Abstract
Increasing atmospheric CO2 levels are driving changes in the seawater carbonate system, resulting in higher pCO2 and reduced pH (ocean acidification). Many studies on marine organisms have focused on short-term physiological responses to increased pCO2, and few on slow-growing polar organisms with a relative low adaptation potential. In order to recognize the consequences of climate change in biological systems, acclimation and adaptation to new environments are crucial to address. In this study, physiological responses to long-term acclimation (194 days, approx. 60 asexual generations) of three pCO2 levels (280, 390 and 960 µatm) were investigated in the psychrophilic sea ice diatom Nitzschia lecointei. After 147 days, a small reduction in growth was detected at 960 µatm pCO2. Previous short-term experiments have failed to detect altered growth in N. lecointei at high pCO2, which illustrates the importance of experimental duration in studies of climate change. In addition, carbon metabolism was significantly affected by the long-term treatments, resulting in higher cellular release of dissolved organic carbon (DOC). In turn, the release of labile organic carbon stimulated bacterial productivity in this system. We conclude that long-term acclimation to ocean acidification is important for N. lecointei and that carbon overconsumption and DOC exudation may increase in a high-CO2 world.
Keywords: climate change, polar, algae, primary production, bacterial production, Southern Ocean
1. Introduction
The projected increase of atmospheric pCO2 from present day (approx. 400 µatm) to the end of the century (approx. 1000 µatm) will reduce the pH of the oceans by approximately 0.3 units [1]. High-latitude marine environments and ecosystems are particularly susceptible to ocean acidification as the solubility of CO2 is higher in cold water [2,3]. The Southern Ocean has a naturally low carbonate saturation state and is believed to be one of the first oceans to become persistently undersaturated with respect to aragonite [3,4]. The ongoing ocean acidification is believed to affect organisms on all systematic levels [5], and especially in polar ecosystems [2].
Due to the low affinity of CO2 to ribulose bisphosphate carboxylase/oxygenase (RuBisCO), ocean acidification has been suggested to stimulate primary productivity [6,7]. Antarctic sea ice algae are known to have widespread range of carbon concentrating mechanisms (CCMs) [8] that facilitate carbon fixation [9]. Furthermore, up to 40% of marine phytoplankton primary production is generally released as dissolved organic carbon (DOC) [10], although values over 70% have been observed [11]. Sea ice algae are known to excrete high amounts of extracellular polymeric substances (EPS) that constitute a substantial fraction of the DOC pool in sea ice [12,13]. EPS are produced as a normal cell function and used for motility, aggregation, desiccation, cryoprotection and protection against ultraviolet radiation [13–15]. The release of DOC is an important nutrient source for heterotrophic bacteria and may therefore affect microbial ecology and biogeochemistry [16].
Sea ice is one of the most productive polar ecosystems, acting both by seeding pelagic phytoplankton blooms and as an important direct food source for higher trophic levels [17]. In addition, sea ice algae and bacteria are important in carbon biogeochemistry in polar areas. For instance, ice algae may contribute up to 25% of the annual primary production of ice-covered waters [18] and provide an important food source for grazers, such as krill [19]. In addition, approximately 20–30% of the primary production in sea ice is cycled through heterotrophic bacteria [20]. Diatoms are major primary producers in sea ice and play an important role in the sea ice microbial ecosystem [18], and could be sensitive to environmental change considering that they already grow in a stressful environment. Short-term responses in sea ice algae to increased pCO2 are highly variable, ranging from negative [21,22] to positive effects [9,22]. Results may also depend on temperature conditions [9] and manipulation technique [22]. Generally, effect sizes from ocean acidification experiments are quite small for sea ice algae. However, the potential of acclimation may be especially important in slow-growing organisms as short-term experiments may fail to address proper acclimation to high CO2.
Many recent studies that investigated physiological effects of ocean acidification on phytoplankton have been performed on a short-term basis, normally ranging from 7 to 15 days [9,21–23]. More specifically, short-term changes in pCO2 affect growth, fatty acid concentration and species competition of Antarctic microalgae [9,22,24,25]. Still, no long-term study (longer than one month) has previously been published on polar microalgae. In phytoplankton from other regions, long-term acclimation and adaptation have shown to affect community structure, growth, fatty acid and amino acid concentrations [26–28]. However, physiological responses to increased pCO2 are both strain- and species-specific [24,26,27,29,30]. In order to understand the impact of climate change, more long-term studies need to be performed to address the potential for acclimation and adaptation to ocean acidification. The aims of this study were to investigate growth and productivity in the Antarctic sea ice diatom Nitzschia lecointei van Heurck 1909 and its associated heterotrophic bacteria during long-term acclimation to different pCO2. Cells were maintained in semi-continuous cultures at three different CO2 levels (280, 390 and 960 µatm pCO2) over a period of 194 days, and sampled regularly throughout the experiment.
2. Material and methods
(a). Long-term acclimation set-up
The diatom N. lecointei was isolated from sea ice collected in the Amundsen Sea in January 2011 and was cultured at –1.8°C for 18 months before the experiment started. The diatoms were inoculated (3 × 106 cells l−1) under non-axenic conditions in f/2 medium [31], prepared from 0.2 µm filtered seawater with a salinity of 33. The culture was subsequently split into 15 identical, 1 l borosilicate flasks, and randomly placed (n = 5 per CO2 treatment) in a cooling water bath set at –1.8°C. The pCO2 manipulation and agitation in each flask were performed by constant bubbling (10–15 ml min−1 flask−1) of synthetic air (Air Liquide, Malmö, Sweden) with different pCO2 (280, 390 and 960 µatm), using a system similar to that in Torstensson et al. [9]. The experiment was conducted under a 16 L : 8 D cycle at 30 µmol photons m−2 s−1 of photosynthetic active radiation (PAR 400–700 nm), provided by fluorescent tubes (Osram Lumilux Cool Daylight L36 W/865). Temperature was monitored throughout the experiment using HOBO Pendant data loggers (Onset Computer Corp., Pocasset, MA, USA). The long-term treatment lasted for 194 days and repeated sampling was conducted throughout the experiment. The cultures were maintained in active growth by semi-continuous dilution to a desired cell concentration (between 1 and 6 × 107 cells l−1, electronic supplementary material, figure S1) with pre-equilibrated f/2 medium from respective CO2-treatment. Dilutions were based on daily cell concentration in each flask, calculated and performed individually by carefully weighing (±0.1 g) the removed culture and fresh medium. The positions of the flasks were rotated on a weekly basis to ensure equal radiation exposure. To reduce diatom and bacterial wall growth, the culture flasks were replaced with new, autoclaved and acid-washed flasks once a month (day 32, 62, 91, 125 and 159).
