Abstract
Experimental studies to determine the nature of ecological interactions between invasive and native species are necessary for conserving and restoring native species in impacted habitats. Theory predicts that species boundaries along environmental gradients are determined by physical factors in stressful environments and by competitive ability in benign environments, but little is known about the mechanisms by which hydrophytes exclude halophytes and the life history stage at which these mechanisms are able to operate. The ongoing invasion of the South American Spartina densiflora in European marshes is causing concern about potential impacts to native plants along the marsh salinity gradient, offering an opportunity to evaluate the mechanisms by which native hydrophytes may limit, or even prevent, the expansion of invasive halophytes. Our study compared S. densiflora seedling establishment with and without competition with Phragmites australis and Typha domingensis, two hydrophytes differing in clonal architecture. We hypothesized that seedlings of the stress tolerant S. densiflora would be out-competed by stands of P. australis and T. domingensis. Growth, survivorship, biomass patterns and foliar nutrient content were recorded in a common garden experiment to determine the effect of mature P. australis and T. domingensis on the growth and colonization of S. densiflora under fresh water conditions where invasion events are likely to occur. Mature P. australis stands prevented establishment of S. densiflora seedlings and T. domingensis reduced S. densiflora establishment by 38%. Seedlings grown with P. australis produced fewer than five short shoots and all plants died after ca. 2 yrs. Our results showed that direct competition, most likely for subterranean resources, was responsible for decreased growth rate and survivorship of S. densiflora. The presence of healthy stands of P. australis, and to some extent T. domingensis, along river channels and in brackish marshes may prevent the invasion of S. densiflora by stopping the establishment of its seedlings.
Keywords: Brackish marshes, Inter-specific competition, Invasion, Salt marshes, Phragmites australis, Intra-specific competition, Typha domingensis
Introduction
Competition between native and invasive plant species has been broadly studied in marshes (Ungar, 1998), however experimental studies to determine the nature of ecological interactions between invasive and native species are necessary for conserving and restoring native species in impacted habitats (Parker, Simberloff & Lonsdale, 1999; Byers & Goldwasser, 2001).
Theory predicts that species boundaries along environmental gradients are determined by physical factors in stressful environments and by competitive ability in benign environments (Crain & Bertness, 2006; Maestre et al., 2009; Engels, Rink & Jensen, 2011). Engels & Jensen (2010) showed that such a relationship controls plant zonation in estuaries. Plants transplanted from low salinity environments (hydrophytes) to salt marshes performed poorly regardless of whether neighbouring vegetation was present or not, and conversely, plants growing in high salinities (halophytes) had low biomass and high mortality rates in the presence of neighbors when transplanted to freshwater marshes. Without neighbors, biomass of halophytes in freshwater wetlands was similar to or higher than that in salt marshes. These results showed a shift in the importance of competition along the estuarine salinity gradient. Still, little is known about competitive outcomes among native and invasive plants differing in salinity tolerance or the life history stage at which these mechanisms operate—information central to managing plant invasions in coastal and estuarine environments.
The South American cordgrass, S. densiflora Brongn. (Poaceae), is a clonal plant invading estuaries in Europe (Nieva et al., 2001) and North America (Kittelson & Boyd, 1997), but the impacts to these systems and mode of invasion are poorly known. In Europe, S. densiflora invades a wide range of habitats, including brackish marshes and river banks (Nieva et al., 2001; Curado et al., 2010) and is interacting with native plants over a strong salinity gradient that may influence competition among species. The native congener S. maritima Curtis (Fernald) may be succumbing to S. densiflora invasion at middle marsh elevations (Castillo et al., 2008; Castillo & Figueroa, 2009), but invasion has not occurred in intact stands of indigenous freshwater and brackish marsh hydrophytes, such as the clonal dominant wetland plants Typha domingensis Pers. (southern cattail) and Phragmites australis (Cav.) Trin. ex Steud. (common reed). Spartina densiflora invades bare sediments in new areas by seedlings established from numerous seeds dispersed by water (Kittelson & Boyd, 1997; Nieva et al., 2001). Thus, S. densiflora invasion offers an opportunity to analyze the mechanisms by which native hydrophytes would limit the expansion of invasive halophytes in mid to high marsh habitats.
