Abstract
Terrestrial plants and mammals, although separated by a great evolutionary distance, have each arrived at a highly conserved body plan in which universal allometric scaling relationships govern the anatomy of vascular networks and key functional metabolic traits. The universality of allometric scaling suggests that these phyla have each evolved an ‘optimal’ transport strategy that has been overwhelmingly adopted by extant species. To truly evaluate the dominance and universality of vascular optimization, however, it is critical to examine other, lesser-known, vascularized phyla. The brown algae (Phaeophyceae) are one such group—as distantly related to plants as mammals, they have convergently evolved a plant-like body plan and a specialized phloem-like transport network. To evaluate possible scaling and optimization in the kelp vascular system, we developed a model of optimized transport anatomy and tested it with measurements of the giant kelp, Macrocystis pyrifera, which is among the largest and most successful of macroalgae. We also evaluated three classical allometric relationships pertaining to plant vascular tissues with a diverse sampling of kelp species. Macrocystis pyrifera displays strong scaling relationships between all tested vascular parameters and agrees with our model; other species within the Laminariales display weak or inconsistent vascular allometries. The lack of universal scaling in the kelps and the presence of optimized transport anatomy in M. pyrifera raises important questions about the evolution of optimization and the possible competitive advantage conferred by optimized vascular systems to multicellular phyla.
Keywords: allometry, scaling, phloem, Laminariales, kelp, Macrocystis pyrifera
1. Introduction
Biological transport networks are a key innovation in the evolution of modern complex organisms. Across the tree of life, there is evidence that vascular transport networks are optimized, balancing maximum speed and integrity of resource delivery with minimal resource investment in transport and infrastructure. West et al. [1] posited that the observed universal allometric scaling relationships between key anatomical traits and metabolism were attributable to optimization in biological resource networks. Their work fuelled a flurry of inquiry into the mechanisms and implications of vascular scaling relationships. In the plant kingdom, robust and universal allometric scaling between functional and anatomical properties of transport cells has been observed, from angiosperms to gymnosperms to bryophytes [2,3]. Even the simplest ‘one-dimensional’ conifer leaves display size scaling of xylem vessel elements, achieving a modelled 10% reduction in transport energy loss relative to a structure with size-invariant vessels [4]. In mammals, strong power-law relationships have been observed between vascular path length and body size across multiple venous and arterial networks of different skeletal muscles and organs [5]. The universality of the scaling exponents within each of these phyla suggests that both terrestrial plants and animals have separately arrived upon a similar optimal strategy for delivering resources to support structure, function and metabolism.
This idea that diverse kingdoms have independently evolved optimized allometric scaling of their vascular networks led us to examine organisms of another kingdom altogether—the Chromista, in which the brown algae (Phaeophyceae, Ochrophyta) reside. Kelps (Laminariales) are as distantly related to plants as metazoans; the basal clades of the Ochrophyta are flagellated unicellular eukaryotes such as diatoms [6,7]. Despite this evolutionary distance, they possess a remarkable convergent physiology. On a macroscopic level, brown algae are often plant-like, bearing specialized analogues of plant organs such as blades (leaves), stipes (stems) and sporangia (reproductive organs). At the cell and tissue level, a few orders of the brown algae also possess a phloem-like long-distance transport network of trumpet-shaped sieve elements (SEs) separated by perforated sieve plates (figure 1). This long-distance transport system moves carbohydrates (mannitol, glucose and mannose), amino acids, heavy metals and perhaps signalling compounds [8,9]. A survey of translocation velocities in the Laminariales found that transport rates of radiolabelled C14 range from 50 to 780 mm h−1 [10]. While macroalgal SEs resemble those of plants, they have no structures analogous to xylem or companion cells, and the mechanism of SE loading is still unclear.
Figure 1.
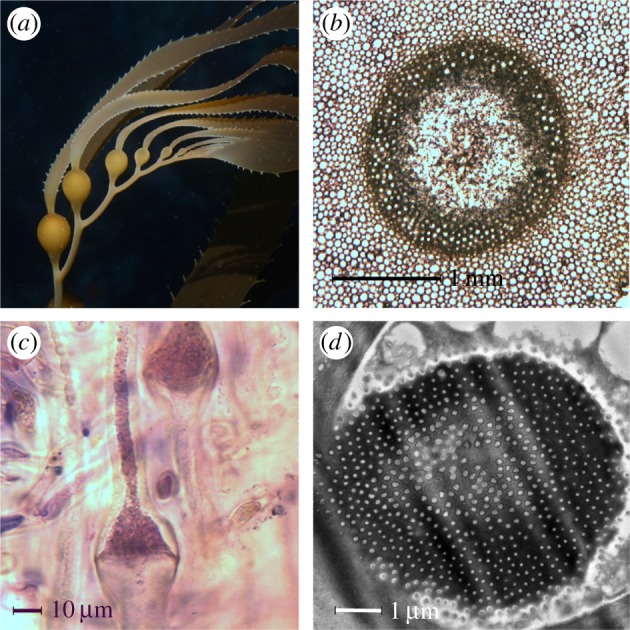
(a) Macrocystis pyrifera sporophyte. Credit: S. Lonhart. (b) Cross-section of N. luetkeana medulla (dark circle) containing SEs. (c) Occluded A. marginata SE in longitudinal section. (d) Alaria marginata sieve plate (transmission electron microscopy, TEM). Credit: S.T.D. (Online version in colour.)
