Abstract
Chk1, an essential checkpoint kinase in the DNA damage response pathway (DDR), is tightly regulated by both ATR-dependent phosphorylation and proteasome-mediated degradation. Here we identify ubiquitin hydrolase USP7 as a novel regulator of Chk1 protein stability. USP7 was shown before to regulate other DDR proteins such as p53, Hdm2 and Claspin, an adaptor protein in the ATR-Chk1 pathway required for Chk1 activation. Depletion or inhibition of USP7 leads to lower Chk1 levels. The decreased Chk1 protein after USP7 knock down cannot be rescued by simultaneously elevating Claspin levels, demonstrating that the effect of USP7 on Chk1 is independent of its known effect on Claspin. Conversely, overexpression of USP7 wild type, but not a catalytic mutant version, elevates Chk1 levels and increases the half-life of Chk1 protein. Importantly, wild type, but not catalytic mutant USP7 can deubiquitinate Chk1 in vivo and in vitro, confirming that USP7 directly regulates Chk1 protein levels. Finally we show that USP7 catalytic mutant is (mono-)ubiquitinated, which suggests auto-deubiquitination by this ubiquitin hydrolase, possibly important for its regulation.
Keywords: Chk1, claspin, ubiquitin hydrolase, USP7
Abbreviations
- CI
catalytic inactive
- DDR
DNA damage response
- DUB
deubiquitylating enzyme
- USP
ubiquitin specific peptidase
- WT
wild type
Introduction
The DNA damage response (DDR) is essential to maintain genomic stability and functions as a first defense in the early stages of cancer development.1 The ATR-Chk1 branch of this response is activated by single-stranded DNA that occur at stalled replication forks or through resection of DNA double strand breaks.2 Tight regulation of effector kinase Chk1 at different levels is critical for correct functioning of this response. Phosphorylation of Chk1 on Ser317 and Ser345 by ATR upon genotoxic stress activates the enzyme, which in turn phosphorylates downstream substrates to mediate an arrest in cell cycle progression, stabilizes replication forks and signals to DNA repair.3 In addition, ATR-mediated phosphorylation also regulates dissociation of Chk1 from the chromatin which is thought to facilitate the transmission of DNA damage signals to downstream targets.4,5 Finally, phosphorylation of Chk1 triggers Chk1 polyubiquitination and subsequent proteasome-mediated degradation at later times after damage induction, thereby terminating the checkpoint.6,7
Protein ubiquitination has emerged as an important regulatory mechanism in the surveillance machinery controlling genomic stability. Mono- or polyubiquitin modifications can target protein stability, localization, or activity and several ubiquitin ligases have been identified to function in the DDR. In contrast, ubiquitin removal by deubiquitylating enzymes (DUBs) is less well characterized but equally important. In fact, aberrant DUB activity was identified in human cancers.8 The DUB ubiquitin specific peptidase 7 (USP7/HAUSP) has many substrates among DDR proteins. Although initially p53 was thought to be the primary substrate of USP7,9 later studies showed that USP7 actually has a much higher affinity for MDM2/HDM2, an E3 ligase promoting p53 degradation.10 Subsequently, many other substrates were identified, for example phosphatase PTEN, a suppressor of the PI3K-AKT pathway, transcription factor FOXO4 and Claspin, a mediator protein in the ATR-Chk1 pathway, critical for Chk1 activation.11-13
Here we identify Chk1 as a novel substrate of USP7. Depletion or inhibition of USP7 decreases Chk1 protein levels. On the other hand, overexpression of USP7 wild type, but not a catalytic mutant, elevates Chk1 levels and increases the half-life of Chk1 protein. Finally, USP7 can deubiquitinate Chk1 in vivo and in vitro, demonstrating that USP7 directly regulates Chk1 protein levels by cleavage of the poly-ubiquitination chain.
