Abstract
In female mice, despite the presence of slight DNA double-strand breaks (DSBs), fully grown oocytes are able to undergo meiosis resumption as indicated by germinal vesicle breakdown (GVBD); however, severe DNA DSBs do reduce and delay entry into M phase through activation of the DNA damage checkpoint. But little is known about the effect of severe DNA DSBs on the spindle assembly checkpoint (SAC) during oocyte maturation. We showed that nearly no first polar body (PB1) was extruded at 12 h of in vitro maturation (IVM) in severe DNA DSBs oocytes, and the limited number of oocytes with PB1 were actually at telophase. However, about 60% of the severe DNA DSBs oocytes which underwent GVBD at 2 h of IVM released a PB1 at 18 h of IVM and these oocytes did reach the second metaphase (MII) stage. Chromosome spread at MI and MII stages showed that chromosomes fragmented after GVBD in severe DNA DSBs oocytes. The delayed PB1 extrusion was due to the disrupted attachment of microtubules to kinetochores and activation of the SAC. At the same time, misaligned chromosome fragments became obvious at the first metaphase (MI) in severe DNA DSBs oocytes. These data implied that the inactivation of SAC during the metaphase-anaphase transition of first meiosis was independent of chromosome integrity. Next, we induced DNA DSBs in vivo, and found that the number of superovulated oocytes per mouse was significantly reduced; moreover, this treatment increased the percentage of apoptotic oocytes. These results suggest that DNA DSBs oocytes undergo apoptosis in vivo.
Keywords: apoptosis, DNA double-strand breaks, meiosis, oocyte, spindle assembly checkpoint
Abbreviations
- DSBs
DNA double-strand breaks
- GVBD
germinal vesicle breakdown
- SAC
spindle assembly checkpoint
- PB1
first polar body
- IVM
in vitro maturation
- MII
the second metaphase
- MI
the first metaphase
- DDR
DNA damage response
- ICL
interstrand crosslinks
- PBE
PB1 extrusion
Introduction
DNA damage which can be induced endogenously or exogenously is a precipitating factor of mutagenesis, carcinogenesis and infertility.1,2 To cope with it, cells have evolved an elaborate molecular response to sense, respond to, and repair the damaged DNA called DNA damage response (DDR). DDR is activated primarily at the G1/S and G2/M-phase transition.3 When DNA damage is very slight, it can be repaired soon without cell cycle arrest, while when the damage becomes severe, the cell cycle is arrested to allow more time for repair; finally, if the damage is too severe to repair, apoptosis will be initiated. ATM (ataxia telangiectasia mutated) and ATR (ataxia telangiectasia and Rad3-related) are 2 major DNA damage sensor proteins.4-6 Once DNA damage occurs, H2AX, the histone H2 A variant, is phosphorylated on serine residue 139.7 This phosphorylated form is concentrated at DNA damage sites, therefore, it has been used as a marker of DNA damage.
DNA damage in germ cells may induce infertility or lead to propagate genetic abnormalities in embryos.8,9 Especially in females, oocytes are arrested at the GV stage in primordial follicles for months or decades dependent on the species; this renders oocytes susceptible to the environmental DNA damage factors. Moreover the expectation for fertility preservation in women undergoing cancer therapy is increasing. It is important and meaningful to uncover the DDR mechanism in oocytes. Two kinds of DNA damage have recently been studied in fully grown oocytes. Interstrand crosslinks (ICL) in oocytes induced by mitomycin C has been well studied by Jones et al.10 Unexpectedly ICL has no effect on the completion of either meiosis I, meiosis II or parthenogenetic activation; however, it does inhibit parthenogenetic embryo development. DNA DSBs induced by neocarzinostatin fragments DNA and blocks meiosis I, but the molecular mechanism of it has not been clarified in this study.10 Another study has focused on the effect of DNA DSBs on meiotically competent oocytes.11 It was found that DNA DSBs induced by etoposide, a topoisomerase II inhibitor, does not prevent entry into the M phase unless the damage level is severe.11 Severe DNA DSBs activates an ATM/Chk1-dependent DNA damage checkpoint to block the cell cycle by increasing the inhibition of phosphorylation of Cdc25B but not Cdc25 A degradation. But compared to blastocysts, oocytes have a limited ability to activate ATM. ATM activation remains at a base level in oocytes before exposure to the maximal concentration of etoposide.3,11 Taken together, severe DNA DSBs but not ICL affect the process of oocyte maturation in vitro. This is confirmed by 2 other studies,12,13 but the molecular mechanisms, particularly the DDR mechanisms after germinal vesicle breakdown (GVBD), remain to be elucidated.
Zeocin is a member of the bleomycin family of antibiotics. It is water-soluble and membrane-permeable. When Zeocin enters cells, it is activated and will intercalate into DNA and cleave it directly. In this study, we carefully examined the ability of the oocytes with severe DNA DSBs induced by zeocin to progress to GVBD and extrude the PB1. Spindle assembly, chromosome alignment and fragmentation, kinetochore-microtubule attachment and spindle assembly checkpoint protein (Bub3) localization were also examined at metaphase I (MI) or at the first metaphase-anaphase transition (MI-AI). These results indicated an effect of severe DNA DSBs on the spindle assembly checkpoint (SAC) in meiosis I in vitro. Furthermore, we analyzed the effect of DNA DSBs on oocyte maturation in vivo by intraperitoneal injection of zeocin in mice.
