Abstract
Both hereditary factors (e.g., BRCA1) and nicotinamide adenine dinucleotide (NAD)-dependent metabolic pathways are implicated in the initiation and progression of ovarian cancer. However, whether crosstalk exists between BRCA1 and NAD metabolism remains largely unknown. Here, we showed that: (i) BRCA1 inactivation events (mutation and promoter methylation) were accompanied by elevated levels of NAD; (ii) the knockdown or overexpression of BRCA1 was an effective way to induce an increase or decrease of nicotinamide phosphoribosyltransferase (Nampt)-related NAD synthesis, respectively; and (iii) BRCA1 expression patterns were inversely correlated with NAD levels in human ovarian cancer specimens. In addition, it is worth noting that: (i) NAD incubation induced increased levels of BRCA1 in a concentration-dependent manner; (ii) Nampt knockdown-mediated reduction in NAD levels was effective at inhibiting BRCA1 expression; and (iii) the overexpression of Nampt led to higher NAD levels and a subsequent increase in BRCA1 levels in primary ovarian cancer cells and A2780, HO-8910 and ES2 ovarian cancer cell lines. These results highlight a novel link between BRCA1 and NAD. Our findings imply that genetic (e.g., BRCA1 inactivation) and NAD-dependent metabolic pathways are jointly involved in the malignant progression of ovarian cancer.
Keywords: BRCA1, NAD, NADH, Nampt, ovarian cancer
Abbreviations
- BRCA1
breast cancer type 1 susceptibility protein
- CtBP
C-terminal binding proteins
- NAD
nicotinamide adenine dinucleotide; Nampt, nicotinamide phosphoribosyltransferase
- PCR
polymerase chain reaction
- shRNAs
short hairpin ribonucleic acids
Introduction
Ovarian cancer is characterized by a high mortality rate among gynecological malignancies worldwide.1 Accumulating evidence indicates that hereditary factors (e.g., BRCA1)2 and energy metabolism3 are implicated in the initiation and progression of ovarian cancer. However, crosstalk between genetic and metabolic mechanisms has not been extensively studied. BRCA1 is a tumor suppressor gene that plays a key role in numerous cellular processes.4 Recent research has confirmed that BRCA1 is an important transcriptional regulator, and BRCA1 depletion shows changes to approximately 7% of the mRNAs expressed in cancer cells.4 Moreover, our recent study also indicated that there are wide ranges of transcriptional regulation, epigenetic patterns, and metabolic differences between BRCA1 dysfunction and the basal phenotype.5-9 Nicotinamide adenine dinucleotide (NAD) is a crucial molecule of energy production and signal transduction processes that have been linked to cancer development.10 To date, the direct link(s) between BRCA1 and NAD metabolism remain largely unknown, but NAD-dependent signaling pathways and enzymes are many and varied, and are involved in a number of fundamental events such as transcription, DNA repair, cell cycle progression, apoptosis and metabolism.11 More specifically, sirtuins are a family of NAD-dependent protein deacetylases that play an important part in transcriptional and epigenetic regulation,12,13 and are also responsible for the changes in metabolic homeostasis.14 For this reason, the present study was undertaken to investigate the regulation of NAD biosynthesis after BRCA1 inactivation events (mutation, promoter methylation, or knockdown), and to provide new insights into BRCA1 dysfunction-mediated abnormal NAD metabolism in ovarian cancer progression.
Results
BRCA1, rather than BRCA2, may be involved in NAD synthesis
Intracellular NAD levels were increased in non-BRCA1-mutated and BRCA1-mutated ovarian cancer compared with their adjacent normal tissue (Fig. 1A). It is interesting to note that BRCA1-mutated ovarian cancer showed dramatically increased intracellular levels of NAD compared with the other 3 groups (Fig. 1A). However, NADH levels were not affected by BRCA1 patterns (Fig. 1B). Therefore, the elevated NAD/NADH ratio was mainly dependent on the increased NAD levels in ovarian cancer tissue (Fig. 1C). In addition, there was a significant inverse trend between BRCA1 mRNA levels and NAD levels in A2780, HO-8910, and ES2 ovarian cancer cell lines (Fig. 1D). Of potential clinical relevance, the relationship between BRCA1 mRNA levels and NAD levels was studied in 40 non-BRCA1-mutated ovarian cancer specimens. Our results showed a significant negative association between BRCA1 mRNA levels and NAD levels (Fig. 1E). Although intracellular NAD levels were increased in non-BRCA2-mutated and BRCA2-mutated ovarian cancers compared with their adjacent normal tissue, there was no significant difference in NAD levels between the non-BRCA2-mutated and BRCA2-mutated groups, including ovarian cancer and normal ovarian tissue (Fig. 1F–H).
Figure 1.
