Abstract
While pathogens are often assumed to limit the growth of wildlife populations, experimental evidence for their effects is rare. A lack of food resources has been suggested to enhance the negative effects of pathogen infection on host populations, but this theory has received little investigation. We conducted a replicated two-factor enclosure experiment, with introduction of the bacterium Bordetella bronchiseptica and food supplementation, to evaluate the individual and interactive effects of pathogen infection and food availability on vole populations during a boreal winter. We show that prior to bacteria introduction, vole populations were limited by food availability. Bordetella bronchiseptica introduction then reduced population growth and abundance, but contrary to predictions, primarily in food supplemented populations. Infection prevalence and pathological changes in vole lungs were most common in food supplemented populations, and are likely to have resulted from increased congregation and bacteria transmission around feeding stations. Bordetella bronchiseptica-infected lungs often showed protozoan co-infection (consistent with Hepatozoon erhardovae), together with more severe inflammatory changes. Using a multidisciplinary approach, this study demonstrates a complex picture of interactions and underlying mechanisms, leading to population-level effects. Our results highlight the potential for food provisioning to markedly influence disease processes in wildlife mammal populations.
Keywords: Bordetella bronchiseptica, co-infection, factorial experiment, food supplementation, population limitation, vole
1. Background
Pathogens can limit the growth of wildlife populations. In the most extreme cases, epizootic outbreaks may cause large-scale, stochastic population die-offs [1,2]. A small but growing body of research has identified more subtle effects of pathogens on host reproduction and survival [3–6]. However, experimental evidence for pathogen-induced population limitation in natural settings is rare (but see [3,7]).
In boreal Europe, vole populations are mostly cyclic [8–11]. Almost a century ago, Charles Elton proposed that infectious disease outbreaks ended periodic overpopulation of cyclic rodent species [12,13]. Since then most mechanistic research has focused on dominant predation theories [11]. However, past decades have seen interest in the role of pathogens in cyclic dynamics reignited [14], with recent studies demonstrating negative effects of endemic viruses on vole survival [5,6].
A survey of vole populations in central Finland during a cyclic winter decline found that 31% (range 11–73%, depending on the winter month) of field voles (Microtus agrestis) were infected with Bordetella bronchiseptica [14]. This bacterium causes respiratory disease in its mammalian hosts [15,16], and a concomitant increase in lymphoid tissue in vole lungs was considered evidence of a possible negative effect of B. bronchiseptica infection on survival [14]. Bordetella bronchiseptica has also been linked to a population crash of montane voles (Microtus montanus) in North America [17]. However, the involvement of other bacteria and viruses as causative agents for the disease processes could not be dismissed in either study.
Peak densities of cyclic vole populations occur in late autumn every 3–5 years, and are followed by a severe density crash during winter that usually continues into the following summer [8,18]. Winter food depletion has been identified as a factor that limits vole population growth at high densities and potentially initiates the cyclic decline [19,20]. However, a complementary role of pathogen infection has not been investigated.
A lack of food resources is likely to diminish an individuals' physiological condition prior to starvation-induced mortality. A vicious circle between poor physiological condition and increased infection risk has been proposed for vole and other wildlife species [21,22]. This relationship is thought to be mediated by trade-offs in the allocation of resources between costly immunological defences and other pertinent processes [23,24], and is consistent with an annual reduction in non-specific immunological investment observed in voles during winter [25].
Here we report on a factorial experiment using B. bronchiseptica introduction and food supplementation to investigate the individual and interactive effects of pathogen infection and resource availability on replicated, high-density field vole populations maintained in large outdoor enclosures over a boreal winter. We hypothesized that B. bronchiseptica introduction and low food availability (no food supplementation) would each limit vole population growth, and that populations subjected to both low food availability and B. bronchiseptica introduction would suffer higher mortality and reduced population growth than populations exposed to either factor alone. To the best of our knowledge, this is the first study to employ microparasite introduction as an experimental tool to investigate population limitation.
2. Material and methods
(a). Enclosures and abundance monitoring
The experiment was conducted in 32 adjoining field enclosures (each 20 × 25 m) near the town of Suonenjoki in central Finland (62°37′30″ N, 27°7′30″ E). This area is characterised by a relatively continental climate, with mean long-term summer temperatures of 15°C and mean long-term winter temperatures of −8°C. In the experimental winter, the permanent snow cover arrived at the enclosures in mid-November 2012 and lasted until the end of April 2013. The maximum snow depth during this period was approximately 60 cm. The enclosures were made of sheet metal, which rose approximately 1 m above ground and extended 50 cm underground. Voles were contained within the enclosures, and access by mammalian predators, particularly mustelids, was largely prevented. As a net did not cover the enclosures, avian predation was possible and signs of owl strikes were occasionally observed on the snow surface. Each enclosure contained eight sheet-metal shelter boxes (40 × 40 × 50 cm), with two entrance holes at the base, approximately 10 m apart. An Ugglan Special live trap (Grahnab, Sweden) was placed in each shelter box.
In early October 2012, five male and seven female wild-caught adult (<8 weeks) field voles were released into each enclosure (total n = 384). This male–female ratio was dictated by the numbers of trapped voles, and therefore, probably reflected natural proportions at the time. It resulted in enclosure densities of 417 voles ha−1, and is within the density range reported during high peak phases of Microtus vole cycles in Fennoscandia (400–500 voles ha−1) [26]. Additionally, a variable number of field voles were already present in some of the enclosures. Live trapping was conducted two weeks after vole introduction, in mid-October, to obtain baseline abundance estimates representative of established individuals. Thereafter, prior to B. bronchiseptica introduction, populations were monitored on three occasions at six- to eight-week intervals (November 2012, January 2013 and early March 2013), and twice more at two-week intervals after B. bronchiseptica introduction (end of March and early April 2013). Food supplementation was discontinued immediately before each trapping occasion. Traps were baited with oats and left unset for 2–3 days. They were then set and checked consecutively at 7.00 h, 14.00 h and 21.00 h, totalling eight or nine times over the course of 3 days. Food supplementation was immediately continued after each trapping occasion.
(b). Experiment implementation
Following baseline trapping in October, all 32 enclosures were randomized to either food supplementation (F+) or no food supplementation (F−) treatments. Food supplementation, in the form of rodent pellets (22.5% crude protein, 5% crude fat, 4.5% crude fibre and 6.5% crude ash; Altromin 1314F; Altromin Spezialfutter GmbH & Co., Lage, Germany), was provided ad libitum from a wire mesh feeder and an open aluminium tray that were placed in each shelter box. Voles could remove and cache pellets from the open trays, whereas from the feeders they had to consume the pellets in situ. Feeders and trays were placed into the shelter boxes of enclosures assigned to no food supplementation treatment, but left empty.