Results
Oocytes with severe DNA DSBs caused by zeocin progressed beyond prophase
To induce severe DNA DSBs, GV oocytes were treated with zeocin (200 μg/ml for 1 h), a member of the bleomycin family, which can bind DNA and cleave it directly. Zeocin caused severe DNA DSBs in oocytes at this concentration as evidenced by the strong signal of phosphorylated H2AX at Ser 139 (Fig. 1A). This signal continued after GVBD; moreover, severe DNA DSBs caused chromosome fragmentation in oocytes beyond GVBD (Fig. 1B). According to the result of a previous study,11 fully grown oocytes could enter the M phase in the presence of severe DNA damage, although the GVBD rate was reduced and delayed. In our study, the percentage of GVBD increased beyond 2 h of IVM, and this number reached 65.0% at 5 h of IVM (Fig. 1C), which was equal to the effect of 10 μg/ml etoposide treatment for 3 h in the previous study.11 This indicated that we successfully induced severe DNA DSBs in oocytes. The molecular mechanisms underlying the fact that oocytes appeared not to launch a powerful DNA damage checkpoint to block cell cycle had been well studied,11 so we set out to investigate the oocyte maturation progress during later times.
Figure 1.
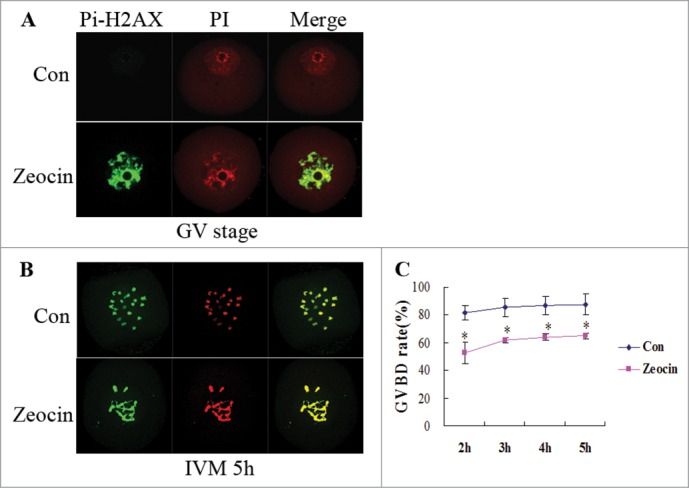
Germinal vesicle breakdown (GVBD) in severe DNA DSBs oocytes induced by zeocin. (A) Zeocin induced severe DNA DSBs at a high concentration. Fully grown GV oocytes were treated with 200 μg/ml zeocin for 1 h in M2 medium supplemented with 2.5 μM milrinone, while oocytes blocked for 1 h by milrinone were used as control. Oocytes were immunostained with anti-Pi-H2AX antibody immediately after 1 h treatment with or without zeocin (A), or after additional 5 h of IVM in M2 medium (B). (C) The GVBD rates were analyzed in the 2 groups from 2 h of IVM to 5 h of IVM. Asterisks indicate significant differences compared to the control group (P < 0.05).
Oocytes with severe DNA DSBs did not reach the MII stage at 12 h of IVM
Since GVBD of oocytes with severe DNA DSBs was delayed, only the oocytes that underwent GVBD at 2 h of IVM were selected to analyze the time for PB1 extrusion during further development. Normally fully grown GV oocytes release the PB1 and reach the MII stage by 12 h of IVM; however, nearly no oocyte with severe DNA DSBs extruded a PB1 at 12 h of IVM, and the effect of severe DNA DSBs on the PB1 extrusion rate reached a highly significant level (P < 0.01) (Figs. 2A and B). Three replicates were performed for this analysis, and we only observed very limited numbers of oocytes with severe DNA DSBs releasing the PB1 at 12 h of IVM. Moreover, all of the PB1-extruded oocytes in the DSBs group were actually at telophase with misaligned chromosomes, while most of the PB1-extruded oocytes reached the MII stage in the control group (Fig. 2C). Most of the DNA DSBs oocytes (93.6%) did not release a PB1 and they were held at the first metaphase (MI) at 12 h of IVM displaying misaligned chromosomes (Fig. 2C).
Figure 2.
