMA mice. Tubulin was used as internal loading control. (L) Semi-quantification of SMN protein using Odyssey Infrared Imaging System application software. *P < 0.05; **P < 0.01; ***P < 0.001.
Muscle strength in young mice was measured by their ability to perform the righting reflex (Fig. 2C). While the untreated type I SMA mice had a significantly delayed righting reflex from PND5 onwards, low-dose PMO25 SC injection at PND0 significantly improved this motor function compared with the untreated type I SMA mice (P < 0.01). The therapeutic effect of low-dose PMO25 SC injection on motor function in these mice was visible until ∼ PND 13, when the motor function started to decline sharply. This was accompanied by the onset of hindlimb paralysis.
Skeletal muscle histopathology was assessed in tibialis anterior (TA) muscles dissected from different types of SMA mice at PND 10. Significant fibre size improvement was observed in the low-dose PMO25 SC treated type I SMA mice compared with untreated type I SMA mice, but the mean fibre diameter was still significantly smaller than that of the type III and heterozygous control mice (Fig. 2D and E). The microarchitecture of neuromuscular junctions (NMJs) was also examined in the four different types of SMA mice using antibodies that recognize the nerves and synapses (NF200 and synaptophysin) and the endplate (α-bungarotoxin). Accumulation of neurofilaments was observed not only in the endplates in untreated type I SMA mice but also in the low-dose PMO25 treated SMA mice (Fig. 2F and G). Quantification of endplate size in low-dose PMO25 SC treated SMA mice demonstrated intermediate area changes between the type I and type III mice (Fig. 2H). Full denervation was very occasionally observed in TA muscles in these mice at PND10. There was no significant difference in denervation in all four groups of mice. However, the percentages of fully innervated endplates in untreated type I SMA and low-dose PMO25 treated type I SMA are significantly lower than in the mild SMA-III and heterozygous control due to the higher percentage of partial innervation (Fig. 2I). The effect of systemic administration of the single low-dose PMO25 on the splicing of SMN2 exon 7 in the spinal cord was measured by quantitative real-time PCR. Ten days after the PND0 injection, a significant 1.6-fold increase of exon 7 inclusion was detected in the single 10 µg/g PMO25 treated SMA mice, compared to the untreated mice. The SMA mice that received a single 40 µg/g high dose PMO25 showed a 2.5-fold increase in exon 7 inclusion (Fig. 2J). At the protein level, a slight but significant increase of SMN protein was measured in the single low-dose treated mice compared with the untreated mice (Fig. 2K and L).
Given the fact that a 10 µg/g single SC administration of PMO25 at PND0 can moderately improve the survival and pathology of the severe type I SMA mice, we then performed additional experiments in these low-dose PMO25 treated SMA mice which displayed a chronic intermediate SMA phenotype and allowed us to further assess the time window of therapeutic intervention.
Assessment of the therapeutic window in low-dose PMO25 generated intermediate SMA mice
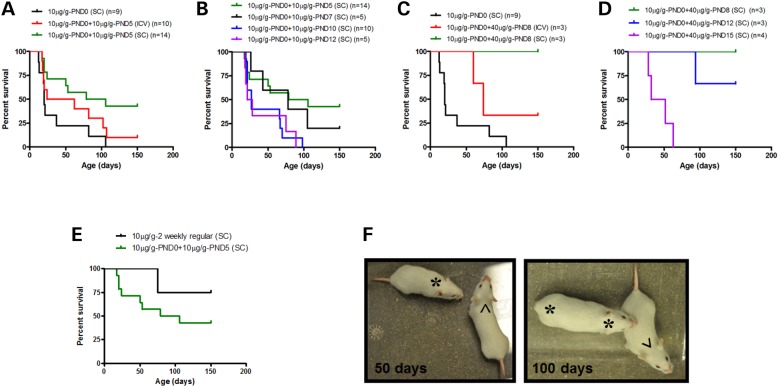
The severe SMA mice treated with 10 µg/g PMO25 at PND0 were given a second dose of 10 µg/g at PND5, by SC or ICV injection. We found that the second administration of 10 µg/g PMO25 by SC injection significantly increased the median survival from 22 to 92.5 days (P = 0.0072) in comparison to a single dose low-dose treatment. The median survival of mice that received the second administration by ICV injection was also increased but only to 43 days (P = 0.098) (Fig. 3A).
Figure 3.
Response to different doses of PMO25 treatment and the therapeutic window in low-dose PMO25 pretreated SMA mice. (A) Survival curves of SMA mice that received the second 10 µg/g low-dose PMO25 at PND5 by either ICV (n = 10) or SC (n = 14) delivery. (B) Survival curves of SMA mice that received the second 10 µg/g by SC injection at PND5 (n = 14), PND7 (n = 5), PND10 (n = 10) and PND12 (n = 5). (C) Survival curves of SMA mice that received the second PMO25 treatment at 40 µg/g at PND8 by either ICV (n = 3) or SC delivery (n = 3). (D) Survival curves of SMA mice that received the second PMO25 treatment at 40 µg/g by SC injection at PND 8 (n = 3) or PND12 (n = 3) or PND 15 (n = 4). (E) Survival curves of SMA mice that received 10 μg/g PMO25 by SC injections at PND0 and PND5 (n = 14, 10 μg/g-PND0+10 μg/g-PND5) and of SMA mice that continued to receive the low-dose treatment at 2 weekly intervals afterwards (n = 4, 10 μg/g-2 weekly regular). (F) Representative pictures of treated SMA mice at 50 days and 100 days old. The mice that received a single high-dose 40 μg/g PMO25 by SC injection at PND0 displayed ear necrosis at 50 days old (*) and complete ear and tail disappearance at 100 days old (* *). The mice that received regular systemic injections of low-dose 10 μg/g PMO25 at 2 weekly intervals up to 100 days old showed no sign of tail or ears necrosis (^).
We then investigated the effect of an additional PMO25 SC administration at different postnatal days, to evaluate its therapeutic efficacy. The severe SMA mice which had received 10 µg/g at PND0, received a second administration of 10 µg/g PMO25 at PND7, PND10 or PND12. The median survival in these groups of animals was extended to 78 days, 27 days and 24 days, respectively (Fig. 3B).
We also tested the effect of high dose 40 µg/g PMO25, in addition to the 10 µg/g low dose, on the survival of the SMA mice with chronic intermediate SMA phenotype. The administration of 40 µg/g PMO25 given at PND8 by SC injection (in mice which had already received 10 µg/g PMO25 at PND0) dramatically increased the survival of the mice, with all the three treated mice living longer than 150 days (n = 3). The mice that received the second administration of 40 µg/g at PND8 by ICV injection, however, had a slightly shorter median survival, with only one mouse living beyond 150 days and the other two dying at 60 and 74 days, respectively (Fig. 3C). Mice treated with 40 µg/g high-dose PMO25 by SC injection at PND12, followed the first 10 µg/g PMO25 SC injection at PND0, had a median survival of over 150 days, with two mice still alive at 150 days and only one mouse that died at 94 days of age. Finally, the administration of 40 µg/g PMO25 at PND15 resulted in a median survival of only 42 days (Fig. 3D).