After the early March trapping occasion, vole populations were ordered from largest to smallest according to population size (minimum number of voles alive) [27], separately for both food treatment groups. Thereafter, populations in both food manipulation groups were assigned in alternating order into either B. bronchiseptica introduction (B+) or no B. bronchiseptica introduction (B−) treatments. Only enclosures containing four or more voles were included in the bacterial manipulation (n = 19 enclosures). Thus, the latter phase of the experiment employed a two-factor study design with four treatment groups: (i) food supplementation + B. bronchiseptica introduction (F + B +; n = 6), (ii) food supplementation alone (F + B−; n = 5), (iii) B. bronchiseptica introduction alone (F−B+; n = 4), and (iv) control (F−B−; n = 4). Vole monitoring was then conducted twice more at two-week intervals.
(c). Vole sampling
Each vole was subcutaneously injected with a passive-induced transponder (PIT) (EID Aalten BV, Aalten, The Netherlands) upon first capture, and the unique identification number was recorded at each encounter. Sex and reproductive status of females (subadult, mature, pregnant and/or lactating, post-mature) were determined through external examination, and body mass and head width were measured to the nearest 0.1 g and 0.1 mm, respectively. To minimize vole handling time and associated stress, only the vole identification number was recorded during the trapping occasion two weeks after B. bronchiseptica introduction.
During the early March and early April trapping occasions (before and after B. bronchiseptica introduction), voles were placed into ventilated buckets and taken to an on-site laboratory. Approximately 150 µl of blood was collected from the retro-orbital sinus (including only individuals greater than or equal to 15 g) with heparinized capillary tubes, and a blood smear was prepared and fixed with ethanol. During the early March trapping occasion, voles were released after sampling into the same shelter box from which they were captured. During the final trapping occasion (early April, one month after B. bronchiseptica introduction), all trapped voles (total n = 111) were euthanized via cervical dislocation after blood sampling. Voles were immediately dissected and their organs removed. A lung sample from each animal was frozen (less than −20°C) and later cultured to determine the presence or absence of B. bronchiseptica. Lung, liver, spleen and kidney samples were fixed in 10% non-buffered formalin for histological assessment.
(d). Introduction of Bordetella bronchiseptica into vole populations
Bordetella bronchiseptica was obtained and cultured (see below) from the lung of a single wild field vole captured in the surrounding area in autumn 2009. A laboratory pilot study was conducted to compare B. bronchiseptica administration routes (subcutaneous injection and oral/nasal) and suspension doses (107 and 108 colony forming units (CFU) ml−1). Following the demonstration of onward transmission to cage mates, a 100 µl suspension of B. bronchiseptica (108 CFU ml−1) in saline, pipetted into the nostrils and mouth of each individual, was selected as the most field deployable method.
To confirm that B. bronchiseptica was not already circulating in the laboratory population prior to the experiment, six laboratory-born voles (aged four to eight weeks) were randomly selected, euthanized and tested via lung culture for B. bronchiseptica infection; all were negative. Voles of the same cohort then underwent an acclimatization process, involving progressively longer periods in outdoor cages, to facilitate adjustment to winter conditions.
A second inoculation suspension was prepared from bacteria isolated from a single field vole experimentally infected during the pilot study. For disease introduction, two of the laboratory-reared voles were experimentally infected as described above, and introduced within 1 h to each B+ enclosure. One vole was placed into each of the two shelter boxes from which the highest number of voles had been captured during the preceding trapping occasion (to maximize the likelihood of transmission). In addition, one established vole from each B+ enclosure was experimentally infected in the field using the same protocol as for the laboratory infected voles. The combination of inoculating two laboratory-originating voles and one resident vole from the enclosure populations was chosen to maximize the likelihood of infected voles spreading the bacteria, while minimizing disruption to the enclosure populations through inoculations and the introduction of foreign individuals. We aimed to experimentally infect as few voles as possible, so that natural cycles of infection would spread the bacterium through the experimental populations. Two voles, treated with saline solution alone, were introduced into each B− enclosure using the same approach. Similarly, one established vole from each B− enclosure was administered saline in the field.
(e). Condition indices
Studentized residuals of a linear regression of body mass on head width were employed as an index of body condition [28]. Identity of the head width measurer was entered as a random factor in the model to adjust for possible systematic measurement error. Pregnant or lactating voles, and juveniles weighing under 20 g, were excluded from the analysis of body condition.
Plasma IgG titres were determined using a microplate enzyme-linked immunosorbent assay (ELISA) [29]. Plasma albumin was measured using a mouse ELISA kit (catalogue number 6300; Alpha Diagnostics International, San Antonio, TX, USA), following the manufacturer's instructions. White blood cell counts were carried out on May-Grünwald-Giemsa stained blood smears by a veterinary laboratory (Vetlab, Tampere, Finland). The total number of leucocytes was determined as the mean of three counts of leucocytes in a ×10 magnification field-of-view. Differential counts were made on 200 leucocytes using ×50/× 100 magnification under oil immersion.
(f). Bacteria cultures
The inocula for the pilot and experimental infections were prepared using a 24 h blood agar plate culture of B. bronchiseptica, as described below. Bacteria from the plate were suspended in saline, the optical density (620 nm) being 0.011. A 10-fold serial dilution was immediately prepared from the bacterial suspension and cultivated on blood agar plates. After incubation for 24 h at 37 ± 1°C, the number of colonies was observed and counted to be 8.6 × 107 CFU ml−1. Within 4 h, the inocula were transferred at room temperature from the site of culture to the site of experimental infection.
(g). Bacteriological examination
Frozen lung samples were thawed and cultured on blood agar (CASO, Merck, incorporated with 5% bovine blood) and bromthymol blue lactose agar plates (Merck). Growths were assessed after 48 h incubation at 37 ± 1°C, and after 6 days, colonies suspected to be B. bronchiseptica were sub-cultured on blood agar, MacConkey agar (Merck) and bromthymol blue lactose agar plates to identify colony morphology and colour reactions consistent with Bordetella bacteria. Six isolates were examined using the following tests: Gram stain, oxidase, motility, fermentation of glucose and API 20 NE (bioMérieux). Fewer tests were used for the remaining isolates. Identification of B. bronchiseptica was based on the following results: typical colony morphology and colour reactions on blood agar, bromthymol blue lactose agar and MacConkey, small Gram-negative rods, oxidase positive, motile, non-fermentative, urea and nitrate reduction positive, assimilation of adipate, malate, citrate and phenylacetate positive, assimilation of caprate and other tests in API 20 NE negative.