Results
USP7 depletion reduces Chk1 protein levels
Recent studies have shown that Chk1 protein levels are controlled by ubiquitin-mediated proteasomal degradation. Although Cul1-Cul4A E3 ligases were described to be involved in this process,6,7 a ubiquitin hydrolase has not been identified yet. While studying the effect of USP7 on the stability of Claspin, a mediator protein in the ATR-Chk1 pathway, we noticed that depletion of this ubiquitin hydrolase not only affected the levels of Claspin but also Chk1.14 This was studied in more detail by downregulating USP7 in U2OS cells by 3 different siRNA oligos, all of which resulted in a decrease in Chk1 protein levels (Fig. 1A). Chk1 levels were reported to vary during the cell cycle.15 As USP7 knock down did not affect the cell cycle progression as determined by flow cytometry analysis (data not shown), the effect of USP7 downregulation on Chk1 protein levels could not be explained by an aspecific effect on cell cycle progression. As depletion, inhibition of USP7 by small molecule inhibitor P2207716 resulted in decreased levels of Chk1 in both 293T and U2OS cells (Fig. 1B). As expected, inhibition of USP7 also lowered Claspin protein levels (Fig. 1B). We considered it therefore a possibility that the effect of knock down of USP7 on Chk1 might be indirectly due to lower Claspin levels as suggested before by others.17,18 However, although Claspin knock down has a minor impact on (decreasing) Chk1 levels, depleting USP7 lead to lower Chk1 protein levels as compared to the Chk1 levels after Claspin knock down (Fig. 1C). Importantly, depletion of USP29, a recently described regulator of Claspin stability by deubiquitination,14 resulted in decreased Claspin levels without affecting Chk1 protein (Fig. 1D). Finally, elevated Claspin protein levels by overexpressing Flag-Claspin in USP7-depleted cells did not rescue the low Chk1 levels, demonstrating that lower Claspin levels are not the cause of decreased Chk1 levels upon USP7 knock down (Fig. 1E). Together these experiments indicate that USP7 regulates Chk1 protein levels, an effect that is independent on the control of USP7 on Claspin stability.
Figure 1.

USP7 depletion results in decreased Chk1 protein levels. (A) U2OS cells were transfected twice with Luciferase (Luc) or different USP7 siRNA oligos. 72 h later, cells were lysed for analysis by western blot with the indicated antibodies. (B) 293T and U2OS cells were treated with PD22077. At the indicated time points, cells were lysed for analysis by western blot with the indicated antibodies. (C) U2OS cells were transfected twice with the indicated siRNA oligos (20 μM). Of Claspin siRNA oligos, lower amounts were used (0.1 or 0.02 μM). Cells were lysed and analyzed as in (A). (D) U2OS cells were transfected twice with the indicated siRNA oligos. 72 h later, cells were lysed for analysis by western blot with the indicated antibodies. Right panel demonstrates USP29 downregulation in representative samples. (E) U2OS cells were transfected with Luc or USP7 siRNA oligos. After 24 h, cells were simultaneously transfected with empty vector (EV) or HA-Claspin and Luc or USP7 siRNA oligos. The next day, cells were lysed and analyzed as in (A). In all panels: the arrow points to Chk1. The asterisk indicates an aspecific band.
Elevated Chk1 levels after overexpression of USP7
If the regulation of Chk1 by USP7 is via deubiquitination, we expected that USP7 overexpression would have the opposite effect on Chk1 levels than knock down of USP7. Indeed, overexpression of wild type USP7 lead to increased Chk1 protein levels (Fig. 2A). This effect is due to the catalytic activity of USP7, as expression of a catalytic mutant of this enzyme (C223S) did not increase, but rather decreased Chk1 levels (Fig. 2A). An in vitro activity assay with HA-Ub-VS as artificial substrate demonstrated that this version of USP7 displayed significant lower catalytic activity as compared to the wild type version (Fig. 2B). As a control the reaction was carried out in the presence of N-ethylmaleimide (NEM), a DUB inhibitor, which completely inhibited the activity of USP7 wild type (Fig. 2B). To study the effect of USP7 on Chk1 protein stability in more detail, the half-life of Chk1 was determined by blocking protein synthesis with cycloheximide (CHX) for different times. As shown in Fig. 2C, the half-life of Chk1 increased upon overexpression of USP7 wild type, but not the catalytic mutant. Together these results indicate that USP7 regulates Chk1 protein stability by its catalytic activity.