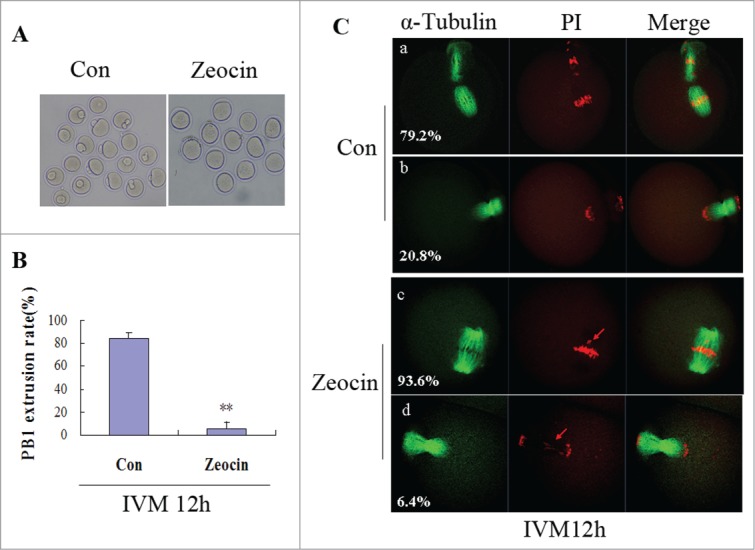
Severe DNA DSBs oocytes did not reach the MII stage at 12 h of IVM. (A) Oocytes with or without zeocin treatment were maturated in M2 medium for 12 h. (B) The percentage of PB1 extrusion in oocytes undergoing GVBD at 2 h of IVM was analyzed at 12 h of IVM. Two asterisks indicate dramatically significant difference compared to the control group (P < 0.01). (C) Spindle assembly and chromosome alignment at 12 h of IVM. Oocytes in the 2 groups were stained with anti-α-tubulin-FITC antibody and PtdIns. The percentages of each type are indicated.
Severe DNA DSBs oocytes extruded a PB1 after an extended time in culture
To investigate if severe DNA DSBs oocytes could extrude a PB1, we prolonged the maturation time. The result showed that severe DNA DSBs oocytes released a PB1 from 14 h to 16 h of IVM; and the PB1 extrusion rate reached 57.2% at 18 h of IVM (Fig. 3A). Oocytes with PB1 reached the MII stage at this time while oocytes without a PB1 were still at the MI stage displaying chromosome misalignment Fig. 3B). Our chromosome spread result showed chromosome fragments in MII oocytes with severe DNA DSBs (Fig. 3C). So the PB1 extrusion was delayed and reduced in severe DNA DSBs oocytes.
Figure 3.
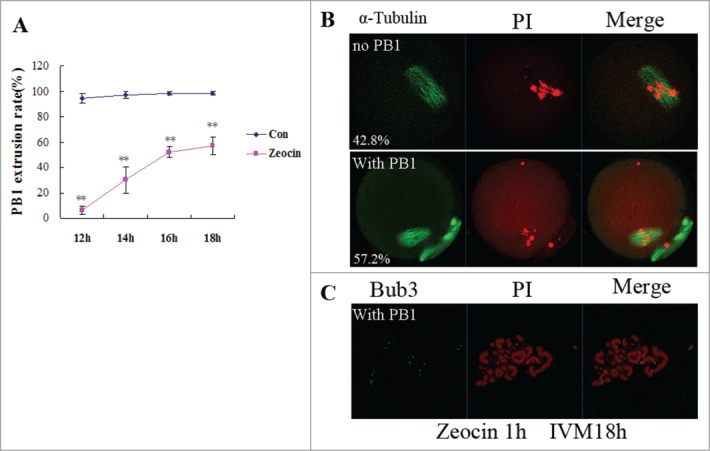
In vitro maturation of severe DNA DSBs oocytes was delayed. (A) PB1 extrusion rates were analyzed from 12 h of IVM to 18 h of IVM. Two asterisks indicate dramatically significant differences compared to the control group (P < 0.01). (B) Spindle assembly and chromosome alignment at 18 h of IVM in zeocin-treated oocytes. Oocytes were stained with anti-α-tubulin-FITC antibody and PI. The percentages of each type were indicated. (C) Chromosome spread of MII oocyte at 18 h of IVM in the DNA DSBs group. Chromosome fragments were observed after staining with anti-Bub3 antibody and PtdIns.
Severe DNA DSBs activated SAC in meiosis I
Having found that PB1 extrusion was delayed and reduced in severe DNA DSBs oocytes, we next sought to define the metaphase-anaphase transition during meiosis I. First we checked spindle assembly and chromosome alignment at MI. All the DNA DSBs oocytes displayed chromosome fragments which were misaligned at 8 h of IVM (Fig. 4A). Then we tested the kinetochore-microtubule attachment by cold treatment of oocytes. In the control group, kinetochore microtubules were cold-stable, whereas in the zeocin-treated group kinetochore microtubules degraded and few kinetochore microtubules were observed in the oocytes (Fig. 4B). This result implied severe DNA DSBs disrupted kinetochore-microtubule attachment during meiosis I. Taken together; we speculated the SAC should be activated. To further test our speculation, we determined Bub3 localization, a SAC protein, at 9.5 h of IVM. As expected, Bub3 still localized at the kinetochores of the chromosome fragments in severe DNA DSBs oocytes, while very weak signal for Bub3 was detected in the control group (Fig. 4C). This result demonstrated that SAC inactivation was inhibited or delayed by severe DNA DSBs.
Figure 4.