(b). Switched treatment assay
After the long-term acclimation, the treatments were switched during a short-term assay to investigate potential sustained long-term effects. Four flasks from the ambient (390 µatm) and four from the high pCO2 treatments (960 µatm) were chosen randomly and retained for the short-term assay. Cell suspensions from each flask were inoculated in two new flasks with f/2 medium. One of the two new flasks was then assayed under the same conditions as the long-term treatment (control), whereas the second flask was switched to the opposite condition (electronic supplementary material, figure S2), creating four assay treatments with four samples in each group. The short-term assay lasted for 13 days.
(c). Cell density and growth rate
Diatom cell density was counted in live samples using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA) prior to dilution. For flow estimation, CountBright absolute counting beads (Invitrogen, Eugene, OR, USA) were added to each sample as an internal standard. Samples were analysed until a minimum of 300 cells were counted (minimum 1000 cells from day 32 and forward). The specific growth rate (μ, d−1) and number of asexual generations (n, number of times the cell population doubles during the time interval) generated was calculated using equations (2.1) and (2.2):
| 2.1 |
and
| 2.2 |
where Dx is the cell concentration at day x (before dilution), Dy is the cell concentration the previous day of measurement (after dilution), tx is the time in days at day x (before dilution) and ty is the time in days of the previous measurement (after dilution). Accumulation of generations for each day was subsequently used to estimate cumulative growth over time.
(d). Primary productivity
Primary productivity was measured using the radiocarbon technique. Ten millilitres of sample was incubated for 1 h with 3–4 µCi 14C-sodium bicarbonate (PerkinElmer, Waltham, MA, USA) at experimental irradiance and temperature. A blank from each treatment was incubated in darkness to estimate dark uptake of 14C. After incubation, formaldehyde (2%, final concentration) was added to all samples to stop carbon uptake. At day 147 and 187, primary productivity was separated into primary productivity of dissolved organic carbon (PPDOC) and particulate organic carbon (PPPOC) by gentle, low-pressure filtration on Whatman GF/F filters. Two drops of 1 M HCl were added to the liquid samples (PPDOC) and the samples were bubbled with N2 for 1 h to remove remaining dissolved inorganic carbon (DIC). Insta-Gel Plus scintillation cocktail (PerkinElmer) was added at a 1 : 1 ratio to the liquid samples and vials were shaken rigorously. The filters (PPPOC) were fumigated overnight after addition of 200 μl of 1 M HCl. Five millilitres of scintillation cocktail was added to the filters and samples were analysed in a liquid scintillation counter (Packard Tri-Carb 2900TR Liquid Scintillation Analyzer). Carbon uptake was corrected for dark uptake and calculated using equation (2.3):
| 2.3 |
where 12C is the carbon uptake rate (mg C l−1 h−1), 14Cs the radioactive activity (disintegrations per minute, DPM) in the sample, 12Ca the available DIC (mg l−1), 1.06 is the constant adjusting the fixation rate due to the discrimination of 14C during carbon fixation, 14Ca is the added activity of 14C to the sample (DPM) and t is the incubation time (h).
(e). Bacterial abundance and productivity
In total, 1.5 ml of sample was fixed in 1% glutaraldehyde (final concentration) and stored at –80°C until analysis. Samples were stained with SYBR Green I Nucleic Acid Gel Stain (Invitrogen) for 10 min in darkness. Bacterial abundances were analysed using a FACSCalibur flow cytometer (BD Biosciences) using CountBright absolute counting beads (Invitrogen) as the internal standard in each sample.
Bacterial productivity was measured according to Fuhrman & Azam [32]. In total, 1.7 ml of sample was incubated in darkness at –1.8°C with 3H-thymidine (100 nM final concentration, PerkinElmer) for 1 h. An additional sample from each treatment was used as a blank during the incubation, in which trichloroacetic acid (TCA, 5% final concentration) was added prior to the addition of 3H-thymidine. To stop the incubations, TCA (5% final concentration) was added to all samples. Samples were centrifuged for 10 min at 21 000g and 4°C, and the supernatant was carefully aspirated. The pellets were rinsed with 5% TCA on ice and subsequently with 80% ethanol on ice. After rinsing, 0.5 ml of scintillation cocktail (Insta-Gel Plus, PerkinElmer) was added to the pellets. The 3H-thymidine uptake was measured in a liquid scintillation counter (Packard Tri-Carb 2900TR Liquid Scintillation Analyzer), and converted into bacterial carbon productivity (BCP) by using 1.63 × 1018 cells mole−1 incorporated thymidine [33] and 20 fg C cell−1 [34]. The isotope concentrations were at satisfying levels according to saturation curves.
(f). Particulate organic carbon and nitrogen
A well-mixed sample (30–230 ml) was filtered onto pre-combusted (3 h at 450°C) Whatman GF/F filters. Samples were stored at –20°C until further analysis, subsequently dried at 60°C over a period of 48 h and the dry weight was measured. The filters were ground for 1 min using a Retsch MM301 ball mill (frequency 30 s−1). Analysis of particulate organic carbon (POC) and nitrogen (PON) was performed with an EA 1108 CHNS-O elemental analyser (Fisons Instruments, Milano, Italy), using 2,5-bis(5-tert-butyl-bensoaxzol-2-yl)thiophen as the internal standard (provided by Säntis Analytical AG, Teufen, Switzerland).
(g). Dissolved inorganic nitrogen, phosphorus and silica
Dissolved inorganic nutrients (NO2− + NO3−, PO43− and Si(OH)4) were sampled to confirm that nutrients were not limiting growth. Samples were filtered (0.2 µm) and stored at –20°C until colorimetric analyses [35] were performed at the Sven Lovén Centre for Marine Sciences, Kristineberg, Sweden.
(h). Dissolved inorganic carbon system
pH was measured spectrophotometrically on the total scale (pHT), using m-cresol purple as an indicator [36]. After tempering at 25°C, the samples were analysed in a Shimadzu UV-1800 spectrophotometer (measured in the visible spectrum). The indicator was prepared from 0.2 µm-filtered seawater (salinity 33) and stored in an E.V.A. gastight container (Baxter, Mississauga, Canada) to avoid atmospheric perturbation of the indicator during the experiment.
Samples for total alkalinity (AT) were stored in darkness overnight at 7°C. Prior to analysis, samples were tempered to 25°C and AT and were measured potentiometrically in an open cell using an automatic titration system (Metrohm 888 Titrando, Metrohm Aquatrode Plus Pt1000). Each sample was titrated with 0.05 M HCl and the equivalence point was evaluated using the Gran function [37]. The accuracy for AT data was checked and calibrated against analysed certified reference material (CRM), provided by Andrew G. Dickson, Scripps Institute of Oceanography, CA, USA. The CRM was titrated daily in triplicate with a variation of 0.5%.
Parameters of the carbonate system was calculated at in situ conditions (salinity, temperature and pressure) from measured values of pHT, AT, salinity and temperature using the program CO2SYS [38]. K1 and K2 constants used in calculations were determined by Mehrbach et al. [39] and refitted by Dickson & Millero [40]. The constant for SO4− was determined by Dickson [41].