Our study compared invasive S. densiflora seedling establishment with and without competition with native P. australis and T. domingensis, two hydrophytes with differing clonal architecture: P. australis has a high stem density with narrow stem diameters and T. domingensis has a lower stem density, but thicker diameter stems. We hypothesized that seedlings of the stress tolerant S. densiflora would be out-competed by mature stands of P. australis and T. domingensis under low salinity conditions following the general ecological theory that stress tolerant plants have a lower competitive capacity than stress-intolerant but fast-growing plants. We compared growth, survivorship, biomass allocation patterns and foliar nutrient content of S. densiflora seedlings in response to inter- and intra-specific competition and in the absence of competition to explore the ability of native P. australis and T. domingensis to prevent S. densiflora invasion under low salinity conditions and at early life-stages of the invasion process.
Materials and Methods
Experimental design
Phragmites australis and Typha domingensis rhizomes and S. densiflora seeds were collected in Odiel Marshes (Southwest Iberian Peninsula). Phragmites australis and T. domingensis rhizomes were planted and grown in peat soil in plastic pots (12 cm diameter and 15 cm height; volume of 2.75 l) until they established mature stands with similar densities to those found in wetlands in the Southwest Iberian Peninsula. Spartina densiflora seedlings were obtained for experiments from seeds sown on peat soil in flats in the greenhouse. Seeds and seedlings were watered regularly to maintain moist soils until transplanted into treatments.
The common garden experiment was initiated in January 2008 and conducted over two years in a common garden at the University of Seville, Spain. Four treatments (two interspecific competition treatments, one intraspecific competition treatment, and one no competition treatment) were established using transplanted S. densiflora seedlings of similar size: (1) Five seedlings of S. densiflora transplanted into a pot containing an established P. australis stand (n = 6 pots); (2) Five seeds of S. densiflora transplanted into a pot containing an established T. domingensis stand (n = 10 pots); (3) Five seedlings of S. densiflora transplanted into a pot containing an established S. densiflora stand (n = 5 pots); and (4) one seedling of S. densiflora without intra- and inter-specific competition (n = 5 pots). S. densiflora seedlings were placed at a depth of 0.5 cm; one at the centre of the pot and the other four around it spaced 3 cm apart. Shoot density and height, and above-ground biomass (AGB) and below-ground biomass (BGB) of P. australis and T. domingensis used in our experiment were within the range of those reported previously in field studies (Sobrero, Sabbatini & Fernandez, 1997; Sharma et al., 2008; Engloner, 2009). Initial planting conditions imitated natural conditions during autumn-winter periods in Southwest Iberian Peninsula when S. densiflora seeds germinate inside native stands with most of their aerial biomass senesced. Plants were maintained at ambient light and temperature and watered daily with fresh water, and pots were placed in pools keeping their base permanently flooded to a height of 3 cm.
Abiotic environment
Light intensity, and soil salinity (measured as electrical conductivity), pH, and redox potential were measured for each pot to evaluate if biotic interactions among species changed the abiotic environment. Photosynthetic photon flux density (PPFD) was recorded with a portable photometer (LI-COR Instruments, Inc., Lincoln, Nebraska, USA) at ground level during midday outside and within the stands of P. australis and T. domingensis during summertime (in July 2008) coinciding with maximum biomass accumulation. At the end of the experiment, soil samples were collected from each pot, dried at 60 °C for two days, and then sieved through mesh to remove particles greater than 2 mm. Total soluble salt concentration (salinity) was determined by measuring electrical conductivity (Rhoades, 1996). To determine electrical conductivity, 60 ml of 0.1 M calcium chloride was added to 20 g of soil (3:1 mixture) and mixed on an orbit shaker for 30 min. Conductivity was measured at 21.0 ± 0.5 °C using a CM35+ meter (Crison Instruments, Inc., Barcelona, Spain). To determine soil pH, 30 g of soil was mixed with 30 ml deionized water (1:1 mixture) and mixed on an orbit shaker for 30 min. pH was measured with a CM35+ meter. Redox potential of the soil between 0–5 cm deep was determined with a portable meter and electrode system (Crison Instruments, Inc., Barcelona, Spain).
Survivorship, shoot production, height and biomass
The number of live S. densiflora seedlings, and live P. australis, T. domingensis, and S. densiflora shoots were counted periodically from the beginning of the experiment. Shoot height of Spartina densiflora seedlings was measured from the base of the shoot to the tip of the longest leaf (n = 5–10 pots; 5 shoots of different clones, or per pot).