(h). Histopathological examination
Formalin-fixed lung, liver, spleen and kidney specimens were trimmed and routinely paraffin wax embedded. Sections (3–5 µm) were prepared and stained with haematoxylin–eosin for the histological assessment.
(i). Data analyses
Vole abundance (hereafter referred to as density) was modelled separately for each enclosure with program Capture [30]. Mh models (which incorporate heterogeneity in capture rate), with a jackknife estimator, were used to estimate densities on all trapping occasions except the last. As voles were not released during the final trapping occasion in mid-April, density was estimated with Mbh (removal) models, using Pollock & Otto's estimator [31]. Population growth rates were calculated for each enclosure-based trapping interval using the formula, Rt = ln (Nt−1/Nt), where Nt is the population density at time t [19,32].
Mark v. 7.0 [33] was used to calculate enclosure-based survival rates for each trapping interval prior to the introduction of B. bronchiseptica (October 2012–March 2013). As survival estimates depend partially on recapture rates, Akaike's information criterion (AIC) was used to compare recapture rate models including enclosure, trapping occasion, their interaction, or only the intercept. Survival estimates were obtained from the most parsimonious recapture rate model [34]. Following the introduction of B. bronchiseptica, survival data were obtained by monitoring the presence or absence of each individual captured in early March through the two successive trapping occasions.
(j). Statistical analyses: pre-Bordetella bronchiseptica introduction
Repeated-measures mixed ANOVA models were used to evaluate the effects of treatment on the population-level outcomes of density, growth rate and survival rate between October 2012 and March 2013. Initial fixed effects included food supplementation, trapping occasion, population density (not in the density response model), all interactions and the intercept. Enclosure and enclosure × trapping occasion were included as random factors. Selection of repeated covariate type (unstructured, autoregressive (1), compound symmetry or Toeplitz) was based on AIC of the full model.
Generalized mixed models were used to evaluate treatment effects on population sex ratio (the proportion of males; binomial distribution). Trapping week (continuous variable), food supplementation, density and all interactions were included as initial fixed factors, and enclosure and trapping week as random factors. The individual and interactive effects of week, population density and food availability on individual level condition index were evaluated separately for males and females in random coefficient regression models. New voles (previously not encountered; predominantly juveniles that have left the natal nest since the last trapping occasion) were excluded from analyses. Enclosure, vole identity and week were set as random factors. Owing to the infrequency and highly skewed treatment-wise distribution of pregnant and/or lactating females and new voles, statistical analysis was not possible and instead raw values are reported.
(k). Statistical analyses: post-Bordetella bronchiseptica introduction
Bordetella bronchiseptica-infected voles were not encountered in two B+ enclosures at the end of the experiment (both F+B+). These enclosures were removed from analyses. All voles released into the enclosures as part of B. bronchiseptica introduction were also excluded. Following the introduction of B. bronchiseptica, effects of treatment on population density and growth rates were evaluated with repeated-measures mixed ANOVA models. Initial fixed effects included food supplementation, B. bronchiseptica introduction, trapping occasion, population density (not for the density outcome model), all interactions and the intercept. Enclosure and enclosure × trapping occasion were entered as random factors.
The effects of treatment on individual survival (binomial distribution) and population sex ratio were evaluated with generalized mixed models. Trapping occasion, population density, food supplementation, B. bronchiseptica introduction and all their interactions were entered as initial fixed factors. Enclosure and the intercept were entered as random factors. As vole sex was not recorded during the trapping occasion two weeks after B. bronchiseptica introduction, trapping occasion was omitted as an explanatory variable for the sex ratio model. Again the population proportions of reproducing females and new voles could not be analysed owing to low prevalence and strong treatment bias. Raw values are presented for both outcomes.
Effects of treatment on individual condition index were evaluated separately for males and females using the same method as before B. bronchiseptica introduction. Bordetella bronchiseptica and all its interactions were also included as initial explanatory variables, and time was omitted. To increase statistical power, sexes were pooled for comparisons of individual level haematological indices (blood albumin, total IgG, total leucocytes, neutrophils, lymphocytes, monocytes and the ratio of neutrophils to lymphocytes) before and after B. bronchiseptica introduction. The effects of week, population density, food supplementation, B. bronchiseptica introduction and all their interactions were analysed with random coefficient regression models. Enclosure and vole identity (and ELISA plate number for albumin and IgG models) were included as random factors. New voles were removed from analyses.
To facilitate interpretation of interactions between density and week on the relative proportions of neutrophils and lymphocytes and their ratio, we first constructed mixed models with population density as a fixed explanatory factor and enclosure as a random factor. Residuals from these models were then used as response variables in mixed models with food supplementation, B. bronchiseptica introduction, week and their interactions as explanatory variables. Vole identity and enclosure were set as random factors.
Owing to extremely skewed infection prevalence data (all voles in F+B+ populations were infected), B. bronchiseptica population prevalence (based on lung culture results) could not be compared between F+B+ and F−B+ treatment groups with parametric tests. Instead, raw values are reported and Fisher's exact test was used to assess the degree of association between positive culture prevalence and treatment group. A generalized mixed model was used to compare the prevalence of individual pathological changes in the lungs (binomial distribution) of culture positive voles between F+B+ and F−B+ treatment groups. Treatment group, population density and their interaction were entered as fixed factors, and enclosure was set as a random factor.
In all models (before and after B. bronchiseptica introduction), selection was based on stepwise reduction, guided by AIC value and biological significance. Interaction terms were excluded if their removal did not increase AIC by more than 2 units. Comparisons were made using the maximum-likelihood method and final values obtained from the most parsimonious model with restricted maximum-likelihood, using Kenwood and Roger estimation. Data were analysed in SAS v. 9.3 (SAS Institute Inc., Cary, NC, USA).
3. Results
(a). Pre-Bordetella bronchiseptica introduction
The effect of food supplementation on population density (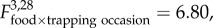 p = 0.001; figure 1) and growth rates (
p = 0.001; figure 1) and growth rates (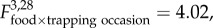 p = 0.024; figure 2) changed with time. All populations declined in size from October until January. However, an increase in growth rate from January to March in populations with food supplementation (F+) resulted in marginally higher densities (p = 0.069), as compared to no food supplementation (F−) populations. Population growth rate was negatively associated with vole population density (
p = 0.024; figure 2) changed with time. All populations declined in size from October until January. However, an increase in growth rate from January to March in populations with food supplementation (F+) resulted in marginally higher densities (p = 0.069), as compared to no food supplementation (F−) populations. Population growth rate was negatively associated with vole population density (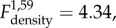 p = 0.041).
p = 0.041).
Figure 1.
