Abstract
The waterflea Daphnia is a model to investigate the genetic basis of phenotypic plasticity resulting from one differentially expressed genome. Daphnia develops adaptive phenotypes (e.g. morphological defences) thwarting predators, based on chemical predator cue perception. To understand the genomic basis of phenotypic plasticity, the description of the precedent cellular and neuronal mechanisms is fundamental. However, key regulators remain unknown. All neuronal and endocrine stimulants were able to modulate but not induce defences, indicating a pathway of interlinked steps. A candidate able to link neuronal with endocrine responses is the multi-functional amine dopamine. We here tested its involvement in trait formation in Daphnia pulex and Daphnia longicephala using an induction assay composed of predator cues combined with dopaminergic and cholinergic stimulants. The mere application of both stimulants was sufficient to induce morphological defences. We determined dopamine localization in cells found in close association with the defensive trait. These cells serve as centres controlling divergent morphologies. As a mitogen and sclerotization agent, we anticipate that dopamine is involved in proliferation and structural formation of morphological defences. Furthermore, dopamine pathways appear to be interconnected with endocrine pathways, and control juvenile hormone and ecdysone levels. In conclusion, dopamine is suggested as a key regulator of phenotypic plasticity.
Keywords: Daphnia, dopamine, phenotypic plasticity, inducible defences, polyploid cells, neurophysiology
1. Background
Predation is a primary force driving adaptation in prey. When predatory threats are fluctuating in natural environments, inducible defences may evolve in prey organisms. For example, behavioural adaptations reduce the chance of predator encounter and life-history changes increase survival chances under size selective predation [1]. Prominent examples of inducible defences are the various defensive morphological traits observed in the model freshwater crustacean Daphnia. Several Daphnia species display spectacular morphological defences [2,3] including crowns of thorns [4], spines [5], crests [6] and helmets [7–9]. All these defensive strategies are induced via predator-specific chemical cues known as kairomones. The chemical perception of kairomones initiates a series of internal physiological reactions including neuronal signal integration [10] and subsequent conversion into endocrine agents [11–13]. These substances in turn modulate developmental changes, which result in the growth of a defended specimen. Interestingly, different predators can induce very different morphological as well as behavioural and/or life-history defences. This enormous flexibility appears to be regulated by the ‘ecoresponsive’ Daphnia genome [14]. This involves different sets of paralogous genes activating evolutionarily conserved and highly derived physiological pathways using different sets of recently diverged genes [14]. These must be carefully regulated within and between different stages and specific cells in order to create the defended phenotype that matches the present environmental condition.
In order to analyse the signalling cascade and transmission of perceived predator cues into molecular responses, an integrative approach combining physiological assays and immunohistochemistry targeting candidate epitopes with RNA expression levels is important. Using the neuroactive stimulant physostigmine, previous work has shown that the perception of the predatory phantom midge larvae Chaoborus is coded via cholinergic signalling [10,15]. In contrast, the perception of fish-specific cues involves GABAergic signals [10]. Moreover, the endocrine system (i.e. juvenile hormones [16] and ecdysone [13]) was also shown to be involved in controlling growth and development of inducible defences in Daphnia. However, all applied agents in these studies were only able to modulate (but not induce) the morphological response. This indicates that several components act complementarily in the underlying pathway. This interaction ultimately results in the observed increase in mitotic activity found in the tissues underlying morphological defence [17]. In addition, microscopic studies revealed a group of large polyploid cells located in close association with the morphological defence structure [17,18] of various daphniid subgenera. The abundance of these cells was species-specific and correlated with the expression of the induced morphological trait. It was therefore suggested that these cells are involved in the development of defensive traits and serve as central control stations secreting proliferation agents [17] responsible for the mitotic activity in the vicinity of these cells. Cell proliferation [19] may lead to the development of defensive traits. Nonetheless, the proliferative agent(s) and its (their) regulatory role in the development of phenotypically plastic morphological defences remain undetermined until now.
In order to gain deeper insights into the signalling pathways of predator-induced morphological defences, we studied the role of the biogenic amine dopamine. Dopamine is a key neurotransmitter involved in many different metabolic pathways and, more importantly, it serves as a neurohormone in arthropods [20]. It is known to be an important signalling molecule in various metabolic pathways in invertebrates (reviewed in [20]). In fact, dopamine can regulate the synthesis and degradation of juvenile hormones [21,22], and thereby we hypothesized that it has the potential to control predator-induced polyphenism.
We tested this hypothesis using an integrative approach applying physiological, immunohistochemical and gene expression techniques. Specifically, we investigated the effects of dopamine on morphological defence formation, its cellular distribution and gene expression levels of the dopamine-synthesizing enzyme dopamine decarboxylase (DDC) by comparing control and predator-exposed Daphnia. We studied animals of two different subgenera (electronic supplementary material, figure S1)—Daphnia (Daphnia pulex) and Ctenodaphnia (Daphnia longicephala)—to test for general validity of this potential key component of phenotypic plasticity. The two Daphnia species are well studied for their morphological defences, but are distantly related phylogenetically (electronic supplementary material, figure S1). Daphnia pulex develops spines (neck teeth) located in the dorsal head region as a defence against the dipteran phantom midge larvae Chaoborus spec. [23] (electronic supplementary material, figure S2A) and D. longicephala forms enlarged crests when threatened by the heteropteran backswimmer Notonecta spec. [6] (electronic supplementary material, figure S2B).
Herein, we show that dopamine alone increased size of both Daphnia species per se. The combined application of dopamine and physostigmine induced the development of morphological defences. Immunolabelling of dopamine in whole mount preparations revealed that it is primarily located in a special type of polyploid cell in close vicinity with the morphological defence structure. In addition, quantitative PCR revealed that the Ddc gene—coding for the enzyme DDC that converts dopa into dopamine—is significantly upregulated in D. pulex. We finally discuss these results in the light of information available from other model species and develop a concept of a potential pathway underlying inducible defences in Daphnia. Here, dopamine is discussed as a pivotal regulator of phenotypic plasticity, transforming predator information into morphological traits.
2. Material and methods
Animals were cultured as described in the electronic supplementary material.
