Abstract
T-cell intracellular antigen 1 (TIA1) and TIA1-related/like protein (TIAR/TIAL1) are 2 proteins discovered in 1991 as components of cytotoxic T lymphocyte granules. They act in the nucleus as regulators of transcription and pre-mRNA splicing. In the cytoplasm, TIA1 and TIAR regulate and/or modulate the location, stability and/or translation of mRNAs. As knowledge of the different genes regulated by these proteins and the cellular/biological programs in which they are involved increases, it is evident that these antigens are key players in human physiology and pathology. This review will discuss the latest developments in the field, with physiopathological relevance, that point to novel roles for these regulators in the molecular and cell biology of higher eukaryotes.
Keywords: cell death, embryogenesis, gene expression control, proliferation, TIA1, TIAR, tumorigenesis, Welander
From Gene to Protein
TIA1 and TIAR possess a modular design characteristic of the classical view of RNA-binding protein (RBP) architecture.1,2 This structure consists of 3 RNA recognition motifs (RRM) of around 100 amino acids each, and a domain that is rich in glutamine and asparagine, of around 90 amino acids located at the C-terminal region (Q-rich domain). Two short peptide domains formed by an amino acid hexamer and octamer, named RNP2 and RNP1, respectively, are conserved in the RRM regions1,2 (Fig. 1).
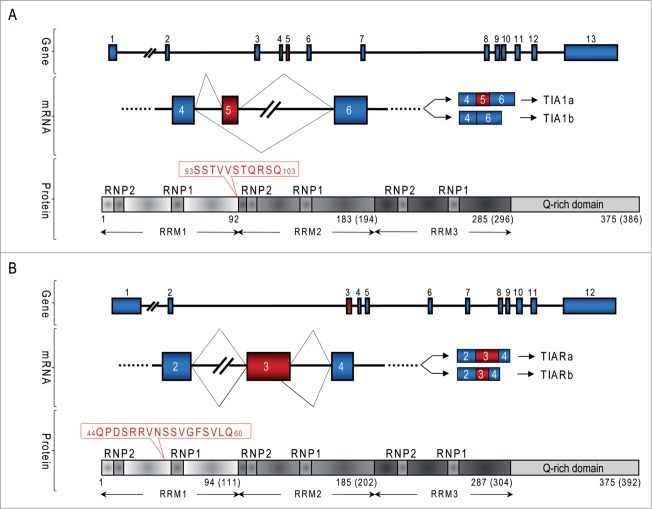
Figure 1.
Diagrams of genes and proteins of TIA1 and TIAR in human and mouse. (A and B) Organization of exons and introns of TIA1 and TIAR genes, the major isoforms a and b generated by alternative pre-mRNA splicing, and functional domains of TIA1 (A) and TIAR (B) proteins. Both proteins contain 3 RNA-recognition motives (RRM) and an auxiliary domain in rich-asparagine and glutamine residues (Q-rich domain). The different amino acid sequences between the isoforms are shown in red for TIA1 and TIAR proteins, respectively.
The human TIA1 gene is located in the chromosomal region 2p13 and contains 13 exons. Exons 1–4, 5–8 and 9–11 encode the RRM1, 2 and 3 domains, respectively. Exons 12 and 13 encode the Q-rich domain (Fig. 1A). The human TIAR gene locates in the chromosomal region 10q and consists of 12 exons. Exons 1–4, 5–7 and 8–10 encode the RRM1, 2 and 3 domains, respectively. Exons 11 and 12 encode the carboxyl-terminal ‘helper’ domain (Fig. 1B).3 Two isoforms of both TIA1 and TIAR exist, generated by the alternative splicing of the pre-mRNAs. The TIA1a isoform (43 kDa) differs from isoform TIA1b (40 kDa) by inclusion of an 11 amino acid residue sequence at the beginning of the RRM2 motif, encoded by exon 5 (Fig. 1A).1,2 Also, isoform TIARa (50 kDa) differs from isoform TIARb (42 kDa) in that it contains a sequence of 17 amino acids between the RNP2 and RNP1 motifs in RRM1, encoded by the last 51 nucleotides of exon 3 (Fig. 1B).3,4 Particular residues contained in isoforms TIA1a and TIARa determine the specificity of their binding to RNA and/or the interaction with other proteins, which expands their regulatory capacity.6 In mice, the TIA1 and TIAR genes are located in the chromosome regions 6D2 and 7F4, respectively. The exon-intron organization is conserved between the murine and human genes, and there is also a high degree of identity between the primary amino acid sequences.4,7
TIA1 and TIAR share 80% identity in their amino acid sequence.2,4 The presence of structural and functional orthologs in different eukaryotic taxonomic groups highlights the biological importance of these proteins in cells, given that they are highly conserved since the first ancestors.8 In fact, the RRM2 domain is highly conserved in all of the reported structural and/or functional orthologs and is the major domain responsible for protein-RNA/DNA binding, which implies a high degree of functional conservation in the mechanism of action of these proteins.9-14 The RRM is a very well characterized domain and a significant amount of structural information exists. Indeed, the structural topology and functional contribution of each of the 3 RRM domains in both TIA1 and TIAR proteins has been the subject of investigations by several laboratories.9-14 Consequently, a detailed text summarizing all important aspects has been recently published by Wilce and coworkers.15 However, no high resolution structure of TIA protein RRM domain in complex with oligonucleotide has yet been reported. Until such data is available, the precise structural basis for the RNA binding specificity of TIA proteins will remain elusive.15
Regulators and/or Modulators of Gene Expression
Gene expression in eukaryotic cells is a vectorial process that encompasses DNA-dependent transcription and post-transcriptional processes such as pre-mRNA processing/splicing, transport, location, stability and/or translation of mature mRNAs. Transcriptional and post-transcriptional regulatory mechanisms occur to orchestrate cellular programs and decisions via modulating the function, half-life and fate of the RNAs and/or proteins inside the cell in a developmental, spatio-temporal and/or environmental-dependent manner. For example, a comprehensive study on the transcriptome of HeLa cells using microarray approaches, where expression of TIA1, TIAR, or both was transiently knocked down, permitted the identification of a large number of mRNAs associated with the processes of inflammation, cellular signaling, immune response, angiogenesis, apoptosis, metabolism and cell proliferation.16 Similar results have been obtained recently in the study of the transcriptome of neural tissues –spinal cord and cerebellum- from an adult mouse lacking TIA1.17 Thus, given their interaction properties with DNA, RNA and other proteins in the cell, TIA proteins, participate in the regulation and/or modulation of many of these processes and networks, via impacting prevalently the pleiotropic roles of specific RNAs and proteins in cell physiology, defining their fates into ribonucleoprotein complexes such as speckles, paraspeckles, messenger ribonucleo/cytoplasmic RNA-protein complexes, processing bodies and/or stress granules (Fig. 2).9-15
Figure 2.
The multifunctional regulatory roles of TIA proteins in the gene expression layers and physio(patho)logical-associated events.
Transcription
The first evidence for the involvement of TIA proteins in transcriptional regulation came from the functional capacity of TIA proteins to bind DNA and the carboxyl-terminal domain of RNA polymerase II.18-21 In the case of TIA1, it has been shown that RRM1 binds to T-rich ssDNA.19 Further, RRM1 and RRM2 of TIAR are able to interact with DNA with micromolar affinity21 and T-rich DNA,11,21 respectively. Thus, this represents an unusual case of an RRM preferentially binding DNA over RNA.15 These interactions suggest a putative co-regulation of the transcription and splicing of pre-mRNAs in the cellular nucleus during early biogenesis of RNAs.15 This process might be facilitating a slowdown in the speed of RNA polymerase II and the coupling of the transcription and the final 3′ processing (Fig. 2).18-22 Some genes, such as Procollagen, type II (COL2A1),20 Insulin-like growth factor binding protein-3 (IGFBP-3)23 and Pituitary adenylate cyclase-activating polypeptide (PACAP)24 could be preferentially regulated by this TIA-dependent pathway.