Improved phenotypic rescue following regular low-dose PMO25 systemic treatment
To assess the effects of chronic systemic administration of low-dose PMO25 on phenotype and survival, 10 µg/g PMO25 was delivered subcutaneously at 2-week intervals, starting from PND5, to the SMA mice originally treated with 10 µg/g at PND0. Three out of four treated SMA mice survived over 150 days (Fig. 3E), compared with 92.5 days median survival of SMA mice that received 10 µg/g PMO25 twice, at PND0 and PND5 respectively, and 22 days median survival of SMA mice that received a single 10 µg/g PMO25 only at PND0.
We have previously reported that type I SMA mice receiving a single 40 µg/g PMO25 developed ear and tail necrosis at around PND 50 (17). In this study, the mice that received regular 10 µg/g PMO25 at two-weekly intervals showed no sign of ear and tail necrosis during the low-dose treatment phase (Fig. 3F).
Repeated 10 μg/g PMO25 injections improve the neuromuscular pathology as efficiently as a single 40 μg/g treatment
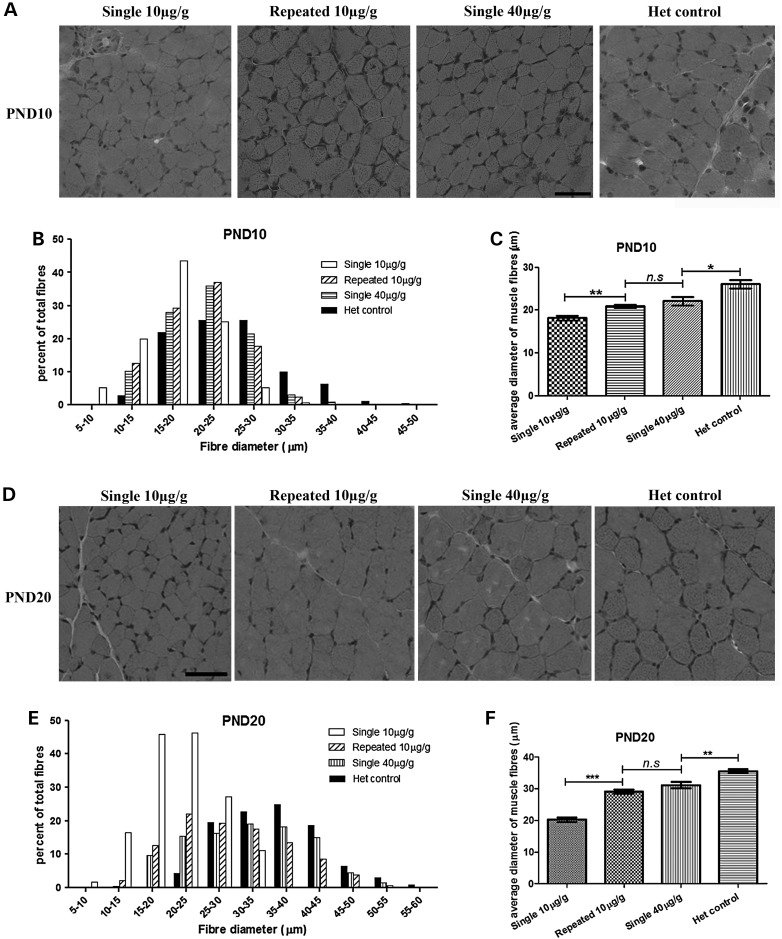
At PND 10, a significant fibre size increase in TA skeletal muscle was detected in repeated 10 μg/g low-dose PMO25 treated SMA mice (20.91 ± 0.4423, n = 5; P < 0.01) compared with the single 10 μg/g treated SMA mice (18.17 ± 0.5118, n = 5; P < 0.0001). There was no difference in fibre diameter between the repeated 10 μg/g PMO25 treated mice and single 40 μg/g PMO25 treated mice (22.13 ± 0.9525, n = 5) at PND10, although the muscle fibres in these mice were smaller than those in the unaffected heterozygous littermate controls (26.15 ± 1.002, n = 5; P < 0.05) (Fig. 4A–C). The histograms of fibre diameter in the repeated 10 μg/g and single 40 μg/g treated SMA mice are shifted from smaller to larger fibres, compared to the mice receiving a single 10 μg/g dose only, indicating the myofibre sizes increase after treatment (Fig. 4B).
Figure 4.
Improvement in muscle pathology after different regimes of PMO25 in SMA mice. (A) H&E staining of transverse sections in TA muscles from 10-day-old SMA mice that received different dosages of PMO25 subcutaneously at PND0. Scale bar = 50 µm. (B) Histogram of myofibre diameters from mice at PND10. (C) Mean diameters of myofibres of 10-day-old SMA mice received a single 10 μg/g (n = 7), a single 40 μg/g (n = 7) or two repeated 10 μg/g PMO25 (n = 6), and unaffected heterozygous littermate control (n = 5). **P < 0.01; *P < 0.05; n.s: non-significant. (D) H&E staining of sections in TA muscle from 20-day-old SMA mice that received different dosages of PMO25 at PND0. Scale bar = 50 µm. (E) Histogram of myofibre diameters from mice at PND 20. (F) Mean diameters of myofibres from 20-day-old SMA mice that received 10 μg/g PMO25 as a single (n = 5) or repeated doses (n = 5), a single 40 μg/g PMO25 (n = 5) and the unaffected heterozygous littermate control (n = 5). **P < 0.01; ***P < 0.001; n.s: non-significant.
The myofibre diameter in TA muscle was also measured at PND 20. There was a significant increase in myofibre size in mice that received repeated 10 μg/g PMO25 (29.11 ± 0.67, n = 5), compared with mice that only received a single 10 μg/g PMO25 (20.23 ± 0.74, n = 5; P < 0.001) (Fig. 4D–F). The fibre size of the mice that received a repeated 10 μg/g PMO25 treatment is comparable to mice that have received a single 40 μg/g PMO25 (31.13 ± 0.98, n = 5), although both are smaller than that of the unaffected heterozygous controls (35.51 ± 0.62, n = 5; P < 0.01). The histogram of fibre diameter in SMA mice which received the two repeated 10 μg/g injections is shifted to larger diameter fibres, compared with the mice which only received a single dose of 10 μg/g PMO25, indicating that the myofibre size is increased in the former group (Fig. 4E).
