Abstract
According to the social decision-making (SDM) network hypothesis, SDM is encoded in a network of forebrain and midbrain structures in a distributed and dynamic fashion, such that the expression of a given social behaviour is better reflected by the overall profile of activation across the different loci rather than by the activity of a single node. This proposal has the implicit assumption that SDM relies on integration across brain regions, rather than on regional specialization. Here we tested the occurrence of functional localization and of functional connectivity in the SDM network. For this purpose we used zebrafish to map different social behaviour states into patterns of neuronal activity, as indicated by the expression of the immediate early genes c-fos and egr-1, across the SDM network. The results did not support functional localization, as some loci had similar patterns of activity associated with different social behaviour states, and showed socially driven changes in functional connectivity. Thus, this study provides functional support to the SDM network hypothesis and suggests that the neural context in which a given node of the network is operating (i.e. the state of its interconnected areas) is central to its functional relevance.
Keywords: social behaviour network, mesolimbic reward system, functional localization, functional connectivity, neural context, zebrafish
1. Introduction
Social decision-making (SDM) involves the integration of multimodal sensory information about social status and social context with previous experience in order to produce an appropriate behavioural response that is adjusted to the perceived social environment. Therefore, social decisions are expected to rely on multiple neural circuits, rather than being controlled by one specific brain region. In line with this argument, an evolutionarily conserved SDM network, composed of two interconnected neural circuits—the social behaviour network [1,2] and the mesolimbic reward circuit [3]—has been proposed to underlie the expression of social behaviour across vertebrates [4,5]. Social information would be encoded in this network of forebrain and midbrain nuclei with reciprocal connections in a distributed and dynamic fashion, such that the expression of a given social behaviour would be better reflected by the overall profile of activation across the different loci in the network rather than by the activity of a single node, and different combinations of activation across nodes and variation in the strength of the connections among them would generate an almost infinite variation in social behaviour [6]. Although the SDM network has been proposed on functional grounds, most of its current support is based on structural evidence, namely on the expression of genetic markers, hormone receptors and neurochemical/neurotransmitter systems that allow the establishment of homologies of its constitutive loci across taxa, as well as on patterns of reciprocal neuronal connections, that confirm the occurrence of structural (anatomical) connectivity among loci [4,7].
From a functional perspective, the establishment of the SDM network as a valid neurobiological construct requires the understanding of how social information is being mapped into the brain. Two hypotheses of brain function are currently available in systems neuroscience: (i) functional specialization, which proposes that different brain regions are engaged in different cognitive functions/behaviours; [8] and (ii) functional connectivity, which postulates that specific cognitive functions/behaviours are mediated by a diffuse network of interacting brain regions [9,10]. Therefore, when two experimental conditions are compared that differ in a specific cognitive function/behaviour, the former hypothesis predicts differences in activity level between the areas relevant for that specific task, whereas the latter hypothesis predicts changes in the covariance in activity levels between different brain areas relevant for the task. Although these two hypotheses have historically been seen as antagonistic, they are not necessarily mutually exclusive as the functional relevance of a specific brain region may depend on the functional state of their connecting areas (i.e. its neural context [11,12]). In the scope of the SDM network, this hypothesis would predict that each node of the network can participate in several social behaviours through its interactions with other nodes. Therefore, both changes in activity levels in specific nodes of the SDM network and changes in its functional connectivity can be predicted in relation to relevant social stimuli. However, the key hypothesis to be tested for the functional validation of the SDM network is the occurrence of flexible functional connectivity across the network.
So far most studies that have mapped social behaviour into patterns of brain activity have only implicitly addressed the functional localization hypothesis by documenting changes in the activity or expression of molecular markers of neuronal activity (e.g. cytochrome oxidase or immediate early genes, respectively) in specific network nodes (e.g. fish [13]; birds [14,15]; mammals [16]). In fact, only a few studies have so far established links between functional connectivity and the expression of social behaviour states. For example, it has been shown that leopard geckos (Eublepharis macularis), a species with temperature-dependent sex determination, incubated at either male- or female-biased temperatures develop more or less aggressive behaviour, which is paralleled by different patterns of functional connectivity across the SDM network [17]. Also, in male green anoles (Anolis carolinensis), exposure to video playbacks of displaying competitors elicited aggressive displays reflected in differentially connected neural networks [18]. Finally, male túngara frogs (Physalaemus pustulosus) exposed to the playback of relevant social calls also show changes in functional connectivity among hypothalamic nuclei [19]. However, these papers pre-date the SDM network proposal, and therefore only nodes from the social behaviour network or subsets of hypothalamic–lymbic nuclei have been considered. This means that the functional validation of the SDM network across vertebrates is still lacking.
In this study, we tested both the functional localization hypothesis and the functional connectivity hypothesis regarding the mapping of social behaviour into brain activity in the SDM network. For this purpose, we characterized the expression of two immediate early genes (c-fos and egr-1), as transient markers of neuronal activity [20,21], across selected nodes of the SDM network in male zebrafish in relation to the outcome of agonistic interactions. Zebrafish were used as a study model given their relevance as potential model organisms in social neuroscience [22]. Adult zebrafish are highly social, expressing a strong preference for shoaling with conspecifics [23–25]. However, despite this affiliative motivation, males also express aggressive behaviour when competing for resources [26,27]. Four social treatments were used: winners and losers of real-opponent interactions; mirror-fighters, which expressed agonistic behaviour towards their own image in the mirror but did not experience either a victory or a defeat; and non-interacting males, as a non-social reference group. In order to test the functional localization hypothesis, we tested for differences in immediate early gene (IEG) expression between each of the three social groups and the non-social reference group at each node of the SDM network. In order to test for the functional localization hypothesis, co-activation matrices (i.e. correlation matrices for the levels of IEG expression across the nodes of the network within each treatment) were compared across social treatments. The use of the mirror treatment was intended to help to discriminate between perceptual and motor influences in the pattern of activity of the SDM network. Fish do not recognize themselves on a mirror, and attack their own image as if it is an intruder [28]. In zebrafish, mirror fights elicit similar levels of aggressive behaviour to those observed in real-opponent fights [29]. However, as submissive behaviour is never expressed by the mirror image, no information on fight outcome is perceived. Thus, as mirror-fighters express a behaviour output similar to that of winners but perceive different responses in the opponent (i.e. submission in the case of the winner; aggression in the case of the mirror-fighter), shared patterns of SDM network between these two groups should reflect motor output, whereas differences should be due to differences in either perception or associative processing of social information.