Pre-mRNA splicing
The first 2 identified events of TIA protein-dependent splicing involved the pre-mRNAs of Fibroblast growth factor receptor 2 (FGFR2)25 and Fas cell death surface death receptor (FAS/CD95/APO-1)26. In both cases, TIA proteins facilitated the recognition between weak 5′ splice-acceptor sites and degenerate sequences for the hybridization of the RNA complementary to U1 snRNP.25-27 In this process, the recognition motif RRM2 in the TIA protein, facilitated by RRM3, attaches to the uridine-rich regions proximal to the intron's 5′ splice site, and the Q-rich domain binds to the N-terminal region of the subunit U1-C in U1 snRNP through a process facilitated by RRM1.11,15,27-29 There are also some studies that point to the participation of TIA proteins in the recruitment of U6 snRNP.30,31 The cellular relevance of TIA proteins in the regulation of constitutive and alternative splicing was confirmed by mapping the binding of these regulators to RNA using an experimental approach in HeLa cells consisting of in vivo irradiation of the cells with UV light and subsequent immunoprecipitation of the RNA-protein complexes.32 These approaches demonstrated that TIA1 and TIAR preferentially bind to sequences rich in uridine and adenosine pentamers, UUUUA or AUUUU, proximal to the 5′ splice-acceptor sites in the introns, to facilitate the recruitment of U1 snRNP and the inclusion of the adjacent exon, enabling also the selection of distant 3′ splice-acceptor sites. Thus, they facilitate splicing of pre-mRNAs via improving the selection of constitutive and atypical 5′ splice sites through shortening the time available for definition of an exon by enhancing recognition of the 5′ splice sites.32 From these studies, it has been estimated that TIA proteins regulate 10% of the constitutive and alternative splicing events in the human genome, whereas ∼20% was initially estimated by an in silico approach.33
Translation of mRNAs
TIA antigens have the ability to regulate and/or modulate the process of cellular translation by limiting the availability of the ribosomal machinery and also the translational efficiency of specific cellular mRNAs, either in stress conditions, to guarantee cell viability, or in conditions of cellular homeostasis (Fig. 2). Under conditions of stress, the α subunit of the translation initiation factor Eukaryotic translation initiation factor 2 (eIF2) is phosphorylated, principally at serine 51, by a family of kinases that includes Eukaryotic translation initiation factor 2-α kinase 1 (HRI), Eukaryotic translation initiation factor 2-α kinase 2 (PKR), Eukaryotic translation initiation factor 2-α kinase 3 (PERK) and Eukaryotic translation initiation factor 2-α kinase 4 (GCN2), depending of the types of stresses and cellular lines involved.34-42 This phosphorylation abolishes the ability of eIF2B to exchange GDP for GTP, which results in a decrease in the levels of eIF2-GTP-tRNAMet ternary complex. In turn, this leads to incorrect formation of the translation pre-initiation complexes. It is at this moment when TIA1 and TIAR play a role, associating with the complex formed by several canonical translational initiation factors such as, eIF4E, eIF4G, eIF4A, eIF4B and eIF3 together with the small ribosomal subunit, in an anomalous 48S complex lacking eIF2 and eIF5.34,35 These inactive translation pre-initiation complexes associated with mRNAs accumulate in the cytoplasm and, due to the aggregation properties of the Q domain of TIA and Poly (A+) binding protein (PABP) proteins, bind among themselves (self-aggregate), creating large foci of mRNA and protein known as stress granules (SG).36-37 SG formation favors cell survival in stress conditions, such as starvation or limitations in amino acid availability, oxidative or osmotic stress, etc., as well as pathophysiological situations, as for instance viral infection37 and Alzheimer disease.38 In these adverse conditions, the cell enters a cellular biology resting state, inhibiting translation in general and allowing energy to be saved for repair of the damage caused by the stressful insult.39-42 Although the appearance of SG is that of stable and poorly-dynamics inactive structures, their size is variable and the majority of their components are in a constant exchange or a dynamic flux of assembly/disassembly.35-43 They appear approximately 15 minutes after the onset of the stressing stimulus and their formation is reversible, disappearing a few hours -between 2 to 6- after the stimulus ends depending on cell type, provided that the stimulus is not lethal.34,35,41-46 It is important to note that controversy exists regarding the process of SG formation mediated by TIA1 and/or TIAR. Indeed, some studies suggest that TIAR cannot form SG without the aid of TIA1.45 However, other results show that only one of the 2 proteins is sufficient for SG formation.47 In addition, recent studies suggest that perhaps the role of these proteins/antigens, including TIA proteins, would not be to facilitate SG formation, but rather the disintegration of these transient structures.48 Further, it has been described that TIA1 and other proteins containing Q-rich domains can form porous hydrogel-nature structures,49 which suggest a model of SG organization whereby SG-proteins and RNA are able to diffuse in and become associated with the hydrogel matrix.15 Given these controversies, we are facing a very interesting challenge with significant implications for cell biology, which requires new experimental approaches to be fully understood. A comprehensive analysis of these possibilities awaits further study.
Immunoprecipitation studies on RNA-protein complexes and identification of immunoprecipitated mRNAs using microarray analysis have permitted the identification of approximately 2 hundred mRNAs associated with TIA1 and/or TIAR.44,47,50 These studies revealed the binding of TIA proteins to motifs rich in uridine, adenosine and cytidine in the 5′ and/or 3′-UTRs of several cellular mRNAs. Also, the participation of TIA proteins in the translational regulation of different mRNAs, such as Tumor necrosis factor α (TNFα),51 pro-inflammatory cytokines,52,53 Cyclooxygenase-2 (COX-2),54 mitochondrial cytochrome c,55 C-MYC oncogene,56 Hypoxia inducible factor 1, α subunit (HIF-1α),57 β-actin subunit,58 some isotype of tubulin,59 some mRNAs implicated in the cell-cycle G2/M transition and DNA repair60 as well as tumor suppressors Breast cancer 1 (BRCA1)61 and Programmed cell death 4 (PDCD4),62 have been suggested.
mRNA stability
mRNA turnover is the process by which an mRNA is degraded before or after translation. This can occur at the 5′ or the 3′ end of the mRNA. Degradation from the 5′ end requires the activity of Dipeptidyl carboxypeptidase 1 (DCP1) and Dipeptidyl carboxypeptidase 2 (DCP2) enzymes, which promote the removal of 7-methyl-guanosine (5′ decapping), and the activity of 5′-3′ exoribonuclease 1 (XRN1) exonuclease.63 Degradation from the 3′ end is mediated by the exosome and is produced by the shortening or deadenylation of the poly(A+) tail of the mRNA and the subsequent recruitment of exonucleases.64 The mRNA regions implicated in this process are sequences rich in adenosine and uridine (ARE sequences) situated in the 3′-UTR, which favor the binding of proteins such as TIA, AU-rich element (ARE) RNA-binding protein 1 (AUF1), KH-type splicing regulatory protein (KSRP) and Tristetrapolin (TTP) and, consequently, the recruitment of proteins associated with the degradation process. Binding of TIA proteins to these regions facilitates the deadenylation of the mRNA and also stimulates cap removal at the 5′ end. In contrast, proteins such as HuR stabilize mRNA, likely due to its inability to recruit exosomes.65,66
microRNAs
A further means to regulate mRNA translation and stability is through binding to micro(mi)RNAs, which leads to their translational repression and/or degradation. miRNAs are small RNA fragments, 19–24 nucleotides in length, that regulate gene expression through base-pairing with complementary sequences, usually in the 3′-UTR regions of mRNAs. The interaction of miRNA with mRNA leads to the recruitment of the RNA-induced silencing complex (RISC) and, subsequently, to mRNA degradation. Several studies suggest that around 20–30% of gene expression is regulated by miRNAs.67 A high-throughput study using microarray analysis revealed an overexpression of 29 miRNAs after transient silencing of TIA1 and TIAR protein expression by RNA interference in HeLa cells. This result was interpreted as a response to counteract the differential expression and phenotypes associated with the short-term reduction of TIA proteins.68 Another study showed that miRNA-579 and miRNA-125b interact with TIAR in the 3′-UTR of TNFα mRNA, leading to its degradation and a decrease in its translation.69 Also, in cellular microvesicles that abundantly express TIA1 and TIAR, which constitutes a mechanism for cell communication in stem cells, 365 miRNAs have been identified whose target mRNAs are related to organ development, survival and differentiation, and also with the regulation of immune responses.70 These observations suggest that TIA proteins are able to regulate/modulate miRNA expression by still unknown mechanisms. Finally, it is important to note that miRNA-15a and miRNA-16–1 have been identified as silencers of TIA1 and TIAR expression,71,72 and adenovirus VA RNA-derived miRNAI-138 targets TIA1.73 These findings indicate that the regulator/modulator can be also regulated/modulated and suggest the existence of autoregulatory loops that could serve to amplify/inhibit complex cellular responses.