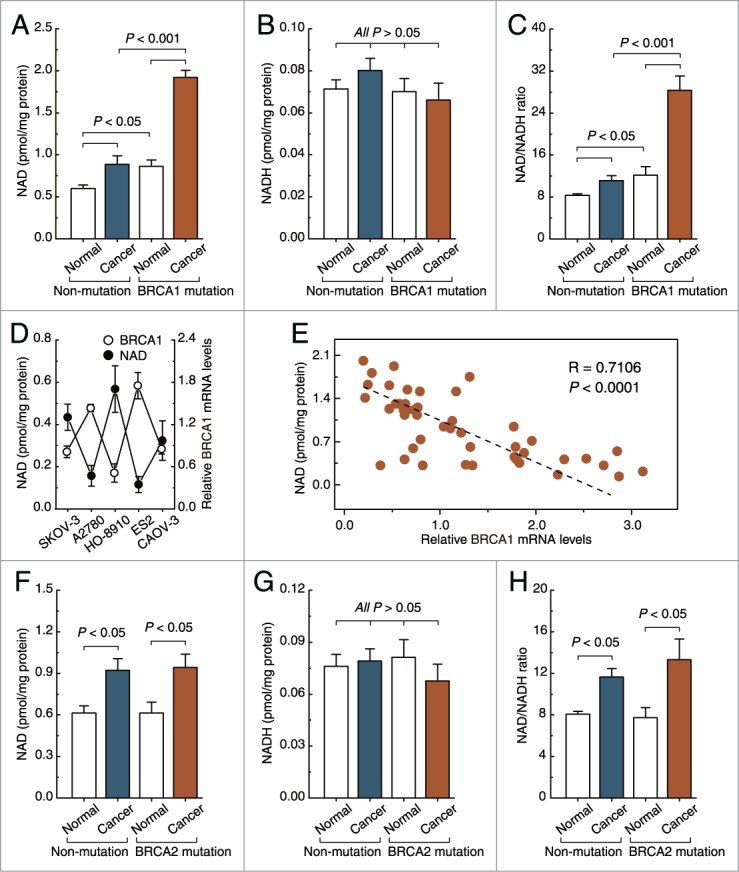
Intracellular NAD levels in non-BRCA1-mutated, BRCA1-mutated, and BRCA2-mutated ovarian cancer. (A–C), NAD levels, NADH levels, and NAD/NADH ratio were measured in 28 pairs of non-BRCA1-mutated and BRCA1-mutated ovarian cancer and their adjacent normal tissue. Bar graphs show mean ±SD. (D) BRCA1 mRNA levels and NAD levels were measured in A2780, SKOV-3, CAOV-3, HO-8910, and ES2 ovarian cancer cell lines (repeated 12 times). Bar graphs show mean ± SD. (E) correlation between BRCA1 mRNA levels and NAD levels in 40 non-BRCA1-mutated ovarian cancer specimens. (F–H) NAD levels, NADH levels, and NAD/NADH ratio were measured in 23 pairs of non-BRCA2-mutated and BRCA2-mutated ovarian cancer and their adjacent normal tissue. Bar graphs show mean ±SD.
Reduced expression of BRCA1 mRNA mediated by promoter hypermethylation is inversely correlated with intracellular NAD levels
In mammals, promoter methylation is an epigenetic modification involved in regulating gene expression.9 Consistent with this idea, we showed that ovarian cancer tissue with a hypermethylated BRCA1 promoter (Figs. 2B and D) displayed reduced expression of BRCA1 mRNA (Fig. 2E) compared with adjacent normal tissue, Fig. 2A shows the location of CpG sites in BRCA1 promoter. However, no significant BRCA1 expression differences (Fig. 2H) were observed in ovarian cancer with an unmethylated BRCA1 promoter (Figs. 2C and G) compared with adjacent normal tissue. Based on these considerations, the low levels of BRCA1 mRNA mediated by promoter hypermethylation were an appropriate model for investigating the physiologic relationship between BRCA1 mRNA and NAD levels. Notably, there was a marked increase in NAD levels (Fig. 2F) along with a hypermethylated promoter-mediated deficiency in BRCA1 mRNA in ovarian cancer (Fig. 2E). However, although NAD levels were also increased in ovarian cancer tissue (Fig. 2I), with no significant difference in BRCA1 promoter methylation or expression (Figs. 2G and H), the increased NAD levels were not significant compared with the levels in ovarian cancer with BRCA1 mRNA deficiency.
Figure 2.
