Abstract
Change in day length is an important cue for reproductive activation in seasonally breeding animals to ensure that the timing of greatest maternal investment (e.g. lactation in mammals) coincides with favourable environmental conditions (e.g. peak productivity). However, artificial light at night has the potential to interfere with the perception of such natural cues. Following a 5-year study on two populations of wild marsupial mammals exposed to different night-time levels of anthropogenic light, we show that light pollution in urban environments masks seasonal changes in ambient light cues, suppressing melatonin levels and delaying births in the tammar wallaby. These results highlight a previously unappreciated relationship linking artificial light at night with induced changes in mammalian reproductive physiology, and the potential for larger-scale impacts at the population level.
Keywords: anthropogenic disturbance, circadian disruption, light pollution, Macropus eugenii, melatonin, trophic mismatch
1. Introduction
Artificial light at night is one of the most common and fastest growing types of environmental pollution, increasing at 6% per year globally, and identified as a key threat to biodiversity [1]. Although the benefit of artificial light at night to humans is clear, for many organisms, anthropogenic sources of night light have the potential to disrupt physiological processes that rely on the daily and seasonal rhythms of light cues, such as emergence time [2,3], foraging behaviour [4], communication [5,6] and the timing of reproduction [7–10]. Additionally, laboratory studies on rodents have shown that artificial light at night can drive other physiological processes that may have fitness and/or survival consequences, such as suppressed immune function, impaired stress responses [11,12] and reduced cognition [13]. However, current research on the effects of light at night in mammals consists of two distinct approaches with little integration: laboratory-based physiological studies and field-based behavioural observations [14].
The physiology and behaviour of mammals follow daily circadian oscillations maintained by an internal timekeeping system within the hypothalamic suprachiasmatic nuclei. This biological clock is set by photic cues perceived by the retina [15]. A key endocrinological player in orchestrating this circadian rhythm is the pineal hormone melatonin (MLT) [16]. The secretion of MLT shows diel variation, with maximal production during the dark phase of the photoperiod and low levels during the light phase. Pinealectomy suppresses these photoperiodic rhythms, and subsequent MLT supplementation reinstates them [17]. In this way, photoperiod and the profile of MLT secretion conveys both time of day and seasonal information in mammals [18]. This information can be used to restrict reproduction to particular seasons to ensure that favourable environmental conditions coincide with increased energetic demands associated with provisioning of young [19,20]. However, artificial light at night can suppress nocturnal MLT production in a range of species, from birds [21,22] to humans [23], with the potential to change the timing of reproduction in species that rely on photoperiod cues [14,24].
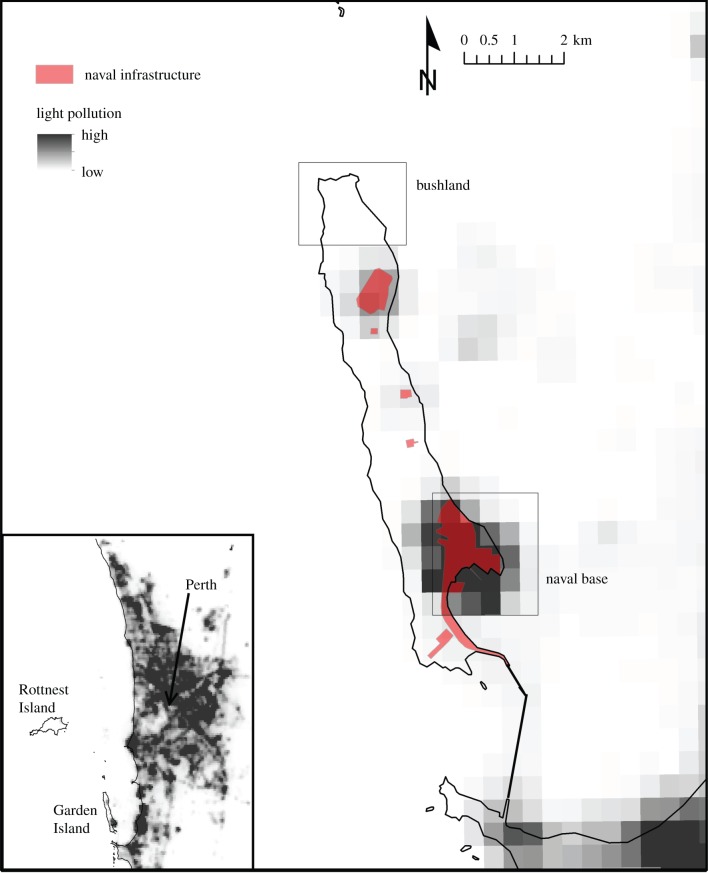
The tammar wallaby (Macropus eugenii) is a small nocturnal macropod marsupial that has been the focus of extensive reproductive research over the last 180 years [25,26]. Free-ranging tammar wallabies are today restricted to small areas of mainland Western Australia and several offshore islands, most notably Kangaroo Island (South Australia) and Garden Island (Western Australia). Garden Island (figure 1) is located 5 km off the coast, approximately 35 km southwest of Perth (115°40′ E, 32°16′ S). Since 1972, Garden Island has been occupied by the Australian Department of Defence for the operation of the country's largest naval base (HMAS Stirling). The naval base infrastructure is fenced, and primarily confined to the southern end of the island, leaving the majority of the remaining island relatively undisturbed. This produces populations of wallabies subject to different levels of anthropogenic disturbance [27,28]. The resulting urbanized naval base population experiences significant night-time light pollution (figure 1), since much of the native vegetation has been replaced with illuminated buildings, footpaths and roads, whereas the bush population is undisturbed and experiences natural levels of light at night.