The administration of a second dose of 10 μg/g PMO25 by SC injection at PND5 completely rescued the righting reflex in the SMA mice until PND20, comparable to mice that received a 40 μg/g PMO25 treatment at PND0 (Fig. 5A). To assess the effect of repeated 10 μg/g low-doses PMO25 on the development of NMJs, we examined the morphology of endplates and the innervation of NMJs in TA muscles collected at PND10. No significant neurofilaments accumulation was observed in SMA mice that received the two repeated 10 μg/g or single 40 μg/g doses (Fig. 5B). The endplate size was significantly increased in the repeated 10 μg/g treated SMA mice (116.2 ± 4.334, n = 5) compared with the single 10 μg/g treated mice (102.6 ± 1.394, n = 5; P = 0.0176). There was no difference in endplate size between mice that received repeated 10 μg/g and a single 40 μg/g treatment (128.3 ± 7.567, n = 5), although they were still smaller in both groups compared with the unaffected heterozygous controls (172.3 ± 9.010, n = 5; P < 0.001) (Fig. 5C). The percentage of fully innervated endplates was significantly increased in severe SMA mice that received either repeated 10 μg/g or a single 40 μg/g PMO25, compared with the single 10 μg/g treated mice (Fig. 5D).
Figure 5.
The improvement in the neuropathology of NMJs at PND10 and PND20. (A) SMA mice that received repeated 10 μg/g PMO25 achieved similar righting reflex until PND20 as the single 40 μg/g treated mice and unaffected heterozygous control. (B) Representative immunofluorescence staining of NMJs in TA muscles at PND10 from mice that had received either two repeated 10 μg/g or a single 40 μg/g PMO25 treatment. (C) Quantification of the mean endplate areas in TA and splenius capitis muscles in SMA mice that received single 10 μg/g PMO25 at PND0 (single 10 µg/g, n = 5), two 10 μg/g PMO25 treatment at PND0 and PND5, respectively (repeated 10 µg/g, n = 5), single 40 µg/g PMO25 at PND0 (single 40 µg/g, n = 5) and the unaffected heterozygous littermate controls (Het control, n = 4). *P < 0.05; **P < 0.01; ***P < 0.001; n.s: non-significant. (D) Quantification of endplate innervation at PND10 in the four groups of mice above. (E) Representative micrographs of the NMJs at PND20 in severe SMA mice that had received a single 10 µg/g PMO25 or unaffected heterozygous controls. Scale bar = 20 µm. (F) Quantification of the mean endplate areas in TA, biceps brachii, splenius capitis and pectoralis muscles in SMA mice that received a single 10 µg/g PMO25 (single 10 µg/g, n = 5), two repeated 10 µg/g PMO25 (repeated 10 µg/g, n = 5) and the unaffected heterozygous littermate controls (Het control, n = 5). **P < 0.01; ***P < 0.001. (G) Quantification of NMJ innervation at PND20 in skeletal muscle samples from SMA mice that had received the different treatments described above. *P < 0.05.
The microarchitecture of NMJs in the splenius capitis muscle was also examined. There was a significant increase in endplate size in single 10 μg/g treated mice (101.0 ± 2.221, n = 5) compared with untreated SMA mice (82.55 ± 0.922, n = 5; P < 0.001). In addition, mice treated by two repeated 10 μg/g administrations (117.7 ± 1.542, n = 5) had increased endplate size compared with single 10 μg/g treated mice (P < 0.001) (Fig. 5C). The two repeated 10 μg/g treated mice had a similar endplate size to the single 40 μg/g treated mice (129.0 ± 5.123, n = 5), although they were still smaller than endplates in unaffected heterozygous controls (169.3 ± 12.38, n = 5; P < 0.001). Full innervation of NMJs was also significantly improved in SMA mice that received either repeated 10 μg/g or single 40 μg/g PMO25, compared with the single 10 μg/g treated SMA mice (P < 0.05) (Fig. 5D).
We further examined the NMJs at PND20 in single and repeated 10 μg/g dose treated SMA mice. The morphology of endplates at PND0 becomes more complex than at PND10, including increased length and branching of the postsynaptic membrane with enlargement of the postsynaptic area. Nevertheless, in single 10 μg/g PMO25 treated SMA mice, NMJs showed poor maturation with little branching of the postsynaptic membrane (Fig. 5E). The endplate size was also significantly reduced in single 10 μg/g PMO25 treated SMA mice compared with those that received two repeated 10 μg/g PMO25 and unaffected heterozygous littermate controls, in all the four muscle groups examined (distal TA muscle, proximal forelimb biceps brachii and axial skeletal muscle splenius capitis and pectoralis) (Fig. 5F). The percentage of fully innervated NMJs in all four skeletal muscles in the single 10 μg/g PMO25 treated SMA mice was significantly lower than that in heterozygous controls. It was corrected to nearly normal level after the repeated 10 μg/g PMO25 treatment. NMJ innervation in another hindlimb muscle, the quadriceps, was also examined and showed the same pattern as the other muscles examined. There was no significant difference in NMJ innervation in all five skeletal muscles examined in this study (Fig. 5G).
