Abstract
For several decades, it was largely assumed that stone tool use and production were abilities limited to the genus Homo. However, growing palaeontological and archaeological evidence, comparative extant primate studies, as well as results from methodological advancements in biomechanics and morphological analyses, have been gradually accumulating and now provide strong support for more advanced manual manipulative abilities and tool-related behaviours in pre-Homo hominins than has been traditionally recognized. Here, I review the fossil evidence related to early hominin dexterity, including the recent discoveries of relatively complete early hominin hand skeletons, and new methodologies that are providing a more holistic interpretation of hand function, and insight into how our early ancestors may have balanced the functional requirements of both arboreal locomotion and tool-related behaviours.
Keywords: Australopithecus, Homo, tool use, dexterity, arboreal locomotion
1. Introduction
‘Now it appears that man-apes—creatures able to run… and with brains no larger than those of apes now living—had already learned to make and to use tools’. Washburn [1, p. 63].
Washburn's [1] (see also [2]) declaration referred to the contemporary discoveries by the Leakey family [3,4] of the robust australopith skull of ‘Zinjanthropus boisei’ in association with a living floor of Oldowan stone tools. Only a few months later the remains of Homo habilis Olduvai Hominid (OH) 7 were discovered [4–7] and quickly deemed the maker of these stone tools, while Zinjanthropus was considered likely to be the prey instead [7]. In the decades following, tool use and tool making were largely considered to be an ability limited to (and, indeed, used to define) the genus Homo (see [8] for a review). However, a growing wealth of palaeontological, archaeological and comparative primate evidence makes clear that Washburn's [1] assertion that pre-Homo ‘ape-men’ were making and using tools still holds true today. In particular, recent, relatively complete fossil hominin hand skeletons [9–11] and archaeological discoveries [12,13] have added greatly to the growing group of palaeoanthropologists and archaeologists open to the idea that enhanced manual dexterity and tool-related behaviours have been a part of our evolutionary history for much longer than traditionally believed [14–22] (see also [23,24]).
Inferences about manipulative ability and potential tool-related behaviours in the earliest hominins must rely largely on morphological fossil evidence. Comparative extant primate studies, showing a dominance of using organic plants as tools in New and Old World monkeys and hominoids, suggests that the modification of plants and tool use were behaviours probably present in the last common ancestor of humans and Pan (chimpanzees and bonobos) [24], and potentially evolved multiple times in extinct fossil primates and hominins [22]. However, evidence of the modification and use of organic materials as tools either does not preserve in the fossil record or is not recognizable as tools [17,22]. Thus, researchers are generally forced to focus their interpretation and understanding of the evolution of human manipulative behaviours on fossilized hand anatomy, especially in the earliest stages of human evolution (e.g. between approx. 7 and approx. 3.5 Ma; see §3) and modified stone tools. Furthermore, the morphological evidence for the majority of our evolutionary history has been limited to isolated hand bones that are not directly associated with taxonomically identifying remains (i.e. craniodental material) or stone tool evidence. Thus, palaeoanthropologists have debated the taxonomic attribution and manipulative capabilities of many early fossil hominins for several decades [14,15,18,25–28]. However, recent palaeontological and archaeological discoveries, as well as advances in methods for analysing morphological remains, have shed new light on the manipulative abilities of early hominins.
There are several reviews of the morphological evidence for manipulative behaviours in human evolution [29–33]. Therefore, this paper focuses on the more recent fossil and archaeological evidence and the results of new methodologies that are helping to broaden our understanding of the evolution of the human hand and, in particular, the potential manipulative abilities of early hominins and how these might also have been balanced with requirements of arboreal locomotion.
2. What makes humans distinct? Manipulative abilities and morphological correlates
For the past few decades, research into the evolution of human manipulative abilities has focused—with good reason—on identifying the manipulative behaviours that are unique to humans compared with other primates, and the morphological features of the human hand that might facilitate these abilities. This research is thoughtfully and thoroughly reviewed most recently by Markze [33] and others [29–32], and thus is briefly summarized below and in figure 1.
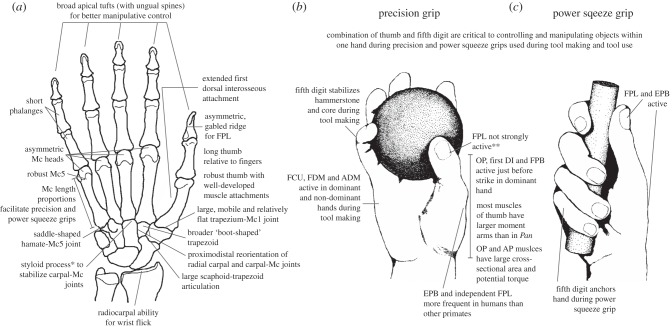
Figure 1.
Bony and soft tissue morphology of the human hand considered advantageous for the manipulative precision and power squeeze grips used during stone tool use and production. (a) The suite of bony features typically considered distinct in the human hand (although some specific features are found in other primates, such as broad apical tufts in baboons) that reflect the ability to forcefully oppose the pads of the thumb and fingers, the well-developed musculature to the thumb and fifth digit, the high external loading of the thumb and distribution of that load across the wrist and palm, and the broad fingertips for control and manipulation of objects, especially within one hand. (b) Human precision grip (shown, a five-jaw chuck precision grip) in which the pads of the thumb and fingers grasp and manipulate the object and (c) a power squeeze grip, in which the fingers grasp the object diagonally and thumb is in line with the forearm. In stone tool behaviours, the thumb and fifth fingers are important for the manipulating and stabilizing objects in both the dominant and non-dominant hands. Muscles of the thumb and fifth digit that are strongly activated during use of these grips in tool making are noted [34]. FPL, flexor pollicis longus; FCU, flexor carpi ulnaris; FDM, flexor digiti minimi; ADM, abductor digiti minimi; OP, opponens pollicis; DI, dorsal interosseus; AP, oblique adductor pollicis; FPB, flexor pollicis brevis; EPB, extensor pollicis brevis; Mc, metacarpal. *The styloid process found at dorsoradial corner of the third metacarpal and thus cannot been seen in palmar view. **Marzke et al. [35] show that the FPL is not strongly active in either the dominant or non-dominant hand, particularly during precision pinch grips, in experienced knappers (but see [36]).
Comparative experimental studies of primate manual manipulation, including those on experienced human stone tool knappers, have revealed three manipulative abilities considered unique to the human hand [37–47]. The first is precision handling: the ability to rotate and manipulate objects within one hand using the thumb and fingertips [37,41]. Other primates typically need to use the palm as well [43] or their other hand, a foot or the mouth to manipulate an object into the desired position [39,41]. The second is forceful precision gripping, in which the pads of the thumb and one or more of the fingers are able to forcefully stabilize or manipulate an object, and at the same time withstand large external forces, such as when knapping a stone tool [29,41] (figure 1b). Other primates are capable of precision grips, typically tip-to-tip or pad-to-side grips between the thumb and index finger, but these are not generally done with strong force (i.e. precision holding; [38–40,45]). However, recent studies have revealed precision pinch grips in wild, habituated macaques [44] and chimpanzees [46,47] that may be forceful, requiring further investigation into whether forceful precision pinch grips are truly unique to humans.
The third uniquely human manipulative ability is power squeeze gripping of cylindrical objects in which the fingers grip the object diagonally across the palm and the thumb is either wrapped around the object or is in line with the forearm, such as when using a hammer [48] (figure 1c). Other primates are capable of power grips (using the palm) or diagonal hook grips (fingers usually stabilized against the palm), but neither provide the same control that the power squeeze grip does in humans [48].