TIA1 and TIAR: Biological Processes, Embryonic Development and Physiopathology
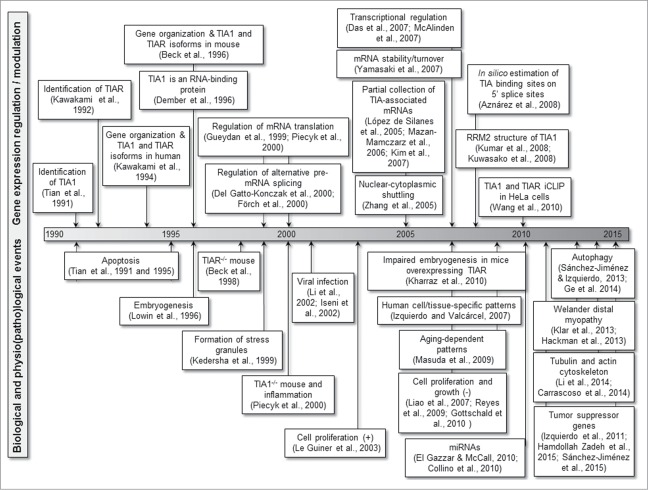
The multifunctional capacity of TIA proteins to regulate/modulate gene expression points to a relevant role for these regulators/modulators in cellular homeostasis, and also in the regulation of several biological and physiopathological processes (Fig. 3).
Figure 3.
Scientific milestones related to the biological processes regulated/modulated by TIA proteins.
Cell death: apoptosis and autophagy
The first experimental evidence for the participation of these proteins in the regulation of the cell death program came from the observation that incubation of (permeabilized) cellular targets of cytotoxic T lymphocytes with TIA1 and TIAR proteins triggered nuclear DNA fragmentation (Fig. 3).1,2 Later, it was demonstrated that TIA proteins regulate the gene expression of several components of the cell death pathways.16,17,24-74-76 However, there are other routes through which TIA proteins could modulate cell death, for example by scavenging of Ribosomal protein S6 kinase, 90 kDa, polypeptide 3 (RSK2) in the SG, facilitating cell survival,77 or activating/repressing the synthesis of other proteins involved in the processes of cellular death/survival.16,17 Recently, we have reported that an increased expression of TIA1 or TIAR in HEK293 cells results in diminished rates of cell proliferation and growth. This is accompanied by cell-cycle arrest at G1/S and cell death through caspase-dependent apoptosis and autophagy. Genome-wide profiling analysis suggest a specific upregulation of p53 signaling pathway-related genes.78 In the same vein, a role for TIA1 in autophagy via interaction with annexin A7 has been described in vascular endothelial cells.79-81
Cell proliferation
TIA1 and TIAR proteins modulate cell proliferation by favoring or inhibiting cell growth. For example, it has been described that decreasing the expression of TIA proteins in the chicken cell line DT40 -lymphoma- promotes a decline in their growth relative to control cells (Fig. 3).82 However, in human tumor cell lines, such as HeLa cells, short- or long-term reduction in TIA1 or TIAR expression, or both, leads to an increase in cell proliferation associated with a tumorigenic phenotype.16,17,83-85 This behavior has also been reported in other human cell lines including K562 (myeloma),56 HCT116 (colorectal carcinoma),83 HEK293 (adenotransformed embryonic kidney cells),78 A549 (lung adenocarcinoma)57 and LS174t colon cancer cells.86 The roles of TIA proteins associated with the cell proliferation could be prevalently linked to the coordinated control of global and specific translational rates.47,53-62,78,85 Taken together, these observations are possibly consistent with the relevance of the genetic background for the expression of the TIA proteins, which determines their functionality in an environmental-dependent way.
Embryonic development
The importance of TIA proteins during embryogenesis was addressed in vivo by studying genetically-modified mice deficient in TIA1 and/or TIAR (Fig. 3).51,87,88 Homozygous mice lacking TIA1 and TIAR died before embryonic day 7 (E7). Experiments performed in several mouse strains revealed that, in the absence of TIA1, 46% of the mice died between E16.5 and 3 weeks after birth. The surviving mice did not show any apparent abnormalities until the end of their lifespan (2 years), which was characterized by a phenotype associated with arthritis51 and a higher sensitivity to dust produced by an exacerbated allergic reaction accompanied by pulmonary inflammation and an increase in Th2/Th17 cytokines in the lymph nodes.89 However, mice lacking TIAR presented different phenotypes that were dependent on the mouse strain studied.88 Thus, in BALB/c mice, the absence of TIAR expression was embryonic lethal in 100% of offspring, whereas in C57BL/6 mice, the lack of TIAR led to death in 90% of the embryos. Intercrosses of BALB/c TIAR+/− with C57BL/6 TIAR+/− resulted in 60% embryonic lethality. Of the remaining mice, half of them survived until adulthood, although they were sterile –mice showed abnormalities in the spermatogenesis and oogenesis processes, and also in the gonad architecture-, obese –despite being born with less body mass- and with neurological disorders –abnormal behavior-. These mice also develop cervical tumors.88 Further, an essential role for TIAR has been described in self-renewal and/or differentiation of mouse embryonic stem cells.90 Additionally, in a transgenic mouse model overexpressing TIAR, 77% of the embryos showed abnormalities at day E7.5.91 This myriad of phenotypes suggests that the equilibrium in the expression of TIA1 and/or TIAR proteins is important, spatially and temporally, for early mouse development.51,88,90-92
Physiopathological implications
The ability of TIA proteins to regulate/modulate gene expression confer on them an important functional role in human pathology given their participation in antiviral, inflammatory, immune, and possibly oncogenic and aging-associated responses, among others (Fig. 3).