(i). Statistical analyses
Individual and combined effects of CO2 treatment and sampling day were analysed with linear mixed effects (LME) models in R v. 3.0.1 [42], using the package nlme [43]. Models were fitted using restricted maximum likelihood using both time and CO2 concentration as fixed factors in a full model with interaction. The individual replicate flask was used as random factor in all analyses to account for pseudoreplication over time. Due to unsuccessful transformation of heterogeneous variances across residuals for cumulative growth data, modelling of the within-error group (per day) was performed to control for heteroscedasticity using the varIdent argument [43,44]. LME models are useful tools that can provide a better fit than generalized linear models (e.g. repeated measure ANOVA) on longitudinal data, especially when dealing with missing data and unequal spacing over time [45]. Multiple comparisons of means were performed on significant effects using generalized linear hypothesis test (glht) and Tukey's (HSD) test in the multcomp package [46]. Interaction in growth data was explored by contrasts defined to compare means between CO2 levels for each day, and Bonferroni correction was applied to account for multiple testing. Statistical significance was determined using a probability level of α < 0.05.
3. Results
(a). Growth rate
During the 194 days of experiment, 55–62 asexual generations of N. lecointei were accumulated, and the accumulation of generations was used to estimate cumulative growth over time. There was a significant interaction between pCO2 treatments and sampling day on accumulated generations (p < 0.0001, LME). Accumulation of generations decreased in the 960 µatm pCO2 treatment relative to the 280 and 390 µatm pCO2 treatments (figure 1). The deviation in accumulated generations from the 280 and 390 µatm treatments started at day 147 (after 40 accumulated generations), and persisted throughout the experiment (p < 0.05, Tukey's test). However, analysis of the specific growth rate did not detect a significant interaction between sampling day and pCO2 (electronic supplementary material, figure S3a, p = 0.084, LME), although the main effects of pCO2 and sampling day were significant (p = 0.038 and p < 0.0001, respectively, LME). The specific growth rate was lower at 960 µatm compared with 280 and 390 µatm pCO2 (p = 0.017 and p = 0.022, respectively, Tukey's test).
Figure 1.
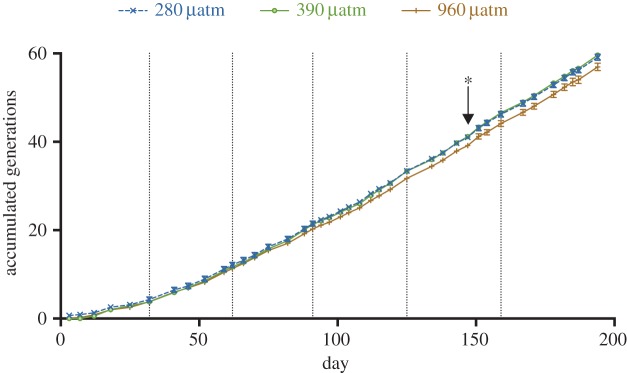
Accumulation of cell generations (cumulative growth) of N. lecointei in three pCO2 treatments over a period of 194 days. Accumulation of generations at 960 µatm was significantly lower compared with 280 and 390 µatm pCO2 at day 147*. Dotted vertical lines show the days where the culture flasks were acid-washed and autoclaved. Error bars represent standard error (n = 5). (Online version in colour.)
(b). Primary productivity
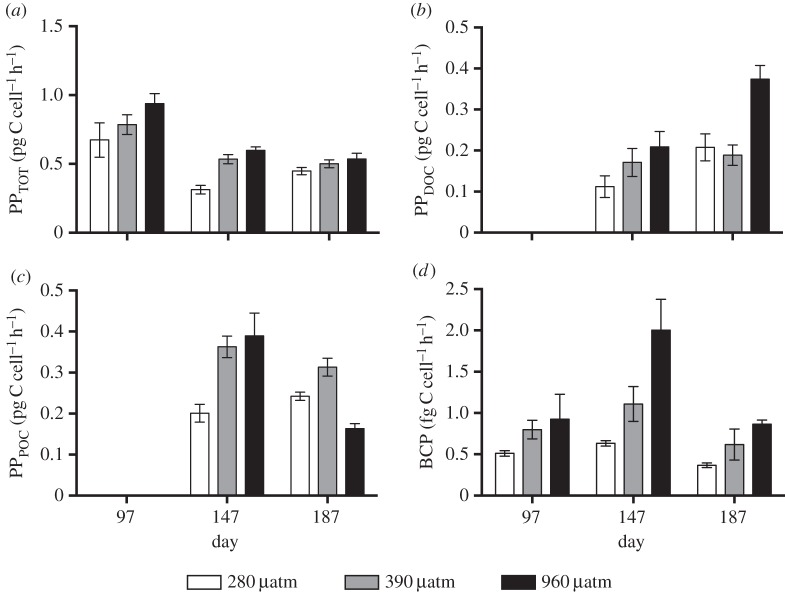
Total primary productivity (PPTOT) was significantly affected by CO2 treatment and sampling day, respectively (figure 2a, p = 0.004 and p < 0.0001, respectively, LME). A significant difference in PPTOT between the 280 µatm and the 960 µatm treatments was detected (p < 0.01, Tukey's test). In addition, the 280 µatm treatment was significantly lower than the 390 µatm level (p = 0.03, Tukey's test). Specific PPTOT was highest at day 97 and decreased significantly at the following two sampling days (p < 0.01, Tukey's test).
Figure 2.
Carbon production rates of N. lecointei cultures during long-term exposure of three pCO2 levels (280, 390 and 960 µatm). (a) Total primary productivity (PPTOT). (b) Dissolved organic carbon primary productivity (PPDOC). (c) Particulate organic carbon primary productivity (PPPOC) and (d) bacterial carbon productivity (BCP). All values are normalized to cell count and error bars represent standard error (n = 5).
When separating PPTOT into PPDOC and PPPOC at day 147 and 187, various effects of treatments and sampling day were found. Significant effects of pCO2 treatment and sampling day were detected on PPDOC (figure 2b, p = 0.006 and p = 0.01, respectively, LME). PPDOC in the 960 µatm pCO2 treatment was significantly higher than the other two treatments (p < 0.02, Tukey's test). In addition, a significant increase in PPDOC was detected from the first to the second sampling day (p < 0.02, Tukey's test).
There was a significant interaction of pCO2 treatment and sampling day on PPPOC (figure 2c, p = 0.003, LME). At day 147, PPPOC was significantly lower at 280 µatm compared with 390 and 960 µatm pCO2 (p = 0.001 and p < 0.001, respectively, Tukey's test). However, at day 187, PPPOC was reduced at 960 µatm compared to 390 µatm pCO2 (p = 0.004, Tukey's test).