(a). Daphnia pulex neurophysiological assay
Induction of D. pulex was using randomly selected black-eyed embryonic Daphnia (fifth embryonic stage 10–12 h before hatching) excised from the mother's brood pouch in a glass dish using preparation needles. For each assay set, individuals of at least five randomly selected mothers were pooled, to avoid maternal bias. Embryonic Daphnia were individually transferred into 50 ml randomly distributed glass vials that contained 40 ml medium holding a distinct concentration of the neurotransmitter. We used neurotransmitter stock solutions of 15 µM dopamine (Sigma Aldrich, Germany) diluted in distilled water that were kept at −20°C and thawed prior to use. A stock solution of the acetylcholine esterase inhibitor physostigmine (Sigma Aldrich) was dissolved in ethanol and stored at −20°C. We applied only a small volume (2 µl diluted in 40 ml) of our stock solution to the individual probes to avoid ethanol effects. Earlier experiments showed that ethanol alone (2 µl) did not significantly affect Daphnia life history and morphology. The lowest physostigmine concentration investigated in [10] was taken for physiological stimulations. Simultaneous stimulation with both neurotransmitters was most effective at a concentration of 15 µM dopamine (similar to that used in [24,25]) and 5 nM physostigmine [10]. The majority of all animals were healthy and capable of moulting into the next instars at concentrations applied. The medium of the individual treatments was exchanged every 48 h to maintain constant stimulation conditions of the drug.
Every trial consisted of four drug treatments and was repeated a minimum of 20 times. For every assay, controls without the drug were performed parallel to the drug treatments.
Neck teeth expression was documented using an Olympus SZX 16 binocular microscope equipped with a Color View III digital camera system (Soft Imaging Solutions; Olympus, Germany) and analysed with Cell D software (Olympus, Germany). Body length at sexual maturity (reached after 6 days when four successful moults were observed in D. pulex) was measured from the upper margin of the eye to the junction of carapace and spine. Neck teeth expression was scored in the second juvenile instar (one moult after being released from the brood pouch, approximately 36 h after excision from the mother's brood pouch) according to Tollrian [23]. These neck scores of the individual specimen were quantified as percentages, with 0% indicating a lack of neck teeth, and 100% maximal expression.
(b). Daphnia longicephala neurophysiological assay
Specimen induction was performed as described for D. pulex. Notonecta kairomone was produced with one Notonecta per litre of charcoal-filtered tap water. Waterbugs were fed with 10 D. longicephala for 24 h. This concentration was defined as a 100% kairomone and was diluted with charcoal-filtered tap water 1 : 4 (25%) and 3 : 4 (75%), respectively. The various neurotransmitter concentrations of 15 µM dopamine and 5 nM physostigmine, as well as the combination of the two, were added as described above. Medium was exchanged every 48 h to ensure a constant neurotransmitter and kairomone concentration. Body length and crest height was measured from behind the eye to the maximal anterior extension of the crest edge once animals bred the first clutch (i.e. 10 days after five successful moults).
(c). Statistics
Daphnia longicephala and D. pulex body length at sexual maturity was normally distributed and the dopamine effect was determined using a Student's t-test. Daphnia longicephala crest height was normalized to body length and followed a normal distribution. Dataset of neck teeth expression was arcsine transformed to fit a normal distribution and to homogenize variances. One-way analysis of variance (ANOVA) was applied to determine kairomone and neurotransmitter effects individually. Differences between kairomone concentrations were determined using a Tukey–Kramer post hoc test. Likewise, we tested for effects between applied neuroactive agents for each kairomone concentration, respectively. Post hoc analysis comparing the effect between the neuroactive agents and the control was performed using a Tukey–Kramer test. All statistics were performed in Statistica v. 12 (Statsoft Inc.). Data are available in the electronic supplementary material.
(d). Induction of animals for histological preparations
Induction was performed using glass beakers containing net cages holding the predators. Experimental specimens were kept outside the net cage to prevent predation. Daphnia longicephala were constantly exposed to Notonecta spec. from the first juvenile instar until they reached sexual maturity. Notonectids were fed with two adult D. longicephala per day. Daphnia pulex holding embryos in the fifth embryonic stage were exposed to 10 Chaoborus larvae (fed with 100 D. pulex juveniles). After juvenile release from the mother's brood pouch, the mothers were removed from the glass beaker and the D. pulex juveniles remained to moult into the second instar. Controls for both species were performed accordingly but in the absence of the predators.
(e). Fluorescent Nissl stain and immunohistochemistry
Immunohistochemistry (D. longicephala and D. pulex) were performed on whole mount preparations as described in [18]. Randomly selected control (n = 20) and induced (n = 20) specimens of both species were preserved in 4% paraformaldehyde for each staining technique. Whole mount preparations were performed as described in [18] using dopamine antibody (DOP 11 s in rabbit; Acris Germany) diluted 1 : 100 in PBS-TX and 5 h in Nissl fluorescence by Neurofluor (Life Technologies; see the electronic supplementary material).
(f). Alexa electroporation of polyploid cell
Daphnia longicephala were prepared in ringer solution (pH 7.5; Sigma Aldrich). The cuticle of the crest was removed to make polyploid cells accessible for the Alexa-filled microelectrode. Alexa594 (1 mM, Molecular Probes) was applied via electroporation (20–30 V and 30 ms). One/two pulses were applied.
(g). Quantitative real-time PCR
Induction of D. pulex for gene expression analysis was performed in net cages (see the electronic supplementary material). We used published primers of the Ddc gene [NCBI_GNO_336293] by Scoville & Pfrender [26]. Sample preparation, RNA extraction, cDNA synthesis, selection of reference genes, qPCR conditions and gene expression analysis were performed as published earlier [27] (see the electronic supplementary material).