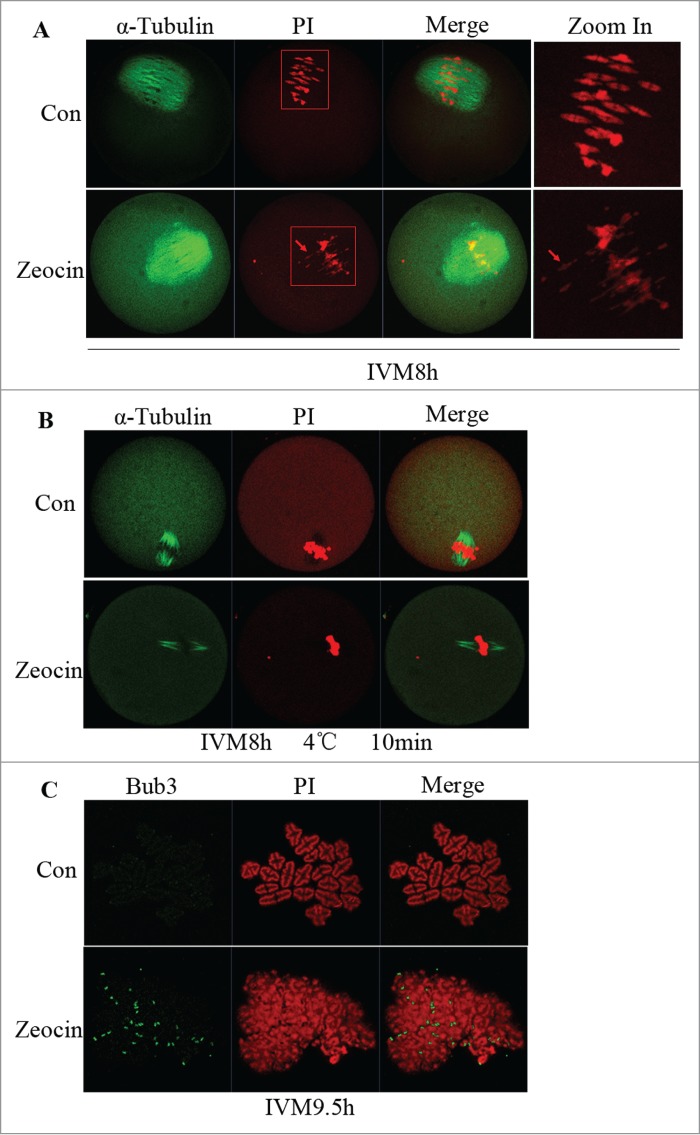
SAC activation and spindle microtubule-kinetochore attachment dsiruption by severe DNA DSBs. (A) Spindle assembly and chromosome alignment at the MI stage. Oocytes in the 2 groups were collected at 8 h of IVM, and then stained with anti-α-tubulin-FITC antibody and PI. Chromosome fragments ware misaligned in DNA DSBs oocytes at this stage (indicated by arrows). (B) Kinetochore-microtubule attachment check at the MI stage. Oocytes with or without zeocin treatment were cultured for 8 h in M2 medium followed by transfer into M2 medium pre-cooled to 4°C and incubated in a refrigerator at 4 °C for 10 min. Finally oocytes were stained with α-tubulin-FITC antibody and PtdIns. Kinetochore microtubules degraded in DNA DSBs oocytes. (C) SAC protein localization analysis at MI-AI stage. Oocytes at 9.5 h of IVM were harvested and chromosome spread was performed followed by immunostaining with anti-Bub3 antibody and PI. SAC was activated in DNA DSBs oocytes.
DNA DSBs induced oocyte apoptosis in vivo
Due to the fact that meiosis I was delayed by severe DNA DSBs in vitro, we wanted to know the effect of DNA DSBs on meiosis in vivo. Zeocin was used to induce DNA DSBs in vivo by intraperitoneal injection. Forty micrograms of zeocin was injected per mouse once every day, the amount of zeocin was determined by the bleomycin dosage used in human cancer therapy adjusted to body weight; then superovulation was performed. Meiosis I was not delayed in vivo, oocytes from the zeocin injection group reached the MII stage at 14 h of hCG injection, similar to the control group (Figs. 5A and C). But more fragmented oocytes were ovulated in the zeocin-injected mice (Fig. 5A). These oocytes were shown to be apoptotic eggs because of high caspase 3 activity indicated by cleaved caspase 3 immunostaining (Fig. 5B). Statistical analysis from 10 mice showed that the total number of ovulation per mouse was significantly reduced, and the rate of apoptotic oocytes was dramatically increased in the zeocin-injected group (Fig. 5D). These data suggest that oocytes with DNA DSBs undergo apoptosis in vivo.
Figure 5.