2. Material and methods
(a). Fish housing
All subjects used in this experiment were adult wild-type (AB) zebrafish bred and held at Instituto Gulbenkian de Ciência (IGC, Oeiras, Portugal). Fish were kept at 28°C with a 14 L : 10 D photoperiod in a recirculating system (ZebraTec, 93 Tecniplast). Fish were fed twice a day, except on the day of the experiments.
(b). Social treatments
To create different social behaviour states we used a previously described short-term agonistic paradigm [27,29]. In brief, adult males were paired in dyads matched for body size (mean ± s.e.m.: 0.4 ± 0.015 g) and placed, as pairs, in an experimental arena (5 × 8 × 6 cm) divided in two halves by one or more removable opaque partition(s) (see below). Therefore, there were two fish per tank, one on each side of the partition, which were kept overnight in visual isolation. At the start of the experiment one or more of the partitions were removed, and the fish were allowed to interact for a period of 30 min. Three social treatments were used: (i) fighting a real-opponent conspecific, where there was a single opaque PVC partition separating the two fish, which was removed; (ii) fighting their own image on a mirror, where there were two mirrors placed back to back, each facing one of the compartments, behind opaque partitions (the partitions were removed to uncover the mirrors); and (iii) no agonistic interaction, where there were three central opaque partitions, and only the outer two were removed (to control for putative stress effects of handling partitions in the experimental tanks). These social treatments generated four social behaviour states: winners (W, n = 12) and losers (L, n = 13) of the real-opponent interaction; fighters of unresolved interactions (i.e. mirror-fighters, M, n = 11); and fish with no social interaction (i.e. visual isolation, I, n = 12). All animals were tested in pairs in order to give them access to conspecific odours, which would otherwise only be present in real-opponent dyads, therefore avoiding confounding effects of putative chemical cues. Behavioural interactions were video-recorded for subsequent behavioural analysis.
(c). Microdissection of regions of interest in the brain
Immediately after the interaction, fish were anaesthetized with an overdose of tricaine solution (MS222, Pharmaq; 500–1000 mg l−1) followed by rapid decapitation. Heads were embedded in mounting media (OCT Compound, Tissue-Tek, Sakura] and rapidly frozen on dry ice. Brains were sectioned in coronal plane at 150 µm on a cryostat (Leica CM 3050 S) and sections were collected onto regular glass slides previously cleaned with 70% ethanol. Regions of interest, identified using the zebrafish brain atlas [30], were then microdissected under a stereoscope (Zeiss Stemi 2000; see the electronic supplementary material for details). For logistical reasons we could not sample the 12 nuclei of the SDM network [4], hence we selected the following subset of five nuclei representative of the two sub-networks: the medial zone of the dorsal telencephalic area (Dm, putative homologue of the mammalian basolateral amygdala) and the lateral zone of the dorsal telencephalic area (Dl, putative homologue of the mammalian hippocampus), from the mesolimbic reward system; the preoptic area (POA) from the social behaviour network; and the ventral nucleus of the ventral telencephalic area (Vv, putative homologue of the mammalian lateral septum) and the supracommissural nucleus of the ventral telencephalic area (Vs, putative homologue of the mammalian medial extended amygdala and the bed nucleus of the stria terminalis), common to both sub-networks [4]. Tissue was collected directly into lysis buffer (RNeasy Lipid Tissue Mini Kit, Qiagen) and stored at −80°C until mRNA extraction.
(d). Gene expression analysis
Total RNA was isolated from brain nuclei using the RNeasy Lipid Tissue Mini Kit with some adjustments to the manufacturer's instructions (see the electronic supplementary material for details). RNA from each sample was then reverse transcribed to cDNA (iScript cDNA Synthesis Kit, Biorad) in accordance with manufacturer's instructions and diluted 1 : 10 before being used as a template for quantitative polymerase chain reactions (RT-PCR) of c-fos and egr-1, using the eukaryotic translation elongation factor 1 alpha 1, like 1 (eef1a1l1) as a reference gene (see the electronic supplementary material for details, especially table S1 for primer sequences). Fluorescence cycle thresholds (CT) were automatically measured (Biosystems 7900HT Fast thermocycler) and the relative expression of the target genes calculated using the 2−ΔCt method [31].
(e). Behavioural observations
Behavioural analysis was conducted using a computerized multi-event recorder (Observer XT, Noldus, Wageningen, The Netherlands), and the zebrafish aggressive behaviour ethogram [27] was used as a reference to identify both aggressive (bite, chase and strike) and submissive (freeze and flee) behaviours. As we were only interested in the behavioural outputs resultant from the different social treatments, and not in the interaction per se, we only analysed the post-resolution phase of the fight (last 5 min of the 30 min interaction), when the different social behaviour states (i.e. winners, losers, mirror-fighters and isolation) can be easily identified.
(f). Statistical analysis
The effect of the relevant social contexts (i.e. mirror-fighters versus winners) in aggressive behaviours was assessed using a t-test.
The overall effects of social behaviour state (winners, losers, mirror-fighters and isolation) and brain nuclei (Dm, Dl, Vv, Vs, POA) in c-fos and egr-1 expression were assessed using linear mixed models (LMMs). As the data for winners and losers come from the same interaction, it cannot be considered independent, and a within-pair design is needed to compare these two social behaviour states [32]. On the other hand, the other two behavioural states (i.e. isolation and mirror-fighters) did not have an opponent, and thus a between-subject design is appropriate. In order to incorporate these two perspectives in the LMM analysis, two random effects were used: one for the subjects and another for the winner–loser dyads. Parametric assumptions were checked using Shapiro–Wilk and Jarque–Bera adjusted multiplier tests to test for normality, Bartlett, Levene and Fligner–Killeen tests to test for homoscedasticity, and plots of the residuals, fitted values and estimated random effects in the LMM. Gene expression data were log-transformed before the analyses to fit parametric assumptions.