Figure 1.
Map of Garden Island, Western Australian showing naval infrastructure and light pollution levels. Light pollution levels are mapped from a cloud-free composite of VIIRS night-time lights from May 2014, produced by the Earth Observation Group, NOAA National Geophysical Data Centre (available at http://ngdc.noaa.cov/eog/viirs/download_monthly.html). The bush population is located at the northern tip of the island in an environment without light pollution, while the naval base population is located at the southeast of the island (shaded red) with light pollution.
The annual cycle of reproduction in the tammar wallaby is highly synchronous and is cued by changes in day length [29–32]. Most births occur in late January, six weeks after the austral summer solstice, and females experience a post-partum oestrus. The embryo conceived post-partum remains dormant during lactation of the current pouch young and is re-activated after the summer solstice [33]. Females breeding for the first time mate in October, yet also hold the resulting embryo dormant until after the summer solstice. This obligatory light-cued breeding is under neuroendocrine control [25]. Briefly, as day length decreases, the increase in MLT causes the pituitary gland to secrete prolactin, which in turn acts on the corpus luteum to produce progesterone, which initiates blastocyst re-activation [34–36]. However, whether increased anthropogenic light at night can influence hormonal signals in wild individuals is unknown. Here, we present the first study linking artificial light at night to changes in endocrinology and reproductive timing in a seasonally reproducing free-ranging mammal.
2. Material and methods
(a). Study population and area
The study was conducted on two populations of free-ranging tammar wallabies on Garden Island, Western Australia, with different levels of artificial lighting. The urbanized naval base population has light pollution, while the natural bush population at the far northern end of the island, approximately 5.6 km from the naval base, is without artificial lighting (figure 1). We have studied these populations since 2005. At each trapping period, wallabies are captured using ‘Thomas traps'—soft-sided traps constructed from shade cloth suspended within a wire frame (450 × 450 × 800 mm; Sheffield Wire Works, Welshpool, Western Australia). At capture, we recorded ear-tag numbers, sex, body mass, long pes (foot) length and reproductive status of females. Previously untagged individuals were given a numbered metal ear-tag in each ear (National Band and Tag Company, Kentucky, USA) for identification on subsequent recaptures. In addition, we made an assessment of the major developmental features (e.g. appearance of whiskers, eyes opening, pigmentation and growth stage of fur), and measured the head length and pes length of all pouch young to calculate age, based on growth tables for the species [37,38], and then back-estimated birth dates (see below).
(b). Measuring individual exposure to light at night
In December 2013, we captured five female wallabies from the naval base and five from the natural bush for individual measurements of light exposure at night in free-ranging wallabies. We used micro-light loggers (weight 2.8 g; custom made by the University of Konstanz, Germany) attached to GPS data-logging collars (Sirtrack G2C171B; Havelock North, New Zealand) with an internal time-release mechanism and VHF transmitter to facilitate collection in April 2014 without the need to recapture each animal. The light loggers recorded and stored light intensity data every 2 min, and the GPS collars recorded and stored position data every 30 min during the night and every 2 h during the day. We additionally deployed 12 light loggers into the environment (six on the base—three directly below streetlights and three at a distance greater than 5 m from a light source—and six in the bush) to measure environmental light. The light loggers had been calibrated against a pyranometer to calculate irradiance (Watt m−2) from frequency values [39]. We recovered light data from 6 of the 10 wallabies (three from each population) and 8 from 12 environment loggers (four from each population); data loss was due to either light logger or VHF failures. To examine the light intensities experienced by wallabies at night, we used the data between astronomical sunset and sunrise (times obtained from the Perth Observatory; available at http://www.perthobservatory.wa.gov.au) to calculate light irradiance for the middle of each night (this excludes twilight) from 14 December 2013 to 15 February 2014. During the month of December, we also calculated median light intensity each night for the environment (bush, base under lights and base away from lights) and the median light experienced by individual wallabies during two time periods: middle of the night (as described above) and dawn/dusk (twilight; sunset to astronomical sunset and astronomical sunrise to sunrise).
(c). Blood collection and melatonin analysis
Blood was obtained from the lateral caudal vein by venipuncture using a 22-gauge needle from female wallabies trapped at night (between astronomical sunset and sunrise) from both populations in December 2009 and 2013 (n = 38 in 2009—29 base, 9 bush; n = 29 in 2013—26 base, 3 bush). Collected blood samples were transferred to vials (BD Vacutainer) containing sodium ethylene diamine tetraacetic and centrifuged at 3000 r.p.m. for 10 min, with the plasma pipetted into Eppendorf tubes and stored frozen at −80°C until assayed.