The effect of low-dose PMO on motor neurons and preservation of proprioceptive synapses
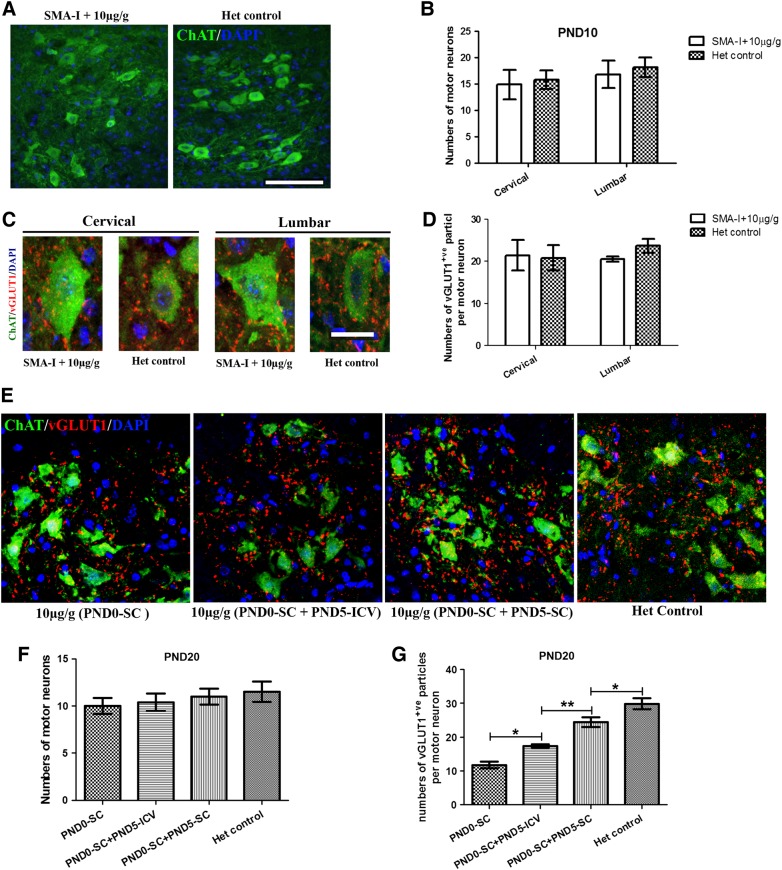
At PND10, the motor neuron numbers in single 10 μg/g PMO25 treated SMA mice did not differ from the heterozygous controls in either cervical or lumbar spinal cord (one-way ANOVA followed by post t-test, P > 0.05) (Fig. 6A and B). vGLUT1 is a marker of synaptic vesicles in primary afferent terminals and has been used to assess the integrity of the synapse of proprioceptive sensory neurons in SMA mice (30,33). Reduction of proprioceptive synapses on the lumbar motor neurons has been reported previously in SMNΔ7 mice (34). At PND10, there was no difference in the number of vGLUT1-positive synapses on choline acetyltransferase (ChAT)-positive motor neurons in the cervical spinal cord sections between the single 10 μg/g PMO25 treated SMA mice and heterozygous controls (Student's t-test, P > 0.05). In lumbar spinal cord sections, the average number of vGLUT1-positive synapses was slightly decreased in the single 10 μg/g PMO25 treated SMA mice than in heterozygous controls, but did not reach statistical significance (Student's t-test, P > 0.05) (Fig. 6C and D).
Figure 6.
The number of motor neurons and the proprioceptive synapses in SMA mice that had received different treatment. (A) ChAT-positive motor neurons in lumbar spinal cord in mice at PND10. Scale bar = 100 μm. (B) The number of motor neurons in cervical and lumbar spinal cord in 10 μg/g PMO25 treated type I SMA mice did not differ from the unaffected heterozygous control mice at PND10 (n = 3 mice/group). (C) Representative micrographs of confocal images of ChAT-positive motor neurons (green) and vGLUT1-positive synapses (red) in cervical and lumbar spinal cord in single 10 μg/g PMO25 treated SMA mice and heterozygous controls. Scale bar = 20 μm. (D) No difference in MN numbers between these two groups of mice at PND10 at both cervical and lumbar levels (n = 3 mice/ group). (E) Distribution of ChAT-positive motor neurons and vGLUT1-positive synapses in lumbar spinal cord in mice that received either a single 10 μg/g PMO25 at PND0 (PND0-SC, n = 5) or two repeated 10 μg/g injections with the second injection by either ICV delivery (PND0-SC + PND5-ICV, n = 5) or SC delivery (PND0-SC + PND5-SC, n = 5) at PND5 and the unaffected heterozygous control mice (Het control, n = 5). (F) Numbers of ChAT-positive motor neurons in SMA mice that received different PMO25 treatment. (G) Quantification of vGLUT1-positive synapses per ChAT-positive motor neurons in the ventral horn of the lumbar spinal cord sections from mice received different treatment. *P < 0.05; **P < 0.01.
The effects of different therapeutic regimens on lumbar spinal cord motor neurons were further studied in PND20 mice that received either a single 10 μg/g at PND0 (PND0-SC), or repeated 10 μg/g at PND0 and PND5, and compared with mice from unaffected heterozygous littermate controls. We have already shown that, following the 10 μg/g PMO25 at PND0, the second 10 μg/g delivered at PND5 is more efficacious by SC (median survival 92.5 days) than by ICV injection (median survival 43 days) (Fig. 3A). To further investigate the potential mechanism leading to this different survival time, we studied the number of motor neurons and proprioceptive synapses at the lumbar spinal cord of SMA mice that received 10 µg/g injection at PND0, with no second treatment (PND0-SC), or with the second 10 μg/g PMO25 at PND5 via SC injection (PND0-SC + PND5-SC) or ICV injection (PND0-SC + PND5-ICV). Lumbar spinal cord sections from unaffected heterozygous littermates were used as controls. There were no significant differences in the number of motor neurons among the four groups (One-way ANOVA, P > 0.05). There was no difference in the number of lumbar spine motor neurons between mice that had received the second 10 μg/g injection at PND5 by either SC or ICV delivery (Fig. 6E and F).
Interestingly, the number of vGLUT1-positive synapses on ChAT-positive motor neurons showed significant differences between the four groups of mice at PND20 (one-way ANOVA followed by post t-test, P < 0.05). Post t-test showed significant more vGLUT1 synapses on ChAT-positive motor neurons in unaffected heterozygous littermate control (P < 0.01) and SMA mice that received two repeated 10 μg/g PMO25 at PND0 (SC) and PND5 (ICV, P < 0.05 or SC, P < 0.01) injections, than those that only received a single 10 μg/g injection at PND0. Furthermore, the number of vGLUT1 synapses in mice that received the second 10 μg/g PMO25 treatment at PND5 was significantly higher in mice treated by SC than by ICV injection (P < 0.05) (Fig. 6G). This suggests that administration of PMO25 systemically rather than by ICV delivery is more effective in preserving the production of proprioceptive synapses and may contribute to the improved survival.
Discussion
SMA animal models, including mouse models with different phenotypic severity and the recently developed pig model (35), are of great value not only in investigating the pathophysiology of the disease but also as in vivo models to evaluate potential therapeutic strategies. There are two commonly used human SMN2 transgenic mouse models, the SMNΔ7 model (26) and the SMN2 Taiwanese mouse model (27), both characterized by fatal SMA-like phenotypes and short lifespans of only 10 or 15 days, respectively. While the severe type I SMA mice from the SMN2 Taiwanese mouse model (TJL005058) have a rapidly progressive condition mimicking type I SMA in infants, the mild type III mice from the same mouse strain do not faithfully recapitulate the chronic SMA variants affecting children. Therefore, mice with intermediate SMA-like phenotypes that mimic the clinical features of type II and III SMA children are needed, and creation of an intermediate SMA mouse model is important for the translational studies in the SMA field.