To test the functional localization hypothesis, planned comparisons were used to measure the effect of each social behaviour state (winners, losers or mirror-fighters versus isolation = reference group) on the activation (i.e. IEGs expression) of each brain nucleus. Planned comparisons among social behaviour states within each brain nucleus were also computed to test for socially driven differential activation.
To test for functional connectivity, Pearson product moment correlations were computed between the IEG expression in each pair of brain nuclei for each social behaviour state. These correlations were considered as indicative of co-activation between nuclei, in that positive correlations correspond to phasic activity and negative correlations to out-of-phase activity. Visual analyses of co-activations between nuclei were performed using heatmaps of the correlation matrices. The occurrence of different patterns of functional connectivity associated with different social behaviour states was assessed by testing the association between any two matrices using the quadratic assignment procedure (QAP) correlation test with 5000 permutations [33]. The null hypothesis of the QAP test is that there is no association between matrices. Thus, a non-significant p-value indicates that the correlation matrices are different. The occurrence of functional sub-networks within the SDM network in each social behaviour state was assessed by clustering analysis of brain areas according to correlations among them. The silhouette-based partitioning around medoids (PAM) method was used to check for clusters, and the strength of a cluster was interpreted from its average silhouette (AS) [34]. The number of clusters to consider was calculated by maximizing the average AS for all possible number of clusters (2, 3 or 4). Finally, we have also estimated two measures of network structure (i.e. centrality and cohesion) to characterize the SDM networks underlying each social behaviour state. Eigenvector centrality, which takes into account the number of direct connections that a node has and how well connected its relations are, was used as a measure of centrality; density, the proportion of all possible connections that are present in the network, was used as a measure of cohesion [35]. In order to compare the density of connections among behavioural states (differences in the mean strengths of the relation between two nuclei), we used a bootstrap t-test approach with 5000 sub-samples.
Sample sizes varied either due to technical problems or to outlier values, identified for each condition with the generalized extreme studentized deviate procedure with p = 0.05 and a maximum number of outliers of 20% of the sample size (see electronic supplementary material, table S2 for detailed information on sample sizes). Statistical analyses were performed on R (www.R-project.org) using the following packages: car (Levene test), cluster (PAM), fBasics (Jarque–Bera test), Hmisc (correlations), lattice (heatmaps), multcomp (planned comparisons) and nlme (LMMs). The network analysis parameters were estimated using UCINET v. 6 [36]. Network representations were produced using Python.
3. Results
(a). Social behaviour states
As expected, the agonistic paradigm produced four social behaviour states. In real-opponent interactions, social hierarchies emerged where fish expressing two social behaviour states can be clearly identified: winners that only display aggressive behaviours (80.2 ± 9.02 acts/5 min) and losers that only display submissive behaviours (46.9 ± 6.19 acts/5 min). Mirror-fighters only displayed aggressive behaviours, at a frequency (59 ± 12.8 acts/5 min) that was not significantly different from that displayed by the winners of the real-opponent interaction (t19 = −1.39, p > 0.05). However, in this treatment, the fight between the focal fish and its mirror image was symmetric and no dominance relationship was established (i.e. the focal expresses as much aggressive behaviour than it receives from the mirror image). Therefore, we considered mirror-fighters as a separate social behaviour state that did not achieve a dominant status despite expressing similar levels of aggression to winners.
(b). Effect of social behaviour state and brain region on immediate early gene expression
There were significant main effects of social behaviour state and brain nuclei both on c-fos and on egr-1 expression levels, and the interaction between these two factors was not significant for either of the genes (table 1). The main effect of social behaviour state on c-fos expression was due to significant differences among all behaviour states (table 1). The main effect of social behaviour state on egr-1 expression was related to a close to significant difference between non-interacting fish and winners, and between non-interacting fish and losers (table 1). For both genes the main effect of brain nuclei was due to significant differences between Vs and all other brain nuclei. For egr-1, there was also a significant difference between Dl and POA (table 1).
Table 1.
Effect of social behaviour state and brain nuclei on c-fos and egr-1 expression. Main effects, interactions and multiple comparisons were calculated using LMMs. I, isolated fish (non-social); M, mirror-fighters; W, winners; L, losers. See table 2 legend for other abbreviations.