Like terrestrial plants, brown macroalgae play a critical role in the ecology of near shore marine systems and in the global carbon cycle. Larger species like the giant kelp (Macrocystis pyrifera) are ecosystem engineers, providing complex three-dimensional structures that form the basis of diverse temperate reefs [11]. Coastal kelp beds in particular are incredibly productive, surpassing the net primary production of tropical rainforests with 1000 g C m−2 yr−1 [12,13]. Despite the ecological importance of kelps in the Laminariales, the structure, function and scaling of their vascular system has been minimally explored. In this paper, we ask the following question: do members of the Laminariales display allometric power-law scaling of their SEs indicative of optimal resource allocation?
We approached this by developing a model for optimized resource transport in brown macroalgae. The model is based on the concept that optimized phloem transport occurs when the osmotic pressure gradient required to drive translocation is minimized, as is the volume of the SEs required to serve the entire organism. Such a network is characterized by the tapering of phloem area in terminal branching units and unloading zones. We then tested the quantitative scaling predictions of the optimal model using the giant kelp, M. pyrifera, a species whose translocation patterns have been carefully studied. Furthermore, we investigated whether the classic vascular plant allometric relationships (SE diameter versus conduit packing, vascular fraction, stipe diameter, biomass and metabolic area) could be observed in M. pyrifera as well as five other members of the family Laminariales ( 1).
Table 1.
Scaling parameters for the log–log relationship between average SE diameter and various functional anatomy traits. (Only significant relationships (p < 0.05) are shown; for all species, see the electronic supplementary material.)
| independent variable | species | slope | intercept | r2 |
|---|---|---|---|---|
| stipe diameter (cm) | L. setchellii | 0.147 | 1.128 | 0.28 |
| M. pyrifera | 0.871 | 1.660 | 0.89 | |
| N. luetkeana | 0.302 | 1.448 | 0.23 | |
| P. californica | 0.197 | 1.137 | 0.11 | |
| conduit packing (SE µm−2) | M. pyrifera | −0.676 | −0.955 | 0.95 |
| vascular fraction | A. marginata | 0.346 | 1.346 | 0.40 |
| E. menziesii | 0.558 | 1.503 | 0.22 | |
| L. setchellii | 0.526 | 1.517 | 0.32 | |
| M. pyrifera | 1.597 | 2.608 | 0.68 | |
| N. luetkeana | 0.735 | 1.991 | 0.46 | |
| P. californica | 0.601 | 1.568 | 0.49 | |
| cumulative blade area (cm2) | A. marginata | 0.108 | 0.833 | 0.37 |
| L. setchellii | 0.229 | 0.366 | 0.46 | |
| M. pyrifera | 0.193 | 0.803 | 0.88 | |
| path length (m) | M. pyrifera | 0.280 | 1.427 | 0.85 |
| cumulative biomass (g) | L. setchellii | 0.239 | 0.747 | 0.25 |
| M. pyrifera | 0.188 | 1.204 | 0.89 |
2. Material and methods
(a). Study species
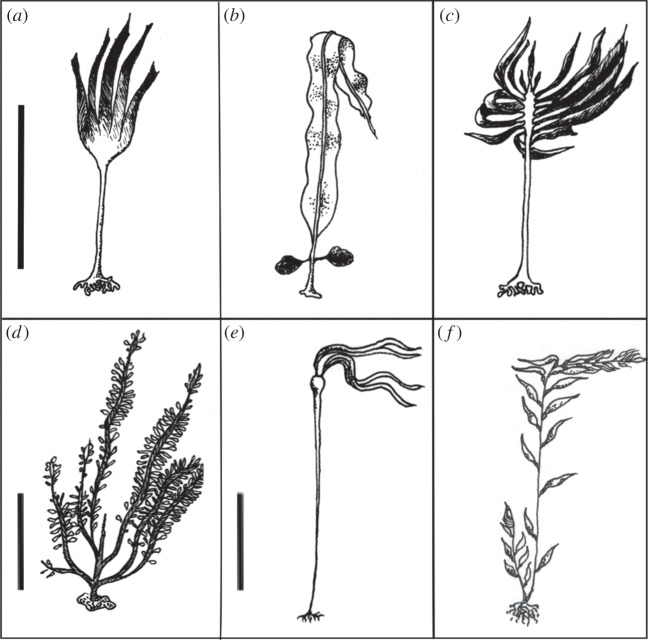
The six species chosen for this study spanned a broad size range (figure 2). Three individuals each of Nereocystis luetkeana and M. pyrifera (large; more than 10 m tall), Egregia menziesii (intermediate; between 2 and 5 m tall), and Laminaria setchellii, Pterygophora californica and Alaria marginata (small; less than 2 m tall) were sampled. These species represent the diversity of macroalgal habits and include taxa that are annual, perennial, upright, prostrate, subtidal and intertidal. These morphologically diverse species were chosen to thoroughly test the hypothesis that observed vascular scaling relationships would be common to all members of the Laminariales. Species were collected intertidally or on SCUBA from the Monterey Bay region of Central California (Davenport Landing, Davenport (37.024663, −122.217210); Lover's Point, Pacific Grove (36.626419, −121.915837); Stillwater Bay, Carmel (36.626419, −121.915837); and Big Creek, Big Sur (36.068229, −121.600362). Three to five individuals of each species were collected, and individuals of each species were collected from the same site. Collections were carried out in summer of 2012 and 2013, as well as November 2013.