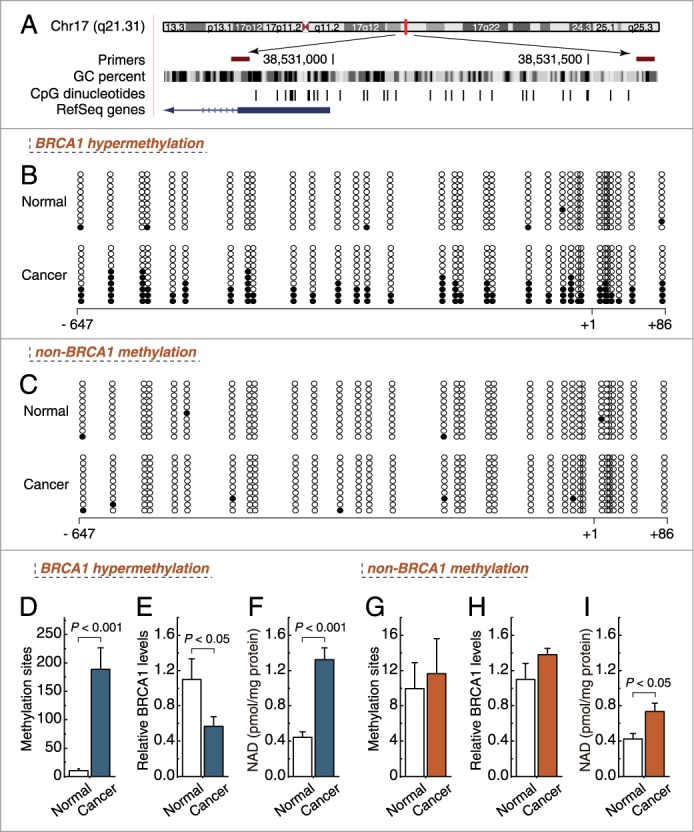
Intracellular NAD levels in ovarian cancer with hypermethylated promoter-mediated BRCA1 inactivation. (A) the location of CpG sites in the core promoter region of BRCA1. Genomic coordinates are shown, along with the primer-amplified fragments, GC percentage, location of individual CpG dinucleotides (dashes), and BRCA1 RefSeq gene (exon 1 is shown as a blue box and the intron is shown as an arrowed line). The arrow indicates the direction of transcription. (B, C) comparative analysis of methylation patterns in the core promoter region of BRCA1 in ovarian cancer and adjacent normal tissue. The circles correspond to the CpG sites denoted by black dashes in (A). Closed circles, methylation; open circles, unmethylated. Ten individual clones were sequenced for each sample. (D, G) summary of the methylation levels of BRCA1 core promoter from the measurements shown in B and C, respectively. (E, H) relative BRCA1 mRNA levels were measured in ovarian cancer with identified hypermethylated or unmethylated BRCA1 promoter, compared with their adjacent normal tissue. (F, I) intracellular NAD levels were measured in ovarian cancer with identified BRCA1 inactivation or not, respectively. Bar graphs show mean ± SD. Each group, n = 19.
BRCA1 can regulate intracellular NAD levels in ovarian cancer cells
To further confirm the role of BRCA1 in the regulation of NAD levels, the effects of knockdown or overexpression of BRCA1 were evaluated in 293T cells, 5 ovarian cancer cell lines (A2780, SKOV-3, CAOV-3, HO-8910, and ES2), and primary ovarian cancer cells with identified BRCA1 mutations or no BRCA1 mutations. The results indicated that there were no significant changes in NAD levels after the knockdown or overexpression of BRCA1 in 293T, SKOV-3, and CAOV-3 cells (Fig. 3). Interestingly, we observed that: (i) overexpression of BRCA1 could effectively reduce the NAD levels in A2780, HO-8910, and ES2 cells and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells; (ii) knockdown of BRCA1 was an effective way to induce an increase of NAD levels in A2780, HO-8910, and ES2 cells and primary non-BRCA1-mutated ovarian cancer cells; and (iii) NAD levels were not sensitive to the BRCA1 knockdown in primary BRCA1-mutated ovarian cancer cells (Fig. 3).
Figure 3.
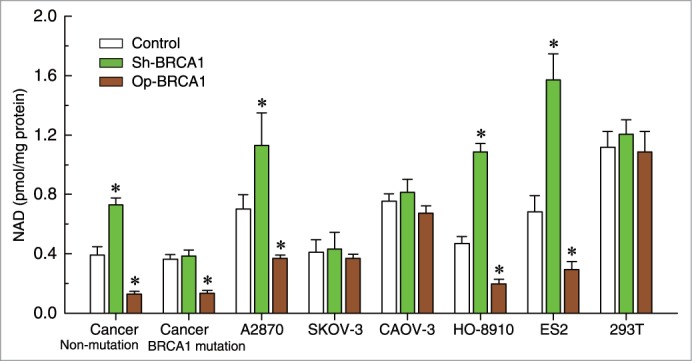
Effects of BRCA1 on intracellular NAD levels. Relative NAD levels after knockdown or overexpression of BRCA1 in 293T cells, A2780, SKOV-3, CAOV-3, HO-8910, and ES2 ovarian cancer cell lines, all wild-type for BRCA1 (repeated 12 times), and primary non-BRCA1-mutated (wild-type for BRCA1) and BRCA1-mutated (mutant-type for BRCA1) ovarian cancer cells (n = 28). Bar graphs show mean ± SD. Sh, shRNAs; Op, overexpression. * P < 0.05 vs. control.
BRCA1 can regulate intracellular Nampt levels in ovarian cancer cells
It is well known that nicotinamide phosphoribosyltransferase (Nampt) is a rate-limiting enzyme in regenerating NAD in mammals. Notably, although intracellular Nampt levels were increased in non-BRCA1-mutated, BRCA1-mutated and BRCA2-mutated ovarian cancer compared with their adjacent normal tissue, BRCA1-mutated ovarian cancer showed significantly increased intracellular Nampt levels compared with non-BRCA1-mutated and BRCA2-mutated ovarian cancer tissues (Figs. 4A and B). In addition, we observed that: (i) overexpression of BRCA1 could effectively reduce the Nampt levels in A2780, HO-8910, and ES2 cells and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells; (ii) knockdown of BRCA1 was an effective way to induce an increase of Nampt levels in HO-8910 and ES2 cells, and primary non-BRCA1-mutated ovarian cancer cells; and (iii) Nampt levels were not sensitive to the BRCA1 knockdown in primary BRCA1-mutated ovarian cancer cells (Fig. 4C). Therefore, these results suggest that Nampt may have an important role in BRCA1-related NAD synthesis.