3. Results
(a). Dopamine effects on body size
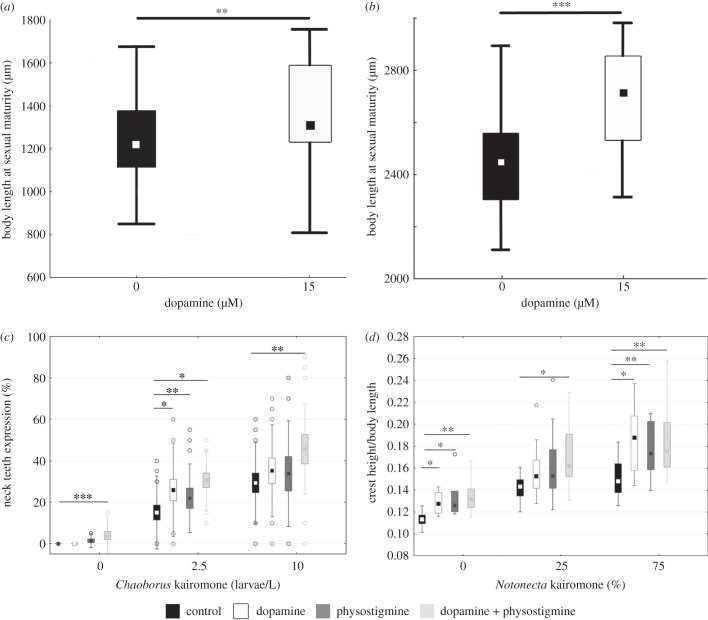
We tested the effect of dopamine on body size in both Daphnia species (figure 1). Dopamine significantly increased the body size of sexually mature D. pulex (t-test, p < 0.01; figure 1a) and D. longicephala (t-test, p < 0.001; figure 1b). Following these significant impacts on Daphnia size, we tested dopamine individually, in combination with physostigmine and with kairomones for effects on the development of inducible morphological defences.
Figure 1.
Neurophysiological induction assay. (a) Daphnia pulex body length of control specimen (black) is increased through the application of dopamine (white: 15 µM DA) at sexual maturity. (b) Daphnia longicephala body length of control specimen (black) is increased by dopamine (white: 15 µM DA) at sexual maturity. (c) Strength of individual neck teeth expression in D. pulex. Comparison of controls (black) and stimulation with physostigmine (dark grey: 5 nM PHY) or dopamine (white: 15 µM DA) did not increase neck teeth expression. Combined stimulation using physostigmine and dopamine in combination (light grey: 5 nM PHY + 15 µM DA) increases neck teeth expression in the absence of the kairomone. (d) Strength of crest expression defined as crest height measured from the posterior eye to anterior crest boundaries. Increase of crest expression in dopamine (white: 15 µM DA) and dopamine + physostigmine (light grey: 15 µM DA + 5 nM PHY) exposed D. longicephala. (c–f) Plotted is the median and interquartile range. Levels of significance: *p < 0.05, **p < 0.01, ***p < 0.001.
(b). Kairomone effects on inducible morphological defences
The application of the Chaoborus kairomone resulted in the expression of neck teeth in D. pulex (figure 1c; electronic supplementary material, table S2; ANOVA, F2,211 = 57.249, p < 0.001). Neck teeth are significantly increased between 0 and 2.5 larvae l−1 (electronic supplementary material, table S3; Tukey–Kramer test, p < 0.001) as well as between 2.5 and 10 larvae l−1 (electronic supplementary material, table S3; Tukey–Kramer test, p < 0.001). The application of the Notonecta kairomone significantly induced the development of crests in D. longicephala (figure 1d; electronic supplementary material, table S4; ANOVA, F2,42 = 29.315, p < 0.001). Crests are significantly increased between 0 and 25% Notonecta (figure 1d; Tukey–Kramer test, p < 0.001) as well as between 0 and 75% (electronic supplementary material, table S5; Tukey–Kramer test, p < 0.001).
(c). Neurophysiological induction assay
In the absence of the kairomone, we observed significant differences in neck teeth expression in D. pulex (ANOVA(0 larvae/L), F3,213 = 7.603, p < 0.001; electronic supplementary material, table S6) and crest development in D. longicephala (ANOVA(0%), F3,36 = 6.22, p < 0.01; electronic supplementary material, table S7) between treatment groups. Dopamine did not significantly affect neck teeth expression in D. pulex (figure 1c; Tukey–Kramer test, p = n.s.; electronic supplementary material, table S8) but we observed crest development in D. longicephala (figure 1d; Tukey–Kramer test, p < 0.04; electronic supplementary material, table S9). The application of physostigmine did not significantly increase neck teeth expression in D. pulex (figure 1c; Tukey–Kramer test, p = n.s.; electronic supplementary material, table S8) but we observed crest development in D. longicephala (figure 1d; Tukey–Kramer test, p < 0.023; electronic supplementary material, table S9). The combination of dopamine and physostigmine increased neck teeth expression in D. pulex and crest development in D. longicephala (figure 1c; Tukey–Kramer test, p < 0.001; electronic supplementary material, table S8; figure 2d; Tukey–Kramer test, p < 0.002; electronic supplementary material, table S9) in the absence of the kairomone.
Figure 2.
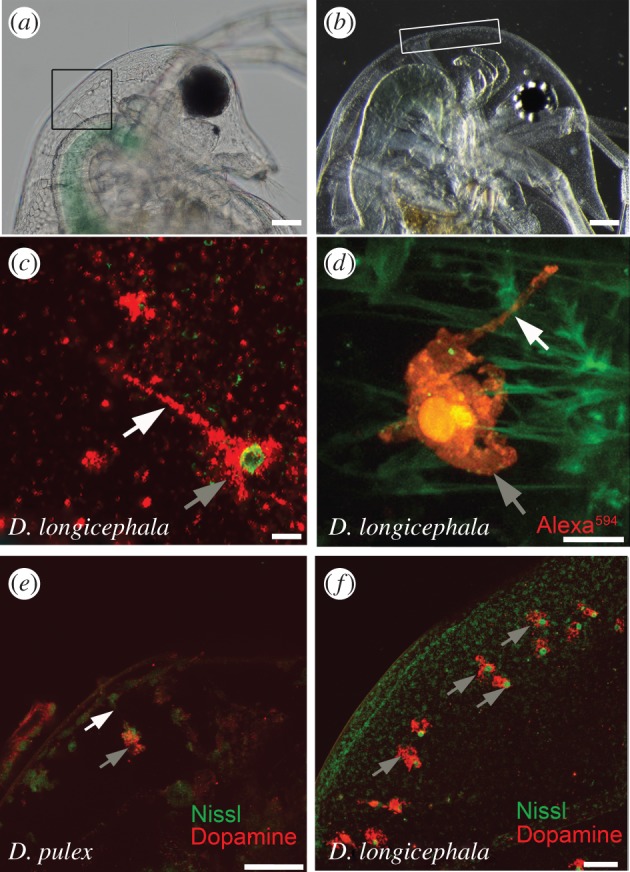
Intracellular localization of dopamine. (a) Daphnia pulex head; box depicts the area of dopamine-containing polyploidy cells. (b) Daphnia longicephala head; box depicts the area of dopamine-containing polyploidy cells. (c) Bulged cell in Notonecta-exposed D. longicephala showing the soma with the nucleus and the cellular extension containing dopamine. Scale bar, 10 µm. (d) Overview of an Alexa594-filled bulged cell with soma and cellular extension. Scale bar, 10 µm. (e) Dopamine immunolabelling of bulged cells in predator-exposed D. pulex. Scale bar, 50 µm. (f) Dopaminergic bulged cells in predator-exposed D. longicephala. Scale bar, 50 µm. (c–f) Grey arrows point to soma of bulged cells, white arrows indicate the cellular extension of the cell.