Figure 2.
Illustration of the six study species: (a–d) L. setchellii, A. marginata, P. californica and E. menziesii; scale bar, 1 m; (e,f) N. luetkeana and M. pyrifera; scale bar, 5 m. Credit: S.T.D.
(b). Sampling methods
After collection, each individual was immediately refrigerated with seawater-soaked paper towels in a closed container for the duration of their dissection. Each individual (or individual frond, if from multi-frond species M. pyrifera and E. menziesii) was cut into 15–25 segments, each representing less than 10% of total thallus length. For each segment, segment length, stipe diameter and attached blade length were measured, as was total photosynthetic area, measured with a LiCOR Li-3100C Area Meter (Lincoln, NE, USA). A subsample of 2–3 cm was reserved in refrigerated seawater for sectioning and microscopic examination; the remaining tissue was dried for biomass analysis. Each subsample was hand sectioned using a razor blade or manual microtome, stained with toluidine blue to provide contrast and photographed on a Motic BA210 microscope mounted with a Moticam digital camera (JH Technologies, San Jose, CA, USA).
Individual SE areas and total medulla (vascular tissue) areas were measured from digital micrographs using Fiji (ImageJ) software [14]. We measured only cells that exhibited the classic ‘trumpet hyphae’ morphology in longitudinal section, identifiable by hallmark swelling of the cell wall in cross-section [15]. We did not measure the interconnected cells known as ‘hyphae’ in the periphery of the medulla; they failed to transport carboxyfluorescein diacetate in preliminary trials and lack the swelling mechanism of pressurized true SE. At least 100 SEs were measured per segment; a total of 37 751 individual SEs were measured in this work. Each segment of an alga was assigned a transport status: ‘loading’, a section of tissue assumed to be only loading photoassimilate into the phloem; ‘unloading’ a section solely unloading from the phloem; and ‘both’, for which bi-directional transport occurs (electronic supplementary material, figure S5). Estimates were based on published C14 tracer studies of study species or congeners sharing their body plans [10,16]. Average SE diameter, conduit packing, vascular fraction and total estimated phloem area were calculated for each segment.
(c). Model development
In our optimal model of phloem functional anatomy, phloem area is expected to taper from large conduits at the start of a loading or unloading zone to smaller conduits at the terminus of transport (distal meristem or storage tissue). This total phloem area taper can manifest as a reduction in the number of SEs (N) or a reduction in individual SE area (A). In the majority of sampled species, N was invariant; thus, we formulated the model to predict variation in A (see the electronic supplementary material).
Below, we develop a one-dimensional phloem transport model for the relationship between SE area and height for macroalgal tissue that exclusively loads or unloads from the phloem conduits. For the full model derivation, see the electronic supplementary material.
Briefly, we assume conservation of carbohydrate mass:
where A(x) = N(x)a(x) is the conductive phloem area, N(x) is the number of conduits, a(x) is the individual conduit area, u(x) is the translocation speed, c is the sap carbohydrate concentration and q is the unloaded volume of sap per unit length. The coordinate x is measured from the start of the unloading zone (ℓ = 1 m from the apex), and we write it as x = ℓ + x* − H, where H is the organism height, and x* is the distance from the holdfast. Using Darcy's law for the relationship between velocity and pressure p: dp/dx = −ηu/k(x), where k is the conductivity, we arrive at an expression for the pressure drop across the unloading zone:
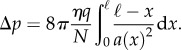 |
Minimizing the pressure drop subject to a constant volume constraint leads to the prediction 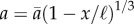 , where
, where 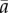 is the area at x = 0. Compared to a uniform collection of pipes (a(x) = const.), the optimum area distribution requires approximately 20% less pressure to drive the same flow. Both this optimal slope (1/3) and the suboptimal slope (0, representing size-invariant SEs) were used as null hypotheses to test for optimality in M. pyrifera, the only brown algal species in which unidirectional transport has been observed [16]. A similar approach was previously used to study architecture of xylem [4] and phloem [17] in pine needles.
is the area at x = 0. Compared to a uniform collection of pipes (a(x) = const.), the optimum area distribution requires approximately 20% less pressure to drive the same flow. Both this optimal slope (1/3) and the suboptimal slope (0, representing size-invariant SEs) were used as null hypotheses to test for optimality in M. pyrifera, the only brown algal species in which unidirectional transport has been observed [16]. A similar approach was previously used to study architecture of xylem [4] and phloem [17] in pine needles.