Viral infections
Several studies suggest a relevant functional role of TIA proteins during viral infections. When viral infection occurs, PKR kinase is activated and phosphorylates eIF2α, inhibiting the translation of cellular and viral proteins by directing the mRNAs to SG, and increasing the expression of proteins involved in the innate immune response to guarantee cell viability.34,35 This is the case in infections caused by Vesicular stomatitis virus (VSV)93 or Transmissible gastroenteritis coronavirus (TGEV).94 Evolution has resulted in many viruses developing mechanisms to evade this response in favor of their own survival.95-97 Indeed, some viruses benefit from TIA proteins to favor their own biology, for example West nile virus (WNV) uses TIA proteins as transcription factors to synthesize its own RNA98,99; Hepatitis C virus (HCV) uses TIA proteins for the replication of the viral genome, assembly and delivery of viral particles100; and Minute virus of mice (MVM) and Human papillomavirus (HPV) use TIA proteins as splicing factors for the synthesis of their own proteins.101,102 Nevertheless, TIA proteins can also act as potent antiviral agents independently of SG formation. Thus, TIA1 can directly bind to the PRE regulatory element in Hepatitis B virus (HBV) and inhibit its function.103 It has been described that in the early stages of hepatitis C infection in chimpanzees, several gene expression changes take place including an increase in TIA1, which allows removal of the virus before chronic infection develops. This constitutes a primary response to eliminate infected hepatocytes.104
Inflammation and immune processes
Regarding the inflammatory processes, TIA1 and TIAR function as gene suppressors in arthritis.105 Consequently, TIA-deficient mice develop arthritis.51 It is important to note that infliximab –a potent anti-inflammatory drug- therapy increases the ratio of TIA1:HuR.106 In fact, TIA proteins can regulate/modulate the expression of inflammatory proteins such as TNFα, IL-1, IL-6, MMP13 or COX-2.5,16,51,52,54,107-109 For example, in women with endometriosis, TIA1 expression in eutopic and ectopic endometrium was reduced compared with TIA1 expression in eutopic endometrium of unaffected control women. Lipopolysaccharide and TNF-α increased TIA1 expression in human endometrial stromal cells (HESCs) in vitro, whereas IL-6 or steroid hormones had no effect. In primary cultured HESCs, down-regulation of TIA-1 resulted in elevated IL-6 and TNF-α expression, whereas TIA-1 overexpression resulted in decreased IL-6 and TNF-α expression. Thus, endometrial TIA1 is regulated throughout the menstrual cycle, TIA1 modulates the expression of immune factors in endometrial cells, and downregulation of TIA1 may contribute to the pathogenesis of endometriosis.109
Regarding cytotoxicity mediated by TIA proteins and, in particular, TIA1 as a component of cytotoxic T lymphocyte granules, there are several reports describing that apoptosis mediated by cytotoxic T-lymphocytes provokes the onset of a series of reactions that are uncomfortable for the patient such as, for example, organ transplant rejection,110,111 food allergy,112 Crohn's disease and ulcerative colitis,113 aplastic anemia114 or platelet inhibition.115 However, an increase in cytotoxic T-lymphocytes is not always negative; it has been demonstrated that a higher percentage of infiltrated CD8+ T cells is associated with better prognosis in cancer patients, suggesting a role for CD8+ T lymphocytes in the anti-tumoral response.116-120
Tumor suppressor genes
The IntOGen-mutations platform (www.intogen.org/mutations) summarizes somatic mutations, genes and pathways involved in tumorigenesis.121 Analysis of this database provides support to link human cancers with somatic mutations in TIA1 and/or TIAR/TIAL1 genes. Accordingly, mutated TIA1 has been found in corpus uteri, kidney, brain, lung, stomach, and breast tumors; while mutated TIAR/TIAL1 has been identified in oropharynx, stomach, liver, lung, breast, corpus uteri, ovary and brain tumors. These cancer mutations associated with TIA1 and TIAR proteins show a heterogeneous distribution across the primary structure of TIA proteins and they are preferentially located on RRMs domains, suggesting a loss-of-function of TIA proteins as DNA/RNA-binding proteins.121 TIA proteins regulate, modulate and/or interact with a large number of mRNAs involved in cell proliferation control, apoptosis, angiogenesis, inflammation, invasiveness and metastasis capacity of tumor cells, and in immune evasion, which suggests a putative role for TIA proteins in preventing tumorigenic processes16,17,25,31,42,51,55-58,60-62,85,86,116-123 (Fig. 3). Thus, TIA proteins can regulate the translation of the C-MYC oncogene32,56 and tumor suppressor gene BRCA1,61 the splicing of FGFR2,25 FAS26,27 or the tumor suppressor genes Neurofibromatosis-1 (NF1)31 and Wilms' tumor suppressor (WT1),122 mRNA stability of tumor suppressor gene PDCD462 or Growth arrest and DNA-damage-inducible protein 45α (GADD45α),124 as well as the expression of inflammatory or angiogenic factors, such as TNFα, COX-2, VEGF, IL-8 or HIF1α 16,51,52,54,57,86 and metastatic factors including MMP13.5 Accordingly, TIA1 and/or TIAR reduction in HeLa cells resulted in an increase in cell proliferation and both anchorage-dependent and independent growth, and also in greater cell migration capacity, facilitating the ability to generate xenotumors in immunocompromised mice. Moreover, studies of TIA1 and/or TIAR expression in a cohort of human epithelial tumors showed a significant reduction in these proteins, pointing to a putative role for TIA1 and/or TIAR as tumor suppressors.57,85,86,123 Remarkably, nude mice injected with doxycycline-inducibe cells expressing TIA1 or TIAR delay, or even abolish, growth of xenotumors. Further, low expressions of TIA1 and TIAR correlate with poor prognosis in patients with lung squamous cell carcinoma.78,123 Collectively, these findings strongly suggest that TIA proteins can act as tumor suppressor genes and cellular gatekeepers.
Welander distal myopathy
The distal myopathies comprise a group of inherited disorders with shared clinical expression involving mainly the functionality of the hands and feet of patients.125,126 To date 20 different distal myopathies have been described and at least 14 of them have a genetic cause.126 Welander Distal Myopathy (WDM; MIM #604454) was one of the first described with such a clinical pathology in the distal myopathies127 group. It is a distal muscular dystrophy, autosomal, dominant and late. This disease manifests itself around 40–50 years.126 A homozygous mutations is associated with a more severe phenotype.
The symptoms usually begin with weakness of the extender of the index fingers, leading to problems in precision movements, progressing to other fingers. This is usually accompanied by weakness in distal areas of the lower extremities, involving the tibialis anterior and the extensor muscles and feet that involve walking difficulties and leads to an equine gait. The pendulum foot movement is also found in patients with sclerosis amyotrophic lateral, multiple sclerosis and Parkinson disease. The disease is more common in the Middle East of Sweden, with a high incidence of 1/100 in local areas, as well as in areas of Finland.129,130 The defect has been associated with a single mutation in WDM supported by a common haplotype in chromosome 2p13 in all the patients of Swedish and Finnish origin.131,132 The haplotype was extended to more than 60 candidate genes, as well as the search for genomic rearrangements, which initially did not result in the identification of the causative mutation. In the first study where a mutation was associated was the genetic analysis of 43 patients of 35 families with clinical and histopathological findings compatible with WDM. In this study the WDM associated 2p13 chromosome haplotype was restricted, and within this region a heterozygote mutation in origin was identified (c.1362G > A; p.E384K) in the gene that encodes TIA1.133 Independently, another study found in a fragment of <806 kb on chromosome 2p13 a single point mutation, G > A (p.E384K) affecting the same gene.134 In the first study, the TIA1 mutation in WDM was associated with alterations in the alternative pre-mRNA splicing of SMN2133 and in the second study with the dynamics of the formation of stress granules.134
The Emerging Picture
In this review, we have addressed how TIA proteins, together with their surrounding regulatory environment regulate/modulate gene expression in eukaryotic cells. We have dissected the multitude of molecular and biological events by which these regulators/modulators contribute to cell physio(patho)logy and discussed how this knowledge can be integrated into cellular decisions, which may represent some therapeutic opportunities. Thus, some progress has been made in studies on the regulatory and functional properties of TIA proteins. Recent technical advances will help to provide reference meta-analyses involving transcriptomes, translatomes, proteomes, ribosomal profiling and/or interactomes for cells, tissues, organisms and individuals, including qualitative and quantitative information about regulatory/modulatory events associated with TIA1 and TIAR proteins and other RNA-binding proteins in different biological situations or pathophysiological conditions. The detailed description of these regulatory phenomena and their cellular and molecular basis will provide important insights in our understanding of many biological processes and networks. For example, the mechanistic nature and molecular events by which components of the p53 pathway and DNA damage response induce cell-cycle arrest and/or cell death during TIA1 or TIAR expression await further exploration. Future studies will elucidate the regulatory characteristics that occur in loss- and gain-of-function models of TIA proteins in genotoxic stresses and under conditions of genomic instability. On the other hand, data from murine models and observations from cancer patients suggest that it may be advantageous for tumors to lose or down-regulate TIA expression, since this reduction could play a role in cancer progression by activating genes involved in neoplastic and malignant transformation, evading the immune system and enhancing the growth and survival of cancer cells. These observations suggest that TIA proteins could be good biomarkers of some cancer types. These proteins could be used to: (i) identify individuals at high risk for metastasis, (ii) differentially diagnose early-late cancer and (iii) assess the efficacy of therapy and chemopreventive agents. Thus, TIA biomarkers may have survival value. However, more studies, for example, cell/tissue specific-conditional TIA1 or TIAR expression, are required to draw firm conclusions about the tumor-suppressive functions of TIA proteins that might be context dependent. Future research should elucidate the specific environmental settings under which TIA proteins act as barrier, i.e. as cellular gatekeepers, to cancer development and/or progression. Indeed, a clearer understanding of the mechanisms controlling cell fate determination by TIA proteins may lead to the identification of novel molecular targets, which could selectively sensitize cancer cells to cell death.