|
c-fos |
egr-1 |
|||
|---|---|---|---|---|
| F | p-value | F | p-value | |
| social behaviour state | 29.52 | <0.001 | 4.17 | 0.006 |
| brain nuclei | 11.41 | <0.001 | 19.77 | <0.001 |
| social behaviour state × brain nuclei | 0.949 | 0.499 | 0.943 | 0.505 |
| z-value | p-value | z-value | p-value | |
|---|---|---|---|---|
| multiple comparisons (social behaviour state) | ||||
| I–M | 4.37 | <0.0001 | 1.44 | 0.14 |
| I–W | 7.26 | <0.0001 | 1.88 | 0.06 |
| I–L | 8.78 | <0.0001 | 2.58 | <0.01 |
| M–W | 2.66 | <0.01 | 0.37 | 0.70 |
| M–L | 4.09 | <0.0001 | 1.05 | 0.29 |
| W–L | 2.35 | <0.05 | 0.70 | 0.48 |
| multiple comparisons (brain nuclei) | ||||
| Dm–Dl | −0.33 | 0.74 | 1.77 | 0.07 |
| Dm–Vv | −0.87 | 0.38 | 0.14 | 0.88 |
| Dm–Vs | 4.47 | <0.0001 | 6.01 | <0.0001 |
| Dm–POA | −0.52 | 0.60 | −1.65 | 0.09 |
| Dl–Vv | −0.53 | 0.59 | −1.64 | 0.10 |
| Dl–Vs | 4.72 | <0.0001 | 4.41 | <0.0001 |
| Dl–POA | −0.19 | 0.84 | −3.43 | <0.0001 |
| Vv–Vs | 5.24 | <0.0001 | 5.98 | <0.0001 |
| Vv–POA | 0.34 | 0.73 | −1.81 | 0.07 |
| POA–Vs | −4.92 | <0.0001 | −7.63 | <0.0001 |
(c). Differences in functional localization among social behaviour states across the social decision-making network
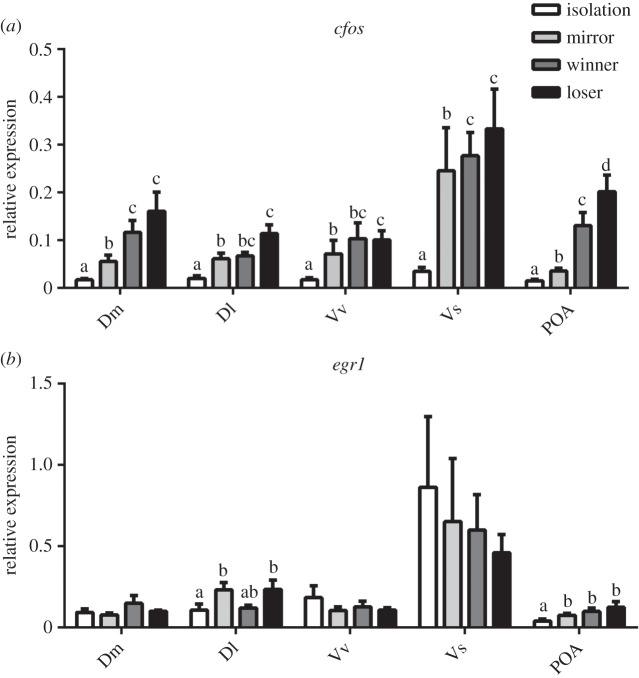
Planned comparison analyses revealed a significant increase in c-fos expression in all nuclei for all social behaviour states (mirror-fighters, winners and losers) when compared with the reference group (i.e. isolation < mirror-fighters, winners, losers; figure 1a). The expression of c-fos in Dm,Vs and POA was higher in both winners and losers than in mirror-fighters, whereas in Dl and Vv mirror-fighter mRNA levels were not significantly different from those of winners, but were different from those of losers (figure 1a).
Figure 1.
Immediate early gene expression in different brain nuclei (Dm, medial zone of the dorsal telencephalic area; Dl, lateral zone of the dorsal telencephalic area; Vv, ventral nucleus of the ventral telencephalic area; Vs, supracommissural nucleus of the ventral telencephalic area; POA, preoptic area) for the different social behaviour states (i.e. isolation, mirror-fighter, winner and loser): (a) c-fos expression; (b) egr-1 expression (normalized to eef1a1l1 in both cases); the graphs represent raw data and error bars represent the standard error of the mean. Different letters indicate social behaviour states that differ significantly from each other within a brain region, using a planned comparisons test (p < 0.05).
Regarding egr-1, planned comparison analyses revealed that only two brain nuclei, Dl and POA, exhibited differential activation in relation to social behaviour state (figure 1b). In Dl, mRNA levels of egr-1 were not significantly different between mirror-fighters, winners and losers, but mirror-fighters and losers were significantly different from the reference group (i.e. isolation < winners, losers; figure 1b). The expression of egr-1 in the POA was significantly higher in all social behaviour states than in the reference group, and there were no significant differences among the three social behaviour states (i.e. isolation < mirror = winners = losers; figure 1b).
(d). Differences in functional connectivity among social behaviour states across the social decision-making network
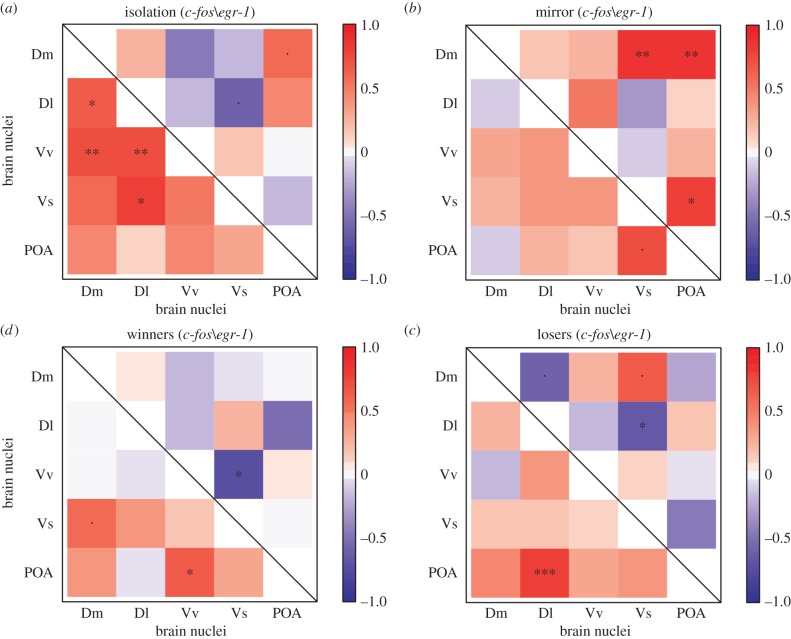
Expression of c-fos revealed distinct co-activation patterns in all social behaviour states (figure 2). The QAP correlations detected a close-to-significant negative relationship between the isolation group and the losers' matrices (r = −0.724, p = 0.054), and all other QAP correlation tests were not significant (isolation versus mirror: r = −0.097, p = 0.369; isolation versus winner: r = −0.119, p = 0.397; loser versus mirror: r = −0.091, p = 0.421; loser versus winner: r = −0.201, p = 0.351; and mirror versus winner: r = 0.048, p = 0.451). From the correlation matrices it can also be seen that each social behaviour state has different sets of significant correlations between different network nodes, which are indicative of behaviour state-specific co-activation patterns (figure 2). Cluster analysis confirmed these different co-activation patterns, as different clusters were found for each social behaviour state (electronic supplementary material, figure S1).