(d). Testing for allometric relationships
We tested three fundamental relationships observed in plant xylem and theoretically expected for phloem. Although xylem and phloem operate under different mechanisms for fluid transport and hydraulic safety, we expected them to exhibit similar allometric trends. Fundamentally, both systems are fluid transport conduits driven by pressure gradients and both are theoretically optimized by the minimization of this gradient.
Relationship 1: SE diameter versus stipe diameter. As in terrestrial plant xylem, we hypothesized that conduit diameter would increase log-linearly as stem diameter increased [2,18–21]. This relationship is primarily a developmental phenomenon and indicates a programmed genetic algorithm for growth. This relationship is universal in angiosperm and gymnosperm trees with an average exponent of 0.27 [2].
Relationship 2: SE area versus conduit packing (transport cells per unit area). We hypothesized that the total conduit area of vascular tissue should remain roughly constant throughout the entire sporophyte of an alga. With this requirement, tapering of individual SE area must be balanced by a denser packing of these small conduits into the medulla to transport the same volume of photoassimilate. In short, small SEs should be more numerous than large SEs in the same medulla area (the packing rule, [18,22]). In the xylem of angiosperm and gymnosperm trees, this relationship is again universal with a scaling exponent of approximately 2.0 [2]. In kelps, we predicted a similar log-linear relationship where conduit packing decreases with increasing conduit size.
Relationship 3: SE area versus biomass, cumulative blade (photosynthetic) area and path length (l-trans, the distance that photosynthate must move from the observed SE). We hypothesized that the diameter of a transport conduit at any given point along the sporophyte would scale exponentially with the photosynthetic area that conduit must support (a proxy for metabolic function). Cumulative blade area is defined as the total blade area ‘supported’ by each dissected segment; this includes any tissue that potentially sends photoassimilate through the segment. Maximum cumulative blade area is usually supported by segments of a macroalga that engage in bi-directional transport (not exclusively sink or source tissue).
The values and universality of the scaling exponents of these log-linear relationships for each study species were analysed using a reduced major axis approach in R (SMATR package) and are fully reported in the electronic supplementary material.
3. Results
The model for optimized phloem transport in brown algae describes an optimal distribution of total phloem area along the length of an individual sporophyte in tissue that is exclusively loading or unloading from the phloem. The model predicts a slope of 1/3 for the linear allometric log-transformed relationship between average SE area (µm2) and distance from the terminus of the unloading zone (m). However, scaling exponents in the range 0.15 < n < 0.5 are within 5% of the efficiency of the optimum value. Macroalgae adhering to this optimization mechanism theoretically conserve 20% of their transport energy expenditure by tapering conduits in this manner (see the electronic supplementary material).
Of the six study species, M. pyrifera was the only species for which we had sufficient physiological data to test the model. N is invariant as a function of sporophyte height for M. pyrifera (figure 3a). However, we detected a strong positive relationship between A and 1 − x/l (inverse height along the sporophyte) for the distal ‘unloading’ zone in M. pyrifera. The slope of this relationship falls within the 95% confidence intervals for the predicted optimal slope of 1/3 (slope (m) = 0.45, p = 0.0003, r2 = 0.276) (figure 3b,c). This slope is significantly different from the suboptimal hypothesis of a zero slope (p < 0.0001).
Figure 3.
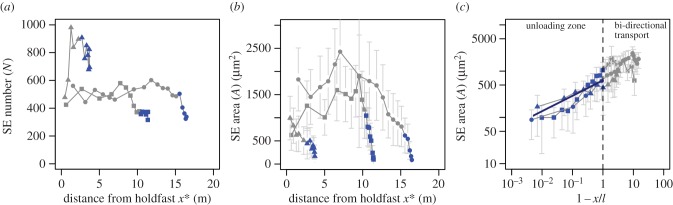
(a) Number of conduits N and (b) average SE cross-sectional area A versus distance from the holdfast x∗ in M. pyrifera. Segments (points) from a single M. pyrifera frond are joined by a line; three individual fronds (symbols) are shown. Points in each frond that purely unload from the phloem are dark blue; segments engaging in bi-directional transport are light grey. (c) Relationship between A and 1 − x/l (inverse height). The data fall along the predicted optimum scaling slope (solid blue line) within a 5% confidence interval. All error bars represent s.d. (Online version in colour.)
In general, the distribution of individual SE areas was normal within each segment of each sporophyte across all species examined. In M. pyrifera, however, segments from the distal 0.5 m of each frond are distinctly skewed towards smaller values. Additionally, when the coefficient of variation is calculated for individual segments of M. pyrifera, it is clear that variance in SE areas increases linearly with increasing height along the sporophyte (i.e. youngest tissue displays the most cell area variance, p < 0.0001, r2 = 0.33) (electronic supplementary material, figure S6).