At the end of the experiment (February 2010), AGB and BGB were recorded for every clone of each species in each treatment (n = 5–10 pots; 5–50 clones per species and treatment). Stems of all plants were harvested, dried at 80 °C for 48 h, and weighed. AGB was divided into dead and live shoots and leaves, and BGB was divided into rhizomes and roots. AGB and BGB for P. australis and T. domingensis stands were also recorded at the beginning of the experiment using extra pots maintained under experimental conditions.
Leaf nitrogen and carbon content
Total leaf carbon (C) and nitrogen (N) content were determined for the three plant species in July 2008, when Spartina seedlings were large enough to contain enough leaf tissue for these analyses. Three leaves per clone within each pot, and for each treatment, were collected and pooled for analysis. The samples were dried in an oven at 80 °C for 48 h, pulverized using a grinder (Cyclotech, Inc., Cypress, California, USA) and filtered using a screen of 80-µm. Total C and N concentration was determined for undigested samples with an elemental analyzer (Leco Instruments, Inc., Saint Joseph, Michigan, USA).
Statistical analysis
Analyses were conducted using SPSS release 12.0 (SPSS Inc., Chicago, IL). Data were tested for normality with the Kolmogorov–Smirnov test and for homogeneity of variance with the Levene test at P > 0.05. When homogeneity of variance between groups was not found, data were transformed using the following functions: ln(x), 1/x and √x. Analysis of variance was used to detect differences in the response variables among competition treatments and Tukey’s Honest Significant Difference (HSD) test was used to detect differences among treatments only if F-test was significant at P = 0.05. Student’s t-test for independent samples was applied to compare AGB and BGB between T. domingensis and P. australis. Deviations were calculated as the standard error of the mean.
Results
Abiotic environment
Ambient Photosynthetic Photon Flux Density (PPFD) measured outside pots with native plant stands averaged 1,660 ± 105 µmol photon m−2 s−1 at full sunlight. PPFD measured within P. australis stands was 55% (928 ± 206 µmol photon m−2 s−1) of full light and was not significantly different than PPFD within T. domingensis stands, which was 63% of full light (1,057 ± 151 µmol photon m−2 s−1; t-test, >0.05). Soil electrical conductivity, pH, and redox potential were not significantly different among treatments (P > 0.05). Mean soil pH was ca. 6 and conductivity varied between 0.30 and 2.32 mS cm−1. Soil redox potential was always positive (ca. +130 mV).
Survivorship, shoot production and height
Phragmites australis consistently had a higher shoot density than T. domingensis over the course of the experiment (Fig. 1). Shoot senescence increased over the winter for both native species, but was higher for P. australis.
Figure 1. Number of live shoots over time.
Temporal variation of the number of live shoots (A) for Phragmites australis, Typha domingensis and (B) S. densiflora, and (C) S. densiflora shoot height (cm)for four treatments: 5 Spartina seedlings within Phragmites australis or within Typha domingensis, 5 Spartina seedlings and 1 Spartina seedling.
Competition treatments during the first 135 days had no effect on the number of live seedlings of S. densiflora. But, by the end of the experiment (ca. two years), no S. densiflora seedlings planted into P. australis stands survived. Seedlings growing within T. domingensis stands were less impacted, having a survival rate of 62%. In contrast, all seedlings planted alone or with other Spartina seedlings survived.
Competitive interaction of Spartina seedlings with both native species caused depressed development, resulting in fewer and short shoots, and lower biomass than in the absence of competition. Average shoot density of S. densiflora seedlings growing alone was 26 ± 3 shoots clone−1, followed by the S. densiflora monoculture with intra-specific competition (11 ± 1 shoots clone−1), seedlings growing within T. domingensis (ca. 7 ± 0 shoots clone−1), and seedlings growing with P. australis(with 2 ± 0 live shoots clone−1 just before dying) (P < 0.05) (Fig. 1). At the end of the experiment, the shortest S. densiflora seedlings were those that had died within P. australis stands and averaged 23.0 ± 2.5 cm, followed by those growing in monoculture (51.3 ± 3.4 cm) and by those growing alone or within T. domingensis stands (68.8 ± 5.5 cm tall) (P < 0.05) (Fig. 1).