Figure 2.
Functional connectivity in the SDM network as measured by Pearson correlations (r) of c-fos (below the diagonal) and egr-1 (above the diagonal) expression between pairs of brain nuclei (Dm, medial zone of the dorsal telencephalic area; Dl, lateral zone of the dorsal telencephalic area; Vv, ventral nucleus of the ventral telencephalic area; Vs, supracommissural nucleus of the ventral telencephalic area; POA, preoptic area) for each social behaviour state: (a) isolated fish (non-social); (b) mirror-fighters; (c) winners; (d) losers; colour scheme represents r values from −1 (blue) to 1 (red); asterisks indicate significant correlations after p-value adjustment: dot (.) p < 0.1; *p < 0.05; **p < 0.01; ***p < 0.001.
The structural characterization of the c-fos SDM networks revealed that in the isolation group there was no evident central nucleus as the values for all areas were very similar, except for POA, which was the most peripheral nucleus. This network appears to have very similar connections (number of relations between the nodes) given no extra weight to any specific area in the expression of this neutral behaviour state (table 2). For mirror-fighters, Vs was the most central nucleus and the Dm was the most marginal one. For winners, POA and Vs were the most connected ones and Dl the less associated nucleus (table 2). Finally, in losers, POA was the most central area (table 2). Regarding cohesion, the density of the c-fos SDM network was significantly higher in the isolation group than in any of the other social behaviour states (versus mirror-fighters: t = 2.831, p = 0.0002; versus winners: t = 2.947, p = 0.0018; versus losers: t = 1.929, p = 0.0184; table 2), and all the other comparisons were not statistically significant.
Table 2.
Quantitative characterization of the SDM network for each social behaviour state, using c-fos or egr-1 as reporters of neuronal activity. Values correspond to centrality measures (eigenvalues) for each network node: Dm, medial zone of the dorsal telencephalic area; Dl, lateral zone of the dorsal telencephalic area; Vv, ventral nucleus of the ventral telencephalic area; Vs, supracommissural nucleus of the ventral telencephalic area; POA, preoptic area and cohesion (density) for each behaviour state.
|
c-fos |
egr-1 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| brain nuclei | isolation | mirror-fighter | winner | loser | isolation | mirror-fighter | winner | loser | |
| eigenvalues | Dm | 0.481 | 0.321 | 0.452 | 0.372 | 0.498 | 0.552 | 0.201 | 0.514 |
| Dl | 0.489 | 0.402 | 0.248 | 0.541 | 0.50 | 0.251 | 0.445 | 0.512 | |
| Vv | 0.484 | 0.427 | 0.402 | 0.353 | 0.326 | 0.265 | 0.581 | 0.208 | |
| Vs | 0.463 | 0.566 | 0.534 | 0.313 | 0.414 | 0.536 | 0.577 | 0.563 | |
| POA | 0.284 | 0.483 | 0.536 | 0.589 | 0.474 | 0.524 | 0.302 | 0.336 | |
| density | 0.529 | 0.319 | 0.269 | 0.332 | 0.318 | 0.414 | 0.205 | 0.338 | |
Expression of egr-1 also showed distinct co-activation patterns for each social behaviour state (figure 2), as indicated by the lack of significant QAP correlations between any two matrices (isolation versus loser: r = 0.211, p = 0.250; isolation versus mirror: r = 0.013, p = 0.497; isolation versus winner: r = 0.083, p = 0.383; loser versus mirror: r = 0.307, p = 0.158; loser versus winner: r = −0.343, p = 0.155; and mirror versus winner: r = −0.653, p = 0.009). The correlation matrices for egr-1 expression also show different sets of significant correlations between different network nodes for each social behaviour state, which is suggestive of behaviour state-specific co-activation patterns (figure 2). Cluster analysis also supports the occurrence of different functional connectivity patterns in different social behaviour states, as different clusters were found for each social behaviour state (electronic supplementary material, figure S2).
The structural characterization of the egr-1 SDM networks showed variation in the most central areas across social behaviour states. In the isolation group they were the Dl and Dm, whereas in mirror-fighters, winners and losers the most well-connected areas were the Dm, Vv and Vs, and Vs, respectively (table 2). Concerning network cohesion, the more densely connected egr-1 network was observed in mirror-fighters (0.414), which was significantly more connected than that of winners (t = 2.055, p = 0.0280), and that of winners was also more densely connected than that of isolated fish (t = 1.6311, p = 0.0428).
Egr-1 and c-fos expression patterns and clusters for the same social behaviour states also showed clear distinctions (figure 2; electronic supplementary material, S1 and S2). Correlation analyses between c-fos and egr-1 expression for the same brain nuclei and social behaviour state showed a general lack of association between the expression of these two IEGs (electronic supplementary material, figure S3). Notable exceptions were the expression of c-fos and egr-1 in Vs in the mirror group and in Vv in the isolation group (with r = 0.94 and r = 0.72, respectively).
(e). Association between immediate early gene expression and behaviour
Correlation analyses between aggressive and submissive behaviour and IEG expression in different brain nuclei for different social contexts showed no significant results either for c-fos or egr-1 (electronic supplementary material, figure S4). However, for c-fos there was a tendency for negative correlations between aggressive behaviour in winners and expression levels in Dl, Vv and Vs (r = −0.54, p = 0.072; r = −0.51, p = 0.088; and r = −0.59, p = 0.071, respectively). For egr-1, we found a single close-to-significant positive correlation between submissive behaviour in losers and expression in Vv (r = 0.61, p = 0.063).