In the presence of low and high kairomone concentrations, neuroactive substances had significant effects on neck teeth expression (ANOVA(5 larvae/L), F3,314 = 4.816, p < 0.01; ANOVA(10 larvae/L), F3,193 = 4.154, p < 0.01; electronic supplementary material, tables S10 and S11) and crest development (ANOVA(25%), F3,60 = 2.771, p < 0.05; ANOVA(75%), F3,71 = 6.285, p < 0.001; electronic supplementary material, tables S12 and S13). Dopamine significantly increased neck teeth expression at low but not at high kairomone concentrations (figure 1c; Tukey–Kramer test(5 larvae/L), p < 0.001; electronic supplementary material, table S14; Tukey–Kramer Test(10 larvae/L), p = n.s.; electronic supplementary material, table S15) in D. pulex. Crest development in D. longicephala was not increased at low (figure 1d; Tukey–Kramer test(25%), p = n.s.; electronic supplementary material, table S16) but was at high Notonecta kairomone concentrations (figure 1d; Tukey–Kramer test(75%), p < 0.004; electronic supplementary material, table S17). The combination of both agents further increased neck teeth expression and crest development in the presence of high and low kairomone concentrations (figure 1d; Tukey–Kramer test(5 larvae/L), p < 0.05; Tukey–Kramer test(10 larvae/L), p < 0.01; electronic supplementary material, tables S14 and S15; figure 1d; Tukey–Kramer test(25%), p < 0.05; Tukey–Kramer test(75%), p < 0.001; electronic supplementary material, tables S16 and S17).
(d). Immunohistochemistry
Using classical and fluorescent Nissl staining, we observed irregularly shaped cells in the region of morphological defence structures (i.e. neck teeth, figure 2a; and crests, figure 2b) in control and predator-exposed D. pulex and D. longicephala. These bulged cells are large (30–50 µm), highly arborized and marked by a star-shaped morphology with distinctive nuclei (electronic supplementary material, figure S3a,b). A thin plasma process targets the epidermis (figure 2c,d). Cells are constant in number (32 ± 2 in D. longicephala, and 4 ± 2 in D. pulex) irrespective of predator exposure.
Nissl fluorescence together with immunolabelling using antibodies raised against dopamine revealed that dopamine is localized within the bulged cells of predator-exposed and control organisms. Soma and extension both show dopamine immunoreactivity (figure 2e,f). All cells that are located in the vicinity of the morphological defence contain dopamine.
(e). Differential gene expression
As we observed dopamine in bulged cells of predator-exposed and control specimens, we aimed to determine dopamine expression levels based on genes identified in the D. pulex genome [14]. We measured gene expression levels in D. pulex of Ddc to be upregulated by a mean factor of 3.25 in Chaoborus-exposed D. pulex (s.e. range 1.62–7.44; p = 0.002).
4. Discussion
With our integrative approach, we demonstrate the role of dopamine in the context of inducible morphological defences in Daphnia.
(a). Neurophysiology of morphological defence structure development
Physiological assays have proven to be a useful tool in obtaining first insights into the neurophysiology of inducible defences [10,15].
Cholinergic signals were shown to modulate the expression of defensive traits in combination with the predator cue [10]. In the absence of the predator cue, plastic traits remained unchanged. This indicated that more than one component is involved in the transmission of predator cues. In our assay, we found that mere dopamine application results in larger individuals at sexual maturity. We therefore anticipate that in Daphnia, dopamine promotes growth per se. Here, dopamine could impact either (i) cell proliferation and/or (ii) increase cellular growth. Evidence suggests that dopamine is likely to act as a proliferative agent [28–30], as increased mitotic activity [17] as well as an increased number of cells forming neck teeth has been observed in D. pulex [19]. However, dopamine-dependent cellular growth increase cannot be excluded.
With respect to the signalling cascade underlying predator-induced morphological defences, our induction assay showed that cholinergic as well as dopaminergic stimulation is involved.
We found that in D. pulex both agents increase the expression of neck teeth when applied in conjunction with the Chaoborus kairomone. In the absence of the kairomone, independent cholinergic and dopaminergic stimulation did not induce neck teeth expression. Only the combined application of physostigmine and dopamine induced the expression of neck teeth.
These results were also verified in D. longicephala. Here, however, both agents applied individually and in conjunction induced crest development. This indicates that there are yet undetermined physiological differences underlying the development of crests in D. longicephala and neck teeth in D. pulex. In fact, defences of D. pulex and D. longicephala are different in their derivation. Whereas neck teeth can be regarded as instar-specific de novo structures, crests in D. longicephala can be seen as an exaggeration of already existing morphologies that built from moult to moult depending on a continuous induction. Moreover, these defences are formed during different developmental time frames. Daphnia pulex is prone to predation only in the juvenile instars. Thus, defences are formed during embryogenesis [31] to be effective upon birth. With age, D. pulex outgrows its gape-limited predators and defences become unnecessary. Alternatively, due to its proboscis, with which Notonecta pierces through the prey's carapace for suction feeding, this predator is not gape-limited and D. longicephala needs to be protected in its larger stages. Notonecta is a visual–tactile predator and has higher chances for successful prey detection when the prey is large. As D. longicephala has to reach a certain size in order to reproduce, they develop crests that increase escape chances by enforcing handling difficulties on the predator.