Figure 2.
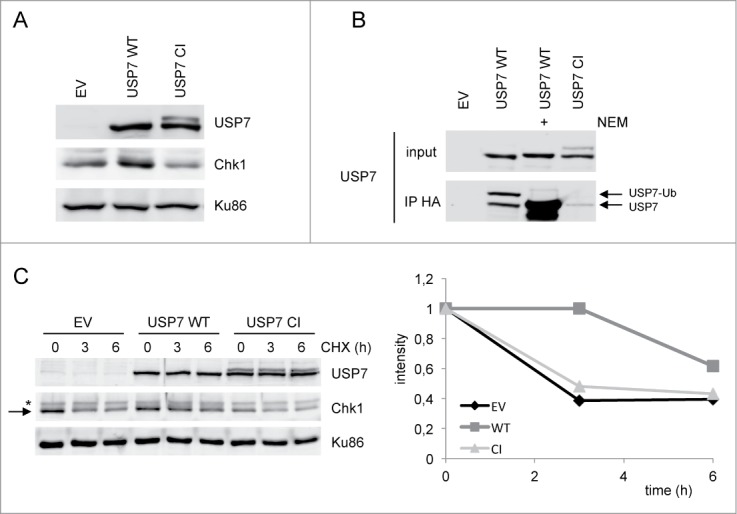
Overexpression of USP7 stabilizes Chk1 levels. (A) 293T cells were transfected with empty vector (EV), USP7 wild type (WT) or USP7 catalytic mutant (CI) expression vector. After 40 h, whole cell extracts were prepared and analyzed by western blotting using the indicated antibodies. (B) 293T cells were transfected with the different USP7 expression vectors. After 40 h, cells were lysed, incubated with HA-Ub-VS for 1 h at 37°C, in presence or absence of NEM, followed by an anti-HA immunoprecipitation and western blotting with the indicated antibodies. USP7-Ub is the active form of the enzyme. (C) 293T cells were transfected as in (A). After 40 h, cells were left untreated or incubated with CHX for the indicated times. Whole cell extracts were prepared and analyzed by western blotting using the indicated antibodies. The arrow points to Chk1, the asterisk indicates an aspecific band. Right panel represents the quantification of the Chk1 western. Chk1 was compared to loading control Ku86 and the amount of Chk1 at t = 0 h was put to 1, in each of the conditions (EV, USP7 WT and USP7 CI).
USP7 deubiquitinates Chk1 in vivo and in vitro
Our results strongly suggest that USP7 regulates Chk1 protein stability by direct deubiquitination. To demonstrate this, 293T cells were transfected with His-Ubiquitin (His-Ub), with or without Flag-USP7 wild type. Subsequently, ubiquitinated proteins were pulled down. A Chk1 western blot demonstrated abundant Chk1 polyubiquitination, which was significantly decreased upon expression of USP7 (Fig. 3A). Importantly, the subsequent in vitro deubiquitination assay using purified proteins demonstrated that USP7 wild type, but not the catalytic mutant, was able to markedly deubiquitinate Chk1 (Fig. 3B). Inhibition of USP7 wild type with NEM also prevented deubiquitination of Chk1 (Fig. 3B). These data demonstrate that USP7 directly acts on Chk1 by deubiquitination.
Figure 3.