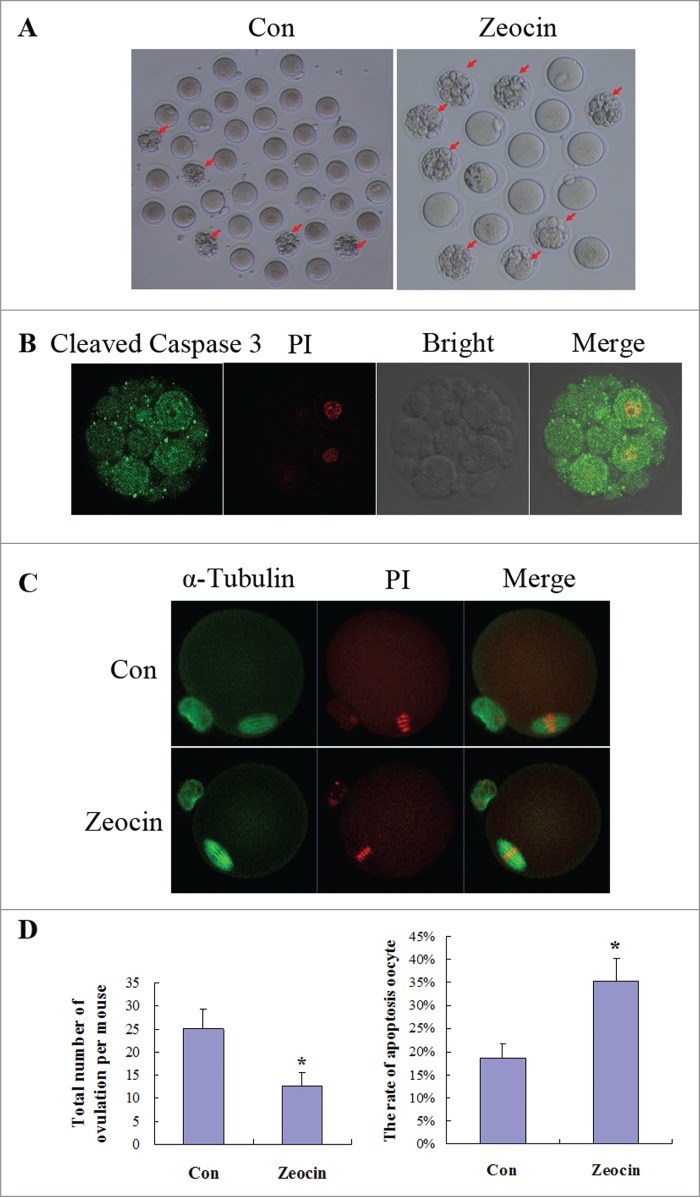
DNA DSBs induced oocyte apoptosis in vivo. (A) Superovulated oocytes in saline or zeocin treated mouse. Ten mice in each group were injected with 0.1 ml saline or 40 μg zeocin in 0.1 ml saline per day for 1 week. Then superovulation was performed. More fragmental oocytes (indicated by arrows) were observed in zeocin-treated mice. Representative oocytes ovulated from one mouse are displayed in (A). (B) Fragmented oocytes showed strong apoptosis signal. Fragmented oocytes were stained with anti-cleaved caspase 3 antibody and PtdIns. (C) Spindle assembly and chromosome alignment of superovulated non-fragmented oocytes. Superovulated non-fragmented oocytes were stained with anti-α-tubulin-FITC antibody and PI. Zeocin-treated mice appeared to ovulate MII oocytes with normal spindle assembly and chromosome alignment. (D) Analysis of total oocyte number of superovulation per mouse and apoptotic oocyte rate. Total number of ovulation per mouse was significantly reduced, and the rate of apoptotic oocytes was dramatically increased in the zeocin injection group. Asterisks indicate significant differences compared to the control group (P < 0.05).
Discussion
Oocytes display a unique cell cycle progression that differs from both somatic cells and male germ cells. Two arrests (prophase arrest in meiosis I and MII arrest) exist during oocyte growth and maturation. Prophase (GV stage) arrest resembles the DNA damage checkpoint at the G2/M transition in somatic cells. High levels of cAMP and cGMP and subsequent sustained activation of PKA are involved in the regulation at this stage.14,15 Different from the physiological arrest in oocytes, DNA DSBs also can induce cell cycle arrest at the G2 stage through activating the DNA damage checkpoint3,16,17 and this is very strict in somatic cells. Surprisingly, fully grown oocytes, which have the responsibility to propagate their genomes to the next generation, have a greatly reduced ability to establish the DNA damage checkpoint, so have the early preimplantation embryos exposed to γ-irradiation (but not X-irradiation).18 Laser microbeam-induced DNA damage in pronucleus or individual blastomere at different stages show poor developmental capability and cleavage cease, failure of incorporate into compacted morulae followed by apoptosis at the blastocyst stage respectively.19 Oocytes entered the M phase with severe DNA DSBs un-repaired in our study, consistent with a previous study.11 This can be partially explained by the reduced ability to activate ATM and the absent degradation of Cdc25 A through DDR in oocytes.11 A mechanism similar to checkpoint adaptation in DNA replication also may be involved,11,20 but the exact adaptation molecule, like Claspin in the DNA replication checkpoint,20 needs to be clarified in the DNA damage checkpoint. There is another mechanism by which the cell cycle checkpoint is abrogated in cancer cells.21,22 Checkpoint kinase 1 (Chk1) down-regulation abrogates doxorubicin-induced G2 arrest followed by mitotic catastrophe and apoptosis.23 Which one works in oocytes still needs to be determined. Our data also indicated that DNA damage was not repaired during the M phase in meiosis I, because oocytes at the MII stage still display chromosome fragments. This result may give a negative answer to the possibility that oocytes are capable of repairing DNA damage during the lengthy meiotic M phase, prior to embryonic development. Thus, the possibility of the alternative way to deal with severe DNA DSBs in oocytes is strong which is degeneration accompanied by follicular atresia instead of repair.