Plasma samples were assayed for MLT using an enzyme-linked immunoassay, ELISA (Wallaby Melatonin Kit, Cat no. KT-61019, Kamiya Biomedical Company, Seattle, USA). Plates were read on an Anthos 2010 plate reader (Anthos Labtec Instruments, Salzburg, Austria) at a wavelength of 450 nm. All plasma samples were assayed in duplicate and the average absorbance calculated. MLT concentration was determined following calculation of the standard curve using the provided calibrators. The sensitivity of the assay is 1.0 pg ml−1. Intra- and inter-assay coefficients of variation were 5.3% and 14.1%, respectively.
(d). Birth schedules
We used our long-term dataset encompassing a 5-year period (2005–2007, 2009–2010) where we had reproductive information to calculate birth schedules for each population (total of 237 births—118 base, 119 bush). The ages of pouch young were determined from aging tables based on major developmental features, head and pes length measurements [37,38]—and their estimated ages used to back-estimate their birth date.
(e). Data analysis
To analyse variation in median light intensities of the environment and those experienced by the wallabies, we built generalized linear mixed-effects models (GLMMs) with a gamma distribution and log-link function using the lme4 package [40] in the R v. 3.0.3 statistical environment [41]. Median light intensity was the response variable, and population of wallabies (base or bush)/location of environment loggers (base under lights, base away from lights or bush) and time of night (dawn/dusk or middle of the night) were fixed effects. The logger was modelled as a random effect to account for non-independency of light intensity values collected on the same logger on different days. Models were built including an interaction between population/location and time of night. Pairwise contrasts were examined using least square means and incorporating Tukey's adjustment for multiple comparisons with the R package lsmeans [42]. We checked model assumptions using Q–Q plots (normality of variance) and by plotting residuals against fitted values (homogeneity of variance).
A preliminary analysis of MLT concentrations using analysis of variance in JMP 11 (SAS Institute, Cary, USA) revealed no significant within-population effect of year (F1,63 = 0.178, p = 0.675); therefore, we pooled the data for the 2 years together. Mean MLT concentration was then analysed for population differences by a pooled t-test. Data were tested for normality (Shapiro–Wilk test) and homogeneity of variances (Levene's test) prior to analysis. MLT data were log-transformed to meet the assumptions of parametric tests. Birth schedules for the two populations were compared using a non-parametric Kolmogorov–Smirnov two-sample test in JMP v. 11 (SAS Institute).
We determined home ranges from the location data recorded by the GPS collars using the time-based local convex hull method (T-LoCoH) with the T-LoCoH package [43] in the R v. 3.0.3 statistical environment [41]. The GPS data were first refined to remove all locations that were determined to be using less than three satellites and those locations with a horizontal dilution of precision value greater than 10. We used the k method to determine the number of nearest neighbours [44], which we set at 15, and the space–time balance was set at s = 0.04. Home ranges were defined as 95% isopleths and used all locations recorded between 14 December and 31 March. The number of locations used ranged between 1850 and 2800.
3. Results
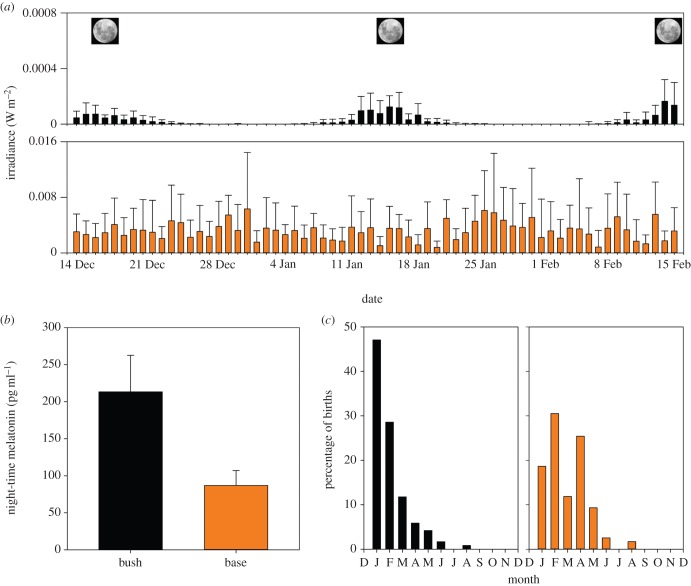
Wallabies living in the bush and on the naval base experienced very different light environments at night. Animals in the bush were exposed only to astronomical sources of light, including a clear lunar cycle (figure 2a). Conversely, such natural cues were not apparent on the base owing to an order of magnitude increase in night-time light intensity (figure 2a). Consistent with other studies showing a suppressive effect of light on MLT secretion [21–23], night-time levels of MLT in naval base wallabies were significantly lower than those of bush animals (t65 = 3.5, p = 0.0009; figure 2b). Wallabies from the bush had mean (±s.e.) December night-time MLT concentrations of 213.24 ± 49.3 pg ml−1, consistent with summer night-time MLT concentrations described by McConnell [45], while base animals had mean (±s.e.) night-time MLT concentrations of 86.91 ± 20 pg m1−1, consistent with diurnal MLT concentrations [45]. Interestingly, the distribution of birth dates is significantly different between the bush and base populations (Kolmogorov–Smirnov two-sample test; D = 0.351, p < 0.001; figure 2c), with a month-long delay in median birth date on the naval base. The median birth date for bush wallabies in this study is six weeks after the summer solstice, consistent with all previous studies, including ones on this population published 38 and 185 years ago [25,46]. In stark contrast to the bush animals, the median birth date for base wallabies is 10 weeks after the summer solstice (figure 2c).