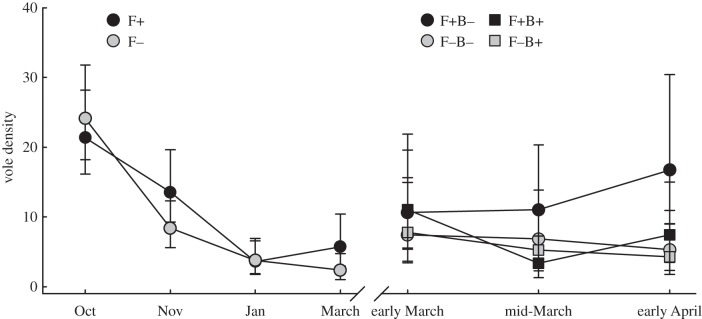
Population density before and after B. bronchiseptica introduction (ls means ± 1 s.e.). The line break in March represents the point when the lowest density populations were removed (hence the increase in average abundance) and B. bronchiseptica was introduced to establish a two-factor experiment design with four treatment groups.
Figure 2.
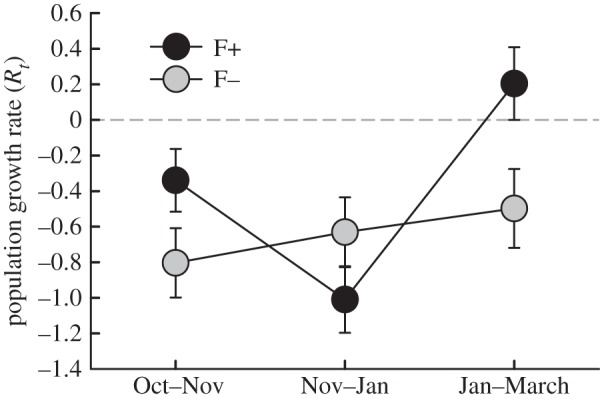
Population growth rates before B. bronchiseptica introduction (ls means ± 1 s.e.). Black symbols denote food supplemented populations (F+), and grey symbols (F−) denote non-food supplemented populations.
Population survival rates varied with time (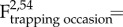
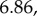 p = 0.002), but not in response to food supplementation or population density (electronic supplementary material, table S1). Sex ratio (expressed as the proportion of males) did not change with time, food supplementation or density (electronic supplementary material, table S1). Only nine pregnant or lactating voles were encountered from October to March, eight of which were from F+ populations. In January, six recently born voles were encountered, three from each treatment group. In March, 33 new voles were recorded across nine enclosures. All were from F+ populations.
p = 0.002), but not in response to food supplementation or population density (electronic supplementary material, table S1). Sex ratio (expressed as the proportion of males) did not change with time, food supplementation or density (electronic supplementary material, table S1). Only nine pregnant or lactating voles were encountered from October to March, eight of which were from F+ populations. In January, six recently born voles were encountered, three from each treatment group. In March, 33 new voles were recorded across nine enclosures. All were from F+ populations.
Male condition index was higher in F+ than F− populations (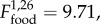 p = 0.004; electronic supplementary material, tables S2 and S3). The effect of food supplementation on female condition index changed with time (
p = 0.004; electronic supplementary material, tables S2 and S3). The effect of food supplementation on female condition index changed with time ( p = 0.003), such that from November until March, female condition index was higher in F+ than F− populations (electronic supplementary material, table S3).
p = 0.003), such that from November until March, female condition index was higher in F+ than F− populations (electronic supplementary material, table S3).
(b). Post-Bordetella bronchiseptica introduction
Lung samples from 111 voles were cultured to determine B. bronchiseptica infection status (total numbers: 40 F+B−, 29 F+B+, 21 F−B+, 21 F−B−). One positive individual was detected in both F+B− and F−B− treatment groups. In B+ groups, prevalence was higher in F+B+ (29 out of 29, 100%) than in F−B+ (10/21, 48%; enclosure prevalence 25–100%) treatment groups (Fisher's exact test, two-sided p < 0.001).
The effects of B. bronchiseptica introduction on population density changed with time (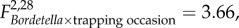 p = 0.04; figure 1). Densities declined considerably in F+B+ populations in the initial two-week period following B. bronchiseptica introduction, while densities in the other treatment group remained relatively stable. Food supplemented populations, especially those without B. bronchiseptica introduction, increased in size during the latter two weeks (
p = 0.04; figure 1). Densities declined considerably in F+B+ populations in the initial two-week period following B. bronchiseptica introduction, while densities in the other treatment group remained relatively stable. Food supplemented populations, especially those without B. bronchiseptica introduction, increased in size during the latter two weeks (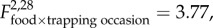 p = 0.04; figure 1). Higher population growth rates were observed in F+B− populations than in the other three treatment groups, and food supplementation similarly had an increasingly positive influence on population growth during the latter two weeks of the post-B. bronchiseptica introduction period, most pronouncedly in B– populations (
p = 0.04; figure 1). Higher population growth rates were observed in F+B− populations than in the other three treatment groups, and food supplementation similarly had an increasingly positive influence on population growth during the latter two weeks of the post-B. bronchiseptica introduction period, most pronouncedly in B– populations ( p = 0.03; figure 3). Variation in population growth was not associated with density (electronic supplementary material, table S1).
p = 0.03; figure 3). Variation in population growth was not associated with density (electronic supplementary material, table S1).
Figure 3.
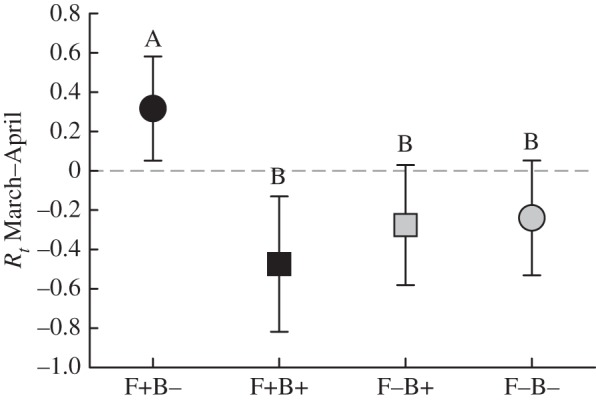
Population growth rates after B. bronchiseptica introduction (ls means ± 1 s.e.). Black symbols denote food supplementation, and squares represent treatment groups with B. bronchiseptica introduced. Different letters above error bars show a statistically significant difference between treatment groups.