To potentially infer when these unique manipulative abilities may have evolved in the human lineage, many have tried to identify the distinctive features, or suites of features, in human bony and soft tissue anatomy that facilitate these gripping behaviours [29,30,32,49–51]. These anatomical features are summarized in figure 1. The most obvious and critical difference about the human hand compared with other primates is our robust and long thumb relative to the length of the fingers. Experimental studies have demonstrated that the thumb of the dominant hand [52,53] and, although much less so, the non-dominant hand [34], incur substantial external force during stone tool making and particularly during tool use, such as when using a flake [53,54] (but see [52] and §5). The bony morphology and musculature of the thumb reflect its importance in human manipulative behaviours. All but one of the muscles of the human thumb have a significantly larger moment arm (i.e. better mechanical advantage or leverage) than that of chimpanzees [35,55]. Two muscles in particular, the opponens pollicis and adductor pollicis, also have larger cross-sectional area and potential torque that together provide better leverage and limit fatigue when opposing the thumb to the pads of the other fingers [55]. Larger muscle attachments on the first metacarpal (Mc1) for the opponens pollicis and first dorsal interosseous muscles also help to increase leverage and stabilize the joint at the base of the thumb during opposition [32,56]. Much attention has been paid to the independent and well-developed flexor pollicis longus (FPL) muscle of humans that helps to flex and stabilize the tip of the thumb [19,29,30,51,57]. Although this muscle is important for precision control and manipulation, it is particularly active during power squeeze grips [35] (but see [36]), rather than precision pinch grips, and other primates also have an independent FPL (e.g. hylobatids [51]) or a similar gabled-attachment on the pollical distal phalanx (e.g. orangutans [57]; figure 1a). Distinctive changes in carpal bone morphology, such a broader and flatter trapezium-Mc1 joint, the reorientation of the radial carpal (e.g. scaphoid-trapezoid) and carpometacarpal (e.g. capitate-Mc2) articulations, and the development of a styloid process on the base of the Mc3, together help to better distribute across the wrist and palm the large loads incurred by the thumb during tool-related behaviours [31,58–61].
The fifth digit is also particularly important during stone tool-related behaviours, but its morphology has been largely ignored in comparative and experimental studies [33,35,55]. The fifth digit stabilizes the dominant hand during power squeeze grips and precision grips (e.g. of the core during the strike of the hammerstone), as well as during precision grips of the non-dominant hand when manoeuvring an object within the hand to find the desired position [35]. In humans, the fifth metacarpal is the most robust of the digits 2–5 and has a unique saddle-shaped joint with the hamate that helps to rotate the fifth digit towards the thumb [31,35,50].
Several other distinctive features of the human bony and soft tissue hand anatomy have also been correlated with forceful precision and power squeeze grips (figure 1), but the functional roles of the marginal aspects of the hand—the thumb and fifth digit—have been shown to be particularly important for the dexterity required to make and use stone tools [35,55]. Looking at the morphology of these two digits, and their associated wrist bones, in fossil hominins may be the most informative way of inferring the evolution of manipulative abilities and tool-related behaviours in human evolutionary history [33].
3. Manipulative abilities of the earliest fossil hominins
Functional interpretations of hand remains in the earliest fossil hominins have focused not only on their potential manipulative abilities, but also on how the morphological requirements for manipulation may have been balanced with those of arboreal locomotion. Perhaps the most critical aspect to the unique manipulative abilities of humans is our intrinsic hand proportions (relative length of the thumb and fingers). However, we are extremely limited in what we can say about hand proportions in the early hominins because it requires the preservation of multiple bones from the same individual. The earliest relatively complete hand is that of Ardipithecus ramidus [62]. With hand proportions that are described as more Old World monkey-like than chimp-like (i.e. short metacarpals, long fingers, robust thumb), the authors describe the functional morphology only in relation to locomotion [62]. They do not discuss potential manipulative abilities, presumably because, at 4.4 Ma, Ardipithecus appears about a million years before the earliest evidence of stone tool use and tool making [12,13,63] (see §5).
However, others have not been deterred from inferring human-like manipulative abilities in the absence of stone tool evidence, and from much less fossil evidence [19,21,64]. Among the two hand bones preserved from one of the earliest bipedal hominins, Orrorin tugenensis (ca 6 Ma), one is a distal pollical phalanx [65,66]. Its surprisingly human-like shape and FPL muscle attachment has led some to conclude that Orrorin possessed human-like precision grip abilities that evolved for the manipulation of organic objects, not stone tool making, with the relaxation of locomotor requirements on the forelimbs [19] (but see [66]).
Similar claims have been made for Australopithecus afarensis [18,28,50]. Though there is a wealth of hand fossils attributed to Au. afarensis [67–69], only a few metacarpals can be reliably associated with the same individual [50] (figure 2a). Hand proportions have been estimated in Au. afarensis by several researchers and range from potentially gorilla-like [27] to very similar [50], if not equal, to modern humans [18,28]. Functional interpretations based on these differences in reconstructed hand proportions, as well as other wrist and hand morphology, also vary: Rolian & Gordon [18] conclude that Au. afarensis could not have produced precision grips with the same efficiency as modern humans; Marzke [29,50] suggests Au. afarensis was capable of pad-to-side as well as three-jaw chuck precision grips, but likely had less effective precision handling and power squeeze grips; while Alba and co-workers [18,28] allow for the distinctly human-like pad-to-pad precision grips. Importantly, especially given the absence of evidence for stone tool behaviours prior to 2.6 Ma [63] until recently [12,13] (see §5), some researchers have supported the idea that the evolution of precision grip ability in Au. afarensis (and earlier hominins) should not be restricted to the context of stone tool behaviours, but may have initially evolved for non-lithic tool use or tool making, or any other complex manipulative behaviours that are required of extant non-human primates [14,17–20,24,28,29].
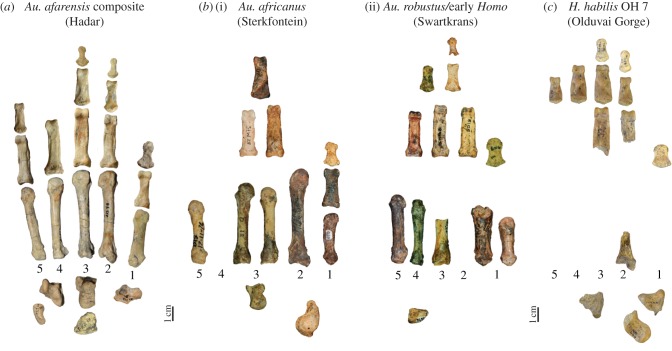
Figure 2.
Fragmentary or unassociated early hominin hand remains. (a) The composite hand of Au. afarensis from various sites in Hadar, Ethiopia. Only metacarpals 2–5 articulate well together and are presumed to be from the same individual. (b) A sample of isolated hand bones from (i) Sterkfontein, generally attributed to Au. africanus and (ii) Swartkrans, associated with either Au. robustus or early Homo. None of the fossils can be associated with the same individual and several elements are not represented in the fossil record. (c) The associated juvenile hand fossils of H. habilis OH 7. Although the phalanges are well represented in the OH 7 hand, little of the thumb and palm is preserved making functional inferences challenging. All known wrist bones shown at bottom and numbers indicate digits 1–5. Note that the phalanges of rays 2–5 cannot be attributed to any particular ray with certainty. All bones are shown in palmar view (apart from the wrist bones) and to the same scale although siding varies. (Online version in colour.)
The inference of human-like manipulative ability in early hominins is generally inherently linked to the relaxation of selective pressures on the hands for locomotion. There have been decades of debate regarding the significance of arboreal locomotion in the early hominin locomotor repertoire and the functional importance of ‘arboreal’ features such as long and curved fingers (see review by Ward [70]). The most common evolutionary scenario is one in which, with the advent of bipedalism, long, curved fingers are needed less, or not at all, for grasping in trees, freeing the fingers to shorten in length (either via neutral or positive selection) and the thumb, somewhat by default, to become relatively long [18,71]. Alternatively, it has been suggested that human hand proportions may have been a pleiotropic by-product of reducing the length of our toes for bipedalism [18,72] or that they are actually symplesiomorphic, more similar to the Miocene apes, and that early hominins never had the long fingers typical of our living chimpanzee and bonobo cousins [28,73]. In all of these scenarios, human hand proportions can be viewed, at least partly, as an exaptation, rather than adaptation, for enhanced dexterity [18,28]. The key to choosing between these scenarios relies on being able to accurately reconstruct intrinsic hand (and potentially foot) proportions in early hominins, which is particularly challenging due to the poor preservation and lack of associated hand skeletons (see below). For example, the estimated human-like hand proportions reconstructed for Au. africanus can only be estimated from unassociated metacarpals [74] (figure 2b). Hand proportions in Paranthropus/early Homo (e.g. Swartkrans specimens; figure 2c), the ‘handy-man’ H. habilis OH7 hand (contra [2]; figure 2d) and Homo erectus are completely unknown because there is simply not enough fossil evidence. The fossil hominin sites at Sierra de Atapuerca, Spain, preserve numerous hand bones, ranging from a single early Homo phalanx from Sima del Elefante [75], to dozens of hand bones from Gran Dolina (Homo antecessor) [76] and Sima de los Huesos (Homo heidelbergensis), the latter of which remain unpublished [77]. All show morphology that can broadly be described as modern human-like, but they cannot be associated with any particular individual to directly quantify intrinsic hand proportions or provide an overall functional interpretation of the hand in these Homo species.