Biomass
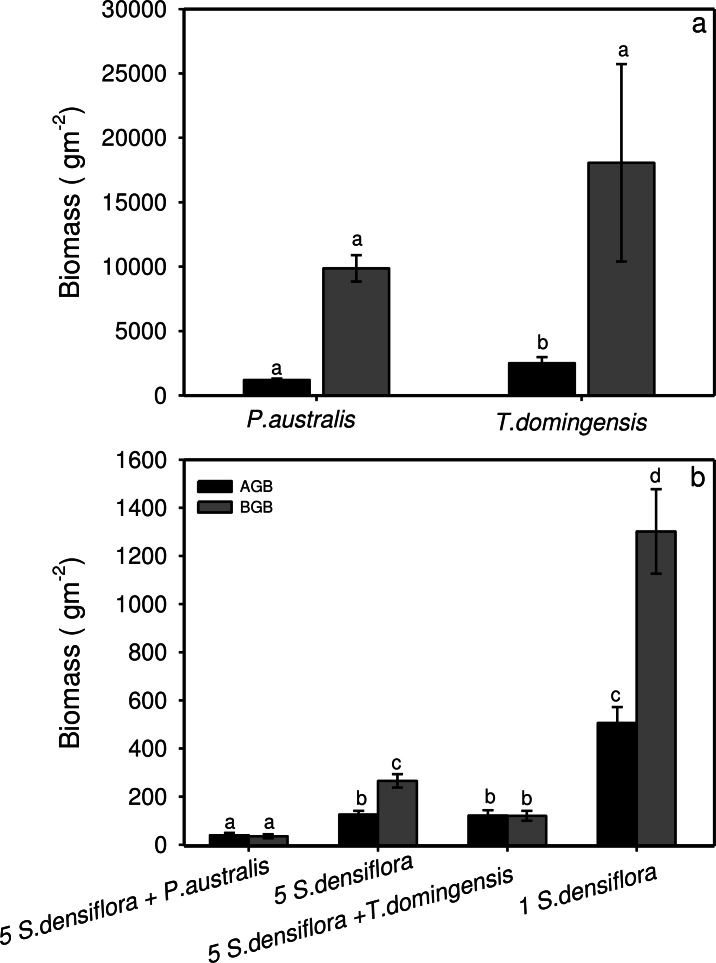
Both P. australis and T. domingensis had lower allocation to above-ground than to below-ground structures, but T. domingensis did have a higher AGB (ca. 2,500 g m−2) than P. australis (ca. 1,200 g m−2) at the end of the experiment (t-test, P < 0.05). BGB of T. domingensis and P. australis stands was similar and averaged ca. 14,000 g m−2 for each species (t-test, P > 0.05) (Fig. 2).
Figure 2. Above- and below-ground biomass for Phragmites australis and Typha domingensis stands, and S. densiflora seedlings in four different competition treatments.
Above- (AGB; black bars) and below-ground (BGB; gray bars) biomass (g m−2) for (A) Phragmites australis and Typha domingensis stands and (B) for S. densiflora seedlings growing in four different competition treatments Different letters indicate significant difference among treatments (ANOVA or t-test, P < 0.05).
At the end of the experiment, AGB and BGB of Spartina seedlings growing alone were significantly higher (AGB: 500 ± 66 g m−2; BGB: 1,300 ± 175 g m−2) than for the other treatments (AGB: ca. 125 g m−2; BGB: ca. 200 g m−2) (AGB: P < 0.05; BGB: P < 0.05). In addition, S. densiflora seedlings growing in the intraspecific competition treatment had greater BGB than those growing within T. domingensis stands. S. densiflora seedlings growing within P. australis had the lowest AGB (40 ± 9 g m−2) and BGB (36 ± 8 g m−2; Fig. 2).