(c). Bacterial abundance and productivity
Bacterial cell concentrations ranged between 0.3 and 8.1 × 106 cells ml−1 during the semi-continuous culturing of N. lecointei (electronic supplementary material, figure S3b). Cell-specific BCP was significantly affected by pCO2 treatment (p < 0.001, LME). Post hoc test indicated that all treatments differed and that bacterial productivity was stimulated by increased pCO2 (figure 2d, p < 0.02, Tukey's test). Heterotrophic bacteria consumed on average 5.6% of the daily DOC produced by N. lecointei in all treatments (figure 3).
Figure 3.
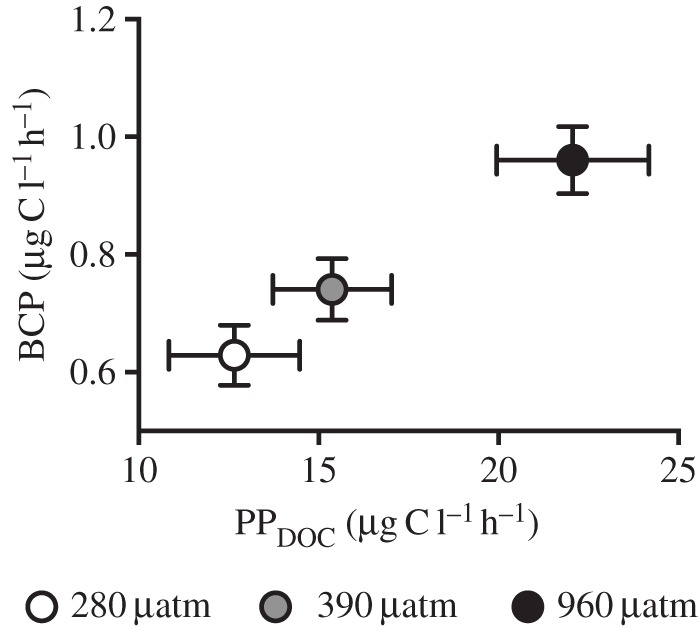
Relationship between volumetric bacterial production and primary productivity of dissolved organic carbon. Average primary productivity of DOC (PPDOC) and bacterial carbon productivity (BCP) are calculated from all data points during the experiment. Error bars represent standard error (n = 10).
(d). Particulate organic carbon and nitrogen
No difference in cellular POC between pCO2 treatments was detected (p > 0.05, LME), however, there was a difference over time. Cellular POC at day 18 was significantly higher compared with all other sampling occasions (figure 4a, p < 0.01, Tukey's test). In addition, there was no difference in cellular dry weight (250 ± 107 pg cell−1, average ± s.d. throughout the experiment) between the CO2 treatments (p > 0.05, LME), indicating that pCO2 treatment did not affect the cell size of N. lecointei.
Figure 4.
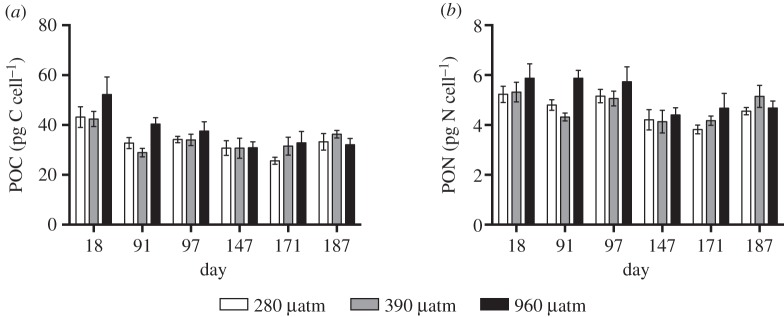
Particulate organic carbon (POC) and nitrogen (PON) of N. lecointei under the long-term acclimation to three pCO2 levels (280, 390 and 960 µatm). (a) Cellular POC values. (b) Cellular PON values. Error bars represent standard error (n = 5).
Cellular PON varied significantly throughout the long-term exposure (figure 4b, p < 0.0001, LME). PON values at day 147 and 171 were significantly lower than at day 18 and 97 (p < 0.002, Tukey's test). However, no difference between pCO2 treatments was detected (p > 0.05, LME). The PON of three samples were below the detection limit and thereby omitted from the analysis. CN ratios varied between 5.5 and 10.2 in all samples, with the highest levels at day 18. No difference in CN ratio between pCO2 treatments was detected (p > 0.05, LME).
(e). Sustained effect of dissolved organic carbon excretion after switched treatment assay
To determine the possibility of sustained effects from long-term exposure of elevated pCO2, long-term acclimated cells from the 390 and 960 µatm pCO2 treatments were assayed under switched pCO2 conditions in a 13-day short-term assay (electronic supplementary material, figure S2). Although PPDOC were higher compared with the long-term study, there was a significant interaction in the PPDOC fraction between long-term pCO2 treatments and assay treatments (figure 5, p = 0.048, LME). For diatoms acclimated to 960 µatm, the short-term assayed cells at 960 µatm had a higher proportion of PPDOC compared with cells assayed at 390 µatm pCO2 (figure 5). However, there was no difference between the assays for cells that were acclimated to 390 µatm pCO2. No differences between treatments were detected in PPTOT, PPPOC, POC, PON or BCP (p > 0.05, LME).
Figure 5.
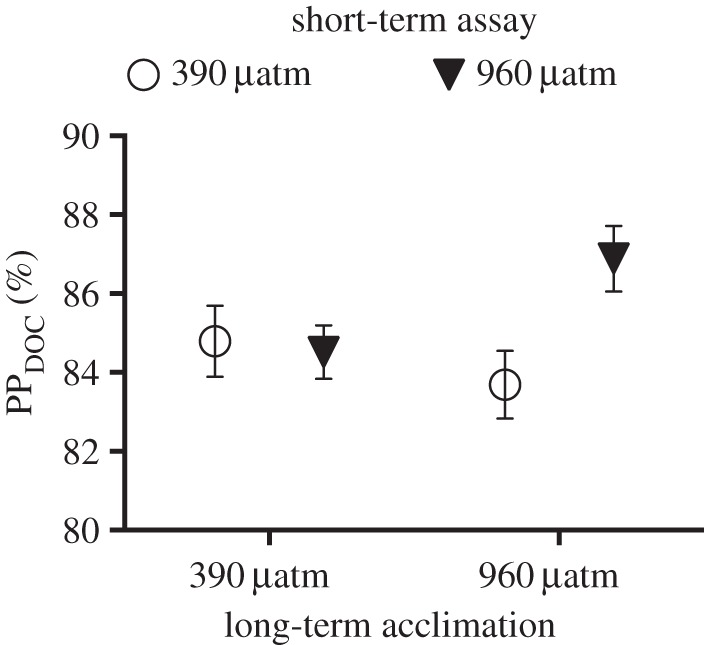
Percentage of primary productivity released as dissolved organic carbon (PPDOC) after the switched treatment assay. Cultures have been acclimated to 390 and 960 µatm pCO2 for 194 days, and subsequently short-term assayed for 13 days with the opposite treatment to study sustained effects of pCO2 acclimation. Error bars represent standard error (n = 4).