We further anticipate that both agents act in concert with further components (e.g. endocrine; see below) that are involved in cue perception and the defence activation pathway. This is supported by the fact that morphological defences formed after the combined application of dopamine and physostigmine are not as profoundly developed when compared with the naturally (i.e. kairomone) induced defences.
At high Chaoborus kairomone concentrations, as well as at low Notonecta kairomone concentration, only the combination of dopamine and physostigmine increased defence development. This observation may result from physiological stimulation differences that depend on the ‘strength’ of the predator cue, which is defined by the cue's concentration and the organism's sensitivity. In turn, this determines the neuroactive agent's impact. Therefore, the precise molecular neurophysiology that explains predator cue perception and signal integration involving dopaminergic and cholinergic signals will be target of future studies.
(b). Cellular localization and dopamine synthesis
Our immunohistochemical analyses revealed the presence of dopamine in both predator-exposed and control Daphnia of both species. It was primarily observed, using Nissl fluorescence, in bulged cells of characteristic shape that line the region of the inducible defence structure. The staining characteristics of these cells (i.e. an intensely stained, large nucleus and a lighter-stained surrounding soma) indicate a high degree of polyploidy, which has been observed previously [17,18]. The fact that we found dopamine in polyploid cells in the vicinity of the morphological defence structures in predator-exposed and control Daphnia of both species suggests that the pathways underlying the formation of inducible defences may be permanently ‘running on standby’. Thereby, upon predator presence, dopamine can be quickly released from bulged cells and initiate the relevant pathways, to minimize lag time until defence structure development. However, in order to maintain constant dopamine stimulation, which is essential for defence structure development, a permanent supply or even a slightly increased level of dopamine is anticipated. We therefore performed quantitative real-time PCR expression analyses targeting the dopamine-synthesizing enzyme gene dopamine decarboxylase (Ddc). Here, Ddc gene expression was upregulated in Chaoborus-exposed D. pulex, which indicates increased levels of dopamine synthesis in the presence of a predator, which in turn points to increased dopamine-associated activity levels. As only the genome of D. pulex is currently available [14] and primers obtained for D. pulex do not amplify reliably in the phylogenetically distant species D. longicephala, we did not assess gene response patterns in the latter.
5. Signalling pathway of environmentally induced polyphenisms
The integration of our results within known elements of hormonal regulation contributes to a more complete understanding of kairomone recognition in Daphnia.
(a). Counterbalanced hormonal control of phenotypic plasticity
Many arthropods can cope with environmental challenges, moulting into one of several adaptive phenotypes after the perception of changes. The key to this is the temporal and spatial regulation of growth and moulting through the counterbalanced actions of juvenile hormones and ecdysteroids. Whereas juvenile hormones control growth and suppress reproduction, ecdysone derivatives induce moulting and embryogenesis [31–34]. Thus in Crustacea, a proper balance between methyl farnesoate (a juvenile hormone) and 20-hydroxyecdysone (the active form of ecdysone) is important for the normal progression of oogenesis. An imbalance towards ecdysone would result in life-history shifts where somatic growth is traded with reproduction. In consequence, animals moult earlier at a smaller size. Conversely, an overabundance of juvenile hormones increases somatic growth and postpones the moulting event.
From an evolutionary perspective, it appears that juvenile hormone signalling has emerged as an important, highly conserved endocrine pathway in arthropod development that regulates the response to changing environmental conditions [13]. These include caste determination in social insects [35], photoperiodic adaptation of reproductive modes in aphids [36], seasonal pattern formation in butterflies [37], as well as the development of colour in caterpillars [38], horns in scarab beetles [39,40] and morphological defences in Daphnia [11,16]. All of these are mediated by juvenile hormones and often dopamine-signalling pathways [41–44], as supported by the data presented here.
(b). Neuronal control of predator-induced phenotypic plasticity
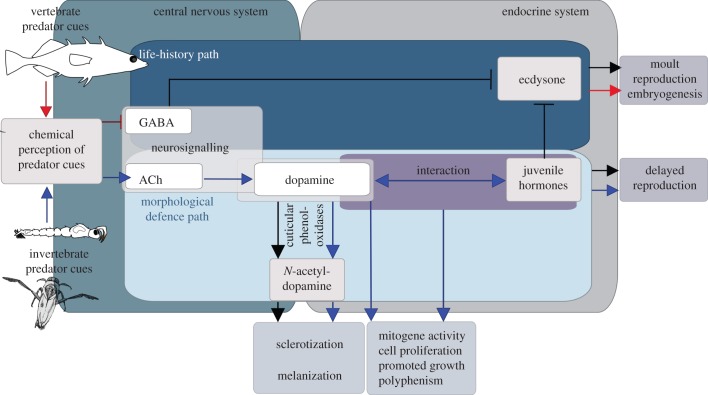
Ultimately, hormones serve as the switch that controls alternative developmental pathways of phenotypic plasticity [41]. This switch is controlled by the central nervous system, which actually perceives and codes the environmental cues. In Daphnia specifically, we anticipate a conceptual network in which dopamine is a central component regulating predator-induced defences of different functionality (figure 3). In the case of a predator-specific chemical cue (e.g. the kairomone), it is perceived, neuronally integrated and transformed into hormonal changes that result in the development of a defended phenotype. Variously, chemoreceptors specific for predator cues, neurotransmitters for chemical information transfer and hormones that ultimately have the capability to regulate gene expression are used to change the phenotype. The only chemoreceptors described in Daphnia to date are 58 gustatory receptors (Grs) that belong to the insect chemoreceptor superfamily in Daphnia [45]. A change in membrane conductance in the dendrites ultimately causes neuron excitation in the brain, integration and subsequently excitation/inhibition of neurosecretory cells to control hormone release, resulting in adaptive phenotypes including neck teeth, greater body size and/or changes in life history. Therefore, the phenotypic outcome depends on the differential regulation of neuronal and hormonal agents.
Figure 3.