Leaf nitrogen and carbon content
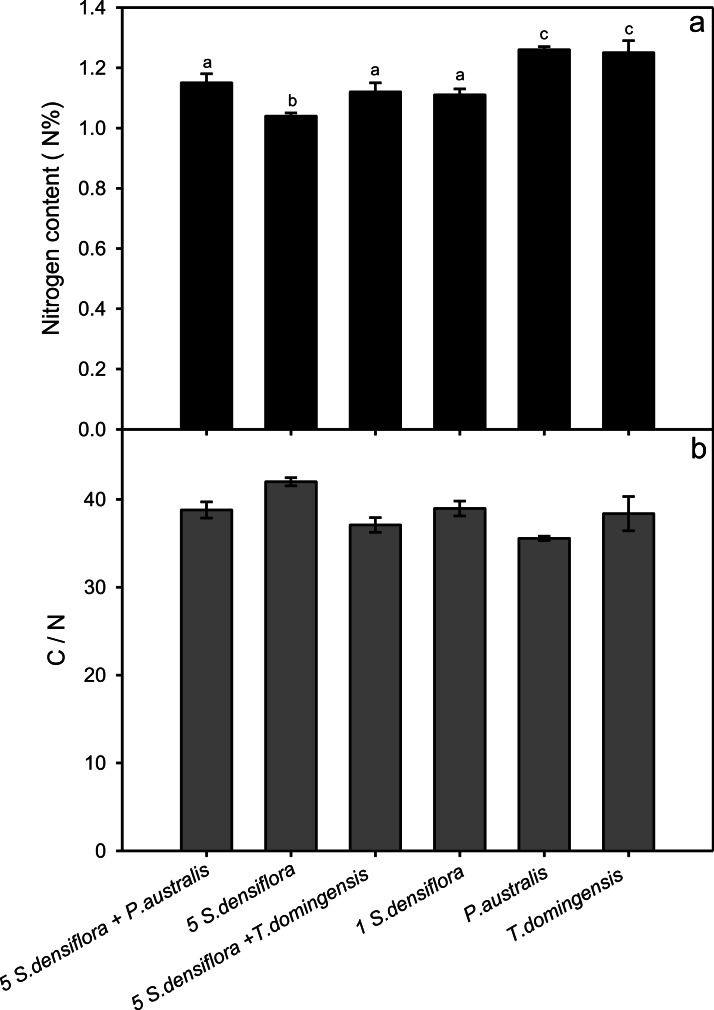
Spartina densiflora had lower leaf N content in all treatments compared with P. australis or T. domingensis. Leaf N content was lowest for S. densiflora seedlings growing in monoculture (1.04 ± 0.02% N), 1.11 ± 0.02%, for seedlings growing alone, 1.15 ± 0.03% for seedlings growing with P. australis, and 1.12 ± 0.03% for seedling growing with T. domingensis. The P. australis and T. domingensis only treatments had significantly higher leaf N content (ca. 1.25%) than all treatments with S. densiflora seedlings (P < 0.05; Fig. 3). As expected, C:N reflected leaf N content among the treatments (Fig. 3).
Figure 3. Nitrogen content and C:N ratio.
(A) Nitrogen content (%), and (B) C: N ratio for S. densiflora in four different competition treatments (see Fig. 1) and for Phragmites australis and Typha domingensis adult stands. Different letters indicate significant difference among treatments (ANOVA, P < 0.05).
Discussion
We hypothesized that mature native hydrophytes would be competitively superior under low salinity conditions and prevent the invasion of S. densiflora. Phragmites australis did effectively exclude S. densiflora seedlings, but T. domingensis stands were not able to completely stop establishment and growth. These differences in the survivorship and the growth of S. densiflora among treatments were likely related to contrasting biomass distribution patterns within the stands of the two native hydrophytes and not changes in the abiotic environment since soil characteristics and PPFD were similar among treatments. P. australis seemed to prevent the colonization of S. densiflora due to its very high biomass allocation to belowground structures, similar to that of T. domingensis, but showed more uniform occupation of the subterranean space than T. domingensis. Phragmites australis has a dense network of shallow rhizomes and roots, with corresponding high aboveground stem density, as opposed to the deep, sparse root structure of T. domingensis (JM Castillo, pers. obs., 2007). The more regular and dense occupation of the subterranean space just below the soil surface by P. australis may have prevented the establishment of S. densiflora seedlings, presumably by blocking the establishment of the subterranean rooting system. Empty space in the below-ground neighbourhood is often a key factor for plant establishment (McConnaugha & Bazzaz, 1991; Casper & Jackson, 1997). Nevertheless, Minchinton, Sympson & Bertness (2006) found that P. australis also effectively excluded other plant species by increased shoot and litter production rather than by changing soil properties or by below-ground competition.