In this study, we describe the generation of intermediate SMA mice by a single 10 μg/g low-dose morpholino antisense oligomer PMO25 administration in the existing severe SMN2 Taiwanese transgenic mice. The treated SMA mice exhibited moderately extended survival and slightly improved but still defective neuromuscular pathology (Figs 1 and 2). Skeletal muscle and NMJ pathologies of these mice showed intermediate features between the severe type I and the mild type III SMA mice (Fig. 2). In these “intermediate” mice, the appearance of the SMA-related features occurred between 15 and 17 days of age, significantly delayed compared with the severe type I SMA mice, in which these features appeared as early as 7–8 days of life (Fig. 2A and C). One of the characteristic features of these “intermediate” SMA mice is that more than 60% mice presented hindlimb paralysis (Fig. 2B). This phenotype has been previously reported by Hsieh-Li et al. (27) with only a 1/10 incidence in the type II-like SMA mice they described. However, it is rarely seen in the type I SMA mice and is never present in type III SMA mice in our laboratory. It is not clear why mice that were of an identical genotype, with two copies of the human SMN2 transgene, exhibited such variable severities with either severe type I or intermediate type II phenotypes (27). A possible explanation could be that the original SMA-like mice reported by Hseih-Li were not on a fully congenic FVB/N background, when this model was originally established, while the strain used in our laboratory is congenic following continuous inbreeding. In order to characterize further the mechanism responsible for the hindlimb paralysis observed in the SMA mice, we performed detailed pathological studies of various hindlimb muscles and of the proprioceptive synapses in cervical and lumbar spinal cord. We found no difference in NMJ innervation between forelimb and hindlimb muscles at PND10 and PND20 (Fig. 2D and G). At PND10, prior to the onset of hindlimb paralysis, there was no difference in vGLUT1-positive synapses between cervical and lumbar spinal cord in single 10 μg/g PMO25 treated SMA mice and the heterozygous controls (Fig. 5D). However, at PND20, significantly reduced numbers of vGLUT1-positive synapses were present in the lumbar spinal cord in single 10 μg/g PMO25 treated SMA mice (Fig. 5G). We did not detect any severe denervation in the axial or distal skeletal muscles that we examined, in contrast to previous findings in the SMNΔ7 SMA mouse model (36). It is possible that the hindlimb paralysis phenotype we have observed in the intermediate SMA mice is mouse strain-dependent, and may explain why it is dissimilar to the clinical feature observed in human SMA patients where proximal muscle groups are affected first.
A narrow therapeutic window has been previously observed in studies in the severe SMNΔ7 mouse model, for treatments including phosphorodiamidate morpholino oligomer (PMO) antisense and scAAV9-SMN viral gene therapy by ICV delivery (24,28). Porensky et al. (24) reported that while the ICV delivery of a therapeutic PMO can significantly increase the median survival of the severe SMNΔ7 mice from 15 to 112 days when treatment was commenced at PND0, the median survival was dramatically reduced to only 41 days when the injection was delayed to PND4. For scAAV9-SMN viral gene therapy treatment, a nearly complete rescue could be achieved when the same treatment was delivered at P2 but only 40% extension in survival was obtained when the treatment was delayed to P8 (28). A narrow therapeutic window was also detected in the severe type I SMA mice of the SMN2 model for PMO25 treatment in our laboratory. While nearly 30-fold extension in survival could be achieved when 40 μg/g PMO25 was administered at PND 0, only 20% extension in survival was gained when the same dose was delivered at PND5 (unpublished data). We here show that there is more than 20% additional extension in survival when the second 10 μg/g low-dose PMO25 was delivered at PND10 to mice that had received their first 10 μg/g PMO25 at PND0 (Fig. 3). An even wider time window was observed when 40 μg/g high-dose PMO25 was delivered in mice following the first 10 μg/g PMO25 administered at PND0. An approximate 90% extension in survival was achieved when the boost 40 μg/g dose was delivered at PND15 (Fig. 3D). This suggests that a wider therapeutic window may be expected in the milder type II and III SMA patients than in the severe type I SMA infants.
Our data also show that while a single bolus injection of high dose PMO25 at 40 μg/g at PND0 can increase the median survival over 200 days, regular systemic administration of PMO25 at doses as low as 10 μg/g initiated at PND0 achieved a similar efficacy on survival, improvement in neuromuscular pathology and early stage motor function (Figs 3–5). The late onset distal tail necrosis observed in single bolus 40 μg/g high-dose PMO25 treated SMA at PND0 was completely suppressed in mice that received chronic low-dose administration systemically (Fig. 3). This suggests that, at least in the mouse model, there is an advantage of the regular low-dose over the single bolus high-dose administration in the late complications of SMA. It also implies that peripheral restoration of SMN is required at the later stage in order to obtain protection from developing the phenotype in SMA mice.
SMN protein is ubiquitously expressed. While SMA is widely known as a lower motor neuron disease with spinal motor neurons being the primary pathological target, increasing numbers of clinical and experimental reports indicate the involvement of additional peripheral organs contributing to the pathogenesis of the disease, at least in severe cases (37). In this study, by using the low-dose PMO generated intermediate SMA mice, we showed that peripheral SMN restoration is required in order to avoid the chronic disease progression. This is supported by a recent report from Krainer's group that restoration of SMN exclusively in peripheral tissues by ASO10-27 completely prevented distal necrosis in mild SMA mice and improved the survival in severe SMA mice (38). While in some studies the liver has been proposed as the most likely candidate organ involved in disease progression in SMA (14,38), some clinical features indicate a vascular component. Indeed digital necrosis and distal vascular thrombosis, similar to the distal necrosis observed in mice, have also been reported in severe SMA infants (39,40). The underlying mechanism responsible for distal necrosis in SMA is still unknown. Hypotheses include autonomic dysregulation, altered expression of angiogenic factors, inflammation and infection, primary or secondary microvascular abnormality. Further investigation on the mechanism of this phenotype is needed.