Additionally, many exciting questions remain: How do TIA proteins function as epigenetic regulators/modulators, for example, transcription and/or splicing enhancers, translational repressors and/or stabilizer activators? What are TIA-associated epigenetic and/or post-translational modifications? Can TIA proteins be found that control biomedical relevant regulatory events with sufficient specificity? Will mutated TIA proteins be sufficient to circumvent functional reprogramming linked to cellular transformation, Welander distal myopathy, or aging and other related diseases? What fraction of TIA1 and/or TIAR regulatory events and layers contributes to phenotypic differences relevant for conserved or prevalent changes between cells, tissues and/or species? We are still far from understanding the regulatory message associated with the TIA proteins, but their secrets and potential constantly invites us to try.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to numerous colleagues whose relevant contributions to the gene expression field could not be cited because of the specific focus of this review and space constraints. We wish to thank J Alcalde, JM Sierra and J Valcárcel for encouragement and helping us with different tips.
Funding
Research in the Izquierdo laboratory is supported by the Spanish Ministry Economic Affairs and Competitiveness through FEDER funds (BFU2008–00354, BFU2011–29653 and BFU2014–57735-R). The CBMSO receives an institutional grant from Fundación Ramón Areces.
References
- 1.Tian Q, Streuli M, Saito H, Schlossman SF, Anderson P. A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 1991; 67:629-39; PMID:1934064; http://dx.doi.org/ 10.1016/0092-8674(91)90536-8 [DOI] [PubMed] [Google Scholar]
- 2.Kawakami A, Tian Q, Duan X, Streuli M, Schlossman SF, Anderson P. Identification and functional characterization of a TIA-1-related nucleolysin. Proc Natl Acad Sci 1992; 89:8681-85; PMID:1326761; http://dx.doi.org/ 10.1073/pnas.89.18.8681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawakami A, Tian Q, Streuli M, Poe M, Edelhoff S, Disteche CM, Anderson P. Intron-exon organization and chromosomal localization of the human TIA-1 gene. J Immunol 1994; 152:4937-45; PMID:8176212 [PubMed] [Google Scholar]
- 4.Beck AR, Medley QG, O'Brien S, Anderson P, Streuli M. Structure, tissue distribution and genomic organization of the murine RRM-type RNA binding proteins TIA-1 and TIAR. Nucleic Acids Res 1996; 24:3829-35; PMID:8871565; http://dx.doi.org/ 10.1093/nar/24.19.3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu Q, Cok SJ, Zeng C, Morrison AR. Translational repression of human matrix metalloproteinases-13 by an alternatively spliced form of T-cell-restricted intracellular antigen-related protein (TIAR). J Biol Chem 2003; 278:1579-84; PMID:12426321; http://dx.doi.org/ 10.1074/jbc.M203526200 [DOI] [PubMed] [Google Scholar]
- 6.Izquierdo JM, Valcárcel J. Two isoforms of the T-cell intracellular antigen 1 (TIA-1) splicing factors display distinct splicing regulation activities. Control of TIA-1 isoform ratio by TIA-1 related protein. J Biol Chem 2007; 282:19410-17; PMID:17488725; http://dx.doi.org/ 10.1074/jbc.M700688200 [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Delestienne N, Huez G, Kruys V, Gueydan C. Identification of the sequence determinants mediating the nucleo-cytoplasmic shuttling of TIAR and TIA-1 RNA-binding proteins. J Cell Sci 2005; 118:5453-63; PMID:16278295; http://dx.doi.org/ 10.1242/jcs.02669 [DOI] [PubMed] [Google Scholar]
- 8.Gal-Mark N, Schwartz S, Ram O, Eyras E, Ast G. The pivotal roles of TIA proteins in 5′ splice-site selection of Alu exons and across evolution. PLoS Genet 2009; 5:e1000717; PMID:19911040; http://dx.doi.org/ 10.1371/journal.pgen.1000717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dember LM, Kim ND, Liu KQ, Anderson P. Individual RNA recognition motifs of TIA-1 and TIAR have different RNA binding specificities. J Biol Chem 1996; 271:2783-88; PMID:8576255; http://dx.doi.org/ 10.1074/jbc.271.5.2783 [DOI] [PubMed] [Google Scholar]
- 10.Kumar AO, Swenson MC, Benning MM, Kielkopf CL. Structure of the central RNA recognition motif of human TIA-1 at 1.95A resolution. Biochem Biophys Res Commun 2008; 367:813-819; PMID:18201561; http://dx.doi.org/ 10.1016/j.bbrc.2008.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwasako K, Takahashi M, Tochio N, Abe C, Tsuda K, Inoue M, Terada T, Shirouzu M, Kobayashi N, Kigawa T, et al.. Solution structure of the second RNA recognition motif (RRM) domain of murine T cell intracellular antigen-1 (TIA-1) and its RNA recognition mode. Biochemistry 2008; 47:6437-50; PMID:18500819; http://dx.doi.org/ 10.1021/bi7024723 [DOI] [PubMed] [Google Scholar]
- 12.Aroca A, Diaz-Quintana A, Diaz-Moreno I. A structural insight into the C-terminal RNA recognition motifs of T-cell intracellular antigen-1 protein. FEBS Lett 2011; 585:2958-64; PMID:21846467; http://dx.doi.org/ 10.1016/j.febslet.2011.07.037 [DOI] [PubMed] [Google Scholar]
- 13.Bauer WJ, Heath J, Jenkins JL, Kielkopf CL. Three RNA recognition motifs participate in RNA recognition and structural organization by the pro-apoptotic factor TIA-1. J Mol Biol 2012; 415:727-40; PMID:22154808; http://dx.doi.org/ 10.1016/j.jmb.2011.11.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang I, Hennig J, Jagtap PK, Sonntag M, Valcárcel J, Sattler M. Structure, dynamics and RNA binding of the multi-domain splicing factor TIA-1. Nucleic Acids Res 2014; 42:5949-66; PMID:24682828; http://dx.doi.org/ 10.1093/nar/gku193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waris S, Wilce MC, Wilce JA. RNA Recognition and Stress Granule Formation by TIA Proteins. Int J Mol Sci 2014; 15:23377-88; PMID:25522169; http://dx.doi.org/ 10.3390/ijms151223377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reyes R, Alcalde J, Izquierdo JM. Depletion of T-cell intracellular antigen proteins promotes cell proliferation. Genome Biol 2009; 10: R87; PMID:19709424; http://dx.doi.org/ 10.1186/gb-2009-10-8-r87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heck MV, Azizov M, Stehning T, Walter M, Kedersha N, Auburger G. Dysregulated expression of lipid storage and membrane dynamics factors in Tia1 knockout mouse nervous tissue. Neurogenetics 2014; 15:135-44; PMID:24659297; http://dx.doi.org/ 10.1007/s10048-014-0397-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Das R, Yu J, Zhang Z, Gygi MP, Krainer AR, Gygi SP, Reed R. SR proteins function in coupling RNAP II transcription to pre-mRNA splicing. Mol Cell 2007; 26:867-81; PMID:17588520; http://dx.doi.org/ 10.1016/j.molcel.2007.05.036 [DOI] [PubMed] [Google Scholar]