USP7 deubiquitinates Chk1 and itself in vivo. (A) In vivo Chk1 ubiquitination. 293T cells were transfected with EV or His-Ub, alone or together with Flag-USP7 WT. Cells were incubated for 16 h with MG132 before lysis. Then a pull down was performed with Ni-NTA beads followed by western blot analysis with the indicated antibodies. (B) In vitro Chk1 deubiquitination assay. 293T cells were transfected with EV or His-Ub. Cells were incubated for 16 h with MG132, lysed and a Ni-NTA pull down was performed. Subsequently, beads were washed and incubated with purified USP7 WT or CI, in the absence or presence of NEM. (C) 293T cells were transfected with USP7 WT or CI, together with His-Ub. Cells were lysed and an anti-Flag immunoprecipitation was performed followed by western blot analysis with the indicated antibodies.
USP7 is (mono)ubiquitinated
Upon expression of USP7 catalytic mutant, we noticed the appearance of an additional band in a USP7 western blot (Fig. 2A–C, Fig. 3B). This band was also observed by others and suggested to be (mono-)ubiquitinated USP7.13 To demonstrate USP7 ubiquitination, cells were transfected with USP7 WT or catalytic mutant, together with His-Ub, to increase potential USP7 ubiquitination. USP7 was subsequently immunoprecipitated using the Flag-tag, followed by western blotting for USP7 and conjugated ubiquitin (FK2; Fig. 3C). After immunoprecipitating USP7, the extra band was clearly visible in the USP7 catalytic mutant lane and corresponds to a specific band at the same height and in the same sample of the conjugated ubiquitin western, showing that USP7 indeed is (mono)ubiquitinated (Fig. 3C). In addition, the absence of ubiquitination of the wild type version of USP7 strongly suggests that this DUB deubiquitinates itself, possibly in a trans mechanism.
Discussion
Tight regulation of checkpoint kinase Chk1 is essential for a correct cellular signaling in response to genotoxic stress and therefore for preventing tumorigenesis. Phosphorylation of Chk1 by upstream kinase ATR at Ser345 is critical for the activation of Chk1, but has been shown to also mark the protein for ubiquitination and subsequent degradation via the proteasome at later time points after damage.6 Early work identified E3 ligase complexes containing Cul1 or Cul4A as the E3 ligases that polyubiquitinate Chk1, later this was specified as the Skp1-Cul1-Fbx6 complex.6,7 Although protein ubiquitination is a reversible process, a DUB that antagonizes Chk1 polyubiquitination was not known yet. Here we identify USP7 as a novel ubiquitin hydrolase for Chk1. Depletion or inhibition of USP7 decreases Chk1 protein levels, whereas overexpression of this DUB has the opposite effect. It should be noted that USP7 was described as a novel regulator of Claspin protein levels.13 Claspin, also tightly regulated by ubiquitin-mediated degradation, acts as an adaptor protein between ATR and Chk1, binds to Chk1 and is required for ATR-mediated phosphorylation of Chk1.19 Complex formation was suggested to stabilize both proteins.18 Indeed, Chk1 was shown to be required to maintain Claspin protein stability17 and our experiments show that depletion of Claspin by siRNA somewhat decreases Chk1 levels (Fig. 1C). The observed effect of lower Chk1 levels after depletion of USP7 could therefore be the result of lower Claspin levels in these conditions. However, our experiments evidently demonstrate that the effect of USP7 on Chk1 is direct and not via Claspin. First, downregulation of USP7 leads to lower Chk1 levels than knock down of Claspin, whereas Claspin levels are lower upon transfection of Claspin siRNA oligos than after USP7 downregulation (Fig. 1C). Second, depletion of USP29, another ubiquitin hydrolase recently described to regulate Claspin protein levels14 does not affect Chk1 protein (Fig. 1D). Third, elevating Claspin protein levels in USP7-depleted cells by transfection of a Claspin expressing plasmid does not rescue the lower Chk1 levels induced by USP7 knock down (Fig. 1E). Finally, purified wild type USP7 deubiquitinates Chk1 in an in vitro deubiquitination assay (Fig. 3B). The fact that USP7 controls both Chk1 and Claspin levels independently and that these 2 proteins are closely related in the ATR pathway, indicates that control of their levels by (de)ubiquitination is critical for correct functioning of the DDR and possibly additionally required for a normal cell cycle progression.20