Conceptual pathway of elements controlling the development of inducible defences based on [10,13,16] and this study. The network consists of successive components: chemical perception of predator cues, changes in neuronal signalling in the central nervous system, and neurohormonal changes. In the absence of predator cues, the moult cycle is under the control of the nervous system, which regulates the release of ecdysone and juvenile hormone. Here, GABA inhibits the release of ecdysone, and thereby controls moult, reproduction and embryogenesis. The regulatory control of dopamine with juvenile hormones stimulates growth and delays reproduction. Furthermore, dopamine itself can be converted via cuticular phenoloxidases into N-acteyl-dopamine, which is a component involved in the cross-linking of orthoquinones responsible for cuticle sclerotization, as well as melanization processes after moulting. Ecdysone and dopamine × juvenile hormone counterbalance growth and reproduction. A fish-induced imbalance towards ecdysone, for example, via relief of the GABAergic inhibition of ecdysone secretion, results in life-history shifts, where animals mature earlier at a smaller size. In contrast, Chaoborus/Notonecta cues are mediated by acetylcholine, dopamine and juvenile hormones, which results in somatic growth. This appears to be controlled via differential cholinergic transmission, which could stimulate additional dopamine release. Dopamine release from polyploid cells can be converted by cuticlar phenoloxidases, which enhances sclerotization and results in the strengthening of the body armour. At the same time, dopamine can serve as a proliferation agent (alone or with juvenile hormones). Being released from the polyploid cells, dopamine stimulation results in the development of morphological defences structures (e.g. neck teeth or crests). At the same time, dopamine × juvenile hormone interaction increases overall body size and postpones reproduction. Black arrows indicate physiological pathways running without environmental predator cues. Blue arrows mark the individual components of the pathway transforming predator cues (e.g. Chaoborus/Notonecta into morphological defences). Red arrows mark the individual components of the pathway transforming predator cues (e.g. fish into life-history shifts). The arrows do not indicate any interaction between the signalling components, but rather refer to their involvement in the respective cascade.
If released onto the epidermis, dopamine is an intermediate and enhances the cross-linking of orthoquinones resulting in cuticle sclerotization, a process known from many arthropod taxa [46–48]. This is very likely to be the mechanism leading to the observed increase in D. pulex carapace stability [49]. Moreover, dopamine has been shown to influence the degree of cuticle melanization [46,48]. For example, Daphnia under fish predation were shown to increase their transparency, and thereby decrease the chance of visual detection by the predator [50]. ‘Large-scale’ (shape and growth) adaptations requiring extensive growth of morphological structures can be explained by localized dopaminergic stimulation, which is anticipated to require juvenile hormone interaction and initiate cell proliferation. Indeed, the involvement of juvenile hormones has previously been shown in neck teeth development in D. pulex [16], as well as helmet elongation in Daphnia galeata through the exposure to one of the juvenile hormone-mimicking pesticides fenoxycarb [11]. The precise mechanisms by which dopamine and juvenile hormones interact to regulate localized growth of morphological structures are yet to be determined in detail. In fact, dopamine can inhibit the degradation [22] and stimulate the biosynthesis [51] of the juvenile hormone, which is known to promote growth and postpone reproduction, which could explain, for example, life-history plasticity.
Here and previously [10], we have shown that in predator-induced morphological defences in Daphnia acetylcholine acts as stimulatory agent in the regulation of morphological defences and GABAergic signalling is involved in the development of life-history shifts. In general, acetylcholine in the brain alters neuronal excitability, influences synaptic transmission, induces synaptic plasticity and coordinates firing of groups of neurons [52]. As a result, it changes the state of neuronal networks throughout the brain, and modifies their response to internal and external inputs, which is the classical role of a neuromodulator.
The neurotransmitter GABA modulates life-history responses against fish predation [10], including those we previously found: reduced body length and clutch size, but increased delay until maturity [10]. This indicates the presence of an underlying GABAergic neuronal control of the neurophysiology of fish kairomone transmission. The observed responses could potentially be explained by a relieved inhibition, which was re-established by the experimental application of GABA. In general, GABA is known to have inhibitory functions. In the absence of vertebrate predators, Daphnia life-history shifts could be inhibited by GABAergic signals by inhibiting the release of ecdysone [53,54]. This results in a ‘general purpose’ life history. Upon the perception of fish cues, this GABAergic inhibition appears to be relieved and specific life-history parameters change. This would elicit the adjustment of life-history parameters in a fast and time-efficient manner.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Alfredo Rago for statistical advice, Felix Felmy for help on the production of Alexa488 electroporation of the illustrated polyploid cell, and Thomas White for editing and proofreading of the manuscript.
Authors' contributions
L.C.W., C.L. and R.T. conceived and designed the study. L.C.W. performed the induction assay. L.C.W. and C.L. performed immunohistochemistry. F.L. conducted qPCR and phylogenetic analysis. L.C.W. and R.T. wrote the manuscript. All authors contributed to the final version of the manuscript.
Competing interests
We have no competing interests.
Funding
L.C.W. was supported by the Ruhr University Research School, funded by Germany's Excellence Initiative (DFG GSC 98/1).