- 19.Suswam EA, Li YY, Mahtani H, King PH. Novel DNA-binding properties of the RNA-binding protein TIAR. Nucleic Acids Res 2005; 33:4507-18; PMID:16091628; http://dx.doi.org/ 10.1093/nar/gki763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McAlinden A, Liang L, Mukudai Y, Imamura T, Sandell LJ. Nuclear protein TIA-1 regulates COL2A1 alternative splicing and interacts with precursor mRNA and genomic DNA. J Biol Chem 2007; 282:24444-54; PMID:17580305; http://dx.doi.org/ 10.1074/jbc.M702717200 [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Headey SJ, Yoga YM, Scanlon MJ, Gorospe M, Wilce MC, Wilce JA. Distinct binding properties of TIAR RRMs and linkers region. RNA Biol 2013; 10:579-89; PMID:23603827; http://dx.doi.org/ 10.4161/rna.24341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danckwardt S, Gantzert AS, Macher-Goeppinger S, Probst HC, Gentzel M, Wilm M, Grone HJ, Schirmacher P, Hentze MW, Kulozik AE. p38 MAPK controls prothrombin expression by regulated RNA 3′ end processing. Mol Cell 2011; 41:298-10; PMID:21292162; http://dx.doi.org/ 10.1016/j.molcel.2010.12.032 [DOI] [PubMed] [Google Scholar]
- 23.Subramaniam K, Ooi LL, Hui KM. Transcriptional down-regulation of IGFBP-3 in human hepatocellular carcinoma cells is mediated by the binding of TIA-1 to its AT-rich element in the 3′-untranslated region. Cancer Lett 2010; 297:259-68; PMID:20599318; http://dx.doi.org/ 10.1016/j.canlet.2010.05.019 [DOI] [PubMed] [Google Scholar]
- 24.Tominaga A, Sugawara H, Futagawa T, Inoue K, Sasaki K, Minamino N, Hatakeyama M, Handa H, Miyata A. Characterization of the testis-specific promoter region in the human pituitary adenylate cyclase-activating polypeptide (PACAP) gene. Genes Cells 2010; 15:595-06; PMID:20500521 [DOI] [PubMed] [Google Scholar]
- 25.Del Gatto-Konczak F, Bourgeois CF, Le Guiner C, Kister L, Gesnel MC, Stevenin J, Breathnach R. The RNA-binding protein TIA-1 is a novel mammalian splicing regulator acting through intron sequences adjacent to a 5′ splice site. Mol Cell Biol 2000; 20:6287-99; PMID:10938105; http://dx.doi.org/ 10.1128/MCB.20.17.6287-6299.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Förch P, Puig O, Kedersha N, Martinez C, Granneman S, Seraphin B, Anderson P, Valcarcel J. The apoptosis-promoting factor TIA-1 is a regulator of alternative pre-mRNA splicing. Mol Cell 2000; 6:1089-98; PMID:11106748; http://dx.doi.org/ 10.1016/S1097-2765(00)00107-6 [DOI] [PubMed] [Google Scholar]
- 27.Förch P, Puig O, Martínez C, Seraphin B, Valcárcel J. The splicing regulator TIA-1 interacts with U1-C to promote U1 snRNP recruitment to 5′ splice sites. EMBO J 2002; 21:6882-92; PMID:12486009; http://dx.doi.org/ 10.1093/emboj/cdf668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz-Gallardo I, Aroca Á, Gunzburg MJ, Sivakumaran A, Yoon JH, Angulo J, Persson C, Gorospe M, Karlsson BG, Wilce JA, Díaz-Moreno I. The binding of TIA-1 to RNA C-rich sequences is driven by its C-terminal RRM domain. RNA Biol 2014; 11:766-76; PMID:24824036; http://dx.doi.org/ 10.4161/rna.28801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang I, Hennig J, Jagtap PK, Sonntag M, Valcárcel J, Sattler M. Structure, dynamics and RNA binding of the multi-domain splicing factor TIA-1. Nucleic Acids Res 2014; 42:5949-66; PMID:24682828; http://dx.doi.org/ 10.1093/nar/gku193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu H, Hasman RA, Young KM, Kedersha NL, Lou H. U1 snRNP-dependent function of TIAR in the regulation of alternative RNA processing of the human calcitonin/CGRP pre-mRNA. Mol Cell Biol 2003; 23:5959-71; PMID:12917321; http://dx.doi.org/ 10.1128/MCB.23.17.5959-5971.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu H, Hinman MN, Hasman RA, Mehta P, Lou H. Regulation of neuron-specific alternative splicing of neurofibromatosis type 1 pre-mRNA. Mol Cell Biol 2008; 28:1240-51; PMID:18086893; http://dx.doi.org/ 10.1128/MCB.01509-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Kayikci M, Briese M, Zarnack K, Luscombe NM, Rot G, Zupan B, Curk T, Ule J. iCLIP predicts the dual splicing effects of TIA-RNA interactions. PLoS Biol 2010; 8:e1000530; PMID:21048981; http://dx.doi.org/ 10.1371/journal.pbio.1000530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aznarez I, Barash Y, Shai O, He D, Zielenski J, Tsui LC, Parkinson J, Frey BJ, Rommens JM, Blencowe BJ. A systematic analysis of intronic sequences downstream of 5′ splice sites reveals a widespread role for U-rich motifs and TIA1/TIAL1 proteins in alternative splicing regulation. Genome Res 2008; 18:1247-58; PMID:18456862; http://dx.doi.org/ 10.1101/gr.073155.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kedersha NL, Gupta M, Li W, Miller I, Anderson P. RNA-binding proteins TIA-1 and TIAR link the phosphorylation of eIF-2 alpha to the assembly of mammalian stress granules. J Cell Biol 1999; 147:1431-42; PMID:10613902; http://dx.doi.org/ 10.1083/jcb.147.7.1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedersha N, Anderson P. Stress granules: sites of mRNA triage that regulate mRNA stability and translatability. Biochem Soc Trans 2002; 30:963-69; PMID:12440955; http://dx.doi.org/ 10.1042/BST0300963 [DOI] [PubMed] [Google Scholar]
- 36.Moutaoufik MT, El Fatimy R, Nassour H, Gareau C, Lang J, Tanguay RM, Mazroui R, Khandjian EW. UVC-induced stress granules in mammalian cells. PLoS One 2014; 9:e112742; PMID:25409157; http://dx.doi.org/ 10.1371/journal.pone.0112742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Albornoz A, Carletti T, Corazza G, Marcello A. The stress granule component TIA-1 binds tick-borne encephalitis virus RNA and is recruited to perinuclear sites of viral replication to inhibit viral translation. J Virol 2014; 88:6611-22; PMID:24696465; http://dx.doi.org/ 10.1128/JVI.03736-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ash PE, Vanderweyde TE, Youmans KL, Apicco DJ, Wolozin B. Pathological stress granules in Alzheimer's disease. Brain Res 2014; 1584:52-58; PMID:25108040; http://dx.doi.org/ 10.1016/j.brainres.2014.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scheu S, Stetson DB, Reinhardt RL, Leber JH, Mohrs M, Locksley RM. Activation of the integrated stress response during T helper cell differentiation. Nat Immunol 2006; 7:644-51; PMID:16680145; http://dx.doi.org/ 10.1038/ni1338 [DOI] [PubMed] [Google Scholar]
- 40.Damgaard CK, Lykke-Andersen J. Translational coregulation of 5′TOP mRNAs by TIA-1 and TIAR. Genes Dev 2011; 25:2057-68; PMID:21979918; http://dx.doi.org/ 10.1101/gad.17355911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012; 485:109-13; PMID:22552098; http://dx.doi.org/ 10.1038/nature11083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miloslavski R, Cohen E, Avraham A, Iluz Y, Hayouka Z, Kasir J, Mudhasani R, Jones SN, Cybulski N, Ruegg MA, Larsson O, Gandin V, Rajakumar A, Topisirovic I, Meyuhas O. Oxygen sufficiency controls TOP mRNA translation via the TSC-Rheb-mTOR pathway in a 4E-BP-independent manner. J Mol Cell Biol 2014; 6:255-66; PMID:24627160; http://dx.doi.org/ 10.1093/jmcb/mju008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santangelo PJ, Lifland AW, Curt P, Sasaki Y, Bassell GJ, Lindquist ME, Crowe JE Jr. Single molecule-sensitive probes for imaging RNA in live cells. Nat Methods 2009; 6:347-49; PMID:19349979; http://dx.doi.org/ 10.1038/nmeth.1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mazan-Mamczarz K, Lal A, Martindale JL, Kawai T, Gorospe M. Translational repression by RNA-binding protein TIAR. Mol Cell Biol 2006; 26:2716-27; PMID:16537914; http://dx.doi.org/ 10.1128/MCB.26.7.2716-2727.