Compared to DDR at the GVBD stage, knowledge about the meiotic M phase DDR is limited.3 Interestingly, ICL induced by mitomycin C does not affect the first and second meiotic division,10 while DSBs induced by neocarzinostatin,10 etoposide or bleomycin13 reduced PB1 extrusion (PBE). It appears that whether or not to activate DDR at the meiotic M phase depends on the type of damage. But another report found that PBE is not affected in GVBD oocytes with DSBs induced by bleomycin.24 We examined the effect of severe DNA DSBs on oocytes at the first meiotic M phase paying attention to SAC. Similar to its effect on GVBD, severe DNA DSBs delayed and reduced PBE. Chromosomes fragmented and SAC was activated by severe DNA DSBs. Our recent report indicated that degradation of cyclin B1 was delayed in DNA DSBs oocytes24 which also indirectly implied that SAC was activated. These data show that if those chromosome fragments with kinetochores aligned tidily at the equatorial plate, SAC can be inactivated and the PB1 is released. The integrity of chromosomes is not essential for SAC inactivation. The delay of PBE may be due to the fact that oocytes with severe DNA DSBs must spend a longer time to align chromosome fragments with kinetochores. Cells may eventually exit the mitotic SAC arrest by a process termed mitotic adaptation during prolonged mitotic SAC arrest.25 SAC in meiosis I was robust at least at 18 h of IVM since 42.8% of the oocytes were still at MI.
Radiotherapy and chemotherapy is common in cancer treatment, both treatments can induce DSBs in germ cells. The side effects of these treatments on reproduction are well characterized,8,26 but women who suffer from cancer expect to preserve fertility after treatment. The mechanisms of germ cells responding to DNA damage should be elucidated. Doxorubicin can cause severe DSBs in oocytes and granulosa cells associated with apoptotic oocyte death and ATM activation.26 Another study found that a significant delay of MI is not observed after injection of etoposide into female mice.12 Consistent with these findings, in our result meiosis I was not delayed and oocytes underwent apoptosis after zeocin treatment in mice. These data indicate that chemotherapy is harmful to female reproduction, and induces acceleration of ovarian aging. New avenues will need to be explored to solve this problem in cancer therapy.
Different responses to DNA DSBs in vitro and in vivo were observed in oocytes in our study. Oocytes with severe DNA DSBs still maintained viability; moreover, they could undergo GVBD and complete meiosis I reaching the MII stage in vitro. However, oocyte apoptosis was induced by DNA DSBs in vivo. Why don't oocytes suffering severe DNA damage in vitro quickly initiate apoptosis? Which step is blocked in the apoptosis pathway? What molecules are involved? More research needs to be undertaken to answer these questions. A possible explanation is that the apoptosis pathway is blocked in the oocyte alone in vitro, and it can be activated in vivo by interacting with granulosa cells. Studies show that genotoxic stress activates Tap63-dependent oocyte apoptosis,2,9 which is a transcription factor related to p53. But it is found to be expressed in primordial, primary and early pre-antral follicles, and it is completely lost in the more mature follicles.3 This may partially explain why fully grown oocytes do not undergo apoptosis in vitro. But why p53 does not work at this stage, and which molecules promote apoptosis in fully grown oocytes in vivo is not known.
Materials and Methods
ICR mice care and manipulations were handled according to the Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences.
Oocyte collection and in vitro maturation
Fully grown GV oocytes were isolated from 6–8 week-old female mouse ovaries in M2 medium containing 2.5 μM milrinone to arrest them at the GV stage. Following specific treatments, oocytes were washed 3 times with M2 medium, and then cultured in M2 medium for in vitro maturation, covered with liquid paraffin oil and maintained in a humidified incubator with 5% CO2 at 37°C. The GVBD was observed at 2 h of IVM and PB1 extrusion was analyzed at 12 h and 18 h of IVM, respectively. Only the oocytes undergoing GVBD at 2 h of IVM were selected for analysis during further development.
DNA DSBs induction by zeocin in vitro
To induce severe DNA DSBs, fully grown GV oocytes were treated with zeocin (200 μg/ml, invitrogen) for 1 h in M2 medium supplemented with 2.5 μM milrinone. Oocytes blocked by the same concentration of milrinone for 1 h were used as control. After 1 h of treatment with or without zeocin, oocytes were washed and cultured in fresh M2 medium for maturation to MI (8 h), MI-AI (9.5 h), 12 h, or 18 h.
Immunofluorescent microscopy
Oocytes at the appropriate stages were harvested and fixed in 4% paraformaldehyde in PBS for 30 min, followed by permeabilization in 0.5% Triton-X 100, then blocked in 1% BSA-supplemented PBS for 1 h at room temperature. After these steps, oocytes were incubated with anti-phosphorylated H2AX at Ser 139 (1:200, Bioworld Technology, Beijing), anti-cleaved caspase 3 (1:200, Cell Signaling Technology, Danvers) antibody overnight at 4°C or anti-α-tubulin-FITC antibody for 2 h at room temperature, respectively. Washed 3 times in PBS containing 1% BSA, oocytes were labeled with FITC-conjugated IgG (1:200) for 1 h at room temperature (for α-tubulin staining, this step was omitted). After washing 3 times, propidium iodide (PI, 10 μg/ml) was used to label the nucleus. Finally, oocytes were examined with a confocal laser scanning microscope (Zeiss LSM 710 META).
Cold treatment of oocytes
Oocytes with or without zeocin treatment were first cultured for 8 h in M2 medium in a humidified incubator with 5% CO2 at 37°C, then they were transferred into M2 medium pre-cooled to 4°C and incubated in a refrigerator at 4°C for 10 min. Finally these oocytes were collected and stained for immunofluorescent analysis with α-tubulin-FITC antibody and PtdIns.