Moving beyond the allometry predicted by the model, we explored three additional functional relationships that we expected to display allometric scaling (and thus optimization).
(a). Relationship 1
In kelps, there is no single universal scaling relationship observed in the log–log plot of stipe diameter versus average SE diameter. Those that do scale, however, do so in a manner similar to terrestrial plants, where SE diameter increases proportionally with stipe diameter. Laminaria setchellii and P. californica are not statistically different from one another (m = 0.15, p = 0.0003, r2 = 0.28; and m = 0.20, p = 0.03, r2 = 0.11, respectively). Nereocystis luetkeana also displays a significant relationship (m = 0.30, p = 0.0006, r2 = 0.23). These three species have low r2 values, indicating high variability around the slope, whereas M. pyrifera displays a much stronger positive relationship (m = 0.87, p < 0.0001, r2 = 0.81) (figure 4a).
Figure 4.
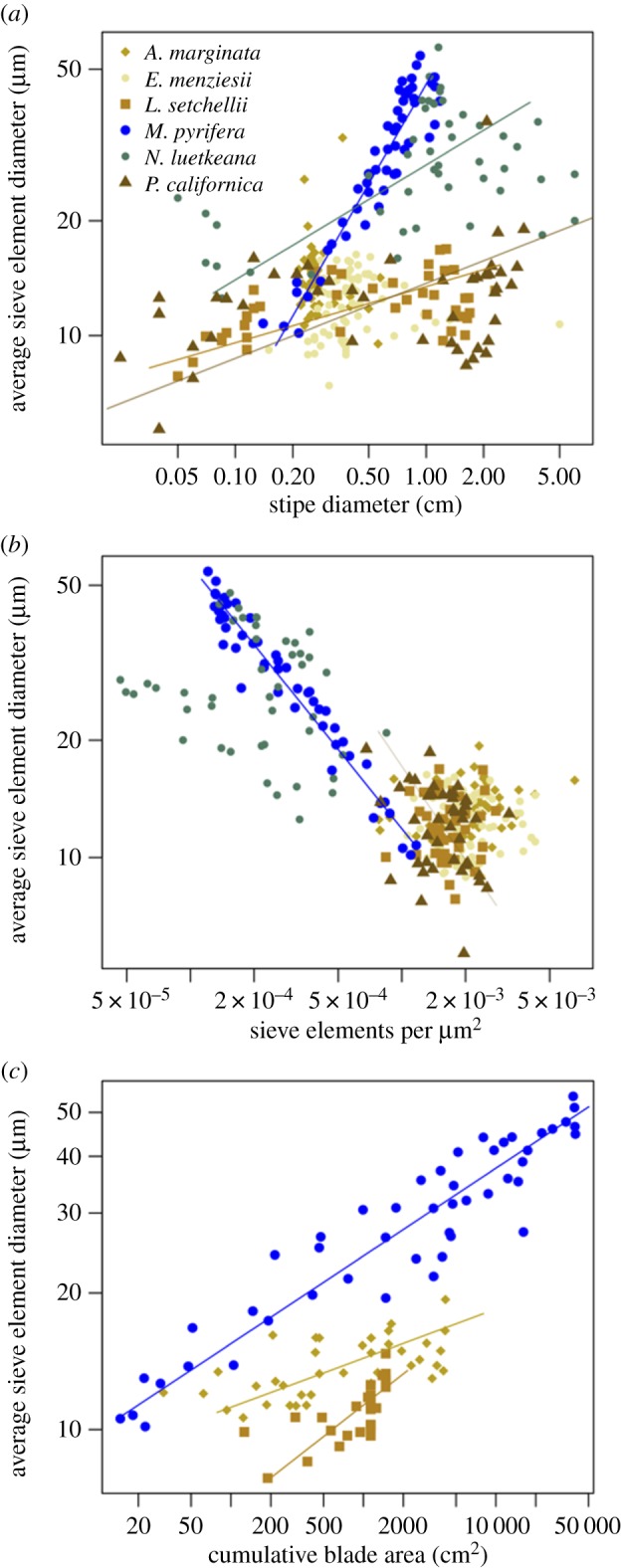
Axes are log-transformed. Each point represents a segment of an individual sporophyte. (a) The developmental scaling relationship between SE diameter and stipe diameter in six kelp species. Four of the six species display significant relationships: for M. pyrifera, m = 0.87; for N. luetkeana, m = 0.30; P. californica and L. setchellii are indistinguishable with m = 0.20 and 0.15, respectively. (b) The packing rule in kelp. Average SE diameter is plotted as a function of conduit packing (number of SE per µm2); only M. pyrifera displays a significant relationship, m = −0.53). (c) The allometry of SE size and supported metabolic tissue. Average SE diameter is plotted as a function of cumulative blade area supported by each segment. Three species scale: A. marginata, m = 0.11; L. setchellii and M. pyrifera are statistically indistinguishable with m = 0.23 and 0.19, respectively. (Online version in colour.)