4. Discussion
Here we provide functional evidence that supports the SDM network hypothesis in zebrafish by confirming its implicit assumption that SDM relies on integration across different regions of the network, rather than on regional specialization of specific network nodes. Specifically, we showed that there were no specific patterns of localized activity in a given node associated with specific social behaviour states, whereas the expression of socially driven behavioural behaviour states was associated with specific patterns of functional connectivity across the SDM network. These results suggest that the neural context in which a given node of the network is operating (that is, the state of its interconnected areas) is central to its functional relevance. Interestingly, IEG expression for c-fos and egr-1 showed distinct neuronal activation patterns for all the considered social contexts (mirror, winners, losers; electronic supplementary material, figure S5), which also suggests that these genes are not working in unity but their activity rather reflects different behaviour state-related processes; c-fos appears to be a good neuronal marker for general brain activity, as all brain nuclei in all conditions responded to social interactions with an increase of c-fos mRNA levels in comparison to the reference non-social group, whereas egr-1 expression seems to be more region- and process-specific.
(a). Functional localization
Although there were main effects of both social behaviour state and brain nuclei on the expression levels of both immediate early genes, in both cases, the interaction between social behaviour state and brain region was not significant, indicating independence between social behaviour state and regional differences in gene expression. The subsequent planned comparisons of neuronal activity, as indicated by IEG expression, confirmed the lack of functional localization of social behaviour states in any of the tested nodes of the SDM network. When comparing each of the three social behaviour states against the non-social reference behaviour state (i.e. isolation) the c-fos data indicated an activation of all brain regions in all behaviour states, whereas egr-1 data only revealed activation of POA for all behaviour states and of Dl for mirror-fighters and losers. Moreover, when comparing the three social behaviour states among themselves (i.e. winners versus losers versus mirror-fighters), different behaviour states shared the same patterns of localized activity. For example, despite the contrasting behaviour states winners and losers had similar levels of c-fos expression in all studied brain regions, and the three social behaviour states (i.e. winners, losers and mirror-fighters) shared similar egr-1 expression levels in the two brain nuclei where this gene responded to social experience (i.e. Dl and POA). Furthermore, winners and mirror-fighters also had similar levels of c-fos expression in Dl and Vv. These results are coincident with those reported for another fish species, the African cichlid Astatotilapia burtoni, where stable dominant and stable subordinate males express different status-specific behavioural profiles, which are also not paralleled by differences in either c-fos or egr-1 expression in any of the studied nodes of the SDM network—which in this case also included, the anterior (ATn) and the ventral tuberal nuclei (VTn) [37]. However, in another study with the same species winners and losers of an acute agonistic interaction show different expression profiles across the network, with localized higher expression of c-fos in the POA and the ATn, and of egr-1 in Dm, Dl, Vv, Vs and VTn of losers [38]. Together these results suggest that socially driven changes in neuronal activation in the SDM network are transient, and that stable social behaviour states do not rely on localized differences in brain activity. Accordingly, the observed behavioural states of winners and losers in this experiment should be seen as stable status-dependent states. This view is supported by the fact that winner and loser effects are observed in zebrafish at least 1 h after a single status establishing fight [27].
Although lacking a behaviour state-specific pattern of activation, from the brain regions studied here, Vs was the one that responded the most to social interactions. This region has been proposed as a teleost putative homologue of the mammalian medial amygdala based on hodological, genomic and functional evidence [4]. However, this view has been recently questioned by a study of molecular markers in the adult zebrafish brain, which suggests that the dorsal and medial Vs are homologous to the central amygdala and its ventral part to the bed nucleus of the stria terminalis [39]. Independent of one-to-one homologies between Vs and specific components of the mammalian amygdala, our study supports the central role of this region in the processing of social information.
Finally, the comparison between winners and mirror-fighters, which share similar behavioural outputs (i.e. both are aggressive) but perceive different behaviours on their opponents (i.e. winners have submissive opponents and mirror-fighters face an aggressive opponent), potentially allows the identification of areas whose activity is better explained either by motor (i.e. when activity is similar between winners and mirror-fighters) or by perceptual information processing (i.e. when activity is different between them). According to this rationale perceptual processes were associated with amygdala (i.e. Dm and Vs) and preoptic c-fos expression, whereas behavioural output was associated with c-fos expression in Dl and Vv and with egr-1 expression in Dl and POA. Thus, this approach revealed a functional differentiation between the two IEGs used in this study, with c-fos more associated with perceptual processes and egr-1 exclusively associated with behavioural output.
(b). Functional connectivity
Our results showed that different social behaviour states exhibited different patterns of functional connectivity, as evidenced by: (i) lack of association between any two correlation matrices that capture the patterns of co-activation of SDM nodes for each social behaviour state (there was only one close to significant association, between isolation and losers for c-fos, and it had a negative sign, indicating opposite and not coincident co-activation patterns); (ii) different clusters (i.e. sub-networks) present in each social behaviour state; (iii) different nodes occupying the central position in the network in each social behaviour state; and (iv) significantly different densities of connections in each social behaviour state. For c-fos, the non-social reference treatment was the one that presented the lowest activity in each node and the most connected SDM network, which breaks apart into different functional networks in the other three social behaviour states without significant differences in connectivity among them (electronic supplementary material, figure S5). This result resembles resting-state functional connectivity networks observed in fMRI human cognition studies, which have been interpreted as intrinsic neural activity reflecting the underlying structural connectivity architecture of the network [40,41]. Similarly, for the SDM network this high functional connectivity in the non-social state may reflect the known reciprocal anatomical connections among the different nodes of the network. For egr-1, mirror-fighters were the behaviour state that presented the most densely connected SDM network, with winners presenting the lowest connectivity.