4. What can we learn from relatively complete hominin hand skeletons?
(a). Little foot
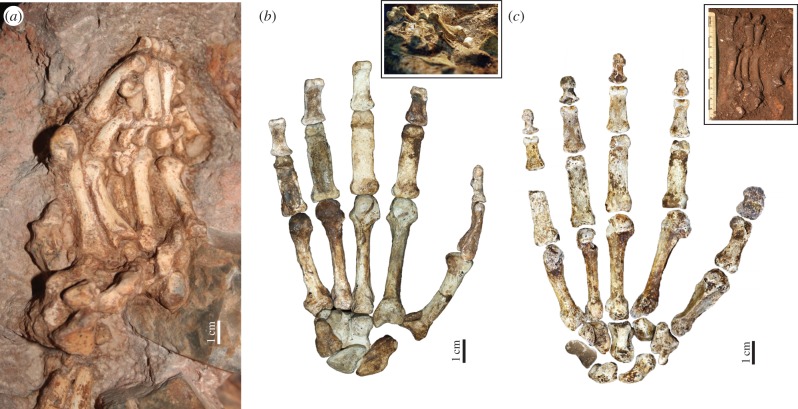
In addition to Ar. ramidus, there are only three relatively complete early hominin hands prior to Neandertals (figure 3). The first is the left hand of Australopithecus prometheus [78] StW 573 or ‘Little foot’, discovered in 1999 in the Silberberg Grotto of Sterkfontein, South Africa [9,78,79] and dated to as early as 3.7 Ma [80] or as late as 2.2 Ma [81] (figure 3a). It is associated with a relatively complete skeleton, allowing the rare opportunity to interpret the hand morphology within the context of the remainder of upper limb and postcranial skeleton, as well as taxonomically identifiable craniodental remains [9,78,79]. Preliminary observations of the hand morphology describe an unusual trapezium-Mc1 articulation that is unlike humans or chimpanzees, a robust, human-like thumb and proximal phalanges that are as curved as Au. afarensis [9]. No inferences about its manipulative capability have yet been made and we still await a full description and morphometric analysis of the left (and right) [79] hand remains. However, curved phalanges suggest use of the hand for locomotor grasping and the current absence of stone tools in Member 2 at Sterkfontein [82] suggests that the evolution of any human-like manipulative capabilities in StW 573 may have been in response to non-lithic tool use.
Figure 3.
The associated hand remains of early hominins. (a) The left hand of Au. prometheus StW 573 [78] in situ; (b) the reconstructed right hand of MH2 (palmar view), with inset image of hand bones in situ; (c) the reconstructed right hand of Hand 1 H. naledi (palmar view) with inset image of hand bones in situ. All hands are shown to the same scale. Photo credit: R. J. Clarke (a) and P. Schmid (b,c). (Online version in colour.)
(b). Australopithecus sediba
In 2010, a relatively complete right hand of the female adult Australopithecus sediba MH2 skeleton from Malapa, South Africa dated to 1.98 Ma was discovered [10,83] (figure 3b). Preserving 20 of the 27 bones in the human hand, MH2 offered the first (published) opportunity to make inferences about potential overall locomotor and manipulative hand function in an early hominin. Furthermore, the MH2 hand is found in association with a complete right arm [84] and much of the postcranial skeleton [83], and post-dates the appearance of stone tools in the archaeological record [13,63]. Although stone tools have not yet been found in direct association with Au. sediba [10], tool making (using stone and bone) has been documented at other local, contemporaneous sites, such as Sterkfontein [82] and Swartkrans [14,16].
The MH2 hand bones present a unique mosaic of features. The proximal and intermediate phalanges are mildly curved, similar to those of Au. africanus and OH 7 [10,11], and display well-developed flexor sheath ridges indicating powerful flexion of the hand during grasping. The wrist bones also show more similarities to other australopiths than to Homo [11] and the metacarpal shafts, especially that of the Mc1, are remarkably gracile (although the Mc3 of the male juvenile MH1 skeleton is more robust, suggesting a strong degree of sexual dimorphism in the hand morphology) [10,11]. However, the distal pollical phalanx is broad with a well-developed FPL attachment, the first metacarpal has a distally extended, human-like positioning of the dorsal interosseous tendon, and the robust base of the fifth metacarpal suggests that the extrinsic and intrinsic musculature to the fifth finger were well developed [10] (figures 1 and 3b). (However, it is important to note that recent evidence has not found a strong correlation between entheses shape and the size of the muscle [85,86].) Perhaps most importantly, the completeness of the Au. sediba MH2 hand offers the first accurate quantification of hand proportions in an early hominin, showing that the thumb is not only long relative to the fingers, but is actually relatively longer than that of modern humans (i.e. falling outside the range of human variation) [10]. Such a long thumb would have greatly facilitated pad-to-pad opposition of the thumb and fingers, and ultimately lead to greater control and manipulation of small objects in particular [29].
With the traditional view that the hand of the last common ancestor of Pan and humans was generally Pan-like (but see [62,87]) with long fingers, one can consider the long thumb of humans to be the result of reducing the length of the fingers, rather than increasing the length of the thumb per se [18]. Such a scenario is expected in a hand that is no longer significantly used for locomotion. However, if the hand were still under forelimb-dominated locomotor selective pressures, then lengthening the thumb would allow a hominin to retain long fingers needed for grasping branches but also a long thumb that is advantageous for pad-to-pad precision gripping [18]. Digit proportions in Au. sediba reveal that the thumb, and particularly the Mc1, is unexpectedly long (relative to the length of the third ray and to hand size) [10,11,28], suggesting perhaps a selection for increased thumb length. However, the length of the proximal and intermediate phalanges (relative to metacarpal length) is similar to that of modern humans, suggesting long fingers were not a functional requirement of its locomotor strategy. Within the context of the remainder of the MH2 skeleton, it may be that the functional requirements of locomotion were fulfilled by the long, ape-like upper limb [84] and mildly curved phalanges [10], while the hand proportions of Au. sediba largely reflect the requirements of enhanced dexterity.
(c). Homo naledi
The recent discovery of a complete right hand (missing only the pisiform) of Homo naledi offers unprecedented insight into how the hominin hand might balance the functional requirements of both locomotor grasping and manipulation [11,88] (figure 3c). The H. naledi right hand was found partially articulated in association with over 150 other isolated hand bones in the Dinaledi Chamber of the Rising Star cave system, South Africa [11,88]. The remains have yet to be dated but present yet another distinct mosaic of morphological features not yet known in any other hominin [11]. The wrist is remarkably derived, demonstrating most of the key features considered advantageous for coping with high external loading of the thumb during tool-related behaviours [27,32,33,59–61] (figure 1). The radial carpal and carpometarcarpal joints show signs of reorientation to a more human-like proximodistal alignment, including a trapezoid that is palmarly expanded to have a human-like ‘boot-shape’. However, the trapezium-Mc1 articulation is remarkably small and there is no styloid process on the Mc3 [11]. The thumb is long relative to the fingers; although not as long as Au. sediba MH2, H. naledi falls only within the variation of modern human males (and outside that of females) [11]. The thumb is robust with a human-like attachment for the FPL muscle and well-developed, flaring muscle attachments for the opponens pollicis and first dorsal interosseous muscles, while the hamate-Mc5 articulation is saddle-shaped like in humans. Together, this morphology suggests enhanced opposition of the thumb to the fingers, particularly while holding and manipulating large objects, as well as efficient precision and precision pinch grips [35]. The suite of features found in the H. naledi wrist and palm have only been found in committed stone toolmakers like Neandertals and H. sapiens, strongly suggesting that H. naledi had enhanced manipulative abilities for tool-related behaviours. Like with Au. sediba, there are no stone tools yet found in association with the H. naledi remains and without knowing the geological age, H. naledi may have used stone and/or organic materials as tools.
In contrast to the derived morphology of the wrist and palm, however, the fingers of H. naledi are strongly curved; more curved than australopiths, including Au. afarensis [11]. Phalangeal curvature is one of the best indicators of function in the hand [89–92]. The degree of longitudinal curvature is strongly correlated with the degree of arboreal locomotion across primates, with climbing and, especially, suspensory taxa showing much stronger curvature than terrestrial quadrupedal or bipedal taxa [89–91]. Phalangeal curvature is also known to be sensitive to changes in locomotion throughout ontogeny, such that more arboreal juveniles have more strongly curved phalanges than their more terrestrial adult counterparts [93]. The strong phalangeal curvature in both the proximal and intermediate phalanges of H. naledi is a clear functional indicator that this hominin still used its hands for locomotor grasping. The combination of such curved phalanges with a largely late-Homo-like wrist and palm demonstrates that (i) locomotor grasping was a functionally significant behaviour [94], not just a primitive, non-adaptive retention that has yet to be lost (contra [95]) and (ii) this hominin was capable of using their hands for both enhanced manipulation and arboreal locomotion, such that one functional role did not necessarily need to be sacrificed for the other.