(b). Relationship 2
Of our six species, only M. pyrifera displays a significant relationship in the log–log plot of average SE diameter versus conduit packing (m = 0.67, p < 0.0001) (figure 4b).
(c). Relationship 3
The relationship between average SE diameter and cumulative blade area (figure 4c) is highly significant and explains substantial portions of the variation in SE diameter for both M. pyrifera (m = 0.19, p < 0.0001, r2 = 0.78) and L. setchellii (m = 0.23, p < 0.0001, r2 = 0.46). Alaria marginata also displays a significant relationship (m = 0.11, p < 0.0001, r2 = 0.37).
4. Discussion
Several surprising results emerge from our analysis of scaling relationships in kelp phloem. The first such result is the lack of universal allometric scaling between SE area and stipe diameter, path length, or metabolic area among study species. Despite morphological diversity, basic uniform allometric relationships have been observed between anatomical parameters of vascular networks and body size/metabolic rate in both terrestrial plants and mammals [2,5]. The theory of metabolic scaling is fundamentally based on the idea that body size and metabolism are constrained by the network that delivers resources to active metabolic tissue [1,23,24].There is no a priori reason to assume that brown macroalgae should possess vascular allometries similar to either terrestrial plant xylem, plant phloem or mammalian cardiovascular systems; however, if these other phyla are any example, we did expect that if an advantageous optimized architecture had sufficiently strong evolutionary drivers and time to emerge in one member of the Laminariales, it would have been adopted throughout the order.
Instead, we observe that members of the Laminariales possess significantly different scaling exponents, as is exemplified by the relationship between SE diameter and stipe diameter (figure 4a). These differences are even more dramatic in other tested allometric relationships, yielding a strong dichotomy between M. pyrifera and the majority of our study species (A. marginata, E. menziesii, L. setchellii, N. luetkeana, P. californica). For every relationship tested, M. pyrifera displays significant allometric relationships indicative of optimal network design, whereas the remaining species display weaker or inconsistent covariation. The data indicate that most kelps possess SE networks that vary little in diameter, and that their vascular anatomy is driven by selective forces other than optimization of carbohydrate transport. These findings lead us to question what ecological and life-history drivers produce the consistent and predictable scaling of M. pyrifera. Is there a body size or sink size threshold over which carbohydrate transport must be optimized? Do smaller kelps retain an ancestral function of the brown algal SE cell network different than that of carbohydrate transport? Finally, is there directionality to the evolution of vascular function within the Laminariales?
The members of the Laminariales that do not display strong vascular allometry may instead possess body plans optimized for other ecologically relevant functions, such as spore dispersal, resistance to hydrodynamic forces or herbivory. Furthermore, most of these macroalgae are less than 2 m tall, so their body size may constrain their maximum SE size and thus variance and detectable allometric scaling. Even in terrestrial plants, smaller plants may not display as broad a range of conduit sizes as large plants [25]. Plant and algal transport pathways may be very complicated, with bi-directional activity and possible relays [19]; it is conceivable that robust scaling of vascular anatomy exists on scales much smaller than those measured in our whole-sporophyte approach. The strength and isolation of carbohydrate sources and sinks is not as extreme in small macroalgae as it is in large M. pyrifera or terrestrial plants. With the exception of A. marginata, these small species co-locate their reproductive tissue with vegetative tissue, minimizing the need for transport. Small kelps have diverse meristem types and placement, and photosynthesize in all parts of the sporophyte. Perhaps, ecological drivers have favoured a flexibility of resource generation over energy-optimized transport in some species.
It is also possible that the ancestral function of the SEs is not to transport energetic subsidies, but rather to facilitate signalling for development or defence. Signalling and development in macroalgae is poorly understood, but there have been studies showing that the intercalary meristem of Laminaria japonica releases a ‘sporification inhibitor’ to mature tissues, preventing tissue from becoming catastrophically reproductive before the proper season [26]. If molecules of this kind are being transported via the SEs, this could provide an alternate view on the early evolutionary role of non-optimized transport networks in brown algae and terrestrial plants.
Evolutionary drivers of optimization are suggested by the unique traits and phenology of M. pyrifera. It is the most plant-like of the Laminariales, with multiple stipes bearing alternately branching blades, and true apical meristems. It is an order of magnitude larger than all but four species in the Laminariales. It is unique among canopy-forming algae in that it has basal meristems and reproductive tissue (i.e. new fronds arise at the base of the alga in a light-limited environment), which require carbon subsidies from surface fronds (electronic supplementary material, figure S4). Increased size, specialization or spatial organ separation could have driven the evolution of transport optimization in giant kelp.