Spartina densiflora did well in the early establishment phase when both native species were dormant and stem density was low, and although above-ground competition is negligible at this point (Engloner, 2009), the rapid decrease in growth over the growing season points to below-ground competition for limited space as the likely mechanism. Spartina densiflora has ruderal characteristics and readily colonizes bare substrates, but is less successful in well established plant communities. Castillo et al. (2008) found that S. densiflora invasion from seeds may be limited in Spanish marshes by inter-specific subterranean competition with the native S. maritima (Curtis). Similarly, tiller expansion of S. densiflora in North American marshes was higher in areas without native competitors (Kittelson & Boyd, 1997). Phragmites australis is a notoriously competitive hydrophyte in brackish and freshwater systems, and may effectively limit the spread of S. densiflora where they co-occur. Engels & Jensen (2010) described competitive displacement of S. anglica C.E. Hubbard by P. australis in North European freshwater marshes, and He et al. (2009) showed that established P. australis stands inhibited the development of Suaeda salsa (L) Pallas seedlings in China. The European P. australis lineage has invaded coastal and freshwater marshes throughout eastern North America (Saltonstall, 2002) where it has formed extensive monocultures and displaced diverse assemblages of native plants (Marks, Lapin & Randall, 1994; Tiner, 1997; Chambers, Meyerson & Saltonstall, 1999; Meyerson, Saltonstall & Windham, 2000), including native populations of Typha spp. (Chun & Choi, 2009), indigenous P. australis (Lambert, Dudley & Saltonstall, 2010), and Spartina spp. (Saltonstall, 2002; Robertson & Weis, 2005; Kimball & Able, 2007).
Nutrient levels and C:N were also generally consistent across treatments so competition for limiting nutrients did not appear to be a factor. Nitrogen content of seedlings was actually higher in the inter-specific competition treatments. We do not know from our study whether P. australis or T. domingensis captured or used nitrogen more efficiently, but other studies have shown that the growth of P. australis is stimulated by nitrogen which it efficiently convert to belowground biomass (Sillman & Bertness, 2004; Lambert, Dudley & Robbins, 2014). Rickey & Anderson (2004) found that P. australis displaced native Spartina pectinata Bosc. ex Link. under high nitrogen conditions.
In view of our results, management of invaded and susceptible marshes should focus on well-conserved communities of native hydrophytes as a way to passively resist invasion by S. densiflora. In some European marshes, P. australis has shown a general dye-back (Ostendorp, 1989) which, if it occurred in estuaries invaded by S. densiflora could open space for further invasion. In addition, planting P. australis in estuaries and river banks in areas already invaded by or susceptible to invasion by S. densiflora should be considered as viable option for creating a barrier to cordgrass expansion.
Supplemental Information
Raw data
Acknowledgments
Many thanks for assistance in the greenhouse: Jorge Carrión, Sameh K. Abd-Elmabod, Ahmed Hassan, Jesús and José.
Funding Statement
This work was supported by PhD scholarship of the Egyptian Government-Ministry of Higher Education (cultural affairs and missions sector). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Ahmed M. Abbas conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Adam M. Lambert analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Alfredo E. Rubio-Casal conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Alfonso De Cires performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Enrique M. Figueroa conceived and designed the experiments.