Survival was higher in F+B− than in F+B+ populations ( p = 0.04; figure 4), but was not associated with population density (electronic supplementary material, table S1). Sex ratio did not vary with treatments or population density (electronic supplementary material, table S1). Twenty-seven reproducing females were recorded at the end of the experiment (total numbers: 21 F+B−, 2 F+B+, 1 F−B+, 3 F−B−), as well as 46 recently born voles (30 F+B−, 15 F+B+, 1 F−B+, 0 F−B−). Both counts were clearly higher in F+ than in F− populations. The condition index of males was inversely related to population density in B+ enclosures, and positively in B− enclosures; female condition index was higher in F+ than F− populations (electronic supplementary material, tables S2 and S3).
p = 0.04; figure 4), but was not associated with population density (electronic supplementary material, table S1). Sex ratio did not vary with treatments or population density (electronic supplementary material, table S1). Twenty-seven reproducing females were recorded at the end of the experiment (total numbers: 21 F+B−, 2 F+B+, 1 F−B+, 3 F−B−), as well as 46 recently born voles (30 F+B−, 15 F+B+, 1 F−B+, 0 F−B−). Both counts were clearly higher in F+ than in F− populations. The condition index of males was inversely related to population density in B+ enclosures, and positively in B− enclosures; female condition index was higher in F+ than F− populations (electronic supplementary material, tables S2 and S3).
Figure 4.
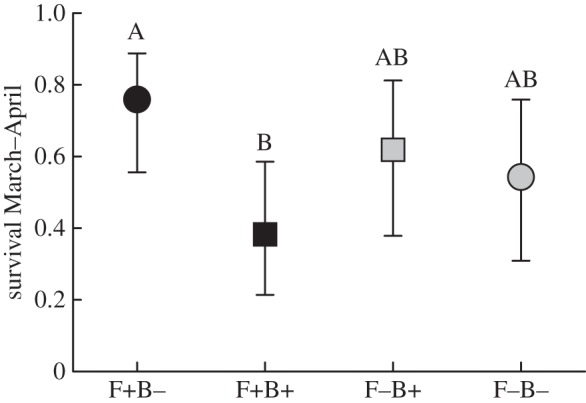
Proportion of voles that survived from immediately prior to B. bronchiseptica introduction until the experiment termination one month later (ls means ± 1 s.e.). Black symbols denote food supplementation, and squares represent treatment groups with B. bronchiseptica introduced. Different letters above error bars show a statistically significant difference between treatment groups.
The organs of 107 voles were assessed histologically for pathological changes. The lungs of a large proportion of voles exhibited a variable amount of bronchus associated lymphatic tissue (BALT), represented by focal and/or circular perivascular and bronchial lymphocyte accumulations. BALT was present in 27 out of 66 (40.9%) B– lungs and 21 out of 41 (51.2%) B+ lungs. BALT was the only pathological finding in all but two B– voles. In the latter, multiple metazoan parasite eggs (possibly cestodes) with surrounding mild mononuclear infiltration and mild alveolar protozoal infestation with mild macrophage infiltration were respectively recorded. Only two B+ positive lungs showed no histological changes (2 out of 41, 4.9%; this included the positive animal from F+B−), and in 10 B+ lungs (10 out of 41, 24.4%; including the positive animal from F−B−) BALT formation was the only finding.
Active inflammatory processes were more common in the lungs of culture positive voles from F+B+ (25 out of 29, 86.2%) populations than from F−B+ (2 out of 10, 20%) populations (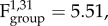 p = 0.025). These were represented by focal to multifocal and coalescing pyogranulomatous (comprised of macrophages and fewer neutrophils) or suppurative (almost exclusively comprising neutrophils) pneumonia that in most cases affected at least one entire lobe. The infiltrates were in some cases associated with chronic changes, such as mild interstitial fibrosis and/or some degree of type II pneumocyte hyperplasia, suggesting previous alveolar damage. No density effect on active inflammatory processes was identified (
p = 0.025). These were represented by focal to multifocal and coalescing pyogranulomatous (comprised of macrophages and fewer neutrophils) or suppurative (almost exclusively comprising neutrophils) pneumonia that in most cases affected at least one entire lobe. The infiltrates were in some cases associated with chronic changes, such as mild interstitial fibrosis and/or some degree of type II pneumocyte hyperplasia, suggesting previous alveolar damage. No density effect on active inflammatory processes was identified (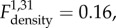 p = 0.69). In 15 of the 41 B. bronchiseptica positive lungs (36.6%), a variable number of apicomplexan protozoan cysts were observed within macrophages or cell free. These were morphologically consistent with the protozoan Hepatozoon erhardovae [35] and were always found within active inflammatory infiltrates. Protozoan parasites were found in only one B– lung sample, and this vole was from a B+ enclosure.
p = 0.69). In 15 of the 41 B. bronchiseptica positive lungs (36.6%), a variable number of apicomplexan protozoan cysts were observed within macrophages or cell free. These were morphologically consistent with the protozoan Hepatozoon erhardovae [35] and were always found within active inflammatory infiltrates. Protozoan parasites were found in only one B– lung sample, and this vole was from a B+ enclosure.
(c). Haematological indices: pre- and post-Bordetella bronchiseptica introduction
Plasma albumin levels did not show any variation in response to treatments, population density or time (electronic supplementary material, table S2). Total IgG levels increased in F− populations, and decreased in F+ populations when comparing before and after B. bronchiseptica introduction ( p = 0.009), and were negatively associated with population density (
p = 0.009), and were negatively associated with population density (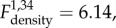 p = 0.018, electronic supplementary material, tables S2 and S3).
p = 0.018, electronic supplementary material, tables S2 and S3).
Total leucocyte levels decreased over time (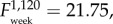 p < 0.001), but were consistently higher in F+ than in F− populations (
p < 0.001), but were consistently higher in F+ than in F− populations (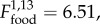 p = 0.024, figure 5a). Monocyte numbers did not change with time (electronic supplementary material, tables S2 and S3), and were similarly higher in F+ than in F− populations (
p = 0.024, figure 5a). Monocyte numbers did not change with time (electronic supplementary material, tables S2 and S3), and were similarly higher in F+ than in F− populations (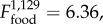 p = 0.012). Neutrophil levels increased in B+ populations after B. bronchiseptica introduction (figure 5b), but remained relatively constant in B− populations (
p = 0.012). Neutrophil levels increased in B+ populations after B. bronchiseptica introduction (figure 5b), but remained relatively constant in B− populations ( p = 0.052). Conversely, lymphocyte levels decreased in B+ populations, while remaining constant in B− populations (
p = 0.052). Conversely, lymphocyte levels decreased in B+ populations, while remaining constant in B− populations ( p = 0.039). As such, the neutrophil/lymphocyte ratio was similar between treatment groups pre-B. bronchiseptica introduction, but approached higher levels in B+ than in B− populations post-introduction (
p = 0.039). As such, the neutrophil/lymphocyte ratio was similar between treatment groups pre-B. bronchiseptica introduction, but approached higher levels in B+ than in B− populations post-introduction ( p = 0.075; electronic supplementary material, tables S2 and S3).
p = 0.075; electronic supplementary material, tables S2 and S3).