Our results add Chk1 to the growing list of USP7 substrates, among which are proteins like p53, HDM2, PTEN and Claspin,21 showing evidence of USP7 being a critical regulator of the DNA damage response and tumorigenesis. The fact that USP7 overexpression has been linked to various cancers such as prostate and lung, whereas its levels are reduced in breast and brain tumors, underscores this theory.8 Understanding the regulation of this ubiquitin hydrolase will therefore contribute to our knowledge regarding the functioning of these important processes. For example, regulation of USP7 enzymatic activity or protein levels likely contributes to the described ubiquitin-mediated degradation of Chk1 at later time points after DNA damage. Our observations with the catalytic mutant version of USP7 suggest that USP7 is (mono-)ubiquitinated and can auto-deubiquitinate. Auto-cleavage by deubiquitinases has been observed before and was suggested to regulate the enzyme in different manners. Auto-deubiquitination of USP1 triggers proteasome-dependent degradation of the cleaved products,22 whereas self-deubiquitination by USP19 stabilizes the protein.23 Auto-deubiquitination regulates the mono-ubiquitination of UCH-L1 and TRE17/USP6, which is thought to contribute to the activity of these enzymes.24,25 Further studies are required to show if USP7 deubiquitinates itself via an intra- or intermolecular reaction and whether this possibly contributes to controlling its catalytic capabilities or protein levels. Decreased USP7 levels after treatment with a catalytic inhibitor (Fig. 1B) suggests that at least the latter is a possibility. As aberrant USP7 activity may promote oncogenesis, USP7 might be a powerful target for therapeutic intervention and studies on the regulation of this DUB are undoubtedly valuable for the development of novel inhibitors.21
Materials and Methods
Cell culture and transfections
293T and U2OS cells were grown using standard procedures.
Plasmid DNA was transfected into cells using the calcium phosphate transfection method. siRNA oligonucleotides (20 μM, unless stated otherwise; Sigma) were transfected into cells using Lipofectamine RNAiMax (Invitrogen). Simultaneous transfection of DNA plasmids and siRNA oligos were performed using JetPRIME (Polyplus) according to the manufacturers instructions.
Plasmids and siRNA oligos
pCMVTag2B Flag-USP7 wild type and the catalytic mutant version (CI, C223S) were kindly provided by R.D. Everett (MRC-University of Glasgow Center for Virus Research, Glasgow, Scotland). pcDNA3 HA-Claspin and pMT107 His-Ubiquitin have been described before.26
Sequences of oligonucleotides were as follows:
Luc CGUACGCGGAAUACUUCGAdTdT
Claspin GCACAUACAUGAUAAAGAAdTdT
USP29 CCCAUCAAGUUUAGAGGAUdTdT
USP7-1 GGCAACCUUUCAGUUCACUdTdT
USP7-2 CCCAAAUUAUUCCGCGGCAAAdTdT
USP7-3 ACCCUUGGACAAUAUUCCUdTdT
Chk1 UCGUGAGCGUUUGUUGAACdTdT
Antibodies and other reagents
Ku86 (C-20) and Chk1 (G-4) were obtained from Santa Cruz Biotechnology, FK2 from Merck-Millipore, β-actin from Sigma-Aldrich and USP7 (ab190183) was purchased from Abcam. Rabbit polyclonal anti-Claspin and anti-USP29 were kind gifts from R. Freire (Unidad de Investigación, HUC, Tenerife, Spain) and have been described previously.14,27
Cycloheximide (CHX, Sigma) was used at 25 μg/ml, MG132 (A.G. Scientific) at 5 μM, USP7 inhibitor P22077 (Calbiochem) at 30 μM and N-ethylmaleimide (NEM, Sigma) at 2 mM.
Whole cell extracts, immunoprecipitations and pull downs
Whole cell extracts were prepared by washing cultures in PBS before boiling cells in Laemmli buffer for 5 minutes. Protein concentrations were determined using the BCA protein assay (Novagen).