Figure 2.
(a) Wallabies on the naval base experience orders of magnitude more light pollution and loss of natural light cues at night. Mean (±s.e) night-time light intensity (irradiance, Watt m−2) from astronomical sunset to sunrise (middle of the night, excludes dawn/dusk) experienced each night from 14 December to 15 February by wallabies inhabiting the bush (black bars) and the naval base (orange bars). Full moons occur on the 17 December, 16 January and the 15 February. Note: y-axes are on different scales. (b) Night-time MLT is significantly suppressed under high light pollution levels. Mean (±s.e.) night-time MLT levels in bush (n = 12; black bar) and naval base wallabies (n = 55; orange bar) around the time of reproductive re-activation (December log-transformed MLT; t65 = 3.5, p = 0.0009). y-axis represents back-transformed log MLT concentrations. (c) Birth is delayed under high light pollution. The distribution of births in the bush (n = 119; black bars) and on the naval base (n = 118; orange bars) are significantly different (Kolmogorov–Smirnov two-sample test; D = 0.351, p < 0.001), with a median birth date of 1 February in the bush and 28 February on the base.
Importantly, the delayed birth dates are directly related to the illuminated night-time environment of the naval base. Indeed, we know that blastocyst re-activation is highly correlated with the summer solstice [32] and stimulated by very small changes in day length, such that an increase in the amount of darkness of about 6 min for Kangaroo Island wallabies initiates re-activation [32]. At the time of the median re-activation date (calculated as 31 days prior to median birth date [26]), the night is only 3 min longer than the summer solstice for bush animals, increasing at a rate of 18 s per night (figure 3). This environmental cue governing re-activation is masked on the base by artificial light at night. Births are therefore delayed and poorly synchronized (figure 2c). The masking effect of light at night can be seen in figure 4. Here, ‘night’ is broken down into the time between sunset to astronomical sunset and astronomical sunrise to sunrise (i.e. dawn and dusk, or twilight), and the time between astronomical sunset and astronomical sunrise (i.e. middle of the night, excludes twilight). In the natural condition of the bush, dawn/dusk is brighter than the darkness of the middle of night (Tukey's test after GLMM, z = −8.93, p < 0.0001; figure 4). Accordingly, wallabies in the bush experience lower light levels in the middle of night than at dawn/dusk (z = −6.01, p < 0.0001) and are better able to perceive slight increases in the duration of the night. Conversely, the intensity of light either under or away from streetlights on the naval base is unchanged by the time of night (under: z = −0.14, p > 0.999; away: z = −0.98, p = 0.993; figure 4). Therefore, wallabies living on the base experience elevated levels of light relative to bush wallabies across the entire night (dawn/dusk: z = 8.91, p < 0.0001; middle of night: z = 8.10, p < 0.0001). Although the light experienced by wallabies on the naval base decreases between dawn/dusk and the middle of the night (z = −8.37, p < 0.0001; figure 4), the increased level of night light experienced by base animals is nevertheless not significantly different from that away from streetlights (dawn/dusk: z = −0.30, p > 0.999; middle of night: z = 1.85, p = 0.702). Wallabies on the base appear to behaviourally avoid night-time light but are limited in their capacity to do so. This increase in light at night compared with bush wallabies is sufficient to mask the cue of increased darkness that triggers blastocyst re-activation.
Figure 3.
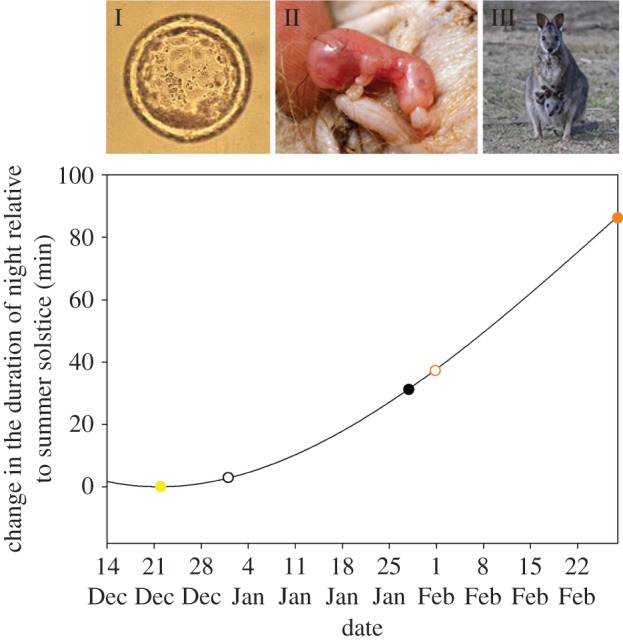
Key reproductive stages are related to changes in the duration of night relative to the austral summer solstice. The longest day of the year occurred on the 22 December (yellow circle, at this stage blastocysts [I] are in diapause), after which there is a progressive increase in the amount of darkness that triggers blastocyst re-activation. Median re-activation dates are plotted as open circles (black for bush, orange for base) followed 31 days later by median birth dates (solid circles). The normal birth of highly altricial young [II] in late January ensures that the time of pouch young emergence [III] and highest daily milk consumption, matches peak resources in August–September following predictable winter rainfall in June–July. Conversely, late re-activation and birth of base animals causes a trophic mismatch later in the year. Photos [I] and [II] with permission Geoff Shaw, [III] Kylie Robert.