Figure 5.
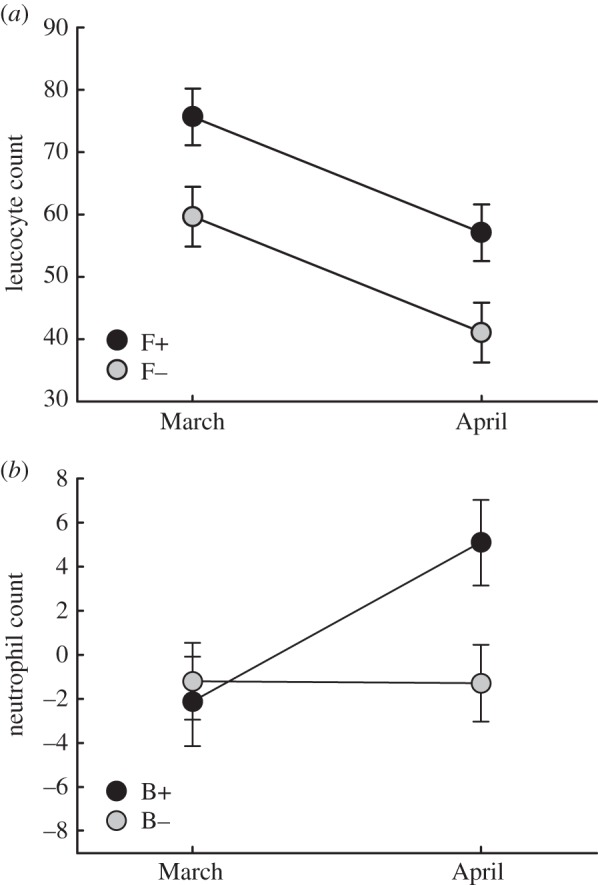
(a) Total leucocyte levels in relation to food supplementation, before and after B. bronchiseptica introduction (ls means ± 1 s.e.). (b) Neutrophil levels, before and after B. bronchiseptica introduction (ls means ± 1 s.e.).
4. Discussion
Contrary to our expectations, both B. bronchiseptica infection prevalence and pathological effects associated with infection were more pronounced in vole populations that received food supplementation than those that didn't. Voles from food supplemented populations displayed lower survival and these effects translated into a reduction in population density and growth. Interestingly, B. bronchiseptica infections were often associated with pulmonary protozoan (H. erhardovae) infection, which potentially contributed to the disease severity. Together these findings provide rare depth into disease processes, and an interesting picture of individual-level interactions and mechanisms leading to population limitation.
Prior to the introduction of B. bronchiseptica, vole populations that received food supplementation displayed faster growth rates and attained larger population size than populations without food supplementation. This is consistent with previous research, which has identified winter food resources as an important limiting factor of folivorous vole populations in boreal Europe [19,20,36]. Mortality rates did not differ between food supplemented and non-supplemented populations, indicating that natural food resources were sufficient for vole survival. However, voles from food supplemented populations displayed consistently higher average body condition, which implies that they were able to allocate resources to reproduction. In line with our findings, voles in best condition have been previously found to initiate seasonal reproduction [25], while high-quality resources can accelerate the onset of spring breeding [37,38].
Food supplementation failed to alleviate the negative effects of B. bronchiseptica on vole population growth. Only populations with food supplementation in the absence of B. bronchiseptica exhibited consistent positive population growth in late winter. Populations without food supplementation, and food supplemented populations with B. bronchiseptica introduced, declined in size. These results provide experimental evidence for the ability of a pathogen, B. bronchiseptica, to limit vole population growth, as previously suggested [14,17]; surpassing the limiting effects of winter food depletion. It is worth noting that these effects were not associated with vole population density, and B. bronchiseptica infection alone is therefore unlikely to lead to the cyclic regulation of vole populations.
Four weeks post-introduction, B. bronchiseptica infection had become more prevalent in food supplemented populations than non-supplemented populations. It is highly likely that aggregation of voles around the feeding stations, or even direct oral contact with the feeding apparatuses, increased transmission of bacteria—analogously to disease transmission associated with garden bird feeding stations [39,40] and other forms of wildlife food provisioning [41]. Similarly, more frequent bacteria re-exposure in the food supplemented populations probably caused higher bacterial burdens and the more severe pathological effects. It remains plausible that at certain times voles will naturally congregate in ways that promote the effects seen here. Voles, like many other small mammal species, exhibit seasonal aggregation or communal nesting to attain, e.g. thermoregulatory or dietary benefits [42–44].
In laboratory rodents, B. bronchiseptica pathogenicity has been associated with co-infection by other parasites [45], which suggests that a relatively severe stress on the immune system is required for the development of clinical B. bronchiseptica-induced disease. In this study, B. bronchiseptica-infected lungs often showed co-infection with protozoa that were morphologically consistent with H. erhardovae. This parasite is found widely in bank voles in northern Europe, but only rarely in field voles [35]. Consistent with previous research [46], this finding demonstrates that one parasite can greatly affect the presence of another within a host. Interestingly, H. erhardovae was previously identified in vole lungs without significant associated inflammation [35], while we observed the parasites in the majority of cases in association with active inflammatory processes. These findings suggest that B. bronchiseptica infection induced inflammatory processes in the vole lungs and thereby rendered them more prone to protozoan infection and further damage.
Prior to the introduction of B. bronchiseptica, haematological indices indicative of chronic immune stimulation (leucocytes and monocytes) were elevated in food supplemented populations. This may be owing to infection by unknown parasites [21], possibly also related to congregation at feeding stations. Nevertheless, voles in food supplemented populations generally displayed better body condition than voles in non-supplemented populations prior to B. bronchiseptica introduction. This indicates that the ability to mount an immune response against infection may be enhanced by the availability of resources, in line with research that has demonstrated higher disease associated mortality in resource-limited populations [7,47]. We found that although non-specific inflammatory cell recruitment occurred following the introduction of B. bronchiseptica (increase in neutrophils and decrease in lymphocytes); its magnitude was not influenced by resource levels.