For immunoprecipitations, cells were lysed in EB150 lysis buffer (50 mM Hepes pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM MgCl2, 0.5% NP40, 10% glycerol) for 20 min on ice. After centrifugation 13000 rpm for 20 min, extracts were incubated with anti-Flag M2 agarose (Sigma) for 2 h at 4°C, followed by 4 washes with lysis buffer. The proteins were eluted with 150 mM Glycine pH 2.3, after which sample buffer was added.
For pull downs of His-tagged ubiquitinated proteins, cells were lysed in buffer A (8M GuHCl, 10 mM Tris-HCl pH 8.0, 100 mM NaH2PO4, 10 mM imidazole). After sonication, the extracts were incubated with Ni-NTA agarose beads (Genescript) for 2 h at RT, followed by 3 washes with buffer A, 2 with a 1:4 mix of buffer A:B (25 mM Tris-HCl pH 6.8, 20 mM imidazole) and finally 2 washes with buffer B. Sample buffer supplemented with 200 mM imidazole was added for elution.
Protein purification
For protein purification of Flag-USP7 WT/CI, 293T cells were transfected with the corresponding expression vectors. Cells were lysed in EB150 lysis buffer (50 mM Hepes pH 7.5, 150 mM NaCl, 5 mM EDTA, 2 mM MgCl2, 0.5% NP40, 10% glycerol) for 20 min on ice. After centrifugation 13000 rpm for 20 min, extracts were incubated with anti-Flag M2 agarose (Sigma) for 2 h at 4°C, followed by 4 washes with lysis buffer and 1 wash in elution buffer (50 mM Tris-HCl pH 7.5). Proteins were eluted in elution buffer supplemented with 330 μg/ml Flag (DYKDDDDK) peptide (Genscript) for 1.5 h at 4°C. Supernatant was collected, 10% glycerol added and aliquots were stored at 20°C.
In vitro deubiquitin assay
Ubiquitinated Chk1, purified on Ni-NTA agarose, and DUB were mixed in buffer (50 mM Tris-HCl pH 7.5 and 4 mM DTT) and incubated for 30 min at 37°C. Sample buffer was added to stop the reaction and samples were analyzed by western blotting.
DUB activity assay
Cells were lysed in lysis buffer (50 mM Tris-HCl pH 7.4, 5 mM MgCl2, 250 mM sucrose, 1 mM DTT, 2 mM ATP and 0.1% NP40) for 20 min on ice. The extracts were centrifuged at 13000 rpm for 20 min and incubated with 50 μM HA-Ubiquitin-Vinyl Sulfone (HA-Ub-VS, Boston Biochemicals) for 1 h at 37°C, when indicated in the presence of NEM. Subsequently the samples were incubated with anti-HA affinity matrix (Roche Diagnostics) for 2 h at 4°C, followed by 4 washes with lysis buffer, after which sample buffer was added and analysis by western blotting for the DUB of interest was performed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Roger Everett for sharing reagents and Raimundo Freire for support and critical reading of the manuscript.
Funding
This work was supported by a grant from the Spanish MINECO (SAF2013-49149). VAJS is a Ramón y Cajal fellow.