Figure 4.
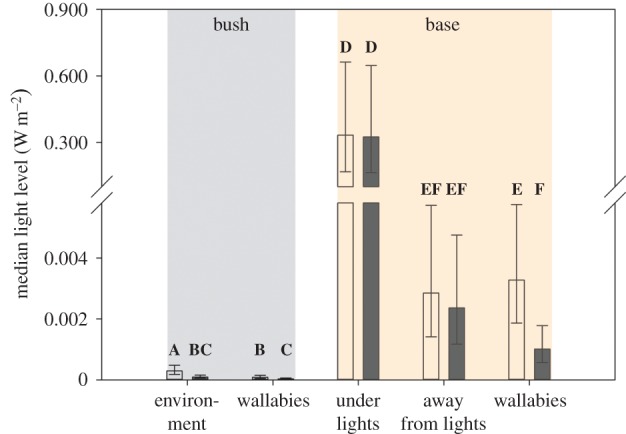
Wallabies on the naval base are restricted in their ability to escape artificial light both during dawn/dusk and within the middle of the night. December median (±95% confidence limits) night-time light intensity (irradiance, Watt m−2) of the available environment (bush, base: under artificial lights, and base: away from human-made light sources) and the light experienced by individual wallabies (bush and base) during two night-time periods; dawn/dusk (open bars) and middle of the night (solid bars). Levels not connected by same letter are significantly different (Tukey's test after GLMM).
4. Discussion
Despite a worldwide increase in anthropogenic light pollution, few studies have assessed the impact of artificial light at night on free-ranging wildlife [47], and to our knowledge no study has examined its impact on the timing of reproduction in a wild mammal. Here, we have demonstrated that anthropogenic light at night modifies nocturnal MLT secretion and delays reproductive activation in a seasonally reproducing free-ranging mammal. These results suggest that urban light pollution could have profound impacts on desynchronizing seasonal physiological processes in wildlife.
Although it is difficult to disentangle the effects of other anthropogenic disturbances, there are no differences in the home range sizes of wallabies inhabiting each population (bush: 3.97 ± 1.96 ha; base: 3.49 ± 0.45 ha; t2.2 = 0.24, p = 0.83), suggesting that the urbanized base environment is not disrupting movements or foraging behaviour. An alternative explanation for our results, however, might propose that resource differences between the populations have relaxed the strictly seasonal breeding of the tammar wallabies on the base, owing to the forage (irrigated lawns) of these animals not being tied to seasonal rains. However, this explanation is unlikely as the timing of birth is remarkably constant from year to year in both captive and wild populations [25,48]—so much so that the median birth date of the Garden Island population was described in studies 147 years apart as 22 January in 1830 [46] and 27 January in 1977 [25]. Moreover, wallabies transported to the USA shifted their timing of births six months out of phase to also fall six weeks after the northern hemisphere summer solstice [29]. Lastly, tammar wallabies do not change their reproductive pattern when held in captivity with unlimited resources (i.e. ad libitum high-quality forage [25]). Instead, photoperiodism and day length are genetically fixed triggers for reproduction in most seasonally reproducing mammals [49].
The full effects of anthropogenic light at night on the fitness consequences of altered timing of reproduction in tammar wallabies are at present largely unknown. Such reproductive timing and resource mismatch has been shown to reduce offspring production fourfold in caribou when calving does not match peak resources [50], and can result in population declines of around 90% in migratory pied flycatchers where peak provisioning of nestlings and food are misaligned [51]. On the naval base, native vegetation persists in clumps, surrounded by lawns of introduced grasses (primarily couch grass, Cynodon dactylon). Until recently, these irrigated grasses were a major part of the diet of the wallabies on the base [27,52] and we believe this access has to date buffered any negative effects [53]. However, the irrigation of these lawns was recently disconnected and the only irrigated area (the oval) has been fenced to exclude wallabies. In the future, late-reproducing base wallabies may suffer a trophic mismatch and food shortages during late lactation, with reduced offspring survival resulting in larger-scale impacts at the population level.
With a need to reduce CO2 emissions globally and meet climate change targets there is an annual growth rate of 30% in energy-efficient light sources such as high-brightness white light-emitting diodes (LEDs) [54]. Despite the energetic benefits of LEDs, there is growing concern that exposure to white LEDs will impact biodiversity [55] in part by suppressing the production of MLT and altering circadian patterns [55]. Accordingly, white LEDs suppress MLT 4.5–5.4 times more than traditionally used high-pressure sodium (HPS) bulbs in humans [56,57]. The replacement of HPS street lighting with white LED street lighting will lead to higher night-time light pollution and greater MLT suppression [57]. Understanding the ecological consequences of this increasing shift to energy-efficient white lights in urban environments requires a greater understanding of the impacts of artificial night light on the biological processes of species in situ.