SMA is characterized by the loss of lower motor neurons and limb and trunk muscle paralysis. Recent studies using transgenic SMA mouse models have also indicated the involvement of spinal and neuromuscular circuitry in the pathogenesis of the disease (30,33). The functional impairment of sensory-motor connectivity is reflected by the reduced proprioceptive reflexes that correlate with decreased number and function of afferent synapses on motor neurons (33). In a proportion of SMA patients, deep tendon reflexes and H reflexes have also been reported to be absent (40,41), and may occur prior to the loss of mobility in some severe patients (Muntoni, personal observation). Clinical-pathological and electrophysiological studies suggest that in the SMNΔ7 mouse model the functional deficit of motor neurons is more severe than the simple reduction of motor neuron numbers. Functional deficit occurs prior to the loss of motor neurons with the reduction of proprioceptive afferent synapses also preceding their loss (29). Our data on the intermediate SMA mice are consistent with this finding, by showing that there was no difference in motor neuron number at PND 20 in SMA mice treated by either single 10 μg/g low-dose, two repeated 10 μg/g low-dose PMO25 or the unaffected heterozygous controls. Instead, a dramatic difference was detected in the integrity of proprioceptive afferent synapses on the lumbar motor neurons in the mice that had received different treatments, suggesting the functional defect of proprioceptive afferent in motor neurons in SMA mice that received only single dose PMO25 at 10 μg/g at PND0 (Fig. 6E–G). In addition, we found a difference in proprioceptive afferent synapses in the mice that were given the second 10 μg/g low-dose PMO25 by the SC or ICV delivery route. Significantly higher numbers of proprioceptive synapses were present in the mice that received the second 10 μg/g PMO25 at PND5 by SC than by ICV injection (Fig. 6G). This is consistent with our earlier finding that the second low-dose administration at PND5 by SC achieved a longer median survival (92.5 days) than via IVC injection (only 43 days) (Fig. 3A). The incomplete efficacy of PMO administration following ICV administration could be due to either insufficient biodistribution of PMO along the spinal cord after the ICV injection in older SMA mice or a peripheral requirement for SMN restoration. Possible candidates for the peripheral involvement could be afferents from skeletal muscle spindles to spinal motor neurons, perisynaptic Schwann cells at NMJs and dorsal root ganglia (DRG). Perisynaptic Schwann cells and DRG are likely to benefit more from the peripheral rather than the exclusive CNS delivery of PMO. Indeed, there is increasing evidence of the importance of SMN in the development of DRG neurons and normal function of Schwann cells. In a zebrafish model of SMN deficiency, the majority of DRG neurons had abnormally short peripheral axons and many of them failed to divide and died (42). A significant reduction in the number of proprioceptive DRG neurons was observed in SMNΔ7 mice compared with control littermates (30). In the SMN deficient zebrafish model, Schwann cells failed to wrap axons tightly due to myelination defects (42). Schwann cells isolated from SMA mice failed to express key myelin proteins following differentiation and had lower level of extracellular matrix protein such as laminin α2. These abnormalities result in myelination defects, delayed maturation of axo-glial interactions and abnormal composition of extracellular matrix in peripheral nerve (43).
Our study suggests that the peripheral correction by systemic administration of AOs is important in the prevention of disease progression in the SMA mice. By using the low-dose PMO25 generated intermediate SMA mice, we provide compelling evidence that regular systemic administration of low-dose AO can provide functional benefit to the severe SMA mice, and restoration of SMN protein in peripheral tissues during the later stage of the disease may still provide therapeutic benefit.
Materials and Methods
Oligonucleotides
The morpholino antisense oligomer was synthesized by Gene Tools LLC with a signed agreement for research use. The morpholino was dissolved to standard concentration and stored according to the manufacturer's instructions. The sequence of PMO25 was described previously (17).
Animals
Mice were bred and experimental procedures were carried out in the Biological Services Unit, University College London Institute of Child Health, in accordance with the Animals (Scientific Procedures) Act 1986. Experiments were performed under Home Office project licence 70/7145.
SMA transgenic mice FVB.Cg-Tg(SMN2)2Hung Smn1tm1Hung/J, the Taiwanese SMA mice, were initially purchased from the Jackson Laboratory (TJL005058). The mild type III SMA mice have four copies of human SMN2 transgene and homozygous knockout of endogenous mouse Smn, with genotype of (SMN2)2+/+; Smn−/− (referred to as ‘SMA-III’). These mice are bred with mice heterozygous for endogenous mouse Smn (Smn+/−). Offspring contain 50% severe type I SMA mice that carry only two copies of human SMN2 transgene with genotype of (SMN2)2+/−; smn−/− (referred to as ‘SMA-I’), and 50% heterozygous non-phenotypic control mice with genotype of (SMN2)2+/−; Smn+/− (referred to as ‘Het control’). The ICV and SC injections in SMA mice were performed as described previously (17). Briefly, oligonucleotides were injected either subcutaneously into the upper back, or intracerebroventricularly using a 10 µl glass capillary (Drummond Scientific Company, Pennsylvania).
Righting ability
The spontaneous righting reflex was evaluated to estimate muscle strength of mice between PND2 and PND20. Mice were placed on their backs and the time (seconds) taken to reposition themselves with all four paws on the ground was recorded. The procedure was repeated three times for each animal, with at least 5 min recovery period between tests. The maximum recording time is 30 s. The data are expressed as the mean time to complete the righting response ± SEM.
RNA and protein analyses
Mouse tissues were homogenized using Precellys Homogenizer (Bertin Technologies) in RLT lysis buffer using RNeasy Mini Kit (Qiagen). Total RNA extraction and reverse-transcription were performed with methods described previously (17,44). Relative quantification of the ratio of full-length to Δ7 SMN2 transcripts was analysed by quantitative real-time PCR as described (17). The expression of human SMN protein and β-tubulin was detected by western blotting, as described previously (17). Probed membranes were detected using the LI-COR Odyssey Imaging System (Biosciences) and quantified by Odyssey Infrared Imaging System application software.
Skeletal muscle histopathology
TA muscles were dissected, embedded in optimal cutting temperature (OCT) (CellPath, UK) on corks and frozen in liquid nitrogen-cooled iso-pentane. Haematoxylin and eosin (H&E) staining was performed on representative 10 µm transverse cryosections. Approximately 500 myofibres from at least five different areas selected randomly from a representative section of each muscle were measured. The diameters and areas of myofibres were measured and quantified using Image J software (http://imagej.nih.gov/ij/).
Neuromuscular junction staining
TA, splenius capitis, biceps brachii and pectoralis muscles were dissected from mice and fixed in 4% paraformaldehyde on ice for 4 h. Whole muscles were incubated in blocking and permeabilizing buffer containing 5% goat serum and 1% Triton X-100 in PBS for 1 h. Muscle samples were then incubated in rabbit polyclonal anti neurofilament antibody (NF200, 1:100; Sigma) and synaptophysin antibody (1:200; Synaptic Systems, Germany) overnight at 4°C, washed and incubated in PBS buffer with Alexa Fluor 488 goat anti-rabbit IgG (1:500; Life Technology) and rhodamine-α-bungarotoxin (α-BT) (1:1000; Life Technology) overnight at 4°C. After thorough washing in PBS-Tween, muscle fibres were teased under a dissecting microscope and mounted using Hydromount mounting medium (National Diagnostics, England). A minimum of 100 NMJs from each muscle section were randomly selected and captured using confocal laser scanning microscopy (Carl Zeiss LSM-710, Germany) and Z-stacks program. The areas of synapse endplates were measured and quantified using Image J software.