5. New insights from new discoveries and methodologies
Decades of research aiming to identify and test the potential morphological features of the human and hominin hand that are adaptive for stone tool behaviours were somewhat undermined by the discovery of the Late Pleistocene Homo floresiensis [96]. Hand bones dated to 17–19 000 BP [97,98] and belonging to at least two, small-bodied individuals (LB1 and LB6) are surprisingly primitive; more similar to extant apes and australopiths than to other Homo species, despite their remarkably young age [98–100]. For example, although H. floresiensis has a broad pollical distal phalanx with a human-like FPL attachment [98,100], the proximal phalanges are curved to a similar degree as in Au. afarensis [100] and the wrist lacks a Mc3 styloid process, a boot-shaped trapezoid, and a reorientation of the radial carpal and metacarpal joints that are found in later Homo (and H. naledi) [98,99]. However, there is a well-documented archaeological sequence at Flores clearly demonstrating that stone tool making and use were part of the behavioural repertoire of H. floresiensis from as early as 840 ka [97,101]. Thus, the direct association between the largely primitive hand of H. floresiensis and stone tools (produced via a simple reduction sequence [97]) calls into question our traditional assumptions about the necessary morphological features and biomechanical consequences of stone tool production [98].
Furthermore, recent archaeological discoveries have changed the traditional perception that stone tool production is an ability limited to the genus Homo (see review in [8]). The first is that of stone cut marks on bone at Dikika, Ethiopia, dated to 3.39 Ma and in deposits currently associated only with Au. afarensis [12,102]. The archaeological evidence demonstrates the use of sharp-edged stones to remove flesh and blunt stones to access marrow [12] (but see [103]). McPherron et al. [12,104] put forth the important message of changing the traditional archaeological paradigm that stone tool use could not have occurred prior to stone tool making, and the need to look for evidence of stone tool use in deposits that predate recognizable stone tools (and predate Homo).
McPherron et al.'s [12,104] somewhat controversial [103] claim of tool-use ability in pre-2.6 Ma hominins is further substantiated by the recent discovery of pre-Oldowan stone tools, currently named Lomekwian, dated to 3.3 Ma from Lomekwi 3 in Kenya [13]. These tools are 700 000 years older than earliest Oldowan technology (2.6 Ma [63]) and over 500 000 years older than the earliest possible fossil evidence of Homo (2.8 Ma [105]). Their techno-morphology suggests they were made using arm and hand motions that are most similar to the hammer-on-anvil technique used by chimpanzees during nut-cracking (but likely requiring greater cognitive ability [106]; see also [107]), rather than the free-hand knapping of the Oldowan [13]. The inferred knapping technique suggests a substantial degree of hand motor control and forceful loading of the hands, but likely less dexterity than Oldowan-making hominins [13]. These tools are found in the same geographical and chronological context of Kenyanthropus platyops, but are also contemporaneous with Au. afarensis [13]. Importantly, both the Lomekwian and Dikika cut marks [12] are too old to be associated with currently known Homo fossils, and thus demonstrate a manipulative (and cognitive) ability of pre-Homo hominins that has not been traditionally recognized.
Biomechanical studies of tool use and tool making have helped to clarify how fossil hominin hands may have been loaded during stone tool-use and tool-making activities [34,52–54]. Rolian et al. [52] created artificial ‘stone tools’ instrumented with force plates to examine how the external forces and joint stress were distributed across the thumb and index finger. They found that the thumb experienced higher external loads and joint stress during tool making than during flake use (but see [53,54] and below). They also found that individuals with longer digits required relatively less muscle force to stabilize the digit joints and experienced relatively less joint stress during stone tool behaviours, because their digits and joints were relatively more robust. The implications of their results, they suggest, are that the gracile pollical metacarpals of chimpanzees and early australopiths could not produce or withstand the high forces that occur during stone tool making [52].
Others [34,53,54] have also investigated external loading of the radial digits during Oldowan stone tool making, but by using pressure strips along the digits, such that force was measured directly on the hand rather than by the tool (as in [52]). In contrast to Rolian et al. [52], Williams et al. [53] found that the loading experienced by the thumb during tool making was actually lower (not higher) than that of the index and middle finger. In other words, the human thumb is ‘over-built’ for Oldowan stone tool making, but appears well adapted for the much higher external forces experienced during flake use [54]. Key & Dunmore [34] further suggest that the robust thumb of humans and other hominins was selected, at least in part, for the loads experienced in the non-dominant hand during stone tool making (although these loads are much lower than those experienced by the thumb of the dominant hand). This variation in experimental results at least partly reflects differences in methodology (portable force plates [52] versus pressure strips [34,53,54], and novice [52] versus expert [34,53,54] knappers), but also demonstrates the challenges of trying to simulate and quantify the biomechanics of tool-related manipulative behaviours in fossil hominins using modern humans (and modern human hand anatomy).
Kinematic modelling of the primate hand also has the potential to make more informed inferences of the manipulative abilities in early hominins. Feix et al. [108] created a kinematic model of thumb and index finger precision grip and manipulative movement based on bony hand morphology in a broad sample of extant primates and fossil hominins. They found that joint mobility and (scaled) digit proportions are critical for determining precision grip and manipulation potential (figure 1), but having a relatively long thumb or high joint mobility alone does not necessarily result in greater dexterity [108]. Despite (potential) differences in digit proportions and joint mobility in australopiths, Au. afarensis and Au. sediba show a manipulation workspace that is similar to that of modern humans [108], supporting previous interpretations of increased dexterity in these taxa [10,18,29] and archaeological evidence of tool-related behaviours in pre-Homo hominins [12,13].
Finally, greater access to three-dimensional scanning techniques, including surface scanning [34–36,53–61,98,99] and microtomography [109,110], has allowed for more comprehensive functional analyses of both the external and internal morphology of hand bones. In particular, analyses of the internal trabecular morphology of hand bones has provided new insights into how early hominins may have actually, rather than potentially, used their hands [110]. Trabecular bone remodels throughout an individual's life in response to mechanical loading, a concept known as bone functional adaptation [111]. Several experimental studies have shown that changes in loading direction or magnitude can be associated with corresponding changes in the orientation of trabeculae struts or relative volume of trabecular bone (e.g. [112,113]). Previous comparative studies show that variation in trabecular structure in extant hominoid (including humans) hand bones correlates well with differences in inferred joint posture and loading during locomotion and manipulation [110]. Within this context, Skinner et al. [21] recently analysed the trabecular structure in several fossil hominin hand bones, and found that Neandertals, early Homo sapiens and modern humans share a distinct asymmetrical pattern in the distribution of trabecular bone in the metacarpals consistent with forceful opposition of the thumb and fingers that is not found in other extant apes (including nut-cracking Taï chimpanzees). Interestingly, Au. africanus also shows the Neandertal- and human-like pattern of trabecular bone, suggesting that this early hominin was also habitually using forceful human-like opposition of the thumb and fingers, such as in the precision and power squeeze grips that are used during tool use and tool making [21]. Although many have proposed that Au. africanus [64] and earlier hominins [18–20,28,29] were potentially capable of human-like precision grips, the trabecular structure provides more direct evidence that Au. africanus was actually loading its hand in a human-like way, despite not having a fully human-like external hand morphology [21,114]. These results provide morphological evidence of enhanced manipulative ability in an australopith and additional support for archaeological evidence of tool use [12] and tool making [13] in pre-Homo hominins.