Infectious agents are an intriguing force in population ecology. Epizootic outbreaks clearly demonstrate the ability of pathogens to decrease the growth of host populations, and are especially pertinent to vulnerable species [48]. Meanwhile, recent investigations show that pathogen effects on wildlife populations may be more pervasive and subtle than traditionally believed [49], and provide an exciting focus for future research. The ability of anthropogenic environmental changes to influence wildlife-pathogen dynamics is increasingly recognized [41], and here we provide experimental evidence that the provision of food resources can alter infectious disease processes in vole populations. We demonstrate the complexity of interactions leading to population-level effects and the use of multidisciplinary biological research for their detection.
Supplementary Material
Acknowledgements
We are very grateful to Eva R. Kallio, Juha Laakkonen and two anonymous reviewers for constructive comments on an earlier version of this paper. We thank the technical staff at the Finnish Centre for Laboratory Animal Pathology, University of Helsinki, for preparing the histological specimens, Ilkka Taponen for maintenance of the laboratory vole colony, Sisko Juutinen and Sami Kyröläinen for conducting the ELISA tests, Arja Väliaho and Minna Nylund for bacteria culturing, Hanna Ruhanen for assistance with the laboratory infections and Geert Meen for help with the fieldwork.
Ethics
All animal procedures used in this study were approved by the Finnish Animal Experiment Board (permit no ESAVI/5879/04.10.03/2012), based on Finnish legislature and the directive of the European Union. The experimental species, the field vole, is not protected in Finland.
Data accessibility
Data are available online from Dryad (http://dx.doi.org/10.5061/dryad.2503h)
Authors' contributions
K.M.F., H.H., T.M., P.S. and O.H. designed the study; K.M.F., T.M., P.S. and O.H. participated in field data collection; V.H.-K. cultured the bacteria; A.K. performed the pathology analysis; K.M.F. and O.H. analysed the data; K.M.F. drafted the manuscript; all authors contributed to revisions of the manuscript and approved the final version.
Competing interests
We have no competing interests.
Funding
This experiment was funded by grants from the Academy of Finland (grant 133495 to O.H., grant 132190 to T.M.). The University of Jyväskylä graduate school financially supported K.M.F.
References
- 1.Young TP. 1994. Natural die-offs of large mammals: implications for conservation. Conserv. Biol. 8, 410–418. ( 10.1046/j.1523-1739.1994.08020410.x) [DOI] [Google Scholar]
- 2.Daszak P, Cunningham AA, Hyatt AD. 2000. Emerging infectious diseases of wildlife: threats to biodiversity and human health. Science 287, 443–449. ( 10.1126/science.287.5452.443) [DOI] [PubMed] [Google Scholar]
- 3.Hudson PJ, Dobson AP, Newborn D. 1998. Prevention of population cycles by parasite removal. Science 282, 2256–2258. ( 10.1126/science.282.5397.2256) [DOI] [PubMed] [Google Scholar]
- 4.Tompkins DM, Begon M. 1995. Parasites can regulate wildlife populations. Parasitol. Today 15, 311–313. ( 10.1016/S0169-4758(99)01484-2) [DOI] [PubMed] [Google Scholar]
- 5.Kallio ER, Voutilainen L, Vapalahti O, Vaheri A, Henttonen H, Koskela E, Mappes T. 2007. Endemic hantavirus infection impairs the winter survival of its rodent host. Ecology 88, 1911–1916. ( 10.1890/06-1620.1) [DOI] [PubMed] [Google Scholar]
- 6.Burthe S, Telfer S, Begon M, Bennett M, Smith A, Lambin X. 2008. Cowpox virus infection in natural field vole Microtus agrestis populations: significant negative impacts on survival. J. Anim. Ecol. 77, 110–119. ( 10.1111/j.1365-2656.2007.01302.x.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen AB, Greives TJ. 2008. The interaction of parasites and resources cause crashes in a wild mouse population. J. Anim. Ecol. 77, 370–377. ( 10.1111/j.1365-2656.2007.01321.x) [DOI] [PubMed] [Google Scholar]
- 8.Hansson L, Henttonen H. 1988. Rodent dynamics as community processes. Trends. Ecol. Evol. 3, 195–200. ( 10.1016/0169-5347(88)90006-7) [DOI] [PubMed] [Google Scholar]
- 9.Korpela K, et al. 2013. Nonlinear effects of climate on boreal rodent dynamics: mild winters do not negate high-amplitude cycles. Glob. Change Biol. 19, 697–710. ( 10.1111/gcb.12099) [DOI] [PubMed] [Google Scholar]
- 10.Cornulier T, et al. 2013. Europe-wide dampening of population cycles in keystone herbivores. Science 340, 63–66. ( 10.1126/science.1228992) [DOI] [PubMed] [Google Scholar]
- 11.Krebs CJ. 2013. Population fluctuations in rodents. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 12.Elton CS. 1924. Periodic fluctuations in the numbers of animals: their causes and effects. J. Exp. Biol. 2, 119–163. [Google Scholar]
- 13.Elton C, Davis DHS, Findlay GM. 1935. An epidemic among voles (Microtus agrestis) on the Scottish border in the spring of 1934. J. Anim. Ecol. 4, 277–288. ( 10.2307/1018) [DOI] [Google Scholar]
- 14.Soveri T, Henttonen H, Rudbäck E, Schildt R, Tanskanen R, Husu-Kallio J, Haukisalmi V, Sakura A, Laakkonen J. 2000. Disease patterns in field and bank vole populations during a cyclic decline in central Finland. Comp. Immunol. Microbiol. Infect. Dis. 23, 73–89. ( 10.1016/S0147-9571(99)00057-0) [DOI] [PubMed] [Google Scholar]
- 15.Woolfrey BF, Moody JA. 1991. Human infections associated with Bordetella bronchiseptica. Clin. Microbiol. Rev. 4, 243–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diavatopoulos DA, Cummings CA, Schouls LM, Brinig MM, Relman DA, Mooi FR. 2005. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 1, e45 ( 10.1371/journal.ppat.0010045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jensen WI, Duncan RM. 1980. Bordetella bronchiseptica associated with pulmonary disease in mountain voles (Microtus montanus). J. Wildl. Dis. 16, 11–14. ( 10.7589/0090-3558-16.1.11) [DOI] [PubMed] [Google Scholar]
- 18.Norrdahl K, Korpimäki E. 2002. Changes in individual quality during a 3-year population cycle of voles. Oecologia 130, 239–249. ( 10.1007/s004420100795) [DOI] [PubMed] [Google Scholar]