Figure 4.
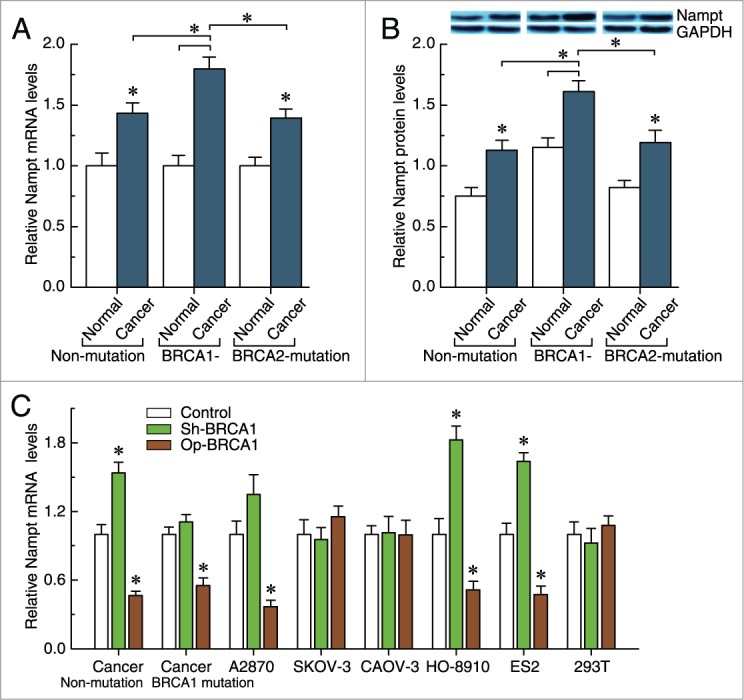
Effects of BRCA1 on intracellular Nampt levels. (A, B) Nampt mRNA and protein levels were measured in 23 pairs of non-mutated, 28 pairs of BRCA1-mutated, and 23 pairs of BRCA2-mutated ovarian cancer and their adjacent normal tissue. (C) Nampt mRNA levels after knockdown or overexpression of BRCA1 in 293T cells, A2780, SKOV-3, CAOV-3, HO-8910, and ES2 ovarian cancer cell lines, all wild-type for BRCA1 (repeated 12 times), and primary non-BRCA1-mutated (wild-type for BRCA1) and BRCA1-mutated (mutant-type for BRCA1) ovarian cancer cells (n = 12). Bar graphs show mean ± SD. Sh, shRNAs; Op, overexpression. * P < 0.05 vs. control.
Intracellular NAD can feedback activate BRCA1 expression in ovarian cancer cells
To confirm the role of NAD in the regulation of BRCA1 levels, the effects of incubation with different concentrations of NAD and knockdown or overexpression of Nampt were evaluated in 293T cells, 5 ovarian cancer cell lines (A2780, SKOV-3, CAOV-3, HO-8910, and ES2), and primary ovarian cancer cells with identified BRCA1 mutations or no BRCA1 mutations. BRCA1 expression was upregulated (Fig. 5B), along with increased levels of intracellular NAD (Fig. 5A) in A2780, HO-8910, and ES2 cells and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells. The non-ovarian cancer cell line 293T was an exception; BRCA1 transcription could not be activated by increased NAD levels. However, intracellular NAD and BRCA1 levels were not sensitive to incubation with extracellular NAD in SKOV-3 and CAOV-3 ovarian cancer cells (Figs. 5A and B).
Figure 5.