Jesús M. Castillo conceived and designed the experiments, wrote the paper, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
References
- Byers & Goldwasser (2001).Byers JE, Goldwasser L. Exposing the mechanisms and timing of impact of non indigenous species on native species. Ecology. 2001;82:1330–1343. doi: 10.1890/0012-9658(2001)082[1330:ETMATO]2.0.CO;2. [DOI] [Google Scholar]
- Casper & Jackson (1997).Casper BB, Jackson RB. Plant competition underground. Annual Review of Ecology, Evolution, and Systematics. 1997;28:545–570. doi: 10.1146/annurev.ecolsys.28.1.545. [DOI] [Google Scholar]
- Castillo & Figueroa (2009).Castillo JM, Figueroa E. Effects of abiotic factors on the life span of the invasive cordgrass Spartina densiflora and the native S. maritima at low marshes. Aquatic Ecology. 2009;43:51–60. doi: 10.1007/s10452-007-9159-2. [DOI] [Google Scholar]
- Castillo et al. (2008).Castillo JM, Mateos-Naranjo E, Nieva FJ, Figueroa E. Plant zonation at salt marshes of the endangered cordgrass Spartina maritima invaded by Spartina densiflora. Hydrobiologia. 2008;614:363–371. doi: 10.1007/s10750-008-9520-z. [DOI] [Google Scholar]
- Chambers, Meyerson & Saltonstall (1999).Chambers RM, Meyerson LA, Saltonstall K. Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Ecology. 1999;64:261–273. doi: 10.1016/S0304-3770(99)00055-8. [DOI] [Google Scholar]
- Chun & Choi (2009).Chun YM, Choi YD. Expansion of Phragmites australis (Cav.) Trin. ex Steud. (P. australis) into Typha spp. (Cattail) Wetlands in Northwestern Indiana, USA. Journal of Plant Biology. 2009;52:220–228. doi: 10.1007/s12374-009-9024-z. [DOI] [Google Scholar]
- Crain & Bertness (2006).Crain CM, Bertness MD. Ecosystem engineering across environmental gradients: implications for conservation and management. Bio. Science. 2006;56:211–218. doi: 10.1641/0006-3568(2006)056[0211:EEAEGI]2.0.CO;2. [DOI] [Google Scholar]
- Curado et al. (2010).Curado G, Rubio-Casal AE, Figueroa E, Castillo JM. Germination and establishment of the invasive cordgrass Spartina densiflora in acidic and metal polluted sediments of the Tinto river. Marine Pollution Bulletin. 2010;60:1842–1848. doi: 10.1016/j.marpolbul.2010.05.022. [DOI] [PubMed] [Google Scholar]
- Engels & Jensen (2010).Engels JG, Jensen K. Role of biotic interactions and physical factors in determining the distribution of marsh species along an estuarine salinity gradient. Oikos. 2010;119:679–685. doi: 10.1111/j.1600-0706.2009.17940.x. [DOI] [Google Scholar]
- Engels, Rink & Jensen (2011).Engels JG, Rink F, Jensen K. Stress tolerance and biotic interactions determine plant zonation patterns in estuarine marshes during seedling emergence and early establishment. Journal of Ecology. 2011;99:277–287. doi: 10.1111/j.1365-2745.2010.01745.x. [DOI] [Google Scholar]
- Engloner (2009).Engloner AI. Structure, growth dynamics and biomass of reed (Phragmites australis)—a review. Flora. 2009;204:331–346. doi: 10.1016/j.flora.2008.05.001. [DOI] [Google Scholar]
- He et al. (2009).He Q, Cui BS, Cai YZ, Deng JF, Sun T, Yang ZF. What confines an annual plant to two separate zones along coastal topographic gradients? Hydrobiologia. 2009;630:327–340. doi: 10.1007/s10750-009-9825-6. [DOI] [Google Scholar]
- Kimball & Able (2007).Kimball ME, Able KW. Nekton utilization of intertidal salt marsh creeks: tidal influences in natural Spartina, invasive Phragmites, and marshes treated for Phragmites removal. Journal of Experimental Marine Biology and Ecology. 2007;346:87–101. doi: 10.1016/j.jembe.2007.03.006. [DOI] [Google Scholar]
- Kittelson & Boyd (1997).Kittelson PM, Boyd MJ. Mechanisms of expansion for an introduced species of cordgrass, Spartina densiflora, in Humboldt Bay, California. Estuaries. 1997;20:773–778. doi: 10.2307/1352250. [DOI] [Google Scholar]
- Lambert, Dudley & Robbins (2014).Lambert AM, Dudley TL, Robbins J. Nutrient enrichment and soil conditions drive productivity in the large-statured invasive grass Arundo donax. Aquatic Botany. 2014;112:16–22. doi: 10.1016/j.aquabot.2013.07.004. [DOI] [Google Scholar]
- Lambert, Dudley & Saltonstall (2010).Lambert AM, Dudley TL, Saltonstall K. Ecology and impacts of the large-statured invasive grasses Arundo donax and Phragmites australis in North America. Invasive Plant Science and Management. 2010;3:489–494. doi: 10.1614/IPSM-D-10-00031.1. [DOI] [Google Scholar]