References
- 1. Bartek J, Bartkova J, Lukas J. DNA damage signalling guards against activated oncogenes and tumour progression. Oncogene 2007; 26:7773-9; PMID:18066090; http://dx.doi.org/ 10.1038/sj.onc.1210881 [DOI] [PubMed] [Google Scholar]
- 2. Smits VAJ, Warmerdam DO, Martín Y, Freire R. Mechanisms of ATR-mediated checkpoint signalling. Front Biosci 2010; 15:840-53; http://dx.doi.org/ 10.2741/3649 [DOI] [PubMed] [Google Scholar]
- 3. Smith J, Tho LM, Xu N, Gillespie DA. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res 2010; 108:73-112; PMID:21034966; http://dx.doi.org/ 10.1016/B978-0-12-380888-2.00003-0 [DOI] [PubMed] [Google Scholar]
- 4. Smits VAJ, Reaper PM, Jackson SP. Rapid PIKK-dependent release of Chk1 from chromatin promotes the DNA-damage checkpoint response. Curr Biol 2006; 16:150-9; PMID:16360315; http://dx.doi.org/ 10.1016/j.cub.2005.11.066 [DOI] [PubMed] [Google Scholar]
- 5. Smits VAJ. Spreading the signal: dissociation of Chk1 from chromatin. Cell Cycle 2006; 5:1039-43; PMID:16721053; http://dx.doi.org/ 10.4161/cc.5.10.2761 [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y-W, Otterness DM, Chiang GG, Xie W, Liu Y-C, Mercurio F, Abraham RT. Genotoxic stress targets human Chk1 for degradation by the ubiquitin-proteasome pathway. Mol Cell 2005; 19:607-18; PMID:16137618; http://dx.doi.org/ 10.1016/j.molcel.2005.07.019 [DOI] [PubMed] [Google Scholar]
- 7. Zhang Y-W, Brognard J, Coughlin C, You Z, Dolled-Filhart M, Aslanian A, Manning G, Abraham RT, Hunter T. The F box protein Fbx6 regulates Chk1 stability and cellular sensitivity to replication stress. Mol Cell 2009; 35:442-53; PMID:19716789; http://dx.doi.org/ 10.1016/j.molcel.2009.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hussain S, Zhang Y, Galardy PJ. DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 2009; 8:1688-97; PMID:19448430; http://dx.doi.org/ 10.4161/cc.8.11.8739 [DOI] [PubMed] [Google Scholar]
- 9. Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature 2002; 416:648-53; PMID:11923872; http://dx.doi.org/ 10.1038/nature737 [DOI] [PubMed] [Google Scholar]
- 10. Hu M, Gu L, Li M, Jeffrey PD, Gu W, Shi Y. Structural basis of competitive recognition of p53 and MDM2 by HAUSPUSP7: implications for the regulation of the p53-MDM2 pathway. PLoS Biol 2006; 4:e27; PMID:16402859; http://dx.doi.org/ 10.1371/journal.pbio.0040027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Song MS, Salmena L, Carracedo A, Egia A, Lo-Coco F, Teruya-Feldstein J, Pandolfi PP. The deubiquitinylation and localization of PTEN are regulated by a HAUSP-PML network. Nature 2008; 455:813-7; PMID:18716620; http://dx.doi.org/ 10.1038/nature07290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Horst A, de Vries-Smits AMM, Brenkman AB, van Triest MH, van den Broek N, Colland F, Maurice MM, Burgering BMT. FOXO4 transcriptional activity is regulated by monoubiquitination and USP7HAUSP. Nat Cell Biol 2006; 8:1064-73; PMID:16964248; http://dx.doi.org/ 10.1038/ncb1469 [DOI] [PubMed] [Google Scholar]
- 13. Faustrup H, Bekker-Jensen S, Bartek J, Lukas J, Mailand N. USP7 counteracts SCFbetaTrCP- but not APCCdh1-mediated proteolysis of Claspin. J Cell Biol 2009; 184:13-9; PMID:19124652; http://dx.doi.org/ 10.1083/jcb.200807137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martín Y, Cabrera E, Amoedo H, Hernández-Pérez S, Domínguez-Kelly R, Freire R. USP29 controls the stability of checkpoint adaptor Claspin by deubiquitination. Oncogene 2014. Mar 17; [Epub ahead of print]; PMID: 24632611; http://dx.doi.org/10391675 10.1038/onc.2014.38 [DOI] [PubMed] [Google Scholar]