Immunohistochemistry
The spine was dissected and fixed in 4% paraformaldehyde at 4°C for 4 h. The spinal cord was then isolated, rinsed in PBS and cryoprotected in 30% sucrose at 4°C for 3 days. The tissues were then embedded in OCT and frozen in liquid nitrogen-cooled iso-pentane. Ten micrometer transverse sections were cut from the lumbar spinal cord. Motor neurons in the spinal cord were stained using an antibody against ChAT (1:100; Millipore). Vesicular glutamine transporter 1 (VGluT1) positive synapses were stained using an antibody against VGluT1 (1:100; Millipore). Sections were imaged using confocal microscopy. Motor neurons and VGluT1-positive synapses were quantified from Z-stack images using image J software.
Statistical analysis
One-way ANOVA and post t-test were used to determine statistical significance. Results presented in this study are displayed as mean ± standard error of the mean (mean ± SEM). Kaplan–Meier survival curves were generated to analyse the survival data, followed by a log-rank test for statistical significance. GraphPad Prism 5.0 software was used for statistical analysis and graph design.
Funding
We thank the University College London (UCL) Therapeutic Innovation Fund (to F.M. and H.Z.) provided by the Wellcome Trust Institutional Strategic Support Fund (grant reference 097815/Z/11/Z), the UCL Biomedical Research Centre (grant reference CBRC 161), the Medical Research Council award grant to F.M. (grant reference MR/L013142/1), and the UCL SLMS Capital Equipment Funding and Wellcome Trust/DOH fund to F.M. The Great Ormond Street Hospital Children's Charity is gratefully acknowledged for its support to J.Mo.; the Great Ormond Street Hospital Biomedical Research Centre support to F.M. is also gratefully acknowledged. H.Z. is UCL Biomedical Research Centre supported senior research fellow. J.Me. is supported by research awards from MRC and AFM.
Acknowledgements
We thank Dr Lucy Feng and Mr Darren Chambers for their help in processing muscle histopathology studies.
Conflict of Interest statement. F.M. is involved as a Principal Investigator in an ISIS Pharmaceutics/Biogen funded clinical trial in SMA; he is also involved in a Roche funded clinical trial on SMA (Moonfish). He previously participated in a Trophos funded clinical trial on SMA. F.M. is a current member of the Pfizer Rare Disease Scientific Advisory Board. F.M. is also involved in translational research projects in Duchenne muscular dystrophy, in collaboration with Prosensa/Biomarin; with PTC Therapeutics; with Sarepta Therapeutics and with Summit Therapeutics.
References
- 1.Moosa A., Dubowitz V. (1973) Spinal muscular atrophy in childhood. Two clues to clinical diagnosis. Arch. Dis. Child, 48, 386–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muralidharan K., Wilson R.B., Ogino S., Nagan N., Curtis C., Schrijver I. (2011) Population carrier screening for spinal muscular atrophy a position statement of the association for molecular pathology. J. Mol. Diagn., 13, 3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lefebvre S., Burglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M. (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell, 80, 155–165. [DOI] [PubMed] [Google Scholar]
- 4.McAndrew P.E., Parsons D.W., Simard L.R., Rochette C., Ray P.N., Mendell J.R., Prior T.W., Burghes A.H. (1997) Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. Am. J. Hum. Genet., 60, 1411–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor J.E., Thomas N.H., Lewis C.M., Abbs S.J., Rodrigues N.R., Davies K.E., Mathew C.G. (1998) Correlation of SMNt and SMNc gene copy number with age of onset and survival in spinal muscular atrophy. Eur. J. Hum. Genet., 6, 467–474. [DOI] [PubMed] [Google Scholar]
- 6.Cartegni L., Krainer A.R. (2002) Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet., 30, 377–384. [DOI] [PubMed] [Google Scholar]
- 7.Kashima T., Manley J.L. (2003) A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat. Genet., 34, 460–463. [DOI] [PubMed] [Google Scholar]
- 8.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. (1999) A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl Acad. Sci. USA, 96, 6307–6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H., McPherson J.D. (1999) A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet., 8, 1177–1183. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez E., Marais T., Chatauret N., Benkhelifa-Ziyyat S., Duque S., Ravassard P., Carcenac R., Astord S., de Pereira M.A., Voit T., Barkats M. (2011) Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum. Mol. Genet., 20, 681–693. [DOI] [PubMed] [Google Scholar]
- 11.Foust K.D., Wang X., McGovern V.L., Braun L., Bevan A.K., Haidet A.M., Le T.T., Morales P.R., Rich M.M., Burghes A.H., Kaspar B.K. (2010) Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat. Biotechnol., 28, 271–274. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Passini M.A., Bu J., Roskelley E.M., Richards A.M., Sardi S.P., O'Riordan C.R., Klinger K.W., Shihabuddin L.S., Cheng S.H. (2010) CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J. Clin. Invest., 120, 1253–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valori C.F., Ning K., Wyles M., Mead R.J., Grierson A.J., Shaw P.J., Azzouz M. (2010) Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci. Transl. Med., 2, 35ra42. [DOI] [PubMed] [Google Scholar]
- 14.Hua Y., Sahashi K., Rigo F., Hung G., Horev G., Bennett C.F., Krainer A.R. (2011) Peripheral SMN restoration is essential for long-term rescue of a severe spinal muscular atrophy mouse model. Nature, 478, 123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passini M.A., Bu J., Richards A.M., Kinnecom C., Sardi S.P., Stanek L.M., Hua Y., Rigo F., Matson J., Hung G. et al. (2011) Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci. Transl. Med., 3, 72ra18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porensky P.N., Mitrpant C., McGovern V.L., Bevan A.K., Foust K.D., Kaspar B.K., Wilton S.D., Burghes A.H. (2012) A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet., 21, 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou H., Janghra N., Mitrpant C., Dickinson R., Anthony K., Price L., Eperon I., Wilton S., Morgan J., Muntoni F. (2013) A novel morpholino oligomer targeting ISS-N1 improves rescue of severe SMA transgenic mice. Hum. Gene Ther., 24, 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Avila A.M., Burnett B.G., Taye A.A., Gabanella F., Knight M.A., Hartenstein P., Cizman Z., Di Prospero N.A., Pellizzoni L., Fischbeck K.H., Sumner C.J. (2007) Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. J. Clin. Invest., 117, 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garbes L., Riessland M., Holker I., Heller R., Hauke J., Trankle C., Coras R., Blumcke I., Hahnen E., Wirth B. (2009) LBH589 induces up to 10-fold SMN protein levels by several independent mechanisms and is effective even in cells from SMA patients non-responsive to valproate. Hum. Mol. Genet., 18, 3645–3658. [DOI] [PubMed] [Google Scholar]