The fact that c-fos and egr-1 expression depict different functional networks (i.e. for the same social behaviour state, the two IEGs show different patterns of co-activation across the SDM network; electronic supplementary material, figure S5) suggests that different socially driven neuromolecular processes are operating in parallel and that different connectivity layers, corresponding to each of these processes, can be simultaneously present in the SDM network. This possibility contradicts the classic view of a single functional connectivity pattern associated with a specific behavioural behaviour state. Indeed different information-processing processes (e.g. attention, memory, decision-making) may contribute to the same social behaviour state, and each of these processes may be differentially represented in the network by different signalling pathways. For example, in the case of egr-1, its expression has been classically associated with the induction of LTP and the expression of long-term memories in mammals [42]. Similarly in the electric fish Apteronotus leptorhynchus, egr-1 expression in the dorsal telencephalon has been associated with the memory of individual conspecifics based on their electric organ discharge frequency, and this memory can last for several days [43]. Thus, the observed expression of egr-1 in Dl and its associated sub-networks may reflect social memory formation in some of the social behaviour states.
5. Conclusion
The results presented here provide functional support to the SDM network hypothesis [4,7], as we have identified functionally connected networks that integrate nodes from both the mesolimbic system and the social behaviour network. Our results also show that the functional relevance of each network node to the social behaviour state depends on the activity in the network nodes to which they are connected, thus highlighting the relevance of neural context for social behaviour states.
Supplementary Material
Acknowledgements
We thank Wayne Korzan for help with the implementation of the microdissection technique.
Ethics
The animal experimentation procedures used in this study followed the institutional guidelines for the use of animals in experimentation and were approved by the internal Ethics Committee of the Gulbenkian Institute of Science and by the National Veterinary Authority (Direção Geral de Alimentação e Veterinária, Portugal; permit number 8954).
Data accessibility
Supporting qPCR and behavioural data can be accessed at Dryad: http://dx.doi.org/10.5061/dryad.826h4.
Authors' contributions
M.C.T. and R.F.O. designed the experiment; M.C.T. and O.A. performed the experiment; M.C.T. and J.S.L. analysed the data; M.C.T. and R.F.O. wrote the paper with contributions from all authors.
Competing interests
We declare we have no competing interests.
Funding
This study was funded by the EU ‘Copewell’ project (grant no. 265957) and by a grant from Fundação para a Ciência e a Tecnologia (FCT, EXCL/BIA-ANM/0549/2012 awarded to R.F.O.), which supported J.S.L.'s post-doc fellowship. M.C.T. and O.A. were supported by individual fellowships from FCT (SFRH/BD/44848/2008 and SFRH/BD/37187/2007, respectively).
References
- 1.Newman SW. 1999. The medial extended amygdala in male reproductive behavior a node in the mammalian social behavior network. Ann. NY Acad. Sci. 877, 242–257. ( 10.1111/j.1749-6632.1999.tb09271.x) [DOI] [PubMed] [Google Scholar]
- 2.Goodson JL. 2005. The vertebrate social behavior network: evolutionary themes and variations. Horm. Behav. 48, 11–22. ( 10.1016/j.yhbeh.2005.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adinoff B. 2004. Neurobiologic processes in drug reward and addiction. Harv. Rev. Psychiatry 12, 305–320. ( 10.1080/10673220490910844) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connell LA, Hofmann HA. 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol. 519, 3599–3639. ( 10.1002/cne.22735) [DOI] [PubMed] [Google Scholar]
- 5.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. ( 10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 6.Goodson JL, Kabelik D. 2009. Dynamic limbic networks and social diversity in vertebrates: from neural context to neuromodulatory patterning. Front. Neuroendocrinol. 30, 429–441. ( 10.1016/j.yfrne.2009.05.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connell LA, Hofmann HA. 2012. Evolution of a vertebrate social decision-making network. Science 336, 1154–1157. ( 10.1126/science.1218889) [DOI] [PubMed] [Google Scholar]
- 8.Posner MI, Petersen SE, Fox PT, Raichle ME. 1988. Localization of cognitive operations in the human brain. Science 240, 1627–1631. ( 10.1126/science.3289116) [DOI] [PubMed] [Google Scholar]
- 9.Friston KJ, Frith CD, Liddle PF, Frackowiak RS. 1993. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 13, 5–14. ( 10.1038/jcbfm.1993.4) [DOI] [PubMed] [Google Scholar]
- 10.Friston KJ. 2004. Functional and effective connectivity in neuroimaging: a synthesis. Hum. Brain Mapp. 2, 56–78. ( 10.1002/hbm.460020107) [DOI] [Google Scholar]
- 11.McIntosh AR. 2000. Towards a network theory of cognition. Neural Netw. 13, 861–870. ( 10.1016/S0893-6080(00)00059-9) [DOI] [PubMed] [Google Scholar]
- 12.McIntosh AR. 2004. Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics 2, 175–182. ( 10.1385/NI:2:2:175) [DOI] [PubMed] [Google Scholar]
- 13.Wai MSM, Lorke DE, Webb SE, Yew DT. 2006. The pattern of c-fos activation in the CNS is related to behavior in the mudskipper, Periophthalmus cantonensis. Behav. Brain Res. 167, 318–327. ( 10.1016/j.bbr.2005.09.018) [DOI] [PubMed] [Google Scholar]
- 14.Ball GF, Balthazar J. 2001. Ethological concepts revisited: immediate early gene induction in response to sexual stimuli in birds. Brain. Behav. Evol. 57, 252–270. ( 10.1159/000047244) [DOI] [PubMed] [Google Scholar]