- 15. Kaneko YS, Watanabe N, Morisaki H, Akita H, Fujimoto A, Tominaga K, Terasawa M, Tachibana A, Ikeda K, Nakanishi M, et al. Cell-cycle-dependent and ATM-independent expression of human Chk1 kinase. Oncogene 1999; 18:3673-81; PMID:10391675; http://dx.doi.org/ 10.1038/sj.onc.1202706 [DOI] [PubMed] [Google Scholar]
- 16. Altun M, Kramer HB, Willems LI, McDermott JL, Leach CA, Goldenberg SJ, Kumar KGS, Konietzny R, Fischer R, Kogan E, et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol 2011; 18:1401-12; PMID:22118674; http://dx.doi.org/ 10.1016/j.chembiol.2011.08.018 [DOI] [PubMed] [Google Scholar]
- 17. Chini CCS, Wood J, Chen J. Chk1 is required to maintain claspin stability. Oncogene 2006; 25:4165-71; PMID:16501606; http://dx.doi.org/ 10.1038/sj.onc.1209447 [DOI] [PubMed] [Google Scholar]
- 18. Lin Y-F, Shih H-Y, Shang Z, Matsunaga S, Chen BP. DNA-PKcs is required to maintain stability of Chk1 and Claspin for optimal replication stress response. Nucleic Acids Res 2014; 42:4463-73; PMID:24500207; http://dx.doi.org/ 10.1093/nar/gku116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freire R, van Vugt MATM, Mamely I, Medema RH. Claspin: timing the cell cycle arrest when the genome is damaged. Cell Cycle 2006; 5:2831-4; PMID:17172868; http://dx.doi.org/ 10.4161/cc.5.24.3559 [DOI] [PubMed] [Google Scholar]
- 20. Sørensen CS, Syljuåsen RG, Lukas J, Bartek J. ATR, Claspin and the Rad9-Rad1-Hus1 complex regulate Chk1 and Cdc25A in the absence of DNA damage. Cell Cycle 2004; 3:941-5; PMID:15190204 [PubMed] [Google Scholar]
- 21. Nicholson B, Suresh Kumar KG. The multifaceted roles of USP7: new therapeutic opportunities. Cell Biochem Biophys 2011; 60:61-8; PMID:21468693; http://dx.doi.org/ 10.1007/s12013-011-9185-5 [DOI] [PubMed] [Google Scholar]
- 22. Huang TT, Nijman SMB, Mirchandani KD, Galardy PJ, Cohn MA, Haas W, Gygi SP, Ploegh HL, Bernards R, D’Andrea AD. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol 2006; 8:339-47; PMID:16531995 [DOI] [PubMed] [Google Scholar]
- 23. Mei Y, Hahn AA, Hu S, Yang X. The USP19 Deubiquitinase Regulates the Stability of c-IAP1 and c-IAP2. J Biol Chem 2011; 286:35380-7; PMID:21849505; http://dx.doi.org/ 10.1074/jbc.M111.282020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meray RK, Lansbury PT. Reversible monoubiquitination regulates the Parkinson disease-associated ubiquitin hydrolase UCH-L1. J Biol Chem 2007; 282:10567-75; PMID:17259170; http://dx.doi.org/ 10.1074/jbc.M611153200 [DOI] [PubMed] [Google Scholar]
- 25. Shen C. CalciumCalmodulin Regulates Ubiquitination of the Ubiquitin-specific Protease TRE17USP6. J Biol Chem 2005; 280:35967-73; PMID:16127172; http://dx.doi.org/ 10.1074/jbc.M505220200 [DOI] [PubMed] [Google Scholar]
- 26. Mamely I, van Vugt MA, Smits VAJ, Semple JI, Lemmens B, Perrakis A, Medema RH, Freire R. Polo-like kinase-1 controls proteasome-dependent degradation of Claspin during checkpoint recovery. Curr Biol 2006; 16:1950-5; PMID:16934469; http://dx.doi.org/ 10.1016/j.cub.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 27. Semple JI, Smits VAJ, Fernaud J-R, Mamely I, Freire R. Cleavage and degradation of Claspin during apoptosis by caspases and the proteasome. Cell Death Differ 2007; 14:1433-42; PMID:17431426; http://dx.doi.org/ 10.1038/sj.cdd.4402134 [DOI] [PubMed] [Google Scholar]