Acknowledgements
Research was conducted with the permission of the Australian Department of Defense. We thank G. Davies and S. Booth for assistance with logistical advice, access to the study sites and use of infrastructure while on the island. We thank K. Bettink, P. Tholen, C. Olejnik and N. Godfrey for help with fieldwork. Our thanks also go to R. Bencini for research advice, loaned equipment and accommodation. We thank D. Dominoni for technical support with the light loggers, S. Watson for statistical advice with the light data and G. Shaw for use of his photographs.
Ethics
All work was conducted according to relevant national and international guidelines. The project was approved by La Trobe University's Animal Ethics Committee (AEC13–46), The University of Western Australia Animal Ethics Committee (RA/3/100/376 and RA/3/100/897) and Department of Environment and Conservation Research Permits (SF007651 and SF009547).
Data accessibility
Data are available from the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.7h08c.
Authors' contributions
K.A.R. and B.C. conceived the study; J.P. provided the light loggers; K.A.R. and B.C. performed the fieldwork and data collection with assistance from those mentioned in the acknowledgements; B.C. generated the maps and calculated home ranges; K.A.R. and J.A.L. performed the data analyses with assistance from those mentioned in the acknowledgements; all authors contributed to writing the manuscript.
Competing interests
We declare that we have no competing interests.
Funding
The research was funded by a La Trobe University Zoology Department Grant (K.A.R.) and a Securing Food, Water and the Environment Grant from La Trobe University (K.A.R., J.A.L., J.P. and B.C.).
References
- 1.Hölker F, Wolter C, Perkin EK, Tockner K. 2010. Light pollution as a biodiversity threat. Trends Ecol. Evol. 25, 681–682. ( 10.1016/j.tree.2010.09.007) [DOI] [PubMed] [Google Scholar]
- 2.Downs NC, Beaton V, Guest J, Polanski J, Robinson SL, Racey PA. 2003. The effects of illuminating the roost entrance on the emergence behaviour of Pipistrellus pygmaeus. Biol. Conserv. 111, 247–252. ( 10.1016/S0006-3207(02)00298-7) [DOI] [Google Scholar]
- 3.Stone E, Jones G, Harris S. 2009. Street lighting disturbs commuting bats. Curr. Biol. 19, 1123–1127. ( 10.1016/j.cub.2009.05.058) [DOI] [PubMed] [Google Scholar]
- 4.Bird BL, Branch LC, Miller DL. 2004. Effects of coastal lighting on foraging behaviour on beach mice. Conserv. Biol. 18, 1435–1439. ( 10.1111/j.1523-1739.2004.00349.x) [DOI] [Google Scholar]
- 5.Krams I. 2001. Communication in crested tits and the risk of predation. Anim. Behav. 61, 1065–1068. ( 10.1006/anbe.2001.1702) [DOI] [Google Scholar]
- 6.Miller MW. 2006. Apparent effects of light pollution on singing behavior of American robins. Condor 108, 130–139. ( 10.1650/0010-5422%282006%29108%5B0130%3AAEOLPO%5D2.0.CO%3B2) [DOI] [Google Scholar]
- 7.Longcore T. 2010. Sensory ecology: night lights alter reproductive behavior of blue tits. Curr. Biol. 20, 893–895. ( 10.1016/j.cub.2010.09.011) [DOI] [PubMed] [Google Scholar]
- 8.Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M. 2010. Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr. Biol. 20, 1735–1739. ( 10.1016/j.cub.2010.08.028) [DOI] [PubMed] [Google Scholar]
- 9.Dominoni D, Quetting M, Partecke J. 2013. Artificial light at night advances avian reproductive physiology. Proc. R. Soc. B 280, 20123017 ( 10.1098/rspb.2012.3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LeTallec T, Théry M, Perret M. 2015. Effects of light pollution on seasonal estrus and daily rhythms in a nocturnal primate. J. Mammal. 96, 438–445. ( 10.1093/jmammal/gyv047) [DOI] [Google Scholar]
- 11.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ. 2011. Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol. Lett. 7, 468–471. ( 10.1098/rsbl.2010.1108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedrosian TA, Aubrecht TG, Kaugars KE, Weil ZM, Nelson RJ. 2013. Artificial light at night alters delayed-type hypersensitivity reaction in response to acute stress in Siberian hamsters. Brain Behav. Immunol. 34, 39–42. ( 10.1016/j.bbi.2013.05.009) [DOI] [PubMed] [Google Scholar]
- 13.Fonken LF, Kitsmiller E, Smale L, Nelson RJ. 2012. Dim nighttime light impairs cognition and provokes depressive-like responses in a diurnal rodent. J. Biol. Rhythms 27, 319–327. ( 10.1177/0748730412448324) [DOI] [PubMed] [Google Scholar]
- 14.Beier P. 2006. Effects of artificial night lighting on terrestrial mammals. In Ecological consequences of artificial night lighting (eds Rich C, Longcore T), pp. 19–42. Washington, DC: Island Press. [Google Scholar]
- 15.Dibner C, Schibler U, Albrecht U. 2010. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu. Rev. Physiol. 72, 517–549. ( 10.1146/annurev-physiol-021909-135821) [DOI] [PubMed] [Google Scholar]
- 16.Prendergast BJ, Nelson RJ, Zucker I. 2002. Mammalian seasonal rhythms: behavior and neuroendocrine substrates. In Hormones, brain and behavior (eds Pfaff DW, Arnold A, Etgen A, Fahrbach S, Rubin R), pp. 93–156. San Diego, CA: Academic Press. [Google Scholar]
