The transfer of proteins or genetic material from exosomes and the subsequent effects on cell fate are well described.1 However lipids effects on cell behavior remain less documented.2 Few years ago Ristorcelli3,4 reported that the human pancreatic cancer SOJ-6 and BxPc-3 cells, 2 differentiated cancer cell lines are sensitive to exosomes whereas poorly differentiated MiaPaCa-2 and Panc-1 cells are (quite) insensitive. SOJ-6 cells expressed exosomes, rich in lipid-forming rafts, which further interacted with cell membrane to disturb the Notch-1 survival pathway. In particular the γ-secretase, cleaving Notch-1 in the active Notch IntraCytoplasmic Domain (NICD), is sensitive to lipid microenvironment.5 These effects on Notch functioning down-regulate the phosphorylation of the pro-apoptotic PTEN and GSK-3β leading to their activation. GSK-3β inhibits mitochondrial PDH, up-regulates pro-apoptotic Bax protein, down-regulates anti-apoptotic Bcl-2, Hes-1 (a nuclear target of the Notch pathway) and cyclin D1 expressions promoting the arrest of the cell cycle in the G0-G1 transition phase. To demonstrate the implication of exosomal lipids in promoting the death of SOJ-6 cells, Beloribi6 synthesized exosome-like nanoparticles (SELN) solely composed of lipids among which lipid-forming rafts predominate. The size of SELN, which appear stable with time, is compatible with a fusion/exchange with rafts in cell membranes. SELN co-localize with Notch-1 and with GangliosideM1 (GM1, a marker of rafts) at the level of plasma membrane and with Notch-1 and Rab5A at the level of early endosomes. Therefore data presented by Beloribi6 fit with a fusion/exchange of lipid SELN with rafts at the plasma membrane followed by endocytosis and/or with a fusion/exchange with rafts of the endosomal membrane. Beloribi6 further showed that SELN mimicked the effects of exosomes and concluded that exosomal lipids alone could be responsible for the death of differentiated pancreatic tumor SOJ-6 cells. To further establish the involvement of rafts lipid the ratio Lo/Ld (i.e., lipids forming rafts versus non-raft lipids) was varied and higher the ratio Lo/Ld (i.e., the amount of raft lipids), greater the detrimental effects on SOJ-6 cells. Beloribi6 concluded that the raft lipids of SELN are responsible for SOJ-6 cells death. On another hand MiaPaCa-2 pancreatic cells, which are considered as a stem-like cancer (or initiating cancer) cell model as they are ALDH-positive and CD44-positive (among others) were insensitive to exosomes3,4 and SELN.6 The difference in sensitivity to SELN could be due to different genetic backgrounds of SOJ-6 and MiaPaCa-2 cells. PTEN (a Notch partner) mutations have been involved in the resistance to Notch inhibition. However PTEN is rarely mutated in pancreatic adenocarcinomas and SOJ-6 and MiaPaCa-2 cells expressed wild-type PTEN. Notch also represses stem/progenitor cell expansion via p53 tumor suppressor protein. However p53 mutations have been observed in DNA specific binding domain in both exosomes-sensitive SOJ-6 and exosomes-insensitive MiaPaCa-2 cells and consequently cannot be the source of observed differences. Therefore the mechanisms of the MiaPaCa-2 cells resistance to exosomal lipids are somewhere else. In a recent paper Beloribi-Djefaflia7 aimed at deciphering the reason(s) of this resistance. In the presence of SELN, the expression of NICD decreases in MiaPaCa-2 cells but neither Hes-1, the nuclear target of NICD, nor the ratio Bax/Bcl-2 were affected. Beloribi-Djefaflia7 concluded that Notch pathway was obviously affected in MiaPaCa-2 cells exposed to SELN without deleterious effects. Authors further showed that in MiaPaCa-2 cells SELN