(f). Dissolved inorganic carbonate system
The pHT and pCO2 were significantly differentiated in the three pCO2 treatments (p < 0.001, LME), indicating that the carbonate system was successfully controlled by the pCO2 manipulations (electronic supplementary material, figure S4a,b). Values of the high pCO2 treatment were in the ranges of predicted atmospheric pCO2 levels by year 2100 [1]. However, high primary productivity resulted in slightly lower pCO2 than the anticipated levels (i.e. 280, 390 and 960 µatm pCO2, respectively). Inorganic nutrient concentrations did not indicate nutrient (NO2− + NO3−, PO43−, Si(OH)4) depletion during the study (electronic supplementary material, tables S1 and S2).
4. Discussion
In this study, we investigated long-term acclimation (194 days, approx. 60 generations) of the sea ice diatom N. lecointei to changes in pCO2. Acclimation and adaptation are crucial in understanding the consequences of ocean acidification, but no published studies have previously addressed long-term responses to increased pCO2 in slow-growing psychrophiles. We observed that long-term exposure to elevated pCO2 affected growth rate and carbon metabolism in N. lecointei.
In comparison with cultures exposed to pre-industrial (280 µatm) and ambient (390 µatm) pCO2, the accumulation of generations was slightly reduced at high pCO2 (960 µatm) after long-term acclimation. These results are complementary to a recent short-term study where no change in specific growth rate was detected in N. lecointei after 14 days exposure to 960 µatm pCO2 at −1.8°C, although increased pCO2 had a positive effect on growth at higher temperatures [9]. Another recent short-term study also concluded that natural sea ice algal communities could be very tolerant to changes in pCO2 and pH [22]. We would like to emphasize that reduction in growth was first observed after 147 days in this study. Hence, it is unlikely that small changes in growth, like the ones illustrated in this study, are detected during short experimental periods. Proper acclimation to different conditions may also influence interpretations of short-term experiments. Therefore, long-term studies are crucial for estimating potential consequences of climate change on algal physiology. Increasing CO2 levels (from approx. 400 to approx. 1000 µatm) seem to have rather limited effects on growth of sea ice associated diatoms, ranging from 0 to 5% ([9,21], this study), but can have more significant effects at higher pCO2 levels (more than 1700 µatm) [22]. Cumulative growth has the advantage of including small changes that do not occur simultaneously in all replicates, which is not unlikely over long experimental durations and could explain why there was no interaction in specific growth rate. Although cumulation is more sensitive towards systematic errors at specific time points, analysis of the specific growth rate suggests that there were no treatment-specific biases at specific sampling days (no interaction). In terms of climate change within the next century, other factors than increased CO2, such as increased temperature [9,47] and sea ice thinning [48], will most likely play a more important ecological role in polar ecosystems. Synergisms between combined environmental stressors may also alleviate effects [9] and are important to consider. However, our study still illustrates the importance of experimental duration in ocean acidification studies, as acclimation may delay certain responses. In addition, changes in algal carbon metabolism (i.e. increased DOC release) may have greater ecological and biogeochemical consequences than a 3–4% reduction of growth.
Earlier long-term studies on marine and freshwater phytoplankton have shown varying growth responses to high pCO2. Schaum & Collins [30] cultured the chlorophyte Ostreococcus for 400 generations and observed high growth rates, although growth rate at high pCO2 was not suppressed until 100 cell cycles. In contrast, Low-Décarie et al. [49] reported increasing growth rates after less than 340 generations (184 days) of high pCO2 conditioning in various freshwater species of diatoms, chlorophytes and cyanobacteria. In addition, no difference in growth was reported after 1000 generations in the chlorophyte Chlamydomonas reinhardtii grown at ambient and high pCO2 [50]. Furthermore, high pCO2 did not promote growth after long-term conditioning of six diatom genera [51], nor various dinoflagellates [27]. In contrast, to other long-term studies, there were considerably fewer cell cycles in our experiment (approx. 60 generations) due to the natural slow growth rate of ice algae. Hence, the potential for biological adaptation is less plausible in our experiment. In contrast, slow-growing organisms will also accumulate less cell cycles within the next century, and acclimation to climate change will be an important factor in determining polar species' responses to climate change.
Species [51] and strain-specific [26,29] responses to elevated pCO2 are surprisingly not occurring and this is probably related to the alga's carbon metabolism and CCM efficiency. In our study, carbon metabolism was altered both during the long-term exposure, and also during the switched treatment assay. For instance, PPDOC was promoted by elevated pCO2 during the long-term acclimation. This may be due to a response referred to as carbon overconsumption, i.e. an increase in carbon relative to nitrogen and phosphorus assimilation as compared to the Redfield ratio of 106C : 16N : 1P [52]. This imbalance generally occurs when nutrients are depleted and often results in increased DOC leakage from the cells. Carbon overconsumption may also occur as a response to increased pCO2 [7,53]. Since nutrient levels were kept replete in this study, the increased DOC excretion is most likely a direct effect of elevated DIC levels. Hence, an imbalance in the C : N : P ratio can have significant effects on the carbon metabolism in N. lecointei. However, it is also important to consider that nutrient levels are much higher in this study than expected in natural samples. Thus, care should be taken when interpreting results from laboratory studies.
In this study, release of DOC was generally high compared with phytoplankton communities. For instance, Engel et al. [53] reported on average 21–23% PPDOC of PPTOT in mesocosms with a natural Arctic phytoplankton community exposed to different pCO2 treatments. Generally, DOC release in natural marine phytoplankton communities does not exceed 40% of the total primary production [10]. Nevertheless, sea ice algae are known to exude high amounts of EPS [12], which is believed to play an important role in cryoprotection in microorganisms [14]. DOC release is also related to the state and age of the culture, and can exceed values measured in the field [54]. Hence, high levels of DOC release can be anticipated in cultured sea ice algae, growing in temperatures close to the freezing point of seawater [13]. As a result, the absolute values of DOC release are probably overestimated compared with natural environments. However, it is unlikely that the relative differences between the treatments are artefacts from culturing conditions.