6. Conclusion
Darwin [115] first proposed that the advent of bipedalism was directly linked to tool use as it freed the hands from the constraints of locomotion. This view was maintained with the earliest discoveries of stone tools and fossil hominin remains in the 1950s and 1960s [1,116]. However, there was a paradigm shift in the 1970s with the discovery of the small-brained, bipedal Au. afarensis 1.5 Myr earlier than the appearance of stone tools [8]. Further discoveries of earlier bipedal hominins dating back to at least approximately 6 Ma [65] only increased the gap between bipedalism and stone tools [8]. Thus, tool-related behaviours have generally no longer been thought to have a cause–effect relationship with the origin of hominin bipedalism [50,117,118]. However, it may be worthwhile to revisit Darwin's original hypothesis. On the one hand, recent discoveries are closing the chronological gap between the origin of bipedalism and evidence for tool-related behaviours. Morphological evidence (albeit limited) suggests the potential for human-like precision grip ability in some of the earliest hominins (Orrorin; [19]). Furthermore, comparative extant primate evidence [22–24] and the recognition of the enhanced manipulative and cognitive abilities required for the production of Oldowan [106,107,119] and, less so, Lomekwian stone tools [13], together suggest that there was likely a long history of experimental tool use and improvements to manual dexterity prior to the first recognizable stone tool behaviours in the archaeological record [17,22]. On the other hand, morphological evidence from relatively complete hand skeletons [9–11] indicates that fossil hominins did not necessarily need to ‘free’ their hands from the functional requirements of locomotion to increase their dexterity. Together recent evidence suggests that pre-Homo hominins were more dextrous than has been traditionally assumed, that tool-related behaviours have played a chronologically deeper and more prominent role in our evolutionary history than previously considered, and that the hands of these early hominins were capable of combining the functional requirements of both arboreal locomotion and enhanced manipulation.
Acknowledgements
I am grateful to Ignacio de la Torre and Satoshi Hirata for the opportunity to participate in the interesting volume. I thank Ron Clarke for sharing his image of the StW 573 hand and Peter Schmid for his images of the Au. sediba and H. naledi hands. I thank the following people for access to original fossils: S. Potze, L. Kgasi (Ditsong Museum), L. Berger, B. Zipfel and F. Thackeray (University of the Witwatersrand), B. Kimbel, T. White, B. Asfaw, G. Semishaw, Y. Assefa, G. Shimelies (National Museum of Ethiopia), A. Gidna and A. Kweka (Museum and House of Culture, National Museum of Tanzania). I am grateful to the following collaborators, whose expertise and discussions over the years have contributed greatly to this review: S. Churchill, L. Berger, C. Orr, M. Tocheri, A. Deane, D. Schmitt, N. Stephens, Z. Tsegai, J.-J. Hublin, S. McPherron, M. Skinner. I thank N. Stephens for his helpful comments on an earlier version of this manuscript.
Competing interests
I have no competing interests.
Funding
This research was supported by the European Research Council Starting Grant no. 336301 and the Max Planck Society.
References
- 1.Washburn SL. 1960. Tools and human evolution. Sci. Am. 203, 62–75. ( 10.1038/scientificamerican0960-62) [DOI] [PubMed] [Google Scholar]
- 2.Napier JR. 1962. The evolution of the hand. Sci. Am. 207, 56–62. ( 10.1038/scientificamerican1262-56) [DOI] [PubMed] [Google Scholar]
- 3.Leakey LSB. 1959. A new fossil skull from Olduvai. Nature 184, 491–493. ( 10.1038/184491a0)13850382 [DOI] [Google Scholar]
- 4.Leakey LSB. 1960. Recent discoveries at Olduvai Gorge. Nature 188, 1050–1052. ( 10.1038/1881050a0) [DOI] [Google Scholar]
- 5.Leakey LSB. 1961. New finds at Olduvai Gorge. Nature 189, 649–650. ( 10.1038/189649a0) [DOI] [PubMed] [Google Scholar]
- 6.Napier JR. 1962. Fossil hand bones from Olduvai Gorge. Nature 196, 409–411. ( 10.1038/196409a0) [DOI] [Google Scholar]
- 7.Leakey LSB, Tobias PV, Napier JR. 1964. A new species of the genus Homo from Olduvai Gorge. Nature 202, 7–9. ( 10.1038/202007a0) [DOI] [PubMed] [Google Scholar]
- 8.de la Torre I. 2011. The origins of stone tool technology in Africa: a historical perspective . Phil. Trans. R. Soc. B 366, 1028–1037. ( 10.1098/rstb.2010.0350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clarke RJ. 1999. Discovery of complete arm and hand of the 3.3 million-year-old Australopithecus skeleton from Sterkfontein. S. Afr. J. Sci. 95, 477–480. [Google Scholar]
- 10.Kivell TL, Kibii JM, Churchill SE, Schmid P, Berger LR. 2011. Australopithecus sediba hand demonstrates mosaic evolution of locomotor and manipulative abilities. Science 333, 1411–1417. ( 10.1126/science.1202625) [DOI] [PubMed] [Google Scholar]
- 11.Kivell TL, Deane AS, Tocheri MW, Orr CM, Schmid P, Hawks J, Berger LR, Churchill SE. In press. The hand of Homo naledi. Nat. Comm. 6, 8431 ( 10.1038/ncomms9431) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA. 2010. Evidence for stone-tool-assisted consumption of animal tissues before 3.39 million years ago at Dikika, Ethiopia. Nature 466, 857–860. ( 10.1038/nature09248) [DOI] [PubMed] [Google Scholar]
- 13.Harmand S, et al. 2015. 3.3-million-year-old stone tools from Lomekwi 3, West Turkana, Kenya. Nature 521, 311–315. ( 10.1038/nature14464) [DOI] [PubMed] [Google Scholar]
- 14.Susman RL. 1988. Hand of Paranthropus robustus from Member 1, Swartkrans: fossil evidence for tool behaviour. Science 240, 781–784. ( 10.1126/science.3129783) [DOI] [PubMed] [Google Scholar]
- 15.Susman RL. 1994. Fossil evidence for early hominid tool use. Science 265, 1570–1573. ( 10.1126/science.8079169) [DOI] [PubMed] [Google Scholar]
- 16.Backwell LR, d'Errico F. 2001. Evidence of termite foraging by Swartkrans early hominids. Proc. Natl Acad. Sci. USA 98, 1358–1363. ( 10.1073/pnas.98.4.1358) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panger MA, Brooks AS, Richmond BG, Wood B. 2002. Older than the Oldowan? Rethinking the emergence of hominin tool use. Evol. Anthropol. 11, 235–245. ( 10.1002/evan.10094) [DOI] [Google Scholar]
- 18.Alba DM, Moyà-Solà S, Köhler M. 2003. Morphological affinities of the Australopithecus afarensis hand on the basis of manual proportions and relative thumb length. J. Hum. Evol. 44, 225–254. ( 10.1016/S0047-2484(02)00207-5) [DOI] [PubMed] [Google Scholar]
- 19.Almécija S, Moyà-Solà S, Alba DM. 2010. Early origin for human-like precision grasping: a comparative study of pollical distal phalanges in fossil hominins. PLoS ONE 5, e11727. ( 10.1371/journal.pone.0011727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Almécija S, Wallace IJ, Judex S, Albar DM, Moyà-Solà S. 2015. Comment on ‘Human-like hand use in Australopithecus africanus'. Science 348, 1101a. ( 10.1126/science.aaa8414) [DOI] [PubMed] [Google Scholar]
- 21.Skinner MM, Stephens NB, Tsegai ZJ, Foote AC, Nguyen NH, Gross T, Pahr DH, Hublin J-J, Kivell TL. 2015. Human-like hand use in Australopithecus africanus. Science 347, 395–399. ( 10.1126/science.1261735) [DOI] [PubMed] [Google Scholar]
- 22.Haslam M, et al. 2009. Primate archaeology. Nature 460, 339–344. ( 10.1038/nature08188) [DOI] [PubMed] [Google Scholar]