induced the activation of NF-kB, thus promoting the expression and the secretion of SDF-1α. Oncogenic Kras (a signature of pancreatic adenocarcinoma) acting as an upstream activator of Akt can stimulate the transcriptional activity of NF-kB which is constitutively activated in almost 70% of pancreatic cancers. Therefore authors showed that MiaPaCa-2 cells harbor the Kras p-Gly12Cys mutation and hypothesized that once the fusion had occurred non-rafts lipids of SELN may freely diffuse within the cell membrane compartment to impact on the constitutively active oncogenic Kras which exhibits stable interaction with the inner leaflet of plasma membrane at saturable non-raft sites. Such interaction is required for efficient signaling of GTP-loaded Ras proteins. Therefore the fusion of SELN with plasma membrane may affect the Ras location and facilitate signaling with the efficient activation of underneath proteins. SELN, impacting on Kras functioning, may lead to a significant over-expression of GSK-3β as observed with MiaPaCa-2 cells upon SELN incubation,6 to the over-activation of NF-kB, to its subsequent transcription activity on SDF-1α expression and secretion. Once secreted the α-chemokine interacts with its dedicated receptor CXCR4 on MiaPaCa-2 cells and activates the Akt survival pathway protecting cells from death. Consequently the SDF-1α-CXCR4 axis is activated by SELN and counteracts their inhibitory effects on the Notch-1 pathway (Fig. 1). MiaPaCa-2 cells are resistant to Gemcitabine, the gold standard for pancreatic adenocarcinoma treatment and Beloribi-Djefaflia7 also reports that SELN were able to reverse the survival inhibition promoted by cyclopamine on MiaPaCa-2 cells via the Hedgehog embryonic pathway. Therefore it can be hypothesized that cancer cells in part those with cancer stem-like cell features take advantage of the predominating CXCR4-SDF-1α axis to resist to drugs and to self-renew. Part of these fates could be maintained via paracrine effects of differentiated cells which may release factors among them lipid vesicles able to promote stem-like (or initiating) cancer cells survival. Future therapies should focus on this dialog.
Figure 1.
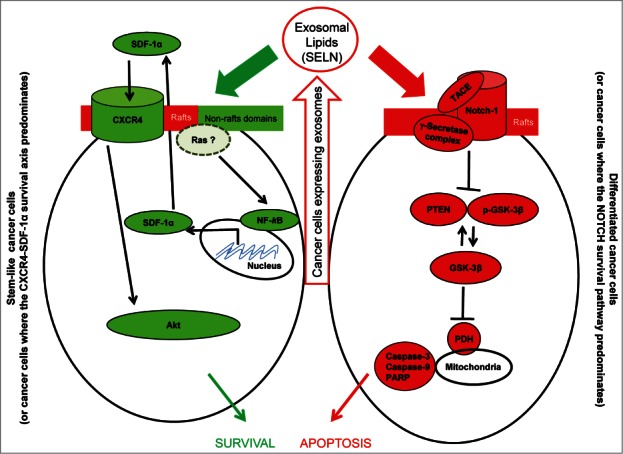
Cells express exosomes comprising lipids however lipids impact on cancer cells according to cell phenotype. On cancer stem-like cells, lipids activate the NF-kB pathway to promote SDF-1α expression/secretion and the activation of the CXCR4-SDF-1α survival axis. On differentiated cancer cells, exosomes inhibit the NOTCH survival pathway to promote apoptosis. Therefore exosomal lipids participate in tumor growth and resistance to drugs.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Zhang Grizzle. Am J Pathol 2014; 184:28-41; PMID:24269592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Record, et al. . Biochim Biophys Acta 2014; 1841:108-20; PMID:24140720, http://dx.doi.org/ 10.1016/j.bbalip.2013.10.004 [DOI] [PubMed] [Google Scholar]
- 3. Ristorcelli, et al. . FASEB J 2008; 22: 3358-69; PMID:18511551; http://dx.doi.org/ 10.1096/fj.07-102855 [DOI] [PubMed] [Google Scholar]
- 4. Ristorcelli, et al. . Int. J. Cancer 2009; 125:1016-26; PMID:19405120; http://dx.doi.org/ 10.1002/ijc.24375 [DOI] [PubMed] [Google Scholar]
- 5. Osenkowski, et al. . J Biol Chem 2008; 283: 22529-40; PMID:18539594; http://dx.doi.org/ 10.1074/jbc.M801925200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beloribi, et al. . PLoS One 2012; 7:e47480; PMID:23094054; http://dx.doi.org/ 10.1371/journal.pone.0047480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beloribi-Djefaflia S, et al. . Oncoscience 2015; 2(1):18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]