- 19.Huitu O, Koivula M, Korpimäki E, Klemola T, Norrdahl K. 2003. Winter food supply limits growth of northern vole populations in the absence of predation. Ecology 84, 2108–2118. ( 10.1890/02-0040) [DOI] [Google Scholar]
- 20.Huitu O, Jokinen I, Korpimäki E, Koskela E, Mappes T. 2007. Phase dependence in winter physiological condition of cyclic voles. Oikos 116, 565–577. ( 10.1111/j.0030-1299.2007.15488.x) [DOI] [Google Scholar]
- 21.Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M. 2008. Poor condition and infection: a vicious circle in natural populations. Proc. R. Soc. B 275, 1753–1759. ( 10.1098/rspb.2008.0147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beldomenico PM, Begon M. 2010. Disease spread, susceptibility and infection intensity: vicious circles? Trends Ecol. Evol. 25, 21–27. ( 10.1016/j.tree.2009.06.015) [DOI] [PubMed] [Google Scholar]
- 23.Sheldon BC, Verhulst S. 1996. Ecological immunity: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 11, 317–321. ( 10.1016/0169-5347(96)10039-2) [DOI] [PubMed] [Google Scholar]
- 24.Lochmiller RL, Deerenberg C. 2000. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos 88, 87–98. ( 10.1034/j.1600-0706.2000.880110.x) [DOI] [Google Scholar]
- 25.Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M, Begon M. 2008. The dynamics of health in wild field vole populations: a haematological perspective. J. Anim. Ecol. 77, 984–997. (doi:0.1111/j.1365-2656.2008.01413.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myllymäki A. 1977. Demographic mechanisms in the fluctuating populations of the field vole Microtus agrestis. Oikos 29, 468–493. ( 10.2307/3543588) [DOI] [Google Scholar]
- 27.Krebs CJ. 1966. Demographic changes in fluctuating populations of Microtus californicus. Ecol. Monogr. 36, 239–273. ( 10.2307/1942418) [DOI] [Google Scholar]
- 28.Schulte-Hostedde AI, Millar JS, Hickling GJ. 2001. Evaluating body condition in small mammals. Can. J. Zool. 79, 1021–1029. ( 10.1139/z2012-055) [DOI] [Google Scholar]
- 29.Forbes KM, Stuart P, Mappes T, Hoset KS, Henttonen H, Huitu O. 2014. Diet quality limits summer growth of field vole populations. PLoS ONE 9, e91113 ( 10.1371/journal.pone.0091113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otis DL, Burnham KP, White GC, Anderson DR. 1978. Statistical inference from capture data on closed animal populations. Wildl. Monogr. 62, 1–135. [Google Scholar]
- 31.Pollock KH, Otto MC. 1983. Robust estimation of population size in closed animal populations from capture-recapture experiments. Biometrics 39, 1035–1049. ( 10.2307/2531337) [DOI] [PubMed] [Google Scholar]
- 32.Sibly RM, Hone J. 2002. Population growth rate and its determinants: an overview. Phil. Trans. R. Soc. Lond. B 357, 1153–1170. ( 10.1098/rstb.2002.1117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White GC, Burnham KP. 1999. Program MARK: survival estimation from populations of marked animals. Bird Study 46, 120–138. ( 10.1080/00063659909477239) [DOI] [Google Scholar]
- 34.Burnham KP, Anderson DR. 2002. Model selection and interference: a practical information-theoretic approach, 2nd edn New York, NY: Springer. [Google Scholar]
- 35.Laakkonen J, Sukura A, Oksanen A, Henttonen H, Soveri T. 2001. Haemogragarines of the genus Hepatozoon (Apicomplexa: Adeleina) in rodents from northern Europe. Folia Parasitol. 48, 263–267. ( 10.14411/fp.2001.043) [DOI] [PubMed] [Google Scholar]
- 36.Forbes KM, Stuart P, Mappes T, Henttonen H, Huitu O. 2014. Food resources and intestinal parasites as limiting factors for boreal vole populations during winter. Ecology 95, 3139–3148. ( 10.1890/13-2381.1) [DOI] [Google Scholar]
- 37.Cole RF, Batzli GO. 1979. Nutrition and population dynamics of the prairie vole, Microtus ochrogaster, in central Illinois. J. Anim. Ecol. 48, 455–470. ( 10.2307/4172) [DOI] [Google Scholar]
- 38.Helle H, Koskela E, Mappes T. 2012. Life in varying environments: experimental evidence for delayed effects of juvenile environment on adult life history. J. Anim. Ecol. 81, 573–582. ( 10.1111/j.1365-2656.2011.01937.x) [DOI] [PubMed] [Google Scholar]
- 39.Brittingham MC, Temple SA. 1986. A survey of avian mortality at winter feeders. Wildl. Soc. Bull. 14, 445–450. [Google Scholar]
- 40.Robinson RA, et al. 2010. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 5, e12215 ( 10.1371/journal.pone.0012215) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker DJ, Streicker GD, Altizer S. 2015. Linking anthropogenic resources to wildlife-pathogen dynamics: a review and meta-analysis. Ecol. Lett. 18, 483–495. ( 10.1111/ele.12428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolff JO, Lidicker WZ. 1981. Communal winter nesting and food sharing in taiga voles. Behav. Ecol. Sociobiol. 9, 237–240. ( 10.1007/BF00299877) [DOI] [Google Scholar]
- 43.Ostfeld RS. 1985. Limiting resources and territoriality in microtine rodents. Am. Nat. 126, 1–15. ( 10.1086/284391) [DOI] [Google Scholar]
- 44.Hayes JP, Speakman JR, Racey PA. 1992. The contribution of local heating and reducing exposed surface area to the energetic benefits of huddling by short-tailed field voles (Microtus agrestis). Physiol. Zool. 65, 742–762. [Google Scholar]
- 45.Bemis DA, Shek WR, Clifford CB. 2003. Bordetella bronchiseptica infection in mice and rats. Comp. Med. 53, 11–20. [PubMed] [Google Scholar]
- 46.Telfer S, Lambin X, Birties R, Beldomenico P, Burthe S, Paterson S, Begon M. 2010. Species interactions in a parasite community drive infection risk in a wildlife population. Science 330, 243–246. ( 10.1126/science.1190333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moret Y, Schmid-Hempel P. 2000. Survival for immunity: the price of immune system activation for bumblebee workers. Science 290, 1166–1168. ( 10.1126/science.290.5494.1166) [DOI] [PubMed] [Google Scholar]
- 48.Daszak P, Cunningham AA. 1999. Extinction by infection. Trends Ecol. Evol. 14, 279 ( 10.1016/S0169-5347(99)01665-1) [DOI] [PubMed] [Google Scholar]
- 49.McCallum H, Dobson A. 2005. Detecting disease and parasite threats to endangered species and ecosystems. Trends Ecol. Evol. 10, 190–194. ( 10.1016/S0169-5347(00)89050-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available online from Dryad (http://dx.doi.org/10.5061/dryad.2503h)