- 23.Luncz LV, Wittig RM, Boesch C. 2015. Primate archaeology reveals cultural transmission in wild chimpanzees (Pan troglodytes verus). Phil. Trans. R. Soc. B 370, 20140348 ( 10.1098/rstb.2014.0348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiten A. 2015. Experimental studies illuminate the cultural transmission of percussive technologies in Homo and Pan. Phil. Trans. R. Soc. B 370, 20140359 ( 10.1098/rstb.2014.0359) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinkaus E, Long JC. 1990. Species attribution of the Swartkrans Member 1 first metacarpals: SK 84 and SKX 5020. Am. J. Phys. Anthropol. 83, 419–424. ( 10.1002/ajpa.1330830403) [DOI] [PubMed] [Google Scholar]
- 26.Hamrick MW, Inouye SE. 1995. Thumbs, tools and early humans. Science 268, 586–587. ( 10.1126/science.7725112) [DOI] [PubMed] [Google Scholar]
- 27.Rolian C, Gordon AD. 2013. Reassessing manual proportions in Australopithecus afarensis. Am. J. Phys. Anthropol. 152, 393–406. ( 10.1002/ajpa.22365) [DOI] [PubMed] [Google Scholar]
- 28.Almécija S, Alba DM. 2014. On manual proportions and pad-to-pad precision grasping in Australopithecus afarensis. J. Hum. Evol. 73, 88–92. ( 10.1016/j.jhevol.2014.02.006) [DOI] [PubMed] [Google Scholar]
- 29.Marzke MW. 1997. Precision grips, hand morphology, and tools. Am. J. Phys. Anthropol. 102, 91–110. () [DOI] [PubMed] [Google Scholar]
- 30.Susman RL. 1998. Hand function and tool behaviour in early hominids. J. Hum. Evol. 35, 23–46. ( 10.1006/jhev.1998.0220) [DOI] [PubMed] [Google Scholar]
- 31.Marzke MW, Marzke RF. 2000. Evolution of the human hand: approaches to acquiring, analysing and interpreting the anatomical evidence. J. Anat. 197, 121–140. ( 10.1046/j.1469-7580.2000.19710121.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tocheri MW, Orr CM, Jacofsky MC, Marzke MW. 2008. The evolutionary history of the hominin hand since the last common ancestor of Pan and Homo. J. Anat. 212, 544–562. ( 10.1111/j.1469-7580.2008.00865.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marzke MW. 2013. Tool making, hand morphology and fossil hominins. Phil. Trans. R. Soc. B 368, 20120414 ( 10.1098/rstb.2012.0414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Key AJ, Dunmore CJ. 2015. The evolution of the hominin thumb and the influence exerted by the non-dominant hand during stone tool production. J. Hum. Evol. 78, 60–69. ( 10.1016/j.jhevol.2014.08.006) [DOI] [PubMed] [Google Scholar]
- 35.Marzke MW, Toth N, Schick K, Reece S, Steinberg B, Hunt K, Linscheid RL, An K-N. 1998. EMG study of hand muscle recruitment during hard hammer percussion manufacture of Oldowan tools. Am. J. Phys. Anthropol. 105, 315–332. () [DOI] [PubMed] [Google Scholar]
- 36.Hamrick MW, Churchill SE, Schmitt D, Hylander WL. 1998. EMG of the human flexor pollicis longus muscle: implications for the evolution of hominid tool use. J. Hum. Evol. 34, 123–136. ( 10.1006/jhev.1997.0177) [DOI] [PubMed] [Google Scholar]
- 37.Marzke MW, Shackley MS. 1986. Hominid hand use in the Pliocene and Pleistocene: evidence from experimental archaeology and comparative morphology. J. Hum. Evol. 15, 439–460. ( 10.1016/S0047-2484(86)80027-6) [DOI] [Google Scholar]
- 38.Boesch C, Boesch H. 1993. Different hand postures for pounding nuts with natural hammers by wild chimpanzees. In Hands of primates (eds Preuschoft H, Chiver D), pp. 31–43. New York, NY: Springer. [Google Scholar]
- 39.Christel MI. 1993. Grasping techniques and hand preferences in Hominoidea. In Hands of primates (eds Preuschoft H, Chiver D), pp. 91–108. New York, NY: Springer. [Google Scholar]
- 40.Jones-Engel LE, Bard KA. 1996. Precision grips in young chimpanzees. Am. J. Primatol. 39, 1–15. () [DOI] [PubMed] [Google Scholar]
- 41.Marzke MW, Wullstein KL. 1996. Chimpanzee and human grips: a new classification with a focus on evolutionary morphology. Int. J. Primatol. 17, 117–139. ( 10.1007/BF02696162) [DOI] [Google Scholar]
- 42.Christel MI, Fragaszy D. 2000. Manual function in Cebus apella: digital mobility, preshaping, and endurance in repetitive grasping. Int. J. Primatol. 21, 697–719. ( 10.1023/A:1005521522418) [DOI] [Google Scholar]
- 43.Crast J, Fragaszy D, Hayashi M, Matsuzawa T. 2009. Dynamic in-hand movements in adult and young juvenile chimpanzees (Pan troglodytes). Am. J. Phys. Anthropol. 138, 274–285. ( 10.1002/ajpa.20925) [DOI] [PubMed] [Google Scholar]
- 44.Gumert MD, Kluck M, Malaivijitnond S. 2009. The physical characteristics and usage patterns of stone axe and pounding hammers used by long-tailed macaques in the Andaman Sea region of Thailand. Am. J. Primatol. 71, 594–608. ( 10.1002/ajp.20694) [DOI] [PubMed] [Google Scholar]
- 45.Pouydebat E, Reghem E, Borel A, Gorce P. 2011. Diversity of grip in adults and young humans and chimpanzees (Pan troglodytes). Behav. Brain Res. 218, 21–28. ( 10.1016/j.bbr.2010.11.021) [DOI] [PubMed] [Google Scholar]
- 46.Marzke MW, Marchant LF, McGrew WC, Reece SP. 2014. Grips and hand movements of chimpanzees during feeding in Mahale Mountains National Park, Tanzania. Am. J. Phys. Anthropol. 156, 317–326. ( 10.1002/ajpa.22651) [DOI] [PubMed] [Google Scholar]
- 47.Neufuss J, Humle T, Deschner T, Robbins MM, Sirianni G, Boesch C, Kivell TL. In press. Diversity of hand grips and laterality in wild African apes. Folia Primatol. 86, 329 ( 10.1159/000435825) [DOI] [Google Scholar]
- 48.Marzke MW, Wullstein KL, Viegas SF. 1992. Evolution of the power (‘squeeze’) grip and its morphological correlates in hominids. Am. J. Phys. Anthropol. 89, 283–298. ( 10.1002/ajpa.1330890303) [DOI] [PubMed] [Google Scholar]
- 49.Landsmeer JMF, Long C. 1965. The mechanism of finger control, based on electromyograms and location analysis. Cells Tissues Organs 60, 330–347. ( 10.1159/000142668) [DOI] [PubMed] [Google Scholar]
- 50.Marzke MW. 1983. Joint functions and grips of the Australopithecus afarensis hand, with special reference to the region of the capitate. J. Hum. Evol. 12, 197–211. ( 10.1016/S0047-2484(83)80025-6) [DOI] [Google Scholar]
- 51.Diogo R, Richmond BG, Wood B. 2012. Evolution and homologies of primate and modern human hand and forearm muscles with notes on thumb movements and tool use. J. Hum. Evol. 63, 64–78. ( 10.1016/j.jhevol.2012.04.001) [DOI] [PubMed] [Google Scholar]
- 52.Rolian C, Lieberman DE, Zermeno JP. 2011. Hand biomechanics during simulated stone tool use. J. Hum. Evol. 61, 26–41. ( 10.1016/j.jhevol.2011.01.008) [DOI] [PubMed] [Google Scholar]
- 53.Williams EM, Gordon AD, Richmond BG. 2012. Hand pressure distribution during Oldowan stone tool production. J. Hum. Evol. 62, 520–532. ( 10.1016/j.jhevol.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 54.Williams EM, Richmond BG. 2012. Manual pressure distribution during stone tool use. Am. J. Phys. Anthropol. 147(Suppl 54), 303. [Google Scholar]
- 55.Marzke MW, Marzke RF, Linscheid RL, Smutz P, Steinberg B, Reece S, An K-N. 1999. Chimpanzee thumb muscle cross sections, moment arms and potential torques, and comparisons with humans. Am. J. Phys. Anthropol. 110, 163–178. () [DOI] [PubMed] [Google Scholar]
- 56.Maki J, Trinkaus E. 2011. Opponens pollicis mechanical effectiveness in Neandertals and early modern humans. PaleoAnthropology 2011, 62–71. ( 10.4207/PA.2011.ART43) [DOI] [Google Scholar]
- 57.Shrewsbury MM, Marzke MW, Linscheid RL, Reece SP. 2003. Comparative morphology of the pollical distal phalanx. Am. J. Phys. Anthropol. 121, 30–47. (doi:10/1002/ajpa.10192) [DOI] [PubMed] [Google Scholar]
- 58.Marzke MW, Marzke RF. 1987. The third metacarpal styloid process in humans: origin and functions. Am. J. Phys. Anthropol. 73, 415–431. ( 10.1002/ajpa.1330730403) [DOI] [PubMed] [Google Scholar]
- 59.Tocheri MW. 2007. Three-dimensional riddles of the radial wrist: derived carpal and carpometacarpal joint morphology in the genus Homo and the implications for understanding the evolution of stone tool-related behaviors in hominins. Dissertation, Arizona State University, Tempe, AZ, USA.