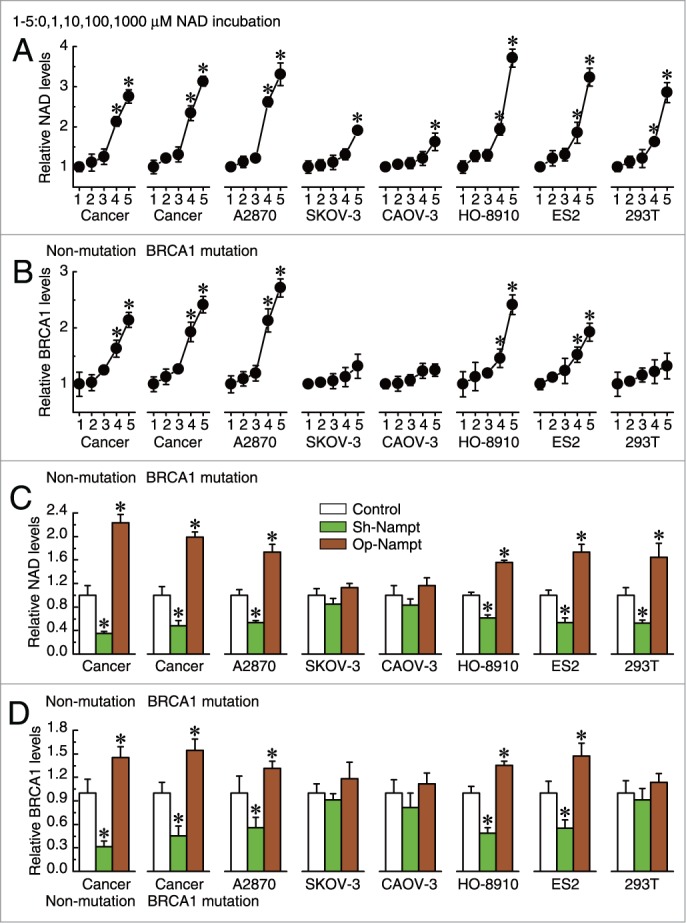
Effects of intracellular NAD on BRCA1 levels. (A, B) relative NAD or BRCA1 levels after incubation with different concentrations of NAD in 293T cells, A2780, SKOV-3, CAOV-3, HO-8910, and ES2 ovarian cancer cell lines (repeated 12 times), and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells (n = 28). One to five: incubation with 0, 1, 10, 100, or 1000 μM NAD. Bar graphs show mean ±SD. * P < 0.05 vs. control. (C, D) relative NAD or BRCA1 levels after knockdown or overexpression of Nampt in 293T cells, A2780, SKOV-3, CAOV-3, HO-8910, and ES2 ovarian cancer cell lines (repeated 12 times), and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells (n = 28). Bar graphs show mean ± SD. Sh, shRNAs; Op, overexpression. *P < 0.05 vs. control.
In addition, knockdown or overexpression of Nampt could effectively reduce or increase NAD levels in A2780, HO-8910, ES2, and 293T cells and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells. However, there were no significant changes in NAD levels after the knockdown or overexpression of Nampt in SKOV-3 and CAOV-3 ovarian cancer cells (Fig. 5C). Notably, we observed that reduced or increased NAD levels mediated by Nampt can moderately inhibit or activate BRCA1 transcription in A2780, HO-8910, and ES2 cells and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells, although there were no significant changes in the BRCA1 levels in SKOV-3, CAOV-3, and 293T cells (Fig. 5D).
Discussion
In this study, we report for the first time that BRCA1 inactivation is responsible for Nampt-related NAD synthesis, and higher NAD levels can feedback-activate BRCA1 expression. Notably, the effects of crosstalk between BRCA1 and NAD metabolism were observed in A2780, HO-8910 and ES2 cells and primary non-BRCA1-mutated and BRCA1-mutated ovarian cancer cells. SKOV-3, CAOV-3 and the non-ovarian cancer cell line 293T were insensitive to the interventions of BRCA1 and NAD. Additionally, Nampt-related NAD synthesis was not affected by BRCA1 knockdown in primary BRCA1-mutated ovarian cancer cells. It can be speculated that mutated BRCA1 genes are transcribed into abnormal mRNA. Therefore, a lack of function of BRCA1 knockdown mediated upregulation of NAD in BRCA1-mutated ovarian cancer, which may be due to BRCA1 itself, has no function along with BRCA1-mutation.
Heretofore, there have been few reports about the link(s) between BRCA1 and NAD metabolism in ovarian cancer cells. It is, however, interesting to note that the evidence accumulated to date suggests a possible link between BRCA1 and NAD metabolism. For example, aberrant proliferation is known to be critical for cancer progression, and it has been shown that the loss of function of the tumor suppressor gene BRCA1 plays an important role in promoting cancer cell proliferation and survival.15,16 The mechanism may involve: (i) inducing insulin-like growth factor 1 (IGF1) expression17,18 in an estrogen receptor (ER) α-dependent manner;17,19 (ii) stimulating progesterone receptor (PR) activity by facilitating progesterone binding to progesterone-response elements;20 and (iii) regulating glucocorticoid receptor (GR) activity through GR phosphorylation on Ser211 by modulating p38 mitogen-activated protein kinase.21 Likewise, NAD-dependent signaling pathways are widely involved in cell proliferation and metabolism.11 Moreover, NAD-dependent enzymes such as poly (ADP-ribose) polymerase 1 and SIRT1 are also potential regulators of IGF1,22 ER,23 PR,23 and GR24.
Of even greater interest, BRCA1 inactivation was shown to increase intracellular levels of NAD, while high NAD levels can feedback activate BRCA1 transcription. These data suggest that the elevation of intracellular NAD-mediated by BRCA1 inactivation may resist the adverse effects of BRCA1 deficiency. It seems beneficial for the dynamic balance between BRCA1-related biologic processes, such as homologous recombination repair, transcription regulation, ubiquitination, apoptosis, cell cycle checkpoints, and NAD-related energy transduction and cellular metabolism. However, to date, the interaction between BRCA1 and NAD at the molecular level is not fully understood. Some preliminary insights have been gained by previous studies, and suggest that transcriptional regulatory activity of C-terminal binding proteins (CtBP) is modulated by the NAD/NADH ratio; increased intracellular NAD/NADH ratios induce the loss of CtBP from the BRCA1 promoter, leading to elevated BRCA1 levels.25,26 In addition, BRCA1 has been shown to serve as a positive regulator of the NAD-dependent histone deacetylase SIRT1 by binding to the promoter region;27,28 decreased levels of SIRT1 triggered by BRCA1 inactivation may reduce the consumption of intracellular NAD, which plays a partial role in elevating NAD levels.