- 20.Hauke J., Riessland M., Lunke S., Eyupoglu I.Y., Blumcke I., El-Osta A., Wirth B., Hahnen E. (2009) Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Hum. Mol. Genet., 18, 304–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kernochan L.E., Russo M.L., Woodling N.S., Huynh T.N., Avila A.M., Fischbeck K.H., Sumner C.J. (2005) The role of histone acetylation in SMN gene expression. Hum. Mol. Genet., 14, 1171–1182. [DOI] [PubMed] [Google Scholar]
- 22.Mohseni J., Zabidi-Hussin Z.A., Sasongko T.H. (2013) Histone deacetylase inhibitors as potential treatment for spinal muscular atrophy. Genet. Mol. Biol., 36, 299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castro D., Iannaccone S.T. (2014) Spinal muscular atrophy: therapeutic strategies. Curr. Treat. Options Neurol., 16, 316. [DOI] [PubMed] [Google Scholar]
- 24.Porensky P.N., Mitrpant C., McGovern V.L., Bevan A.K., Foust K.D., Kaspar B.K., Wilton S.D., Burghes A.H. (2011) A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum. Mol. Genet., 21, 1625–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Naryshkin N.A., Weetall M., Dakka A., Narasimhan J., Zhao X., Feng Z., Ling K.K., Karp G.M., Qi H., Woll M.G. et al. (2014) Motor neuron disease. SMN2 splicing modifiers improve motor function and longevity in mice with spinal muscular atrophy. Science, 345, 688–693. [DOI] [PubMed] [Google Scholar]
- 26.Le T.T., Pham L.T., Butchbach M.E., Zhang H.L., Monani U.R., Coovert D.D., Gavrilina T.O., Xing L., Bassell G.J., Burghes A.H. (2005) SMNDelta7, the major product of the centromeric survival motor neuron (SMN2) gene, extends survival in mice with spinal muscular atrophy and associates with full-length SMN. Hum. Mol. Genet., 14, 845–857. [DOI] [PubMed] [Google Scholar]
- 27.Hsieh-Li H.M., Chang J.G., Jong Y.J., Wu M.H., Wang N.M., Tsai C.H., Li H. (2000) A mouse model for spinal muscular atrophy. Nat. Genet., 24, 66–70. [DOI] [PubMed] [Google Scholar]
- 28.Robbins K.L., Glascock J.J., Osman E.Y., Miller M.R., Lorson C.L. (2014) Defining the therapeutic window in a severe animal model of spinal muscular atrophy. Hum. Mol. Genet., 23, 4559–4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hua Y., Sahashi K., Hung G., Rigo F., Passini M.A., Bennett C.F., Krainer A.R. (2010) Antisense correction of SMN2 splicing in the CNS rescues necrosis in a type III SMA mouse model. Genes Dev., 24, 1634–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cobb M.S., Rose F.F., Rindt H., Glascock J.J., Shababi M., Miller M.R., Osman E.Y., Yen P.F., Garcia M.L., Martin B.R. et al. (2013) Development and characterization of an SMN2-based intermediate mouse model of Spinal Muscular Atrophy. Hum. Mol. Genet., 22, 1843–1855. [DOI] [PubMed] [Google Scholar]
- 31.Osman E.Y., Miller M.R., Robbins K.L., Lombardi A.M., Atkinson A.K., Brehm A.J., Lorson C.L. (2014) Morpholino antisense oligonucleotides targeting intronic repressor Element1 improve phenotype in SMA mouse models. Hum. Mol. Genet., 23, 4832–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahashi K., Ling K.K., Hua Y., Wilkinson J.E., Nomakuchi T., Rigo F., Hung G., Xu D., Jiang Y., Lin R.Z. et al. (2013) Pathological impact of SMN2 mis-splicing in adult SMA mice. EMBO Mol. Med., 5, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mentis G.Z., Blivis D., Liu W., Drobac E., Crowder M.E., Kong L., Alvarez F.J., Sumner C.J., O'Donovan M.J. (2011) Early functional impairment of sensory-motor connectivity in a mouse model of spinal muscular atrophy. Neuron, 69, 453–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ling K.K., Lin M.Y., Zingg B., Feng Z., Ko C.P. (2010) Synaptic defects in the spinal and neuromuscular circuitry in a mouse model of spinal muscular atrophy. PLoS One, 5, e15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duque S.I., Arnold W.D., Odermatt P., Li X., Porensky P.N., Schmelzer L., Meyer K., Kolb S.J., Schumperli D., Kaspar B.K., Burghes A.H. (2014) A large animal model of spinal muscular atrophy and correction of phenotype. Ann. Neurol., 77, 399–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ling K.K., Gibbs R.M., Feng Z., Ko C. (2012) Severe neuromuscular denervation of clinically relevant muscles in a mouse model of spinal muscular atrophy. Hum. Mol. Genet., 21, 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shababi M., Lorson C.L., Rudnik-Schoneborn S.S. (2014) Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? J. Anat., 224, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hua Y., Liu Y.H., Sahashi K., Rigo F., Bennett C.F., Krainer A.R. (2015) Motor neuron cell-nonautonomous rescue of spinal muscular atrophy phenotypes in mild and severe transgenic mouse models. Genes Dev., 29, 288–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araujo A.Q., Araujo M., Swoboda K.J. (2009) Vascular perfusion abnormalities in infants with spinal muscular atrophy. J. Pediatr., 155, 292–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudnik-Schoneborn S., Vogelgesang S., Armbrust S., Graul-Neumann L., Fusch C., Zerres K. (2010) Digital necroses and vascular thrombosis in severe spinal muscular atrophy. Muscle Nerve, 42, 144–147. [DOI] [PubMed] [Google Scholar]
- 41.Iannaccone S.T., Browne R.H., Samaha F.J., Buncher C.R. (1993) Prospective study of spinal muscular atrophy before age 6 years. DCN/SMA Group. Pediatr. Neurol., 9, 187–193. [DOI] [PubMed] [Google Scholar]
- 42.Hao l.T., Duy P.Q., Jontes J.D., Beattie C.E. (2015) Motoneuron development influences dorsal root ganglia survival and Schwann cell development in a vertebrate model of spinal muscular atrophy. Hum. Mol. Genet., 24, 346–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunter G., Aghamaleky S.A., Roche S.L., Symes R.C., Gillingwater T.H. (2014) SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Hum. Mol. Genet., 23, 2235–2250. [DOI] [PubMed] [Google Scholar]
- 44.Owen N., Zhou H., Malygin A.A., Sangha J., Smith L.D., Muntoni F., Eperon I.C. (2011) Design principles for bifunctional targeted oligonucleotide enhancers of splicing. Nucleic Acids Res., 39, 7194–7208. [DOI] [PMC free article] [PubMed] [Google Scholar]