Natural killer (NK) cells are Innate Lymphoid Cells (ILC) involved in the immuno-surveillance of cancers and in the early control of infections by intracellular pathogens.1 They can kill cells recognized as targets through a battery of surface receptors2 and produce large amounts of IFN-γ upon activation.1 They differentiate mainly in the Bone Marrow (BM) where sequential developmental intermediates, from immature to mature, can be defined on the basis of surface expression of the tumor necrosis factor (TNF) superfamily member CD27 and the integrin CD11b: CD11bloCD27hi NK cells, CD11bhiCD27hi and CD11bhiCD27lo.3,4 A hallmark of the NK cell population is their expression of high CD122 levels, the β subunit of the IL-2/IL-15 receptor underlining the fact that the various aspects of NK cell development, homeostasis and activation are conditioned by IL15. Despite this well acknowledged role, the molecular basis for the diversity of effects of IL-15 remains unknown. Metabolic integrators have recently emerged as key molecular players in immune cells differentiation. The metabolic checkpoint kinase mammalian Target Of Rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase integrating various extracellular cues: metabolite and growth factors but also antigenic and inflammatory signals and controlling in return numerous metabolic pathways. We recently reported that mTOR is essential for the proper differentiation and peripheral activation of NK cells and is activated upon NK cell exposure to IL-15.5
Measuring the regulation of nutrient receptors expression as well as changes in respiratory and glycolytic rates, we showed that metabolism is gradually shut down upon NK cell differentiation in the BM leading to metabolically resting mature NK cells. In contrast, peripheral NK cell activation is characterized by an increase in metabolic activity. These results prompted us to analyze the phosphorylation status of mTOR targets by flow cytometry. We observed a perfect correlation between NK cell metabolism and mTOR activity. Submitting NK cells to a wide-range of stimuli in order to identify the signals able to control mTOR activity in NK cells, we showed that mTOR activity is triggered by IL-15 and by IL-18 to a lesser extent. In vivo blocking experiments demonstrated the paramount role of IL-15 in mTOR activation during development and following activation. Of note, mTOR activation necessitated high IL-15 concentrations in vitro; instead, STAT5 phosphorylation was readily triggered by low doses of IL-15.
To establish the physiological relevance of mTOR signaling in NK cells, we deleted mTOR in NK cells by crossing mice bearing loxP-flanked alleles encoding mTOR (Mtorlox/lox) with mice expressing the Cre recombinase from the gene encoding the NK cell specific activating receptor NKp46. mTOR deletion resulted in major effects on NK cell homeostasis. Indeed, Mtor−/− NK cell differentiation in the BM was severely blocked at the CD11bloCD27hi stage. This defect, combined with a substantial decrease in the proliferation rate of immature NK cells, lead to a 6-fold-decrease in the number of NK cells in peripheral organs. Interestingly, survival of mTOR deficient NK cells is not affected in accordance with previous studies suggesting that the pro-survival signals given by IL-15 are mediated by STAT5.6 Of note, IL-15 receptor level was halved in Mtor−/− NK cells and this was associated with a blunted sensing of IL-15 signals in vitro, which could contribute to reinforce the observed phenotype.
We also studied the impact of mTOR deficiency on NK cell activation and found that Mtor−/− NK cells were hyporesponsive to inflammatory stimuli triggered by the dsRNA mimetic poly(I:C) as well as to Mouse CytoMegaloVirus (MCMV) infection. This hyporesponsiveness was characterized by a decrease in cytotoxicity, in metabolism upregulation and in proliferation. In contrast, IFN-γ production by Mtor−/− NK cells in response to a combination of IL-12 and IL-18 was normal, demonstrating that mTOR controls selectively some NK cell effector functions. As the mTOR inhibitor Rapamycin is commonly used in clinical settings, we investigated its effects on NK cell activation in murine and human models and found that it dampens the acquisition of cytotoxicity.
In summary, our findings provide crucial insights into the IL-15 signaling pathway in NK cells, placing mTOR at a key signaling node controlling 2 major checkpoints of NK cell biology: maturation in the BM and peripheral activation. Moreover, showing that mTOR pharmacological inhibitors used in clinic have the capacity to affect NK cell cytotoxicity in mice and humans, we extend their known immunosuppressant role. The fact that low doses of IL-15 trigger STAT5 activation while higher concentrations are needed to activate mTOR, combined with the observation that NK cell survival was intact while proliferation and effector functions were impaired in the absence of mTOR, lead us to propose the model depicted in Figure 1. Open questions still remain. In particular how IL-15R triggering leads to mTOR activation or the identity and the role of the metabolic network activated by mTOR. These questions will be the subject of further investigations.
Figure 1.
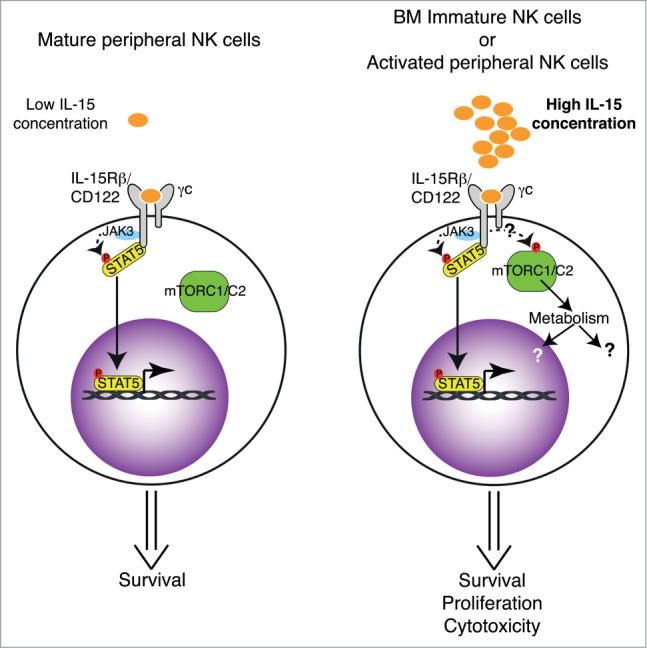
(A) In homeostatic conditions, mature NK cells are exposed to low IL-15 concentration triggering STAT5 phosphorylation and translocation to the nucleus where pro-survival genes are transcribed. (B) Immature NK cells in the BM or mature NK cells following activation are exposed to high IL-15 concentration leading to mTOR activation of metabolism. This induces proliferation and the development of effector functions.
References
- 1. Vivier E, et al. Nat Immunol 2008; 9:503-10; PMID:18425107 [DOI] [PubMed] [Google Scholar]
- 2. Diefenbach A, Raulet DH. Curr Biol CB 1999; 9:R851-3; PMID:10574749 [DOI] [PubMed] [Google Scholar]
- 3. Chiossone L, et al. Blood 2009; 113:5488-96; PMID:19234143 [DOI] [PubMed] [Google Scholar]
- 4. Hayakawa Y, Smyth MJ. J Immunol 2006; 176:1517-24; PMID:16424180 [DOI] [PubMed] [Google Scholar]
- 5. Marçais A, et al. Nat Immunol 2014; 15:749-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckelhart E, et al. Blood 2011; 117:1565-73; PMID:21127177 [DOI] [PubMed] [Google Scholar]