Overall, this study provides new insights into the crosstalk between BRCA1 and Nampt-related NAD metabolism. All of this may improve our understanding of the basic molecular mechanism underlying BRCA1- and NAD-related ovarian cancer.
Materials and Methods
Ethical statements
Investigation has been conducted in accordance with the ethical standards and according to the Helsinki Declaration of 1975.
Patients and tissue collection
This study was approved by the Institutional Review Board at China Medical University. Serous ovarian cancer patients were enrolled between 2010 and 2012, and all patients gave informed consent. Fresh tumor samples, adjacent normal ovarian tissues, ascites, and blood samples were obtained at the time of primary surgery before any chemotherapy or radiotherapy (28 pairs of BRCA1-mutated or not, 23 pairs of BRCA2-mutated or not, and 19 pairs with hypermethylated BRCA1 promoter or not). Hematoxylin and eosin staining of the samples for histopathological diagnosis and grading were determined by 3 staff pathologists using the World Health Organization criteria. All patients were screened for BRCA1 and BRCA2 mutations by multiplex polymerase chain reaction (PCR), their characteristics are given in Tables S1 and 2.
Cell culture and lentiviral transfection
Primary ovarian cancer cells were obtained from the ascites of patients undergoing surgery for ovarian cancer and cultured in RPMI 1640 with 10% fetal bovine serum (Invitrogen) using previously reported.8 Human 293T cells and A2780, SKOV-3, CAOV-3, HO-8910 and ES2 ovarian cancer cells were maintained in DMEM with 10% fetal bovine serum (Invitrogen). Lentiviral vectors expressing short hairpin RNAs (shRNAs) against BRCA1 (NM_007299) were obtained from GeneChem Co., Ltd, and synthesized as follows: Forward: 5′-CCGGAACCTGTCTCCACAAAGTGTGCTCGAGCACACTTTGTGGAGACAGGTTTTTTTG-3′, and Reverse: 5′-aattcaaaaaaaCCTGTCTCCACAAAGTGTGCTCGAGCACACTTTGTGGAGACAGGTT-3′. The non-silencing shRNA sequence was used as a negative control and synthesized as follows: forward, 5′-ccggTTCTCCGAACGTGTCACGTctcgagACGTGACACGTTCGGAGAAtttttg-3′, and reverse, 5′-aattcaaaaaTTCTCCGAACGTGTCACGTctcgagACGTGACACGTTCGGAGAA-3′. The shRNAs lentiviral particles of Nampt (sc-45843-V) were purchased from Santa Cruz Biotechnology. For overexpression of BRCA1 or Nampt, the open reading frame of BRCA1 (NM_007299) or Nampt (NM_005746) were respectively cloned into the lentiviral vector GV287 (Ubi-MCS-3FLAG-SV40-EGFP; GeneChem Co., Ltd). Transfections were performed using polybrene and enhanced infection solution (GeneChem Co., Ltd) according to the manufacturer's recommended protocol. The efficiency of BRCA1 and Nampt knockdown and overexpression was shown in Fig. S1 (Procedure are shown in Supplementary Methods).
Real-time quantitative PCR
Total RNA was extracted using Trizol reagents (Invitrogen) according to the manufacturer's protocol. DNA contamination was removed by adding DNase I (Invitrogen) according to the manufacturer's protocol. Total RNA was then reverse-transcribed from 2 μg of RNA using the PrimeScript RT Master Mix kit (TaKaRa) and amplified by SYBR Premix Ex TaqTM II (TaKaRa) in a Roche LightCycler 2.0 instrument (Roche Diagnostics). The specific primer sequences were as follows: BRCA1, Forward: 5′-GGCTATCCTCTCAGAGTGACATTT-3′, and Reverse: 5′-GCTTTATCAGGTTATGTTGCATGG-3′; Nampt, Forward: 5′-AGGGCTTTGTCATTCCCAGA-3′, and Reverse: 5′-TGGCCACTGTGATTGGATACC-3′; GAPDH, Forward: 5′-AGGTGAAGGTCGGAGTCA-3′, and Reverse: 5′-GGTCATTGATGGCAACAA-3′. GAPDH mRNA was amplified as an internal control for normalization of each sample. All samples were analyzed in triplicate using the 2–ΔΔCT method.
Bisulfite sequencing for BRCA1 promoter
All the tissues were used for bisulfite sequencing from the non-BRCA1-mutated cases. Genomic DNA extracted from ovarian cancer and normal ovarian tissue with a TIANamp Genomic DNA kit (Tiangen Biotech) was subjected to bisulfite conversion using the EZ DNA Methylation-Direct kit (Zymo Research) following the manufacturer's instructions; the conversion efficiency was estimated to be at least 99.6%. It was then amplified by nested PCR. After gel purification, cloning and transformation into E. coli Competent Cells JM109 (TaKaRa), 10 positive clones of each sample were sequenced to ascertain the methylation patterns of each CpG locus. The following primers were used for BRCA1 gene promoter (Accession number: NG_005905): round I, Forward: 5′-TTGTAGTTTTTTTAAAGAGT-3′, and Reverse: 5′-TACTACCTTTACCCAAAACAAAA-3′; round II, Forward: 5′-GTAGTTTTTTTAAAGAGTTGTA-3′, and Reverse: 5′-ACCTTTACCCAAAACAAAAA-3′. The conditions were as follows: 95°C for 2 min, 40 cycles of 30s at 95°C, 30s at 56°C and 45s at 72°C, then 72°C for 7 min.