Although the percentage of DOC release in the switched treatment assay was relatively high, it indicated that increased PPDOC was a sustained alteration of physiology caused by long-term exposure to high pCO2. On the other hand, this sustained effect after the assay treatment was only reflected in PPDOC, and not in growth. Although DOC excretion is a response of algal growth, DOC leakage does not promote growth. Increased cellular DOC excretion has also been correlated to combinations of environmental stressors [55] and carbon overconsumption [52,53]. Acclimation to high pCO2 may have affected the gene expression in N. lecointei, and needs to be further investigated. Sustained alteration of physiology as a result of long-term exposure of high pCO2 has been observed in other studies. Consistent with the results from this study, Lohbeck et al. [26] reported decreased growth rates in the haptophyte Emiliania huxleyi after less than 430 generations of high pCO2 conditions. It could be debated whether this should be considered a positive effect, as the overall growth rate decreased in comparison to E. huxleyi treated at ambient pCO2. However, high pCO2 treated multi-clone samples had higher growth rates compared to the controls when assayed at high pCO2 after 500 generations. In our study, we confirm sustained effects after considerably less cell cycles, suggesting that the response in N. lecointei is rather acclimation, and probably not adaptation. The number of generations is indeed important for adaption to a changing environment, and may be of special concern in slow-growing organisms such as psychrophilic algae.
The mechanisms behind potential stress of ocean acidification on microalgae are not yet fully understood. It is possible that ocean acidification affects the membrane potential in algae by altering intracellular pH [56] and can affect enzymatic processes and energy partitioning in the cells [57,58]. A 5% reduction in growth rate at high pCO2 has previously been reported in the Arctic sea ice diatom Navicula directa [21]. Increased release of DOC in N. lecointei could be interpreted as an indication of physiological stress. One plausible explanation for reduced growth rate and increased DOC exudation could be due to changes in enzyme activities. For instance, elevated CO2 levels have recently been described to affect the activity of carbonic anhydrase and nitrate reductase in the rhodophyte Corallina officinalis [58]. If enzymes related to the assimilation of inorganic nutrients are suppressed in N. lecointei, e.g. nitrate reductase [58], carbon overconsumption and reduced growth rate are expected to occur simultaneously. Although enzymes are highly influenced by pH, there are few published data on enzymatic processes in algae exposed to CO2 levels projected within this century. However, it has been shown that several extracellular hydrolytic enzymes in bacterial communities, including leucine aminopeptidase and lipase, are negatively affected by ocean acidification [59]. The effect of ocean acidification on different enzyme activities and pH homeostasis in algae may be important in algal physiology [56,58] and should be further investigated.
Sea ice algal communities live associated with bacteria in aggregations of EPS predominantly produced by diatoms [12]. Bacterial abundance and BCP in this study were comparable to natural environments [14,47]. In addition, bacterial activity was closely related to PPDOC, suggesting that bacterial growth was limited by labile organic carbon in the cultures. Hence, we assume that carbon overconsumption in N. lecointei nourished heterotrophic bacteria at high pCO2 and therefore had an indirect effect on bacterial productivity. Similar observations have been seen in natural Arctic spring bloom assemblages [53]. DOC release also plays an important role in carbon biogeochemistry. It promotes the microbial loop [16] and aggregation of cells [13], the latter possibly resulting in an enhanced biological carbon pump due to increased sinking speeds. Hence, the coupling between primary producers and heterotrophic bacteria may cause indirect effects of ocean acidification, and may be important to consider when interpreting experimental data. Other factors influencing these observations may be pH sensitive enzymes and bacterial community evolution. It has been shown that extracellular hydrolytic enzymes are negatively affected by ocean acidification [59], which could directly influence bacterial productivity. Still, this does not fully explain an increase in BCP at high pCO2 as we identified a positive effect of increased pCO2. There is also a potential for uncontrolled bacterial evolution and changes in bacterial community composition in our system as it also allows for bacterial growth. Although this is a very important aspect, this needs a different approach, as we do not fully know the origin and history of the bacteria in this study. Therefore, we choose not to discuss bacterial evolution in detail. Carbon dioxide induced changes in the bacterial community composition may, however, influence the feedback mechanisms of carbon metabolism in this study. On the other hand, effects of increased CO2 (up to approx. 1 050 µatm) in Arctic bacterioplankton communities have previously been shown to be rather small [60]. Therefore, we believe that increased BCP was most likely an indirect response of increased DOC release from N. lecointei.
Polar areas are believed to be particularly susceptible to climate change [2], and sea ice algae are a vital part of primary production in polar oceans [17]. This is the first published study that addresses long-term acclimation to elevated pCO2 in psychrophilic organisms and illustrates that long-term acclimation is important in determining mechanistic responses of diatoms to ocean acidification. We conclude that certain, small changes in growth can only be detected over longer periods of time (147 days in this study). Long-term acclimation to high pCO2 may also affect the carbon metabolism of N. lecointei, with increased rates of DOC release as an outcome. In turn, this promotes bacterial growth and productivity, important for carbon biogeochemistry and microbial ecology.
Supplementary Material
Data accessibility
Raw experimental data and R scripts from this work are published in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h838q.
Authors' Contributions
A.T., M.H., M.C. and A.W. designed the study. A.T., M.H. and M.M.B. performed the experiment. A.T. and M.H. analysed the data and wrote the manuscript. A.W., M.C. and M.M.B. contributed to the revision and approved the final version of the manuscript.
Competing Interests
The authors declare no competing interest.
Funding
This project received financial support from the Royal Society of Arts and Sciences in Gothenburg (KVVS), Ymer –80 Foundation, Adlerbertska Reseach Foundation and the Lars Hierta Memorial Foundation. This study is a contribution to the project ‘Greenhouse gases and mercury in a changing Arctic-GreMeCA,’ funded by the Swedish Research Council (no. 2007-8365) and the Swedish Research Council project (2009–2994, Oden Southern Ocean (OSO) 2010/2011).