- 15.Goodson JL, Evans AK, Soma KK. 2005. Neural responses to aggressive challenge correlate with behavior in nonbreeding sparrows. Neuroreport 16, 1719–1723. ( 10.1097/01.wnr.0000183898.47160.15) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kollack-Walker S, Newman SW. 1995. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience 66, 721–736. ( 10.1016/0306-4522(94)00563-K) [DOI] [PubMed] [Google Scholar]
- 17.Sakata JT, Coomber P, Gonzalez-Lima F, Crews D. 2000. Functional connectivity among limbic brain areas: differential effects of incubation temperature and gonadal sex in the leopard gecko, Eublepharis macularius. Brain. Behav. Evol. 55, 139–151. ( 10.1159/000006648) [DOI] [PubMed] [Google Scholar]
- 18.Yang E, Wilczynski W. 2007. Social experience organizes parallel networks in sensory and limbic forebrain. Dev. Neurobiol. 67, 285–303. ( 10.1002/dneu.20347) [DOI] [PubMed] [Google Scholar]
- 19.Hoke KL, Ryan MJ, Wilczynski W. 2005. Social cues shift functional connectivity in the hypothalamus. Proc. Natl Acad. Sci. USA 102, 10 712–10 717. ( 10.1073/pnas.0502361102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuno H. 2011. Regulation and function of immediate-early genes in the brain: beyond neuronal activity markers. Neurosci. Res. 69, 175–186. ( 10.1016/j.neures.2010.12.007) [DOI] [PubMed] [Google Scholar]
- 21.Lanahan A, Worley P. 1998. Immediate-early genes and synaptic function. Neurobiol. Learn. Mem. 70, 37–43. ( 10.1006/nlme.1998.3836) [DOI] [PubMed] [Google Scholar]
- 22.Oliveira RF. 2013. Mind the fish: zebrafish as a model in cognitive social neuroscience. Front. Neural Circuits 7, 131 ( 10.3389/fncir.2013.00131) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Engeszer RE, Ryan MJ, Parichy DM. 2004. Learned social preference in zebrafish. Curr. Biol. 14, 881–884. ( 10.1016/j.cub.2004.04.042) [DOI] [PubMed] [Google Scholar]
- 24.Engeszer RE, Barbiano LADA, Ryan MJ, Parichy DM. 2007. Timing and plasticity of shoaling behaviour in the zebrafish, Danio rerio. Anim. Behav. 74, 1269–1275. ( 10.1016/j.anbehav.2007.01.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buske C, Gerlai R. 2011. Shoaling develops with age in zebrafish (Danio rerio). Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1409–1415. ( 10.1016/j.pnpbp.2010.09.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paull GC, Filby AL, Giddins HG, Coe TS, Hamilton PB, Tyler CR. 2010. Dominance hierarchies in zebrafish (Danio rerio) and their relationship with reproductive success. Zebrafish 7, 109–117. ( 10.1089/zeb.2009.0618) [DOI] [PubMed] [Google Scholar]
- 27.Oliveira RF, Silva JF, Simões JM. 2011. Fighting zebrafish: characterization of aggressive behavior and winner–loser effects. Zebrafish 8, 73–81. ( 10.1089/zeb.2011.0690) [DOI] [PubMed] [Google Scholar]
- 28.Oliveira RF, Carneiro LA, Canário AVM. 2005. No hormonal response in tied fights. Nature 437, 207–208. ( 10.1038/437207a) [DOI] [PubMed] [Google Scholar]
- 29.Teles MC, Dahlbom SJ, Winberg S, Oliveira RF. 2013. Social modulation of brain monoamine levels in zebrafish. Behav. Brain Res. 253, 17–24. ( 10.1016/j.bbr.2013.07.012) [DOI] [PubMed] [Google Scholar]
- 30.Wullimann MF, Rupp B, Reichert H. 1996. Neuroanatomy of the zebrafish brain: a topological atlas. Basel, Switzerland: Birkhäuser. [Google Scholar]
- 31.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. ( 10.1006/meth.2001.1262) [DOI] [PubMed] [Google Scholar]
- 32.Briffa M, Elwood RW. 2010. Repeated measures analysis of contests and other dyadic interactions: problems of semantics, not statistical validity. Anim. Behav. 80, 583–588. ( 10.1016/j.anbehav.2010.06.009) [DOI] [Google Scholar]
- 33.Borgatti SP, Everett MG, Johnson JC. 2013. Analyzing social networks. Beverley Hills, CA: Sage Publications. [Google Scholar]
- 34.Nagpaul PS. 2001. Cluster analysis. See www.unesco.org/webworld/idams/advguide/Chapt7.htm.
- 35.Makagon MM, McCowan B, Mench JA. 2012. How can social network analysis contribute to social behavior research in applied ethology? Appl. Anim. Behav. Sci. 138, 152–161. ( 10.1016/j.applanim.2012.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borgatti SP, Everett MG, Freeman LC. 2002. Ucinet for Windows: software for social network analysis. Harvard, MA: Analytic Technologies. [Google Scholar]
- 37.Maruska KP, Becker L, Neboori A, Fernald RD. 2013. Social opportunity causes rapid transcriptional changes in the social behaviour network of the brain in an African cichlid fish. J. Neuroendocrinol. 25, 145–157. ( 10.1111/j.1365-2826.2012.02382.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maruska KP, Becker L, Neboori A, Fernald RD. 2013. Social descent with territory loss causes rapid behavioral, endocrine and transcriptional changes in the brain. J. Exp. Biol. 216, 3656–3666. ( 10.1242/jeb.088617) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M, Brand M. 2012. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J. Comp. Neurol. 520, 633–655. ( 10.1002/cne.22757) [DOI] [PubMed] [Google Scholar]
- 40.Kelly C, Biswal BB, Craddock RC, Castellanos FX, Milham MP. 2012. Characterizing variation in the functional connectome: promise and pitfalls. Trends Cogn. Sci. 16, 181–188. ( 10.1016/j.tics.2012.02.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE. 2009. Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum. Brain Mapp. 30, 3127–3141. ( 10.1002/hbm.20737) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones MW, et al. 2001. A requirement for the immediate early gene Zif268 in the expression of late LTP and long-term memories. Nat. Neurosci. 4, 289–296. ( 10.1038/85138) [DOI] [PubMed] [Google Scholar]
- 43.Harvey-Girard E, Tweedle J, Ironstone J, Cuddy M, Ellis W, Maler L. 2010. Long-term recognition memory of individual conspecifics is associated with telencephalic expression of Egr-1 in the electric fish Apteronotus leptorhynchus. J. Comp. Neurol. 518, 2666–2692. ( 10.1002/cne.22358) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supporting qPCR and behavioural data can be accessed at Dryad: http://dx.doi.org/10.5061/dryad.826h4.