NAD/NADH assay
For NAD/NADH assay, 20 mg of freezed ovarian tissue, or 20 μl packed cultured cells were homogenized in 400 μl BioVsion NAD/NADH Extraction Buffer (BioVsion). The homogenate was ultrafiltered using BioVsion 10-kD cut-off filters (14000 g, 30 min, 4°C). Assays were performed using the NAD/NADH Quantification Kits according to the manufacturer's instructions (BioVsion).
Statistical analysis
Regression analysis was used to examine the possible relationship between BRCA1 and NAD levels. The data are presented as means ± SD. Statistical differences in the data were evaluated by Student's t-test or one-way ANOVA as appropriate, and were considered significant at P < 0.05.
Funding Statement
This work was supported by the Natural Science Foundation of China (No. 81402130) and the Doctoral Start-up Foundation of Liaoning Province (No. 20141045).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Lech A, Daneva T, Pashova S, Gagov H, Crayton R, Kukwa W, Czarnecka AM, Szczylik C. Ovarian cancer as a genetic disease. Front Biosci 2013; 18:543–63; http://dx.doi.org/ 10.2741/4119 [DOI] [PubMed] [Google Scholar]
- 2. Pruthi S, Gostout BS, Lindor NM. Identification and management of women with BRCA mutations or hereditary predisposition for breast and ovarian cancer. Mayo Clin Proc 2010; 85:1111–20; PMID: 21123638; http://dx.doi.org/ 10.4065/mcp.2010.0414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schouten LJ, van Dijk BA, Lumey LH, Goldbohm RA, van den Brandt PA. Energy restriction during childhood and early adulthood and ovarian cancer risk. PLoS One 2011; 6:e27960; PMID: 22132180; http://dx.doi.org/ 10.1371/journal.pone.0027960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dacheux E, Vincent A, Nazaret N, Combet C, Wierinckx A, Mazoyer S, Diaz JJ, Lachuer J, Venezia ND. BRCA1-dependent translational regulation in breast cancer cells. PLoS One 2013; 8:e67313; PMID: 23805307; http://dx.doi.org/ 10.1371/journal.pone.0067313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li D, Bi FF, Cao JM, Cao C, Li CY, Yang Q. Effect of BRCA1 on epidermal growth factor receptor in ovarian cancer. J Exp Clin Cancer Res 2013; 32:102; PMID: 24321281; http://dx.doi.org/ 10.1186/1756-9966-32-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bi FF, Li D, Cao C, Li CY, Yang Q. Regulation of angiotensin II type 1 receptor expression in ovarian cancer: a potential role for BRCA1. J Ovarian Res 2013; 6:89; PMID: 24321324; http://dx.doi.org/ 10.1186/1757-2215-6-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li D, Bi FF, Chen NN, Cao JM, Sun WP, Zhou YM, Cao C, Li CY, Liu B, Yang Q. Epigenetic repression of phosphatidylethanolamine, N-methyltransferase (PEMT) in BRCA1-mutated breast cancer. Oncotarget 2014; 5:1315–25. PMID: 24675476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li D, Bi FF, Cao JM, Cao C, Li CY, Liu B, Yang Q. Poly (ADP-ribose) polymerase 1 transcriptional regulation: A novel crosstalk between histone modification H3K9ac and ETS1 motif hypomethylation in BRCA1-mutated ovarian cancer. Oncotarget 2014; 5:291–7; PMID: 24448423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li D, Bi FF, Cao JM, Cao C, Liu B, Yang Q. Regulation of DNA methyltransferase 1 transcription in BRCA1-mutated breast cancer: a novel crosstalk between E2F1 motif hypermethylation and loss of histone H3 lysine 9 acetylation. Mol Cancer 2014; 13:26; PMID: 24502362; http://dx.doi.org/ 10.1186/1476-4598-13-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pan LZ, Ahn DG, Sharif T, Clements D, Gujar SA, Lee PW. The NAD synthesizing enzyme nicotinamide mononucleotide adenylyltransferase 2 (NMNAT-2) is a p53 downstream target. Cell Cycle 2014; 13:1–8; PMID: 24231773; http://dx.doi.org/ 10.4161/cc.28128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chiarugi A, Dölle C, Felici R, Ziegler M. The NAD metabolome–a key determinant of cancer cell biology. Nat Rev Cancer 2012; 12:741–52; PMID: 23018234; http://dx.doi.org/ 10.1038/nrc3340 [DOI] [PubMed] [Google Scholar]