- 17.Malpaux B, Thiéry JC, Chemineau P. 1999. Melatonin and the seasonal control of reproduction. Reprod. Nutr. Dev. 39, 355–366. ( 10.1051/rnd:19990308) [DOI] [PubMed] [Google Scholar]
- 18.Arendt J. 1998. Melatonin and the pineal gland: influence on mammalian seasonal and circadian physiology. Rev. Reprod. 3, 13–22. ( 10.1530/ror.0.0030013) [DOI] [PubMed] [Google Scholar]
- 19.Bronson FH. 1985. Mammalian reproduction, an ecological perspective. Biol. Reprod. 32, 1–26. ( 10.1095/biolreprod32.1.1) [DOI] [PubMed] [Google Scholar]
- 20.Cork SJ, Dove H. 1989. Lactation in the tammar wallaby (Macropus eugenii). II. Intake of milk components and maternal allocation of energy. J. Zool. 219, 399–409. ( 10.1111/j.1469-7998.1989.tb02588.x) [DOI] [Google Scholar]
- 21.Yamada H, Oshima I, Sato K, Ebihara S. 1988. Loss of the circadian rhythms of locomotor activity, food intake, and plasma melatonin concentration induced by constant bright light in the pigeon (Columba livia). J. Comp. Physiol. A 163, 459–463. ( 10.1007/BF00604900) [DOI] [PubMed] [Google Scholar]
- 22.Dominoni DM, Goymann W, Helm B, Partecke J. 2013. Urban-like night illumination reduces melatonin release in European blackbirds (Turdus merula): implications of city life for biological time-keeping of songbirds. Front. Zool. 10, 60 ( 10.1186/1742-9994-10-60) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey P. 1980. Light suppresses melatonin secretion in humans. Science 210, 1267–1269. ( 10.1126/science.7434030) [DOI] [PubMed] [Google Scholar]
- 24.Kurvers RHJM, Hölker F. 2015. Bright nights and social interactions: a neglected issue. Behav. Ecol. 26, 334–339. ( 10.1093/beheco/aru223) [DOI] [Google Scholar]
- 25.Tyndale-Biscoe H, Renfree M. 1987. Reproductive physiology of marsupials. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 26.Tyndale-Biscoe H. 2005. Life of marsupials. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 27.Chambers BK. 2009. Human disturbance affects the ecology and population dynamics of the tammar wallaby, Macropus eugenii, on Garden Island, Western Australia. Dissertation, University of Western Australia, Australia.
- 28.Chambers BK, Bencini R. 2010. Impact of human disturbance on the population dynamics and ecology of tammar wallabies on Garden Island, Western Australia. In Macropods: the biology of kangaroos, wallabies and rat-kangaroos (eds Coulson G, Eldridge M), pp. 211–218. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 29.Berger PJ. 1966. Eleven-month ‘embryonic diapause’ in a marsupial. Nature 211, 435–436. ( 10.1038/211435a0) [DOI] [PubMed] [Google Scholar]
- 30.Sharman GB, Berger PJ. 1969. Embryonic diapause in marsupials. Adv. Reprod. Physiol. 4, 211–240. [Google Scholar]
- 31.Renfree MB, Tyndale-Biscoe CH. 1973. Intra-uterine development after diapause in the marsupial Macropus eugenii. Dev. Biol. 32, 28–40. ( 10.1016/0012-1606(73)90217-0) [DOI] [PubMed] [Google Scholar]
- 32.Sadleir R, Tyndale-Biscoe H. 1977. Photoperiod and the termination of embryonic diapause in the marsupial Macropus eugenii. Biol. Reprod. 16, 605–608. ( 10.1095/biolreprod16.5.605) [DOI] [PubMed] [Google Scholar]
- 33.Hinds LA, Tyndale-Biscoe CH. 1985. Seasonal and circadian patterns of circulating prolactin during lactation and seasonal quiescence in the tammar, Macropus eugenii. J. Reprod. Fertil. 74, 173–183. ( 10.1530/jrf.0.0740173) [DOI] [PubMed] [Google Scholar]
- 34.Renfree MB, Lincoln DW, Almeida OFX, Short RV. 1981. Abolition of seasonal embryonic diapause in a wallaby by pineal denervation. Nature 293, 138–139. ( 10.1038/293138a0) [DOI] [PubMed] [Google Scholar]
- 35.McConnell SJ, Tyndale-Biscoe CH. 1985. Response in peripheral plasma melatonin to photoperiod change and the effects of exogenous melatonin on seasonal quiescence in the tammar, Macropus eugenii. J. Reprod. Fertil. 75, 529–538. ( 10.1530/jrf.0.0730529) [DOI] [PubMed] [Google Scholar]
- 36.McConnell SJ, Tyndale-Biscoe CH, Hinds LA. 1986. Change in duration of elevated concentrations of melatonin is the factor in photoperiod response of the tammar, Macropus eugenii. J. Reprod. Fertil. 77, 623–632. ( 10.1530/jrf.0.0770623) [DOI] [PubMed] [Google Scholar]
- 37.Inns RW. 1982. Age determination in the Kangaroo Island wallaby, Macropus eugenii (Desmarest). Aust. Wild. Res. 9, 213–220. ( 10.1071/WR9820213) [DOI] [Google Scholar]
- 38.Poole WE, Simms NG, Wood JT, Luboloa M. 1991. Tables for age determination of the Kangaroo Island tammar wallaby (Macropus eugenii), from body measurements. Technical Memorandum no. 32 Canberra, Australia: CSIRO (Division of Wildlife and Ecology). [Google Scholar]
- 39.Dominoni DM, Carmona-Wagner EO, Hofmann M, Kranstauber B, Partecke J. 2013. Individual-based measurements of light intensity provide new insights into the effects of artificial light at night on daily rhythms of urban-dwelling songbirds. J. Anim. Ecol. 83, 681–692. ( 10.1111/1365-2656.12150) [DOI] [PubMed] [Google Scholar]
- 40.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: linear mixed-effects models using Eigen and S4. R package v. 1.1–7. See http://cran.r-project.org/package=lme4.