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gilks N, Kedersha N, Ayodele M, Shen L, Stoecklin G, Dember LM, Anderson P. Stress granule assembly is mediated by prion-like aggregation of TIA-1. Mol Biol Cell 2004; 15:5383-98; PMID:15371533; http://dx.doi.org/ 10.1091/mbc.E04-08-0715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bley N, Lederer M, Pfalz B, Reinke C, Fuchs T, Glaß M, Möller B, Hüttelmaier S. Stress granules are dispensable for mRNA stabilization during cellular stress. Nucleic Acids Res 2014; 43:e26; PMID:25488811; http://dx.doi.org/ 10.1093/nar/gku1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.López de Silanes I, Galban S, Martindale JL, Yang X, Mazan-Mamczarz K, Indig FE, Falco G, Zhan M, Gorospe M. Identification and functional outcome of mRNAs associated with RNA-binding protein TIA-1. Mol Cell Biol 2005; 25:9520-31; PMID:16227602; http://dx.doi.org/ 10.1128/MCB.25.21.9520-9531.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bounedjah O, Desforges B, Wu TD, Pioche-Durieu C, Marco S, Hamon L, Curmi PA, Guerquin-Kern JL, Piétrement O, Pastré D. Free mRNA in excess upon polysome dissociation is a scaffold for protein multimerization to form stress granules. Nucleic Acids Res 2014; 42:8678-91; PMID:25013173; http://dx.doi.org/ 10.1093/nar/gku582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kato M, Han TW, Xie S, Shi K, Du X, Wu LC, Mirzaei H, Goldsmith EJ, Longgood J, Pei J et al.. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. Cell 2012, 149:753-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim HS, Kuwano Y, Zhan M, Pullmann R Jr, Mazan-Mamczarz K, Li H, Kedersha N, Anderson P, Wilce MC, Gorospe M, Wilce JA. Elucidation of a C-rich signature motif in target mRNAs of RNA-binding protein TIAR. Mol Cell Biol 2007; 27:6806-17; PMID:17682065; http://dx.doi.org/ 10.1128/MCB.01036-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piecyk M, Wax S, Beck AR, Kedersha N, Gupta M, Maritim B, Chen S, Gueydan C, Kruys V, Streuli M, Anderson P. TIA-1 is a translational silencer that selectively regulates the expression of TNF-alpha. EMBO J 2000; 19:4154-63; PMID:10921895; http://dx.doi.org/ 10.1093/emboj/19.15.4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gueydan C, Droogmans L, Chalon P, Huez G, Caput D, Kruys V. Identification of TIAR as a protein binding to the translational regulatory AU-rich element of tumor necrosis factor alpha mRNA. J Biol Chem 1999; 274:2322-26; PMID:9890998; http://dx.doi.org/ 10.1074/jbc.274.4.2322 [DOI] [PubMed] [Google Scholar]
- 53.Yu C, York B, Wang S, Feng Q, Xu J, O'Malley BW. An essential function of the SRC-3 coactivator in suppression of cytokine mRNA translation and inflammatory response. Mol Cell 2007; 25:765-78; PMID:17349961; http://dx.doi.org/ 10.1016/j.molcel.2007.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dixon DA, Balch GC, Kedersha N, Anderson P, Zimmerman GA, Beauchamp RD, Prescott SM. Regulation of cyclooxygenase-2 expression by the translational silencer TIA-1. J Exp Med 2003; 198:475-81; PMID:12885872; http://dx.doi.org/ 10.1084/jem.20030616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kawai T, Lal A, Yang X, Galban S, Mazan-Mamczarz K, Gorospe M. Translational control of cytochrome c by RNA-binding proteins TIA-1 and HuR. Mol Cell Biol 2006; 26:3295-07; PMID:16581801; http://dx.doi.org/ 10.1128/MCB.26.8.3295-3307.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liao B, Hu Y, Brewer G. Competitive binding of AUF1 and TIAR to MYC mRNA controls its translation. Nat Struct Mol Biol 2007; 14:511-18; PMID:17486099; http://dx.doi.org/ 10.1038/nsmb1249 [DOI] [PubMed] [Google Scholar]
- 57.Gottschald OR, Malec V, Krasteva G, Hasan D, Kamlah F, Herold S, Rose F, Seeger W, Hanze J. TIAR and TIA-1 mRNA-binding proteins co-aggregate under conditions of rapid oxygen decline and extreme hypoxia and suppress the HIF-1alpha pathway. J Mol Cell Biol 2010; 2:345-56; PMID:20980400; http://dx.doi.org/ 10.1093/jmcb/mjq032 [DOI] [PubMed] [Google Scholar]
- 58.Carrascoso I, Sánchez-Jiménez C, Izquierdo JM. Long-term reduction of T-cell intracellular antigens leads to increased beta-actin expression. Mol Cancer 2014; 13:90; PMID:24766723; http://dx.doi.org/ 10.1186/1476-4598-13-90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li X, Rayman JB, Kandel ER, Derkatch IL. Functional role of Tia1/Pub1 and Sup35 prion domains: directing protein synthesis machinery to the tubulin cytoskeleton. Mol Cell 2014; 55:305-18; PMID:24981173; http://dx.doi.org/ 10.1016/j.molcel.2014.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carrascoso I, Sanchez-Jimenez C, Izquierdo JM. Genome-wide profiling reveals a role for T-cell intracellular antigens TIA-1 and TIAR in the control of translational specificity in HeLa cells. Biochem J 2014; 461:43-50; PMID:24927121 [DOI] [PubMed] [Google Scholar]
- 61.Podszywalow-Bartnicka P, Wolczyk M, Kusio-Kobialka M, Wolanin K, Skowronek K, Nieborowska-Skorska M, Dasgupta Y, Skorski T, Piwocka K. Downregulation of BRCA1 protein in BCR-ABL1 leukemia cells depends on stress-triggered TIAR-mediated suppression of translation. Cell Cycle 2014; 13:3727-41; PMID:25483082; http://dx.doi.org/ 10.4161/15384101.2014.965013 [DOI] [PubMed] [Google Scholar]
- 62.Wigington CP, Jung J, Rye EA, Belauret SL, Philpot AM, Feng Y, Santangelo PJ, Corbett AH. Post-transcriptional regulation of programmed cell death 4 (PDCD4) mRNA by the RNA binding proteins human antigen R (HuR) and T-cell intracellular antigen 1 (TIA1). J Biol Chem 2015; 290:3468-87; PMID:25519906; http://dx.doi.org/ 10.1074/jbc.M114.631937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eulalio A, Behm-Ansmant I, Schweizer D, Izaurralde E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol Cell Biol 2007; 27:3970-81; PMID:17403906; http://dx.doi.org/ 10.1128/MCB.00128-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Buttner K, Wenig K, Hopfner KP. The exosome: a macromolecular cage for controlled RNA degradation. Mol Microbiol 2006; 61:1372-79; PMID:16968219; http://dx.doi.org/ 10.1111/j.1365-2958.2006.05331.x [DOI] [PubMed] [Google Scholar]
- 65.Chen CY, Gherzi R, Ong SE, Chan EL, Raijmakers R, Pruijn GJ, Stoecklin G, Moroni C, Mann M, Karin M. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell 2001; 107:451-64; PMID:11719186; http://dx.doi.org/ 10.1016/S0092-8674(01)00578-5 [DOI] [PubMed] [Google Scholar]
- 66.Yamasaki S, Stoecklin G, Kedersha N, Simarro M, Anderson P. T-cell intracellular antigen-1 (TIA-1)-induced translational silencing promotes the decay of selected mRNAs. J Biol Chem 2007; 282:30070-77; PMID:17711853; http://dx.doi.org/ 10.1074/jbc.M706273200 [DOI] [PubMed] [Google Scholar]