- 12. Zhang B, Chen J, Cheng AS, Ko BC. Depletion of sirtuin 1 (SIRT1) leads to epigenetic modifications of telomerase (TERT) gene in hepatocellular carcinoma cells. PLoS One 2014; 9:e84931; PMID: 24416313; http://dx.doi.org/ 10.1371/journal.pone.0084931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahajan SS, Scian M, Sripathy S, Posakony J, Lao U, Loe TK, Leko V, Thalhofer A, Schuler AD, Bedalov A, et al. Development of Pyrazolone and Isoxazol-5-one Cambinol Analogues as Sirtuin Inhibitors. J Med Chem 2014; 57:3283–94; PMID: 24697269; http://dx.doi.org/ 10.1021/jm4018064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chang HC, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 2014; 25:138–45; PMID: 24388149; http://dx.doi.org/ 10.1016/j.tem.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burga LN, Tung NM, Troyan SL, Bostina M, Konstantinopoulos PA, Fountzilas H, Spentzos D, Miron A, Yassin YA, Lee BT, Wulf GM. Altered proliferation and differentiation properties of primary mammary epithelial cells from BRCA1 mutation carriers. Cancer Res 2009; 69:1273–8; PMID: 19190334; http://dx.doi.org/ 10.1158/0008-5472.CAN-08-2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Promkan M, Liu G, Patmasiriwat P, Chakrabarty S. BRCA1 modulates malignant cell behavior, the expression of survivin and chemosensitivity in human breast cancer cells. Int J Cancer 2009; 125:2820–8; PMID: 19551867; http://dx.doi.org/ 10.1002/ijc.24684 [DOI] [PubMed] [Google Scholar]
- 17. Kang HJ, Yi YW, Kim HJ, Hong YB, Seong YS, Bae I. BRCA1 negatively regulates IGF-1 expression through an estrogen-responsive element-like site. Cell Death Dis 2012; 3:e336; PMID: 22739988; http://dx.doi.org/ 10.1038/cddis.2012.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Werner H, Bruchim I. IGF-1 and BRCA1 signalling pathways in familial cancer. Lancet Oncol 2012; 13:e537–44; PMID: 23182194; http://dx.doi.org/ 10.1016/S1470-2045(12)70362-5 [DOI] [PubMed] [Google Scholar]
- 19. Wen J, Li R, Lu Y, Shupnik MA. Decreased BRCA1 confers tamoxifen resistance in breast cancer cells by altering estrogen receptor-coregulator interactions. Oncogene 2009; 28:575–86; PMID: 18997820; http://dx.doi.org/ 10.1038/onc.2008.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Katiyar P, Ma Y, Riegel A, Fan S, Rosen EM. Mechanism of BRCA1-mediated inhibition of progesterone receptor transcriptional activity. Mol Endocrinol 2009; 23:1135–46; PMID: 19389812; http://dx.doi.org/ 10.1210/me.2008-0347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vilasco M, Communal L, Hugon-Rodin J, Penault-Llorca F, Mourra N, Wu Z, Forgez P, Gompel A, Bracaps. Loss of glucocorticoid receptor activation is a hallmark of BRCA1-mutated breast tissue. Breast Cancer Res Treat 2013; 142:283–96; PMID: 24166279; http://dx.doi.org/ 10.1007/s10549-013-2722-8 [DOI] [PubMed] [Google Scholar]
- 22. Ng F, Tang BL. When is Sirt1 activity bad for dying neurons? Front Cell Neurosci 2013; 7:186; PMID: 24167473; http://dx.doi.org/ 10.3389/fncel.2013.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ossovskaya V, Koo IC, Kaldjian EP, Alvares C, Sherman BM. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010; 1:812–21; PMID: 21779467; http://dx.doi.org/ 10.1177/1947601910383418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ito K. Impact of post-translational modifications of proteins on the inflammatory process. Biochem Soc Trans 2007; 35:281–3; PMID: 17371260; http://dx.doi.org/ 10.1042/BST0350281 [DOI] [PubMed] [Google Scholar]
- 25. Deng Y, Liu J, Han G, Lu SL, Wang SY, Malkoski S, Tan AC, Deng C, Wang XJ, Zhang Q. Redox-dependent Brca1 transcriptional regulation by an NADH-sensor CtBP1. Oncogene 2010; 29:6603–8; PMID: 20818429; http://dx.doi.org/ 10.1038/onc.2010.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Di LJ, Fernandez AG, De Siervi A, Longo DL, Gardner K. Transcriptional regulation of BRCA1 expression by a metabolic switch. Nat Struct Mol Biol 2010; 17:1406–13; PMID: 21102443; http://dx.doi.org/ 10.1038/nsmb.1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanikawa M, Wada-Hiraike O, Nakagawa S, Shirane A, Hiraike H, Koyama S, Miyamoto Y, Sone K, Tsuruga T, Nagasaka K, et al. Multifunctional transcription factor TFII-I is an activator of BRCA1 function. Br J Cancer 2011; 104:1349–55; PMID: 21407215; http://dx.doi.org/ 10.1038/bjc.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hiraike H, Wada-Hiraike O, Nakagawa S, Koyama S, Miyamoto Y, Sone K, Tanikawa M, Tsuruga T, Nagasaka K, Matsumoto Y, et al. Identification of DBC1 as a transcriptional repressor for BRCA1. Br J Cancer 2010; 102:1061–7; PMID: 20160719; http://dx.doi.org/ 10.1038/sj.bjc.6605577 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