Chromosome spread
Oocytes at the first metaphase-anaphase transition (IVM 9.5 h) or MII stage were collected and exposed to acid Tyrode's solution (Sigma, St. Louis) to remove the zona pellucida (ZP) at room temperature. Once the ZP had disappeared, oocytes were transferred and washed in M2 medium. Then oocytes were transferred continuously onto glass slides one by one and fixed in 1% paraformaldehyde in distilled H2O containing 0.15% Triton X-100 and 3 mM dithiothreitol (pH 9.2). The slides were dried, then blocked with 1% BSA in PBS for 1 h at room temperature and incubated with anti-Bub3 antibody (1:50, Santa Cruz Biotechnology, Dallas) overnight at 4°C. After washing, slides were incubated with FITC-conjugated IgG (1:200) for 1 h followed by stained with PI. Slides were mounted and observed with a confocal microscope.
DNA DSBs induction in vivo
To induce DNA DSBs in vivo, zeocin was injected into the abdominal cavity of female ICR mice, and physiological saline was injected as control. Zeocin (100 mg/ml) was diluted in physiological saline to give a final concentration of 400 μg/ml, and 0.1 ml (40 μg zeocin) was injected per mouse once every day for 1 week. The same amount of normal saline was injected as control and 10 mice were injected in each group. After 1 week of injection, female mice were superovulated by administration of 5 IU PMSG and 48 h later 5 IU hCG. Oocytes were collected from ampullae of the oviduct at 14 h of hCG injection.
Statistical analysis
For each experiment, at least 3 replicates were performed. The statistical analysis was conducted by independent-samples t-test using SPSS software and P < 0.05 is considered significant. Data were expressed as mean ± SEM.
Funding Statement
This work was supported by Major Basic Research Program of China (No 2012CB944404, 2011CB944501) and the National Natural Science Foundation of China (No.30930065) to Qing-Yuan Sun.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Hua Qin and Li-Juan Wang for their assistance with confocal laser microscopy. We also thank the other members in Dr Sun's lab for their kind and fruitful discussions.
References
- 1. De Bont R, van Larebeke N. Endogenous DNA damage in humans: a review of quantitative data. Mutagenesis 2004; 19:169-85; PMID: 15123782; http://dx.doi.org/ 10.1093/mutage/geh025 [DOI] [PubMed] [Google Scholar]
- 2. Kerr JB, Hutt KJ, Michalak EM, Cook M, Vandenberg CJ, Liew SH, Bouillet P, Mills A, Scott CL, Findlay JK, et al. . DNA damage-induced primordial follicle oocyte apoptosis and loss of fertility require TAp63-mediated induction of Puma and Noxa. Mol Cell 2012; 48:343-52; PMID: 23000175; http://dx.doi.org/ 10.1016/j.molcel.2012.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carroll J, Marangos P. The DNA damage response in mammalian oocytes. Front Genet 2013; 4:117; PMID: 23805152; http://dx.doi.org/11248557 10.3389/fgene.2013.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiloh Y. ATM and ATR: networking cellular responses to DNA damage. Curr Opin Genet Dev 2001; 11:71-7; PMID: 11163154; http://dx.doi.org/11248557 10.1016/S0959-437X(00)00159-3 [DOI] [PubMed] [Google Scholar]
- 5. Durocher D, Jackson SP. DNA-PK, ATM and ATR as sensors of DNA damage: variations on a theme? Curr Opin Cell Biol 2001; 13:225-31; PMID: 11248557; http://dx.doi.org/ 10.1016/S0955-0674(00)00201-5 [DOI] [PubMed] [Google Scholar]
- 6. Bakkenist CJ, Kastan MB. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003; 421:499-506; PMID: 12556884; http://dx.doi.org/ 10.1038/nature01368 [DOI] [PubMed] [Google Scholar]
- 7. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273:5858-68; PMID: 9488723; http://dx.doi.org/ 10.1074/jbc.273.10.5858 [DOI] [PubMed] [Google Scholar]
- 8. Jacquet P. Sensitivity of germ cells and embryos to ionizing radiation. J Biol Regul Homeost Agents 2004; 18:106-14; PMID: 15471212 [PubMed] [Google Scholar]
- 9. Suh EK, Yang A, Kettenbach A, Bamberger C, Michaelis AH, Zhu Z, Elvin JA, Bronson RT, Crum CP, McKeon F. p63 protects the female germ line during meiotic arrest. Nature 2006; 444:624-8; PMID: 17122775; http://dx.doi.org/ 10.1038/nature05337 [DOI] [PubMed] [Google Scholar]