References
- 1.IPCC. 2013. Climate change 2013: the physical science basis. In Contribution of working group I to the fifth assessment report of the Intergovernmental Panel on Climate Change (eds Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Bex V, Midgley PM), 1535 pp. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Doney SC, et al. 2012. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 4, 11–37. ( 10.1146/annurev-marine-041911-111611) [DOI] [PubMed] [Google Scholar]
- 3.Steinacher M, Joos F, Frölicher TL, Plattner G-K, Doney SC. 2009. Imminent ocean acidification in the Arctic projected with the NCAR global coupled carbon cycle–climate model. Biogeosciences 6, 1877–1882. ( 10.5194/bg-6-515-2009) [DOI] [Google Scholar]
- 4.Mattsdotter Björk M, Fransson A, Torstensson A, Chierici M. 2014. Ocean acidification state in western Antarctic surface waters: controls and interannual variability. Biogeosciences 11, 57–73. ( 10.5194/bg-11-57-2014) [DOI] [Google Scholar]
- 5.Kroeker KJ, Kordas RL, Crim R, Hendriks IE, Ramajo L, Singh GS, Duarte CM, Gattuso J-P. 2013. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 19, 1884–1896. ( 10.1111/gcb.12179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riebesell U, Wolf-Gladrow DA, Smetacek V. 1993. Carbon dioxide limitation of marine phytoplankton growth rates. Nature 361, 249–251. ( 10.1038/361249a0) [DOI] [Google Scholar]
- 7.Riebesell U, et al. 2007. Enhanced biological carbon consumption in a high CO2 ocean. Nature 450, 545–548. ( 10.1038/nature06267) [DOI] [PubMed] [Google Scholar]
- 8.Tortell PD, Mills MM, Payne CD, Maldonado MT, Chierici M, Fransson A, Alderkamp AC, Arrigo KR. 2013. Inorganic C utilization and C isotope fractionation by pelagic and sea ice algal assemblages along the Antarctic continental shelf. Mar. Ecol. Prog. Ser. 483, 47–66. ( 10.3354/meps10279) [DOI] [Google Scholar]
- 9.Torstensson A, Hedblom M, Andersson J, Andersson MX, Wulff A. 2013. Synergism between elevated pCO2 and temperature on the Antarctic sea ice diatom Nitzschia lecointei. Biogeosciences 10, 6391–6401. ( 10.5194/bg-10-6391-2013) [DOI] [Google Scholar]
- 10.Baines SB, Pace ML. 1991. The production of dissolved organic matter by phytoplankton and its importance to bacteria: patterns across marine and freshwater systems. Limnol. Oceanogr. 36, 1078–1090. ( 10.4319/lo.1991.36.6.1078) [DOI] [Google Scholar]
- 11.Choi CI. 1972. Primary producion and release of dissolved inorganic carbon from phytoplankton in the western North Atlantic Ocean. Deep Sea Res. Pt. I 19, 731–735. [Google Scholar]
- 12.Underwood GJC, Aslam SN, Michel C, Niemi A, Norman L, Meiners KM, Laybourn-Parry J, Patersong H, Thomas DN. 2013. Broad-scale predictability of carbohydrates and exopolymers in Antarctic and Arctic sea ice. Proc. Natl Acad. Sci. USA 110, 15 734–15 739. ( 10.1073/pnas.1302870110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewert M, Deming JW. 2013. Sea ice microorganisms: environmental constraints and extracellular responses. Biology 2, 603–628. ( 10.3390/biology2020603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deming JW. 2010. Sea ice bacteria and viruses. In Sea ice (eds Thomas DN, Dieckmann GS), pp. 247–282. Oxford, UK: Wiley-Blackwell. [Google Scholar]
- 15.Ortega-Retuerta E, Passow U, Duarte CM, Reche I. 2009. Effects of ultraviolet B radiation on (not so) transparent exopolymer particles. Biogeosciences 6, 3071–3080. ( 10.5194/bg-6-3071-2009) [DOI] [Google Scholar]
- 16.Azam F, Fenchel T, Field JG, Gray JS, Meyerreil LA, Thingstad F. 1983. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 10, 257–263. ( 10.3354/meps010257) [DOI] [Google Scholar]
- 17.Lizotte MP. 2001. The contributions of sea ice algae to Antarctic marine primary production. Am. Zool. 41, 57–73. ( 10.1668/0003-1569%282001%29041%5B0057%3ATCOSIA%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 18.Arrigo KR, Thomas DN. 2004. Large scale importance of sea ice biology in the Southern Ocean. Antarct. Sci. 16, 471–486. ( 10.1017/S0954102004002263) [DOI] [Google Scholar]
- 19.O'Brien DP. 1987. Direct observations of the behavior of Euphausia superba and Euphausia crystallorophias (Crustacea: Euphausiacea) under pack ice during the Antarctic Spring of 1985. J. Crust. Biol. 7, 437–448. ( 10.2307/1548293) [DOI] [Google Scholar]
- 20.Staley JT, Gosink JJ. 1999. Poles apart: biodiversity and biogeography of sea ice bacteria. Annu. Rev. Microbiol. 53, 189–215. ( 10.1146/annurev.micro.53.1.189) [DOI] [PubMed] [Google Scholar]
- 21.Torstensson A, Chierici M, Wulff A. 2012. The influence of increased temperature and carbon dioxide levels on the benthic/sea ice diatom Navicula directa. Polar Biol. 35, 205–214. ( 10.1007/s00300-011-1056-4) [DOI] [Google Scholar]
- 22.McMinn A, Müller MN, Martin A, Ryan KG. 2014. The response of Antarctic sea ice algae to changes in pH and CO2. PLoS ONE 9, e86984 ( 10.1371/journal.pone.0086984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boelen P, van de Poll WH, van der Strate HJ, Neven IA, Beardall J, Buma AGJ. 2011. Neither elevated nor reduced CO2 affects the photophysiological performance of the marine Antarctic diatom Chaetoceros brevis. J. Exp. Mar. Biol. Ecol. 406, 38–45. ( 10.1016/j.jembe.2011.06.012) [DOI] [Google Scholar]
- 24.Trimborn S, Brenneis T, Sweet E, Rost B. 2013. Sensitivity of Antarctic phytoplankton species to ocean acidification: growth, carbon acquisition, and species interaction. Limnol. Oceanogr. 58, 997–1007. ( 10.4319/lo.2013.58.3.0997) [DOI] [Google Scholar]
- 25.Wynn-Edwards C, King R, Davidson A, Wright S, Nichols PD, Wotherspoon S, Kawaguchi S, Virtue P. 2014. Species-specific variations in the nutritional quality of Southern Ocean phytoplankton in response to elevated pCO2. Water 6, 1840–1859. ( 10.3390/w6061840) [DOI] [Google Scholar]
- 26.Lohbeck KT, Riebesell U, Reusch TBH. 2012. Adaptive evolution of a key phytoplankton species to ocean acidification. Nat. Geosci. 5, 346–351. ( 10.1038/ngeo1441) [DOI] [Google Scholar]
- 27.Tatters AO, Schnetzer A, Fu F, Lie AAY, Caron DA, Hutchins DA. 2013. Short- versus long-term responses to changing CO2 in a costal dinoflagellate bloom: implications for interspecific competitive interactions and community structure. Evolution 67, 1879–1891. ( 10.1111/evo.12029) [DOI] [PubMed] [Google Scholar]
- 28.Bermúdez R et al. 2015. Long-term conditioning to elevated pCO2 and warming influences the fatty and amino acid composition of the diatom Cylindrotheca fusiformis. PLoS ONE 10, e0123945 ( 10.1371/journal.pone.0123945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kremp A, Godhe A, Egardt J, Dupont S, Suikkanen S, Casablanca S, Penna A. 2012. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecol. Evol. 2, 1195–1207. ( 10.1002/ece3.245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaum CE, Collins S. 2014. Plasticity predicts evolution in a marine alga. Proc. R. Soc. B 281, 20141486 ( 10.1098/rspb.2014.1486) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guillard RRL. 1975. Culture of phytoplankton for feeding marine invertebrates. In Culture of marine invertebrate animals (eds Smith WL, Chanley MH), pp. 29–60. New York, NY: Plenum. [Google Scholar]
- 32.Fuhrman JA, Azam F. 1982. Thymidine incorporation as a measure of heterotrophic bacterioplankton production in marine surface waters: evaluation and field results. Mar. Biol. 66, 109–120. ( 10.1007/BF00397184) [DOI] [Google Scholar]
- 33.Carlson CA, Ducklow HW, Sleeter TD. 1996. Stocks and dynamics of bacterioplankton in the Northwestern Sargasso Sea. Deep Sea Res. II 43, 491–515. ( 10.1016/0967-0645(95)00101-8) [DOI] [Google Scholar]
- 34.Lee S, Fuhrman JA. 1987. Relationship between biovolume and biomass of naturally derived marine bacterioplankton. Appl. Environ. Microbiol. 536, 1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grasshoff K, Kremling K, Ehrhardt M. 1999. Methods of seawater analysis, 3rd edn Weinheim, Germany: Wiley-VHC. [Google Scholar]
- 36.Clayton TD, Byrne RH. 1993. Spectrophotometric seawater pH measurements: total hydrogen ion concentration scale calibration of m-cresol purple and at-sea results. Deep Sea Res. I 40, 2115–2129. ( 10.1016/0967-0637(93)90048-8) [DOI] [Google Scholar]
- 37.Gran G. 1952. Determination of the equivalence point in potentiometric titrations. Part II. Analyst 77, 661–671. ( 10.1039/an9527700661) [DOI] [Google Scholar]
- 38.Pierrot D, Lewis E, Wallace DWR.2006. MS Excel program developed for CO2 system calculations. ORNL/CDIAC-105a. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, US. Department of Energy, Oak Ridge, TN (v. 1.02). See http://cdiac.ornl.gov/ftp/co2sys/CO2SYS_calc_XLS_v2.1/ .