It is possible that the observed scaling relationships between SE cells and gross anatomy are an artefact of development in M. pyrifera. The smallest conducting cells are located in the apical meristem and could simply be immature as opposed to tapered for optimized transport. This interpretation is unlikely to be correct. In M. pyrifera, the largest SEs and stipe diameters are located not at the oldest point of each mature frond (the base), but rather mid-frond, the most productive portion of the sporophyte (figure 3b). This association of large SEs with productivity instead of age indicates that there is a developmental programme for SE size in M. pyrifera, possibly triggered by light availability. Secondly, there is the fact of increased variance in individual SE area in immature apical tissues. While apical tissue contains mostly small SEs (5–10 µm in diameter), it also supports a few SEs that are an order of magnitude larger. These ‘disproportionately’ large SEs have the capacity to support exponentially higher phloem conductivity and velocity [25]. It is likely that these large SEs are allocated for later transport as the stipe elongates, while the more numerous small SEs conduct transport more appropriate to their current loading position.
The Laminariales and other phloem-bearing brown macroalgae are evolutionarily younger than terrestrial plants. The first vascular tracheophytes emerged 419–454 Ma, whereas the common Phaeophycean ancestor of the phloem-bearing lineages is estimated to have evolved 189 Ma, and the Laminariales 84 Ma [27,28]. It is conceivable that the evolution of a functional long-distance transport network is in its infancy in brown algae; in this way, our study of scaling and simple functional anatomy is not only an exploration of algal biology, but also an exploration of the broader process of the evolution of transport structures and network optimization in any phylum.
Supplementary Material
Acknowledgements
We gratefully acknowledge Prof. Innes Cuthill and two anonymous reviewers for handling and improving this manuscript. We are grateful to Alex Baer and Marilyne Allietta for assistance in data collection, and to the Raimondi-Carr Laboratory, Michael Fox and Mark Readdie for specimen collection. The authors also acknowledge Michael Knoblauch and the staff at the Franceschi Microscopy & Imaging Center for assistance with TEM imaging.
Data accessibility
The following datasets are freely accessible on Dryad at the following doi: http://dx.doi.org/10.5061/dryad.d5s92. Phloem anatomy, Laminariales: raw individual SE data table, measured from micrographs. Phloem anatomy (organismal level): averaged SE diameter, variance analyses and gross anatomical data for multiple replicates of all six study species.
Authors' contributions
S.T.D. designed the sampling protocol, acquired data, analysed data and wrote the paper. K.H.J. developed the model of optimal vascular transport in macroalgae and contributed methods. P.P. acquired micrographs and analysed data. J.P. guided study development, data acquisition and analysis. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
The authors were partially funded by National Science Foundation grant IOS 1027410 (to J.P.). S.T.D. was supported by the Graduate Research Fellowship Program of the National Science Foundation. K.H.J. was supported by the Carlsberg Foundation grant no. 2013_01_0449.
References
- 1.West GB, Brown JH, Enquist BJ. 1999. The fourth dimension of life: fractal geometry and allometric scaling of organisms. Science 284, 1677–1679. ( 10.1126/science.284.5420.1677) [DOI] [PubMed] [Google Scholar]
- 2.Savage VM, Bentley L, Enquist BJ, Sperry JS, Smith DD, Reich PB, Allmen EI. 2010. Hydraulic trade-offs and space filling enable better predictions of vascular structure and function in plants. Proc. Natl Acad. Sci. USA 107, 22 722–22 727. ( 10.1073/pnas.1012194108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niklas KJ. 1993. The allometry of plant reproductive biomass and stem diameter. Am. J. Bot. 80, 461–467. ( 10.2307/2445392) [DOI] [Google Scholar]
- 4.Zwieniecki MA, Stone H, Leigh A, Boyce C, Holbrook NM. 2006. Hydraulic design of pine needles: one-dimensional optimization for single-vein leaves. Plant Cell Environ. 29, 803–809. ( 10.1111/j.1365-3040.2005.01448.x) [DOI] [PubMed] [Google Scholar]
- 5.Kassab GS. 2006. Scaling laws of vascular trees: of form and function. Am. J. Physiol. Heart Circ. Physiol. 290, H894–H903. ( 10.1152/ajpheart.00579.2005) [DOI] [PubMed] [Google Scholar]
- 6.Baldauf SL. 2008. An overview of the phylogeny and diversity of eukaryotes. Evolution 46, 263–273. [Google Scholar]
- 7.Riisberg I, Orr RJS, Kluge R, Shalchian-Tabrizi K, Bowers HA, Patil V, Edvardsen B, Jakobsen KS. 2009. Seven gene phylogeny of heterokonts. Protist 160, 191–204. ( 10.1016/j.protis.2008.11.004) [DOI] [PubMed] [Google Scholar]