References
- 1.Tollrian R, Harvell CD. 1999. The ecology and evolution of inducible defenses. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Dodson SI. 2006. Cyclomorphosis in Daphnia galeata mendotae birge and D. Retrocurva forbes as a predator-induced response. Freshw. Biol. 19, 109–114. ( 10.1111/j.1365-2427.1988.tb00332.x) [DOI] [Google Scholar]
- 3.Tollrian R. 1995. Predator-Induced morphological defenses: costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 76, 1691–1705. ( 10.2307/1940703) [DOI] [Google Scholar]
- 4.Petrusek A, Tollrian R, Schwenk K, Haas A, Laforsch C. 2009. A ‘crown of thorns’ is an inducible defense that protects Daphnia against an ancient predator. Proc. Natl Acad. Sci. USA 106, 2248–2252. ( 10.1073/pnas.0808075106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tollrian R. 1994. Fish-kairomone induced morphological changes in Daphnia lumholtzi (Sars). Arch. Hydrobiol. 130, 69. [Google Scholar]
- 6.Grant JWG, Bayly IAE. 1981. Predator induction of crests in morphs of the Daphnia carinata king complex. Limnol. Oceanogr. 26, 201–218. ( 10.4319/lo.1981.26.2.0201) [DOI] [Google Scholar]
- 7.Pijanowska J. 1992. Anti-predator defence in three Daphnia species. Int. Rev. Hydrobiol. Hydrogr. 77, 153–163. ( 10.1002/iroh.19920770111) [DOI] [Google Scholar]
- 8.Herzog Q, Laforsch C. 2013. Modality matters for the expression of inducible defenses: introducing a concept of predator modality. BMC Biol. 11, 113 ( 10.1186/1741-7007-11-113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tollrian R. 1990. Predator-induced helmet formation in Daphnia cucullata (sars). Arch. Hydrobiol. 119, 191–196. [Google Scholar]
- 10.Weiss LC, Kruppert S, Laforsch C, Tollrian R. 2012. Chaoborus and Gasterosteus anti-predator responses in Daphnia pulex are mediated by independent cholinergic and gabaergic neuronal signals. PLoS ONE 7, e36879 ( 10.1371/journal.pone.0036879) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oda S, Kato Y, Watanabe H, Tatarazako N, Iguchi T. 2011. Morphological changes in Daphnia galeata induced by a crustacean terpenoid hormone and its analog. Environ. Toxicol. Chem. 30, 232–238. ( 10.1002/etc.378) [DOI] [PubMed] [Google Scholar]
- 12.Miyakawa H, et al. 2010. Gene up-regulation in response to predator kairomones in the water flea, Daphnia pulex. BMC Dev. Biol. 10, 45 ( 10.1186/1471-213X-10-45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis SR, LeBlanc GA, Beckerman AP. 2014. Endocrine regulation of predator-induced phenotypic plasticity. Oecologia 176, 625–635. ( 10.1007/s00442-014-3102-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colbourne JK, et al. 2011. The ecoresponsive genome of Daphnia pulex. Science 331, 555–561. ( 10.1126/science.1197761) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barry MJ. 2002. Progress towards understanding the neurophysiological basis of predator-induced morphology in Daphnia pulex. Physiol. Biochem. Zool. 75, 179–186. ( 10.1086/339389) [DOI] [PubMed] [Google Scholar]
- 16.Miyakawa H, Gotoh H, Sugimoto N, Miura T. 2013. Effect of juvenoids on predator-induced polyphenism in the water flea, Daphnia pulex. J. Exp. Zool. Part A 319A, 440–450. ( 10.1002/jez.1807) [DOI] [PubMed] [Google Scholar]
- 17.Beaton MJ, Hebert PDN. 1997. The cellular basis of divergent head morphologies in Daphnia. Limnol. Oceanogr. 42, 346–356. ( 10.4319/lo.1997.42.2.0346) [DOI] [Google Scholar]
- 18.Weiss LC, Tollrian R, Herbert Z, Laforsch C. 2012. Morphology of the daphnia nervous system: a comparative study on Daphnia pulex, Daphnia lumholtzi, and Daphnia longicephala. J. Morphol. 273, 1392–1405. ( 10.1002/jmor.20068) [DOI] [PubMed] [Google Scholar]
- 19.Naraki Y, Hiruta C, Tochinai S. 2013. Identification of the precise kairomone-sensitive period and histological characterization of necktooth formation in predator-induced polyphenism in Daphnia pulex. Zool. Sci. 30, 619–625. ( 10.2108/zsj.30.619) [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto K, Vernier P. 2011. The evolution of dopamine systems in chordates. Front. Neuroanat. 5, 21 ( 10.3389/fnana.2011.00021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruntenko NE, Karpova EK, Alekseev AA, Chentsova NA, Saprykina ZV, Bownes M, Rauschenbach IY. 2005. Effects of dopamine on juvenile hormone metabolism and fitness in Drosophila virilis. J. Insect. Physiol. 51, 959–968. ( 10.1016/j.jinsphys.2005.04.010) [DOI] [PubMed] [Google Scholar]
- 22.Gruntenko NE, Karpova EK, Adonyeva NV, Chentsova NA, Faddeeva NV, Alekseev AA, Rauschenbach IY. 2005. Juvenile hormone, 20-hydroxyecdysone and dopamine interaction in Drosophila virilis reproduction under normal and nutritional stress conditions. J. Insect. Physiol. 51, 417–425. ( 10.1016/j.jinsphys.2005.01.007) [DOI] [PubMed] [Google Scholar]
- 23.Tollrian R. 1993. Neckteeth formation in Daphnia pulex as an example of continuous phenotypic plasticity: morphological effects of Chaoborus kairomone concentration and their quantification. J. Plankton Res. 15, 1309–1318. ( 10.1093/plankt/15.11.1309) [DOI] [Google Scholar]
- 24.Peñalva-Arana C, Moore A, Feinberg A, DeWall J, Strickler R. 2007. Studying Daphnia feeding behavior as a black box: a novel electrochemical approach. Hydrobiology 594, 153–163. ( 10.1007/s10750-007-9080-7) [DOI] [Google Scholar]
- 25.Barrozo E, Fowler D, Ingebretson J, Beckman M. 2014. The role of dopaminergic signaling in Daphnia magna swimming. FASEB 28, 839–845. [Google Scholar]
- 26.Scoville AG, Pfrender ME. 2010. Phenotypic plasticity facilitates recurrent rapid adaptation to introduced predators. Proc. Natl Acad. Sci. USA 107, 4260–4263. ( 10.1073/pnas.0912748107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spanier KI, Leese F, Mayer C, Colbourne JK, Gilbert D, Pfrender ME, Tollrian R. 2010. Predator-induced defences in Daphnia pulex: selection and evaluation of internal reference genes for gene expression studies with real-time PCR. BMC Mol. Biol. 11, 50 ( 10.1186/1471-2199-11-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. 2004. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat. Neurosci. 7, 726–735. ( 10.1038/nn1265) [DOI] [PubMed] [Google Scholar]