- Maestre et al. (2009).Maestre FT, Callaway RM, Valladares F, Lortie CJ. Refining the stress-gradient hypothesis for competition and facilitation in plant communities. Journal of Ecology. 2009;97:199–205. doi: 10.1111/j.1365-2745.2008.01476.x. [DOI] [Google Scholar]
- Marks, Lapin & Randall (1994).Marks M, Lapin B, Randall J. Phragmites australis (Phragmites communis): threats, management, and monitoring. Natural Areas Journal. 1994;14:285–294. [Google Scholar]
- McConnaugha & Bazzaz (1991).McConnaugha KDM, Bazzaz FA. Is physical space a soil resource? Ecology. 1991;72:94–103. doi: 10.2307/1938905. [DOI] [Google Scholar]
- Meyerson, Saltonstall & Windham (2000).Meyerson LA, Saltonstall K, Windham L. A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecology and Management. 2000;8:89–103. doi: 10.1023/A:1008432200133. [DOI] [Google Scholar]
- Minchinton, Sympson & Bertness (2006).Minchinton TE, Sympson JC, Bertness MD. Mechanisms of exclusion of native coastal marsh plants by an invasive grass. Journal of Ecology. 2006;94:342–354. doi: 10.1111/j.1365-2745.2006.01099.x. [DOI] [Google Scholar]
- Nieva et al. (2001).Nieva FJ, Díaz-Espejo A, Castellanos EM, Figueroa ME. Field variability of invading populations of Spartina densiflora Brong. grown in different habitats of the Odiel marshes (SW Spain) Estuarine, Coastal and Shelf Science. 2001;52:515–527. doi: 10.1006/ecss.2000.0750. [DOI] [Google Scholar]
- Ostendorp (1989).Ostendorp W. Die-back of reeds in Europe: a critical review. Aquatic Botany. 1989;35:5–26. doi: 10.1016/0304-3770(89)90063-6. [DOI] [Google Scholar]
- Parker, Simberloff & Lonsdale (1999).Parker IM, Simberloff D, Lonsdale WM. Impact: toward a framework for understanding the ecological effects of invaders. Biological Invasions. 1999;1:3–19. doi: 10.1023/A:1010034312781. [DOI] [Google Scholar]
- Rhoades (1996).Rhoades JD. Salinity: electrical conductivity and total dissolved solids. In: Sparks DL, Page AL, Helmke PA, Loeppert RH, editors. Methods of soil analysis. Part 3. Chemical methods. Madison: Soil Science Society of America and American Society of Agronomy; 1996. pp. 417–435. [Google Scholar]
- Rickey & Anderson (2004).Rickey MA, Anderson RC. Effects of nitrogen addition on the invasive grass Phragmites australis and a native competitor Spartina pectinata. Journal of Applied Ecology. 2004;41:888–896. doi: 10.1111/j.0021-8901.2004.00948.x. [DOI] [Google Scholar]
- Robertson & Weis (2005).Robertson TL, Weis JS. A comparison of epifaunal communities associated with the stems of salt marsh grasses Phragmites australis and Spartina alterniflora. Wetlands. 2005;25:1–7. doi: 10.1672/0277-5212(2005)025[0001:ACOECA]2.0.CO;2. [DOI] [Google Scholar]
- Saltonstall (2002).Saltonstall K. Cryptic invasion by a non-native genotype of the P. australis, Phragmites australis, into North America. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:2445–2449. doi: 10.1073/pnas.032477999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma et al. (2008).Sharma P, Asaeda T, Kalibbala M, Fujino T. Morphology, growth and carbohydrate storage of the plant Typha angustifolia at different water depths. Chemistry and Ecology. 2008;24:133–145. doi: 10.1080/02757540801919289. [DOI] [Google Scholar]
- Sillman & Bertness (2004).Sillman BR, Bertness MD. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conservation Biology. 2004;18:1424–1434. doi: 10.1111/j.1523-1739.2004.00112.x. [DOI] [Google Scholar]
- Sobrero, Sabbatini & Fernandez (1997).Sobrero MT, Sabbatini MR, Fernandez OA. Phenology and biomass dynamics of cattail (Typha subulata) in southern Argentina. Weed Science. 1997;45:419–422. [Google Scholar]
- Tiner (1997).Tiner RW. Managing P. australis (Phragmites australis) in Massachusetts: an overview of the species and control techniques. Boston: Massachusetts Wetlands Restoration and Banking Program; 1997. [Google Scholar]
- Ungar (1998).Ungar IA. Are biotic factors significant in influencing the distribution of halophytes in saline habitats? Botanical Review. 1998;64:176–199. doi: 10.1007/BF02856582. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data