- 8.Schmitz K. 1981. Translocation. In The biology of seaweeds (eds Lobban C, Wynne M), pp. 534–558. Botanical Monographs 17 Oakland, CA: University of California Press. [Google Scholar]
- 9.Manley SL. 1983. Composition of sieve tube sap from Macrocystis pyrifera (Phaeophyta) with emphasis on the inorganic constituents. J. Phycol. 19, 118–121. ( 10.1111/j.0022-3646.1983.00118.x) [DOI] [Google Scholar]
- 10.Schmitz K, Lobban C. 1976. A survey of translocation in Laminariales (Phaeophyceae). Mar. Biol. 36, 207–216. ( 10.1007/BF00389281) [DOI] [Google Scholar]
- 11.Reed DC, Brzezinski M. 2009. Kelp forests. In The management of natural coastal carbon sinks (eds Laffoley D, Grimsditch G), pp. 31–51. Gland, Switzerland: IUCN. [Google Scholar]
- 12.Mann KH. 1982. Ecology of coastal waters: a systems approach. Berkeley, CA: University of California Press. [Google Scholar]
- 13.Huston M, Wolverton S. 2009. The global distribution of net primary production: resolving the paradox. Ecol. Monogr. 79, 343–377. ( 10.1890/08-0588.1) [DOI] [Google Scholar]
- 14.Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. ( 10.1038/nmeth.2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoblauch J, Drobnitch ST, Peters WS, Knoblauch M. In preparation Reversible cell wall swelling in sieve elements of brown algae. [Google Scholar]
- 16.Lobban CS. 1978. Translocation of C in Macrocystis pyrifera (giant kelp). Plant Physiol. 61, 585–589. ( 10.1104/pp.61.4.585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ronellenfitsch H, Liesche J, Jensen KH, Holbrook NM, Schulz A, Katifori E. 2015. Scaling of phloem structure and optimality of photoassimilate transport in conifer needles. Proc. R. Soc. B 282, 20141863 ( 10.1098/rspb.2014.1863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enquist BJ, Biology E. 2003. Cope's Rule and the evolution of long-distance transport in vascular plants: allometric scaling, biomass partitioning and optimization. Plant Cell Environ. 26, 151–161. ( 10.1046/j.1365-3040.2003.00987.x) [DOI] [Google Scholar]
- 19.Hölttä T, Mencuccini M, Nikinmaa E. 2009. Linking phloem function to structure: analysis with a coupled xylem–phloem transport model. J. Theor. Biol. 259, 325–337. ( 10.1016/j.jtbi.2009.03.039) [DOI] [PubMed] [Google Scholar]
- 20.Jensen KH, Liesche J, Bohr T, Schulz A. 2012. Universality of phloem transport in seed plants. Plant Cell Environ. 35, 1065–1076. ( 10.1111/j.1365-3040.2011.02472.x) [DOI] [PubMed] [Google Scholar]
- 21.Petit G, Crivellaro A. 2014. Comparative axial widening of phloem and xylem conduits in small woody plants. Trees 28, 915–921. ( 10.1007/s00468-014-1006-1) [DOI] [Google Scholar]
- 22.Chen H, Niklas KJ, Sun S. 2012. Testing the packing rule across the twig–petiole interface of temperate woody species. Trees 26, 1737–1745. ( 10.1007/s00468-012-0742-3) [DOI] [Google Scholar]
- 23.Banavar JR, Moses ME, Brown JH, Damuth J, Rinaldo A, Sibly RM, Maritan A. 2010. A general basis for quarter-power scaling in animals. Proc. Natl Acad. Sci. USA 107, 15 816–15 820. ( 10.1073/pnas.1009974107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.West GB, Brown JH, Enquist BJ. 1997. A general model for the origin of allometric scaling laws in biology. Science 276, 122–126. ( 10.1126/science.276.5309.122) [DOI] [PubMed] [Google Scholar]
- 25.Jensen KH, Lee J, Bohr T, Bruus H, Holbrook NM, Zwieniecki MA. 2011. Optimality of the Münch mechanism for translocation of sugars in plants. J. R. Soc. Interface 8, 1155–1165. ( 10.1098/rsif.2010.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skriptsova AV, Titlyanov EA. 2003. Effect of the meristem on sporification of Laminaria cichorioides. Russ. J. Mar. Biol. 29, 372–377. ( 10.1023/B:RUMB.0000011705.44607.c2) [DOI] [Google Scholar]
- 27.Silberfeld T, Leigh JW, Verbruggen H, Cruaud C, de Reviers B, Rousseau F. 2010. A multi-locus time-calibrated phylogeny of the brown algae (Heterokonta, Ochrophyta, Phaeophyceae): investigating the evolutionary nature of the ‘brown algal crown radiation’. Mol. Phylogenet. Evol. 56, 659–674. ( 10.1016/j.ympev.2010.04.020) [DOI] [PubMed] [Google Scholar]
- 28.Clarke JT, Warnock RCM, Donoghue PCJ. 2011. Establishing a time-scale for plant evolution. New Phytol. 192, 266–301. ( 10.1111/j.1469-8137.2011.03794.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following datasets are freely accessible on Dryad at the following doi: http://dx.doi.org/10.5061/dryad.d5s92. Phloem anatomy, Laminariales: raw individual SE data table, measured from micrographs. Phloem anatomy (organismal level): averaged SE diameter, variance analyses and gross anatomical data for multiple replicates of all six study species.