- 39.Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. ( 10.4319/lo.1973.18.6.0897) [DOI] [Google Scholar]
- 40.Dickson AG, Millero FJ. 1987. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. I 34, 1733–1743. ( 10.1016/0198-0149(87)90021-5) [DOI] [Google Scholar]
- 41.Dickson AG. 1990. Standard potential of the reaction: AgCl(s)+OH2(g)=Ag(s)+HCl(aq), and the standard acidity constant of the ion HSO4− in synthetic seawater from 273.15 to 318.15 K. J. Chem. Thermodyn. 22, 113–127. ( 10.1016/0021-9614(90)90074-Z) [DOI] [Google Scholar]
- 42.R Core Team. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 43.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2014. nlme: Linear and nonlinear mixed effects models. R package v. 3.1–117. See http://CRAN.R-project.org/package=nlme.
- 44.Pinheiro J, Bates D. 2000. Mixed-effects models in S and S-PLUS. New York, NY: Springer. [Google Scholar]
- 45.Quinn GP, Keough MJ. 2002. Experimental design and data analysis for biologists. Cambridge, UK: Cambridge Universtiy Press. [Google Scholar]
- 46.Hothorn T, Bretz F, Westfall P. 2008. Simultaneous inference in general parametric models. Biom. J. 50, 346–363. ( 10.1002/bimj.200810425) [DOI] [PubMed] [Google Scholar]
- 47.Torstensson A, Dinasquet J, Chierici M, Fransson A, Riemann L, Wulff A. 2015. Physicochemical control of bacterial and protist community composition and diversity in Antarctic sea ice. Environ. Microbiol. ( 10.1111/1462-2920.12865) [DOI] [PubMed] [Google Scholar]
- 48.Boetius A, et al. 2013. Export of algal biomass from the melting Arctic sea ice. Science 339, 1430–1432. ( 10.1126/science.1231346) [DOI] [PubMed] [Google Scholar]
- 49.Low-Décarie E, Jewell MD, Fussmann GF, Bell G. 2013. Long-term culture at elevated atmospheric CO2 fails to evoke specific adaptation in seven freshwater phytoplankton species. Proc. R. Soc. B 280, 20122598 ( 10.1098/rspb.2012.2598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Collins S, Bell G. 2004. Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature 431, 566–569. ( 10.1038/nature02945) [DOI] [PubMed] [Google Scholar]
- 51.Tatters AO, Roleda MY, Schnetzer A, Fu F, Hurd CL, Boyd PW, Caron DA, Lie AAY, Hoffmann LJ. 2013. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Phil. Trans. R. Soc. B 368, 20120437 ( 10.1098/rstb.2012.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Toggweiler JR. 1993. Carbon overconsumption. Nature 363, 210–211. ( 10.1038/363210a0) [DOI] [Google Scholar]
- 53.Engel A, Borchard C, Piontek J, Schulz K, Riebesell U, Bellerby R. 2013. CO2 increases 14C-primary production in an Arctic plankton community. Biogeosciences 10, 1291–1308. ( 10.5194/bg-10-1291-2013) [DOI] [Google Scholar]
- 54.Wetz MS, Wheeler PA. 2007. Release of dissolved organic matter by coastal diatoms. Limnol. Oceanogr. 52, 798–807. ( 10.4319/lo.2007.52.2.0798) [DOI] [Google Scholar]
- 55.Gosselin M, Levasseur M, Wheeler PA, Horner RA, Booth BC. 1997. New measurements of phytoplankton and ice algal production in the Arctic ocean. Deep Sea Res. II 44, 1623–1644. ( 10.1016/S0967-0645(97)00054-4) [DOI] [Google Scholar]
- 56.Suffrian K, Schulz K, Gutowska M, Riebesell U, Bleich M. 2011. Cellular pH measurements in Emiliania huxleyi reveal pronounced membrane proton permeability. New Phytol. 190, 595–608. ( 10.1111/j.1469-8137.2010.03633.x) [DOI] [PubMed] [Google Scholar]
- 57.Beardall J, Raven JA. 2004. The potential effects of global climate change on microalgal photosynthesis, growth and ecology. Phycologia 43, 26–40. ( 10.2216/i0031-8884-43-1-26.1) [DOI] [Google Scholar]
- 58.Hofmann LC, Straub S, Bischof K. 2013. Elevated CO2 levels affect the activity of nitrate reductase and carbonic anhydrase in the calcifying rhodophyte Corallina officinalis. J. Exp. Bot. 64, 899–908. ( 10.1093/jxb/ers369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamada N, Suzumura M. 2010. Effects of seawater acidification on hydrolytic enzyme activities. J. Oceanogr. 66, 233–241. ( 10.1007/s10872-010-0021-0) [DOI] [Google Scholar]
- 60.Roy AS, et al. 2013. Ocean acidification shows negligible impacts on high-latitude bacterial community structure in coastal pelagic mesocosms. Biogeosciences 10, 555–566. ( 10.5194/bg-10-555-2013) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw experimental data and R scripts from this work are published in the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.h838q.