- 60.Tocheri MW, Marzke MW, Liu D, Bae M, Jones GP, Williams RC, Razdan A. 2003. Functional capabilities of modern and fossil hominid hands: three-dimensional analysis of trapezia. Am. J. Phys. Anthropol. 122, 101–112. ( 10.1002/ajpa.10235) [DOI] [PubMed] [Google Scholar]
- 61.Tocheri MW, Razdan A, Williams RC, Marzke MW. 2005. A 3D quantitative comparison of trapezium and trapezoid relative articular and nonarticular surface areas in modern humans and great apes. J. Hum. Evol. 49, 570–586. ( 10.1016/j.jhevol.2005.06.005) [DOI] [PubMed] [Google Scholar]
- 62.Lovejoy CO, Simpson SW, White TD, Asfaw B, Suwa G. 2009. Careful climbing in the Miocene: the forelimbs of Ardipithecus ramidus and humans are primitive. Science 326, 70, 70e1–70e8. ( 10.1126/science.1175827) [DOI] [PubMed] [Google Scholar]
- 63.Semaw S, et al. 2003. 2.6-Million-year-old stone tools and associated bones from OGS-6 and OGS-7, Gona, Afar, Ethiopia. J. Hum. Evol. 45, 169–177. ( 10.1016/S0047-2484(03)00093-9) [DOI] [PubMed] [Google Scholar]
- 64.Ricklan DE. 1990. The precision grip in Australopithecus africanus: anatomical and behavioral correlates. In From apes to angels: essays in anthropology in honor of Phillip V. Tobias (eds Sperber GH.), pp. 171–183. New York, NY: Wiley-Liss. [Google Scholar]
- 65.Senut B, Pickford M, Gommery D, Mein P, Cheboi K, Coppens Y. 2001. First hominid from the Miocene (Lukeino formation, Kenya). C. R. Acad. Sci.: Series IIA-Earth Planetary Sci. 332, 137–144. ( 10.1016/S1251-8050(01)01529-4) [DOI] [Google Scholar]
- 66.Gommery D, Senut B. 2006. La phalange distale du pouce d’Orrorin tugenensis (Miocene supérieur du Kenya). Geobios 39, 372–384. ( 10.1016/j.geobios.2005.03.002) [DOI] [Google Scholar]
- 67.Bush ME, Lovejoy CO, Johanson DC, Coppens Y. 1982. Hominid carpal, metacarpal, and phalangeal bones recovered from the Hadar formation: 1974–1977 collections. Am. J. Phys. Anthropol. 57, 651–677. ( 10.1002/ajpa.1330570410) [DOI] [Google Scholar]
- 68.Drapeau MSM, Ward CV, Kimbel WH, Johanson DC, Rak Y. 2005. Associated cranial and forelimb remains attributed to Australopithecus afarensis from Hadar, Ethiopia. J. Hum. Evol. 48, 593–642. ( 10.1016/j.jhevol.2005.02.005) [DOI] [PubMed] [Google Scholar]
- 69.Ward CV, Kimbel WH, Harmon EH, Johanson DC. 2012. New postcranial fossils of Australopithecus afarensis from Hadar, Ethiopia (1990–2007). J. Hum. Evol. 63, 1–51. ( 10.1016/j.jhevol.2011.11.012) [DOI] [PubMed] [Google Scholar]
- 70.Ward CV. 2002. Interpreting the posture and locomotion of Australopithecus afarensis: where do we stand? Am. J. Phys. Anthropol. 119(Suppl 35), 185–215. ( 10.1002/ajpa.10185) [DOI] [PubMed] [Google Scholar]
- 71.Wood Jones F. 1916. Arboreal man. London, UK: Arnold. [Google Scholar]
- 72.Rolian C, Lieberman DE, Hallgrímsson B. 2010. The coevolution of human hands and feet. Evolution 64, 1558–1568. ( 10.1111/j.1558-5646.2009.00944.x) [DOI] [PubMed] [Google Scholar]
- 73.Almécija S, Shrewsbury M, Rook L, Moyà-Solà S. 2014. The morphology of Oreopithecus bambolii pollical distal phalanx. Am. J. Phys. Anthropol. 153, 582–597. ( 10.1002/ajpa.22458) [DOI] [PubMed] [Google Scholar]
- 74.Green DJ, Gordon AD. 2008. Metacarpal proportions in Australopithecus africanus. J. Hum. Evol. 54, 705–719. ( 10.1016/j.jhevol.2007.10.007) [DOI] [PubMed] [Google Scholar]
- 75.Lorenzo C, Pablos A, Carretero JM, Huguet R, Valverdú J, Martinón-Torres M, Arsuaga JL, Carbonell E, Bermúdez de Castro JM. 2015. Early Pleistocene human hand phalanx from the Sima del Elefante (TE) cave site in Sierra de Atapuerca (Spain). J. Hum. Evol. 78, 114–121. ( 10.1016/j.jhevol.2014.08.007) [DOI] [PubMed] [Google Scholar]
- 76.Lorenzo C, Arsuaga JL, Carretero JM. 1999. Hand and foot remains from the Gran Dolina Early Pleistocene site (Sierra de Atapuerca, Spain). J. Hum. Evol. 37, 501–522. ( 10.1006/jhev.1999.0341) [DOI] [PubMed] [Google Scholar]
- 77.Arsuaga JL, Martínez I, Garcia A, Carretero JM, Lorenzo C, García N, Ortega AI. 1997. Sima de los Huesos (Sierra de Atapuerca, Spain). The site. J. Hum. Evol. 33, 109–127. ( 10.1006/jhev.1997.0132) [DOI] [PubMed] [Google Scholar]
- 78.Clarke RJ. 2013. Australopithecus from Sterkfontein Caves, South Africa. In The paleobiology of Australopithecus (eds Reed KE, Fleagle JG, Leakey RE), pp. 105–123. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 79.Clarke RJ. 2008. Latest information on Sterkfontein's Australopithecus skeleton and a new look at Australopithecus. S. Afr. J. Sci. 104, 443–449. ( 10.1590/S0038-23532008000600015) [DOI] [Google Scholar]
- 80.Granger DE, Gibbon RJ, Kuman K, Clarke RJ, Bruxelles L, Caffee MW. 2015. New cosmogenic burial ages for Sterkfontein Member 2 Australopithecus and Member 5 Oldowan. Nature 522, 85–88. ( 10.1038/nature14268) [DOI] [PubMed] [Google Scholar]
- 81.Pickering R, Kramers JD. 2010. Re-appraisal of the stratigraphy and determination of new U-Pb dates for the Sterkfontein hominin site, South Africa. J. Hum. Evol. 59, 70–86. ( 10.1016/j.jhevol.2010.03.014) [DOI] [PubMed] [Google Scholar]
- 82.Kuman K, Clarke RJ. 2000. Stratigraphy, artefact industries and hominid associations for Sterkfontein, Member 5. J. Hum. Evol. 38, 827–847. ( 10.1006/jhev.1999.0392) [DOI] [PubMed] [Google Scholar]
- 83.Berger LR, de Ruiter DJ, Churchill SE, Schmid P, Carlson KJ, Dirks PH, Kibii JM. 2010. Australopithecus sediba: a new species of Homo-like australopith from South Africa. Science 328, 195–204. ( 10.1126/science.1184944) [DOI] [PubMed] [Google Scholar]
- 84.Churchill SE, et al. 2013. The upper limb of Australopithecus sediba. Science 340, 1233477 ( 10.1126/science.1233477) [DOI] [PubMed] [Google Scholar]
- 85.Rabey K, Green DJ, Taylor AB, Begun DR, Richmond BG, McFarlin SC. 2015. Locomotor actrivity influences muscle architecture and bone growth but not muscle attachment site morphology. J. Hum. Evol. 78, 91–102. ( 10.1016/j.jhevol.2014.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Williams-Hatala EM, Hiles S, Hatala KG, Rabey K. 2015. Relationships between muscle architectural anatomy and the morphology of entheses in the thenar and hypothenar regions of modern humans. Am. J. Phys. Anthropol. 156(Suppl 60), 326. [Google Scholar]
- 87.Drapeau MS, Ward CV. 2007. Forelimb segment length proportions in extant hominoids and Australopithecus afarensis. Am. J. Phys. Anthropol. 132, 327–343. ( 10.1002/ajpa.20533) [DOI] [PubMed] [Google Scholar]
- 88.Berger LR, et al. 2015. Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. eLife 4, e09560 ( 10.7554/eLife.09560) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jungers WL, Godfrey LR, Simons EL, Chatrath PS. 1997. Phalangeal curvature and positional behaviour in extinct sloth lemurs (Primates, Palaeopropithecidae). Proc. Natl Acad. Sci. USA 94, 11 998–12 001. ( 10.1073/pnas.94.22.11998) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richmond BG. 2007. Biomechanics of phalangeal curvature. J. Hum. Evol. 53, 678–690. ( 10.1016/j.jhevol.2007.05.011) [DOI] [PubMed] [Google Scholar]
- 91.Deane AS, Begun DR. 2008. Broken fingers: retesting locomotor hypotheses for fossil hominoids using fragmentary proximal phalanges and high-resolution polynomial curve fitting (HR-PCF). J. Hum. Evol. 55, 691–701. ( 10.1016/j.jhevol.2008.05.005) [DOI] [PubMed] [Google Scholar]
- 92.Nguyen NH, Pahr DH, Gross T, Skinner MM, Kivell TL. 2014. Micro-finite element (µFE) modeling of the siamang (Symphalangus syndactylus) third proximal phalanx: the functional role of curvature and the flexor sheath ridge. J. Hum. Evol. 67, 60–75. ( 10.1016/j.jhevol.2013.12.008) [DOI] [PubMed] [Google Scholar]
- 93.Richmond BG. 1998. Ontogeny and biomechanics of phalangeal form in primates. Dissertation, State University of New York at Stony Brook, NY, USA.