- 41.R Development Core Team. 2014. R: a language and environment for statistical computing, v. 3.0.1. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 42.Lenth RV. 2014. Least-squares means. R package v. 2.17. See http://cran.r-project.org/package=lsmeans.
- 43.Lyons A, Turner W, Getz W. 2013. Home range plus: a space-time characterization of movement over real landscapes. BMC Mov. Ecol. 1, 2 ( 10.1186/2051-3933-1-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Getz WM, Fortmann-Roe S, Cross PC, Lyons AJ, Ryan SJ, Wilmers CC. 2007. LoCoH: nonparameteric kernel methods for constructing home ranges and utilization distributions. PLoS ONE 2, e207 ( 10.1371/journal.pone.0000207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McConnell S. 1986. Seasonal changes in the circadian plasma melatonin profile of the Tammar, Macropus eugenii. J. Pineal. Res. 3, 119–125. ( 10.1111/j.1600-079X.1986.tb00734.x) [DOI] [PubMed] [Google Scholar]
- 46.Collie A. 1830. On some particulars connected with the natural history of the kangaroo. Zool. J. 5, 238–241. [Google Scholar]
- 47.Rich C, Longcore T. 2006. Ecological consequences of artificial night lighting. Washington, DC: Island Press. [Google Scholar]
- 48.Inns RW. 1980. Ecology of the Kangaroo Island wallaby (Macropus eugenii) in the Flinders Chase National Park. Dissertation, University of Adelaide, Australia.
- 49.Zerbe P, Clauss M, Codron D. 2012. Reproductive seasonality in captive wild ruminants: implications for biogeographical adaptation, photoperiodic control, and life history. Biol. Rev. 87, 965–990. ( 10.1111/j.1469-185X.2012.00238.x) [DOI] [PubMed] [Google Scholar]
- 50.Post E, Forchhammer MC. 2008. Climate change reduces reproductive success of an arctic herbivore through trophic mismatch. Phil. Trans. R. Soc. B 363, 2369–2375. ( 10.1098/rstb.2007.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Both C, Bouwhuis S, Lessells CM, Visser ME. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441, 81–83. ( 10.1038/nature04539) [DOI] [PubMed] [Google Scholar]
- 52.McMillan A, Coupland G, Chambers BK, Mills HR, Bencini R. 2010. Determining the diet of tammar wallabies on Garden island, Western Australia, using stable isotope analysis. In Macropods: the biology of kangaroos, wallabies and rat-kangaroos (eds Coulson G, Eldridge M), pp. 171–177. Collingwood, Australia: CSIRO Publishing. [Google Scholar]
- 53.Schwanz LE, Robert KA. 2012. Reproductive ecology of wild tammar wallabies in natural and developed habitats on Garden Island, Western Australia. Aust. J. Zool. 60, 111–119. ( 10.1071/ZO12024) [DOI] [Google Scholar]
- 54.Mottier P. 2009. LED for lighting applications. Hoboken, NJ: John Wiley and Sons. [Google Scholar]
- 55.Stone E, Jones G, Harris S. 2012. Conserving energy at a cost to biodiversity? Impacts of LED lighting on bats. Glob. Change Biol. 18, 2458–2465. ( 10.1111/j.1365-2486.2012.02705.x) [DOI] [Google Scholar]
- 56.Falchi F, Cinzano P, Elvidge CD, Keith DM, Haim A. 2011. Limiting the impact of light pollution on human health, environment and stellar visibility. J. Environ. Manage. 92, 2714–2722. ( 10.1016/j.jenvman.2011.06.029) [DOI] [PubMed] [Google Scholar]
- 57.Aubé M, Roby J, Kocifaj M. 2013. Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. PLoS ONE 8, e67798 ( 10.1371/journal.pone.0067798) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Dryad Data Repository: http://dx.doi.org/10.5061/dryad.7h08c.