- 10. Yuen WS, Merriman JA, O'Bryan MK, Jones KT. DNA double strand breaks but not interstrand crosslinks prevent progress through meiosis in fully grown mouse oocytes. PLoS One 2012; 7:e43875; PMID: 22928046; http://dx.doi.org/ 10.1371/journal.pone.0043875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marangos P, Carroll J. Oocytes progress beyond prophase in the presence of DNA damage. Curr Biol 2012; 22:989-94; PMID: 22578416; http://dx.doi.org/ 10.1016/j.cub.2012.03.063 [DOI] [PubMed] [Google Scholar]
- 12. Mailhes JB, Marchetti F, Phillips GL, Jr., Barnhill DR. Preferential pericentric lesions and aneuploidy induced in mouse oocytes by the topoisomerase II inhibitor etoposide. Teratog Carcinog Mutagen 1994; 14:39-51; PMID: 7910418; http://dx.doi.org/ 10.1002/tcm.1770140106 [DOI] [PubMed] [Google Scholar]
- 13. Li XM, Yu C, Wang ZW, Zhang YL, Liu XM, Zhou D, Sun QY, Fan HY. DNA topoisomerase II is dispensable for oocyte meiotic resumption but is essential for meiotic chromosome condensation and separation in mice. Biol Reprod 2013; 89:118; PMID: 24048577; http://dx.doi.org/ 10.1095/biolreprod.113.110692 [DOI] [PubMed] [Google Scholar]
- 14. Conti M, Hsieh M, Zamah AM, Oh JS. Novel signaling mechanisms in the ovary during oocyte maturation and ovulation. Mol Cell Endocrinol 2012; 356:65-73; PMID: 22101318; http://dx.doi.org/ 10.1016/j.mce.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stanford JS, Lieberman SL, Wong VL, Ruderman JV. Regulation of the G2/M transition in oocytes of xenopus tropicalis. Dev Biol 2003; 260:438-48; PMID: 12921744; http://dx.doi.org/ 10.1016/S0012-1606(03)00259-8 [DOI] [PubMed] [Google Scholar]
- 16. Cahill D, Connor B, Carney JP. Mechanisms of eukaryotic DNA double strand break repair. Front Biosci 2006; 11:1958-76; PMID: 16368571; http://dx.doi.org/ 10.2741/1938 [DOI] [PubMed] [Google Scholar]
- 17. Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem 2004; 73:39-85; PMID: 15189136; http://dx.doi.org/ 10.1146/annurev.biochem.73.011303.073723 [DOI] [PubMed] [Google Scholar]
- 18. Yukawa M, Oda S, Mitani H, Nagata M, Aoki F. Deficiency in the response to DNA double-strand breaks in mouse early preimplantation embryos. Biochem Biophys Res Commun 2007; 358:578-84; PMID: 17498656; http://dx.doi.org/ 10.1016/j.bbrc.2007.04.162 [DOI] [PubMed] [Google Scholar]
- 19. Wang ZW, Ma XS, Ma JY, Luo YB, Lin F, Wang ZB, Fan HY, Schatten H, Sun QY. Laser microbeam-induced DNA damage inhibits cell division in fertilized eggs and early embryos. Cell Cycle 2013; 12:3336-44; PMID: 24036543; http://dx.doi.org/ 10.4161/cc.26327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yoo HY, Kumagai A, Shevchenko A, Dunphy WG. Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell 2004; 117:575-88; PMID: 15163406; http://dx.doi.org/ 10.1016/S0092-8674(04)00417-9 [DOI] [PubMed] [Google Scholar]
- 21. Hashimoto H, Sudo T, Mikami Y, Otani M, Takano M, Tsuda H, Itamochi H, Katabuchi H, Ito M, Nishimura R. Germ cell specific protein VASA is over-expressed in epithelial ovarian cancer and disrupts DNA damage-induced G2 checkpoint. Gynecol Oncol 2008; 111:312-9; PMID: 18805576; http://dx.doi.org/ 10.1016/j.ygyno.2008.08.014 [DOI] [PubMed] [Google Scholar]
- 22. Suganuma M, Kawabe T, Hori H, Funabiki T, Okamoto T. Sensitization of cancer cells to DNA damage-induced cell death by specific cell cycle G2 checkpoint abrogation. Cancer Res 1999; 59:5887-91; PMID: 10606229 [PubMed] [Google Scholar]
- 23. Xiao Z, Xue J, Sowin TJ, Zhang H. Differential roles of checkpoint kinase 1, checkpoint kinase 2, and mitogen-activated protein kinase-activated protein kinase 2 in mediating DNA damage-induced cell cycle arrest: implications for cancer therapy. Mol Cancer Ther 2006; 5:1935-43; PMID: 16928813; http://dx.doi.org/ 10.1158/1535-7163.MCT-06-0077 [DOI] [PubMed] [Google Scholar]
- 24. Ma JY, Ou Yang YC, Wang ZW, Wang ZB, Jiang ZZ, Luo SM, Hou Y, Liu ZH, Schatten H, Sun QY. The effects of DNA double-strand breaks on mouse oocyte meiotic maturation. Cell Cycle 2013; 12:1233-41; PMID: 23518501; http://dx.doi.org/ 10.4161/cc.24311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Souza CP, Osmani SA. A new level of spindle assembly checkpoint inactivation that functions without mitotic spindles. Cell Cycle 2011; 10:3805-6; PMID: 22067564; http://dx.doi.org/ 10.4161/cc.10.22.18187 [DOI] [PubMed] [Google Scholar]
- 26. Soleimani R, Heytens E, Darzynkiewicz Z, Oktay K. Mechanisms of chemotherapy-induced human ovarian aging: double strand DNA breaks and microvascular compromise. Aging (Albany NY) 2011; 3:782-93; PMID: 21869459 [DOI] [PMC free article] [PubMed] [Google Scholar]