- 94.Stern JT Jr, Susman RL. 1983. The locomotor anatomy of Australopithecus afarensis. Am. J. Phys. Anthropol. 60, 279–317. ( 10.1002/ajpa.1330600302) [DOI] [PubMed] [Google Scholar]
- 95.Latimer B. 1991. Locomotor adaptations in Australopithecus afarensis: the issue of arboreality. In Origine(s) de la bipédie chez les hominidés (eds Senut B, Coppens Y), pp. 169–176. Paris: CNRS. [Google Scholar]
- 96.Brown P, Sutikna T, Morwood MJ, Soejono RP, Jatmiko, Wayhu Saptomo E, Awe Due R. 2004. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature 431, 1055–1061. ( 10.1038/nature02999) [DOI] [PubMed] [Google Scholar]
- 97.Moore MW, Sutikna T, Morwood MJ, Brumm A. 2009. Continuities in stone flaking technology at Liang Bua, Flores, Indonesia. J. Hum. Evol. 57, 503–526. ( 10.1016/j.jhevol.2008.10.006) [DOI] [PubMed] [Google Scholar]
- 98.Orr CM, Tocheri MW, Burnett SE, Awe Due R, Wahyu Saptomo E, Sutikna T, Wasisto S, Morwood MJ, Jungers WL. 2013. New wrist bones of Homo floresiensis from Liang Bua (Flores, Indonesia). J. Hum. Evol. 64, 109–129. ( 10.1016/j.jhevol.2012.10.003) [DOI] [PubMed] [Google Scholar]
- 99.Tocheri MW, Orr CM, Larson SG, Sutikna T, Wahyu Saptomo E, Awe Due R, Djubiantono T, Morwood MJ, Jungers WL. 2007. The primitive wrist of Homo floresiensis and its implications for hominin evolution. Science 317, 1743–1745. ( 10.1126/science.1147143) [DOI] [PubMed] [Google Scholar]
- 100.Larson SG, Jungers WL, Tocheri MW, Orr CM, Morwood MJ, Sutikna T, Awe Due R, Djubiantono T. 2009. Descriptions of the upper limb skeleton of Homo floresiensis. J. Hum. Evol. 57, 555–570. ( 10.1016/j.jhevol.2008.06.007) [DOI] [PubMed] [Google Scholar]
- 101.Brumm A, Aziz F, van den Bergh GD, Morwood MJ, Moore MW, Kurniawan I, Hobbs DR, Fullagar R. 2006. Early stone technology on Flores and its implications for Homo floresiensis. Nature 441, 624–628. ( 10.1038/nature04618) [DOI] [PubMed] [Google Scholar]
- 102.Alemseged Z, Spoor F, Kimbel WH, Bobe R, Geraads D, Reed D, Wynn JG. 2006. A juvenile early hominin skeleton from Dikika, Ethiopia. Nature 443, 296–301. ( 10.1038/nature05047) [DOI] [PubMed] [Google Scholar]
- 103.Domínguez-Rodrigo M, Pickering TP, Bunn HT. 2011. Reply to McPherron et al.: Doubting Dikika is about data, not paradigms. Proc. Natl Acad. Sci. USA 108, e117. ( 10.1073/pnas.1104647108) [DOI] [Google Scholar]
- 104.McPherron SP, Alemseged Z, Marean CW, Wynn JG, Reed D, Geraads D, Bobe R, Béarat HA. 2011. Tool-marked bones from before the Oldowan change the paradigm. Proc. Natl Acad. Sci. USA 108, e116. ( 10.1073/pnas.1101298108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Villmoare B, Kimbel WH, Seyoum C, Campisano CJ, DiMaggio EN, Rowan J, Braun DR, Arrowsmith JR, Reed KE. 2015. Early Homo at 2.8 Ma from Ledi-Geraru, Afar, Ethiopia. Science 347, 1352–1355. ( 10.1126/science.aaa1343) [DOI] [PubMed] [Google Scholar]
- 106.de la Torre I. 2010. Insights on the technical competence of the early Oldowan. In Stone tools and the evolution of human cognition (eds Nowell A, Davidson I), pp. 45–65. Boulder, CO: University Press of Colorado. [Google Scholar]
- 107.Bril B, Parry R, Dietrich G. 2015. How similar are nut-cracking and stone-flaking? A functional approach to percussive technology. Phil. Trans. R. Soc. B 370, 20140355 ( 10.1098/rstb.2014.0355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feix T, Kivell TL, Pouydebat E, Dollar AM. 2015. Estimating thumb-index finger precision grip and manipulation potential in extant and fossil primates. J. R. Soc. Interface 12, 20150176 ( 10.1098/rsif.2015.0176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kivell TL, Skinner MM, Lazenby R, Hublin J-J. 2011. Methodological considerations for analyzing trabecular architecture: an example from the primate hand. J. Anat. 218, 209–225. ( 10.1111/j.1469-7580.2010.01314.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsegai ZJ, Kivell TL, Gross T, Nguyen NH, Pahr DH, Smaers JB, Skinner MM. 2013. Trabecular bone structure correlates with hand posture and use in hominoids. PLoS ONE 8, e78781. ( 10.1371/journal.pone.0078781) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruff C, Holt B, Trinkaus E. 2006. Who's afraid of the big bad Wolff? ‘Wolff's law’ and bone functional adaptation. Am. J. Phys. Anthropol. 129, 484–498. ( 10.1002/ajpa.20371) [DOI] [PubMed] [Google Scholar]
- 112.Biewener AA, Fazzalari NL, Konieczynski DD, Baudinette RV. 1996. Adaptive changes in trabecular architecture in relation to functional strain patterns and disuse. Bone 19, 1–8. ( 10.1016/8756-3282(96)00116-0) [DOI] [PubMed] [Google Scholar]
- 113.Barak MM, Lieberman DE, Hublin J-J. 2011. A Wolff in sheep's clothing: trabecular bone adaptation in response to changes in joint loading orientation. Bone 49, 1141–1151. ( 10.1016/j.bone.2011.08.020) [DOI] [PubMed] [Google Scholar]
- 114.Skinner MM, Stephens NB, Tsegai ZJ, Foote AC, Nguyen NH, Gross T, Pahr DH, Hublin J-J, Kivell TL. 2015. Response to comment on ‘Human-like hand use in Australopithecus africanus’. Science 348, 1101b. ( 10.1126/science.aaa8931) [DOI] [PubMed] [Google Scholar]
- 115.Darwin CR. 1871. The descent of man, and selection in relation to sex. London, UK: John Murray. [Google Scholar]
- 116.Washburn SL. 1967. Behavior and the origin of man. Proc. Royal Anthropol. Inst. 1967, 21–27. [Google Scholar]
- 117.Richmond BG, Begun DR, Strait DS. 2001. Origin of human bipedalism: the knuckle-walking hypothesis revisited. Am. J. Phys. Anthropol. 116), 70–105. ( 10.1002/ajpa.10019) [DOI] [PubMed] [Google Scholar]
- 118.Lovejoy CO. 2009. Reexamining human origins in light of Ardipithecus ramidus. Science 326, 74e1–74e8. [PubMed] [Google Scholar]
- 119.Stout D. 2011. Stone tool-making and the evolution of human culture and cognition. Phil. Trans. R. Soc. B 336, 1050–1059. ( 10.1098/rstb.2010.0369) [DOI] [PMC free article] [PubMed] [Google Scholar]