- 29.Neckameyer W, O'Donnell J, Huang Z, Stark W. 2001. Dopamine and sensory tissue development in Drosophila melanogaster. J. Neurobiol. 47, 280–294. ( 10.1002/neu.1035) [DOI] [PubMed] [Google Scholar]
- 30.Dawirs RR, Hildebrandt K, Teuchert-Noodt G. 1998. Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J. Neural Transm. 105, 317–327. ( 10.1007/s007020050061) [DOI] [PubMed] [Google Scholar]
- 31.Laforsch C, Tollrian R. 2004. Embryological aspects of inducible morphological defenses in Daphnia. J. Morphol. 262, 701–707. ( 10.1002/jmor.10270) [DOI] [PubMed] [Google Scholar]
- 32.Martin-Creuzburg D, Westerlund SA, Hoffmann KH. 2007. Ecdysteroid levels in Daphnia magna during a molt cycle: determination by radioimmunoassay (RIA) and liquid chromatography-mass spectrometry (LC-MS). Gen. Comp. Endocrinol. 151, 66–71. ( 10.1016/j.ygcen.2006.11.015) [DOI] [PubMed] [Google Scholar]
- 33.Sumiya E, Ogino Y, Miyakawa H, Hiruta C, Toyota K, Miyagawa S, Iguchi T. 2014. Roles of ecdysteroids for progression of reproductive cycle in the freshwater crustacean Daphnia magna. Front. Zool. 11, 60 ( 10.1186/s12983-014-0060-2) [DOI] [Google Scholar]
- 34.Nakatsuji T, Lee CY, Watson RD. 2009. Crustacean molt-inhibiting hormone: structure, function, and cellular mode of action. Comp. Biochem. Physiol. A 152, 139–148. ( 10.1016/j.cbpa.2008.10.012) [DOI] [PubMed] [Google Scholar]
- 35.Robinson GE. 1987. Regulation of honey bee age polyethism by juvenile hormone. Behav. Ecol. Sociobiol. 20, 329–338. ( 10.1007/BF00300679) [DOI] [Google Scholar]
- 36.Le Trionnaire G, et al. 2009. Transcriptomic and proteomic analyses of seasonal photoperiodism in the pea aphid. BMC Genomics 10, 456 ( 10.1186/1471-2164-10-456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Truman JW, Riddiford LM, Safranek L. 1974. Temporal patterns of response to ecdysone and juvenile hormone in the epidermis of the tobacco hornworm, Manduca sexta. Dev. Biol. 39, 247–262. [DOI] [PubMed] [Google Scholar]
- 38.Nijhout HF, Wheeler DE. 1982. Juvenile hormone and the physiological basis of insect polymorphisms. Q. Rev. Biol. 57, 109–133. ( 10.1086/412671) [DOI] [Google Scholar]
- 39.Emlen DJ, Szafran Q, Corley LS, Dworkin I. 2006. Insulin signaling and limb-patterning: candidate pathways for the origin and evolutionary diversification of beetle horns. Heredity 97, 179–191. ( 10.1038/sj.hdy.6800868) [DOI] [PubMed] [Google Scholar]
- 40.Gotoh H, Cornette R, Koshikawa S, Okada Y, Lavine LC, Emlen DJ, Miura T. 2011. Juvenile hormone regulates extreme mandible growth in male stag beetles. PLoS ONE 6, e21139 ( 10.1371/journal.pone.0021139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nijhout HF. 2013. Arthropod developmental endocrinology. In Arthropod biology and evolution (eds Minelli A, Boxshall G, Fusco G), pp. 123–148. Berlin, Germany: Springer. [Google Scholar]
- 42.Sasaki K, Akasaka S, Mezawa R, Shimada K, Maekawa K. 2012. Regulation of the brain dopaminergic system by juvenile hormone in honey bee males (Apis mellifera L.). Insect Mol. Biol. 21, 502–509. ( 10.1111/j.1365-2583.2012.01153.x) [DOI] [PubMed] [Google Scholar]
- 43.Sasaki K, Matsuyama S, Harano K, Nagao T. 2012. Caste differences in dopamine-related substances and dopamine supply in the brains of honeybees (Apis mellifera L.). Gen. Comp. Endocrinol. 178, 46–53. ( 10.1016/j.ygcen.2012.04.006) [DOI] [PubMed] [Google Scholar]
- 44.Koch PB, Keys DN, Rocheleau T, Aronstein K, Blackburn M, Carroll SB. 1998. Regulation of dopa decarboxylase expression during colour pattern formation in wild-type and melanic tiger swallowtail butterflies. Development 125, 2303–2313. [DOI] [PubMed] [Google Scholar]
- 45.Peñalva-Arana DC, Lynch M, Robertson HM. 2009. The chemoreceptor genes of the waterflea Daphnia pulex: many GRs but no ORs. BMC Evol. Biol. 9, 79 ( 10.1186/1471-2148-9-79) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sherald AF. 1980. Sclerotization and coloration of the insect cuticle. Cell Mol. Life Sci. 36, 143–146. ( 10.1007/BF01953696) [DOI] [Google Scholar]
- 47.Watanabe T, Sadamoto H, Aonuma H. 2013. Molecular basis of the dopaminergic system in the cricket Gryllus bimaculatus. Invert. Neurosci. 13, 107–123. ( 10.1007/s10158-013-0153-1) [DOI] [PubMed] [Google Scholar]
- 48.Andersen SO. 2010. Insect cuticular sclerotization: a review. Insect Biochem. Mol. Biol. 40, 166–178. ( 10.1016/j.ibmb.2009.10.007) [DOI] [PubMed] [Google Scholar]
- 49.Laforsch C, Ngwa W, Grill W, Tollrian R. 2004. An acoustic microscopy technique reveals hidden morphological defenses in Daphnia. Proc. Natl Acad. Sci. USA 101, 15 911–15 914. ( 10.1073/pnas.0404860101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tollrian R, Heibl C. 2004. Phenotypic plasticity in pigmentation in Daphnia induced by UV radiation and fish kairomones. Funct. Ecol. 18, 497–502. ( 10.1111/j.0269-8463.2004.00870.x) [DOI] [Google Scholar]
- 51.Nijhout HF, Riddiford LM, Mirth C, Shingleton AW, Suzuki Y, Callier V. 2014. The developmental control of size in insects. Dev. Biol. 3, 113–134. ( 10.1002/wdev.124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Picciotto MR, Higley MJ, Mineur YS. 2012. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron 76, 116–129. ( 10.1016/j.neuron.2012.08.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Käuser G, Brandtner HM, Bidmon H-J, Koolman J. 1988. Ecdysone synthesis and release by the brain-ring gland complex of blowfly larvae. J. Insect Physiol. 34, 563–569. ( 10.1016/0022-1910(88)90060-1) [DOI] [Google Scholar]
- 54.Beckerman AP, de Roij J, Dennis SR, Little TJ. 2013. A shared mechanism of defense against predators and parasites: chitin regulation and its implications for life-history theory. Ecol. Evol. 3, 5119–5126. ( 10.1002/ece3.766) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.