- 67.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009; 136:215-33; PMID:19167326; http://dx.doi.org/ 10.1016/j.cell.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sánchez-Jimenez C, Carrascoso I, Barrero J, Izquierdo JM. Identification of a set of miRNAs differentially expressed in transiently TIA-depleted HeLa cells by genome-wide profiling. BMC Mol Biol 2013; 14:4; http://dx.doi.org/ 10.1186/1471-2199-14-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El Gazzar M, McCall CE. MicroRNAs distinguish translational from transcriptional silencing during endotoxin tolerance. J Biol Chem 2010; 285:20940-51; PMID:20435889; http://dx.doi.org/ 10.1074/jbc.M110.115063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS One 2010; 5:e11803; PMID:20668554; http://dx.doi.org/ 10.1371/journal.pone.0011803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jing Q, Huang S, Guth S, Zarubin T, Motoyama A, Chen J, Di Padova F, Lin SC, Gram H, Han J. Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 2005; 120:623-34; PMID:15766526 [DOI] [PubMed] [Google Scholar]
- 72.Bomben R, Dal-Bo M, Benedetti D, Capello D, Forconi F, Marconi D, Bertoni F, Maffei R, Laurenti L, Rossi D, et al.. Expression of mutated IGHV3-23 genes in chronic lymphocytic leukemia identifies a disease subset with peculiar clinical and biological features. Clin Cancer Res 2010; 16:620-28; PMID:20068100; http://dx.doi.org/ 10.1158/1078-0432.CCR-09-1638 [DOI] [PubMed] [Google Scholar]
- 73.Aparicio O, Carnero E, Abad X, Razquin N, Guruceaga E, Segura V, Fortes P. Adenovirus VA RNA-derived miRNAs target cellular genes involved in cell growth, gene expression and DNA repair. Nucleic Acids Res 2010; 38:750-63; PMID:19933264; http://dx.doi.org/ 10.1093/nar/gkp1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian Q, Taupin J, Elledge S, Robertson M, Anderson P. Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis. J Exp Med 1995; 182:865-74; PMID:7544399; http://dx.doi.org/ 10.1084/jem.182.3.865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Izquierdo JM, Majós N, Bonnal S, Martínez C, Castelo R, Guigo R, Bilbao D, Valcárcel J. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol Cell 2005; 19:475-84; PMID:16109372; http://dx.doi.org/ 10.1016/j.molcel.2005.06.015 [DOI] [PubMed] [Google Scholar]
- 76.Izquierdo JM, Valcárcel J. Fas-activated serine/threonine kinase (FAST K) synergizes with TIA-1/TIAR proteins to regulate Fas alternative splicing. J Biol Chem 2007; 282:1539-43; PMID:17135269; http://dx.doi.org/ 10.1074/jbc.C600198200 [DOI] [PubMed] [Google Scholar]
- 77.Eisinger-Mathason TS, Andrade J, Groehler AL, Clark DE, Muratore-Schroeder TL, Pasic L, Smith JA, Shabanowitz J, Hunt DF, Macara IG, et al.. Codependent functions of RSK2 and the apoptosis-promoting factor TIA-1 in stress granule assembly and cell survival. Mol Cell 2008; 31:722-36; PMID:18775331; http://dx.doi.org/ 10.1016/j.molcel.2008.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sánchez-Jiménez C, Ludeña MD, Izquierdo JM. T-cell intracellular antigens function as tumor supresor genes. Cell Death Dis 2015; 6:e1669; http://dx.doi.org/ 10.1038/cddis.2015.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Townsend KN, Spowart JE, Huwait H, Eshragh S, West NR, Elrick MA, Kalloger SE, Anglesio M, Watson PH, Huntsman DG, et al.. Markers of T cell infiltration and function associate with favorable outcome in vascularized high-grade serous ovarian carcinoma. PLoS One 2013; 8:e82406; PMID:24376535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ge D, Han L, Huang S, Peng N, Wang P, Jiang Z, Zhao J, Su L, Zhang S, Zhang Y, et al.. Identification of a novel MTOR activator and discovery of a competing endogenous RNA regulating autophagy in vascular endothelial cells. Autophagy 2014; 10:957-71; PMID:24879147; http://dx.doi.org/ 10.4161/auto.28363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang S, Liu N, Li H, Zhao J, Su L, Zhang Y, Zhang S, Zhao B, Miao J. TIA1 interacts with annexin A7 in regulating vascular endothelial cell autophagy. Int J Biochem Cell Biol 2014; 57:115-22; PMID:25461769; http://dx.doi.org/ 10.1016/j.biocel.2014.10.015 [DOI] [PubMed] [Google Scholar]
- 82.Le Guiner C, Gesnel MC, Breathnach R. TIA-1 or TIAR is required for DT40 cell viability. J Biol Chem 2003; 278:10465-76; PMID:12533540; http://dx.doi.org/ 10.1074/jbc.M212378200 [DOI] [PubMed] [Google Scholar]
- 83.Izquierdo JM. Hu antigen R (HuR) functions as an alternative pre-mRNA splicing regulator of Fas apoptosis-promoting receptor on exon definition. J Biol Chem 2008; 283:19077-84; PMID:18463097; http://dx.doi.org/ 10.1074/jbc.M800017200 [DOI] [PubMed] [Google Scholar]
- 84.Izquierdo JM. Cell-specific regulation of Fas exon 6 splicing mediated by Hu antigen R. Biochem Biophys Res Commun 2010; 402:324-28; PMID:20951677; http://dx.doi.org/ 10.1016/j.bbrc.2010.10.025 [DOI] [PubMed] [Google Scholar]
- 85.Izquierdo JM, Alcalde J, Carrascoso I, Reyes R, Ludena MD. Knockdown of T-cell intracellular antigens triggers cell proliferation, invasion and tumour growth. Biochem J 2011; 435:337-44; PMID:21284605; http://dx.doi.org/ 10.1042/BJ20101030 [DOI] [PubMed] [Google Scholar]
- 86.Hamdollah Zadeh MA, Amin EM, Hoareau-Aveilla C, Domingo E, Symonds KE, Ye K, Heesom KJ, Salmon A, D'Silva O, Betteridge KB, et al.. Alternative splicing of TIA-1 in human colon cancer regulates VEGF isoform expression, angiogenesis, tumour growth and bevacizumab resistance. Mol Oncol. 2015; 9:167-78; PMID:25224594; http://dx.doi.org/ 10.1016/j.molonc.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lowin B, French L, Martinou JC, Tschopp J. Expression of the CTL-associated protein TIA-1 during murine embryogenesis. J Immunol 1996; 157:1448-54; PMID:8759725 [PubMed] [Google Scholar]
- 88.Beck AR, Miller IJ, Anderson P, Streuli M. RNA-binding protein TIAR is essential for primordial germ cell development. Proc Natl Acad Sci 1998; 95:2331-36; PMID:9482885; http://dx.doi.org/ 10.1073/pnas.95.5.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simarro M, Giannattasio G, Xing W, Lundequist EM, Stewart S, Stevens RL, Orduna A, Boyce JA, Anderson PJ. The translational repressor T-cell intracellular antigen-1 (TIA-1) is a key modulator of Th2 and Th17 responses driving pulmonary inflammation induced by exposure to house dust mite. Immunol Lett 2012; 146:8-14; PMID:22525013; http://dx.doi.org/ 10.1016/j.imlet.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geng Z, Li P, Tan L, Song H. Targeted knockdown of RNA-binding protein TIAR for promoting self-renewal and attenuating differentiation of mouse embryonic stem cells. Stem Cells Int 2015; 2015:657325; PMID:25918534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kharraz Y, Salmand PA, Camus A, Auriol J, Gueydan C, Kruys V, Morello D. Impaired embryonic development in mice overexpressing the RNA-binding protein TIAR. PLoS One 2010; 5:e11352; PMID:20596534; http://dx.doi.org/ 10.1371/journal.pone.0011352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sánchez-Jiménez C, Izquierdo JM. T-cell intracellular antigen (TIA)-proteins deficiency in murine embryonic fibroblasts alters cell cycle progression and induces autophagy. PLoS One 2013; 8:e75127; http://dx.doi.org/ 10.1371/journal.pone.0075127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dinh PX, Beura LK, Das PB, Panda D, Das A, Pattnaik AK. Induction of stress granule-like structures in vesicular stomatitis virus-infected cells. J Virol 2013; 87:372-83; PMID:23077311; http://dx.doi.org/ 10.1128/JVI.02305-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sola I, Galán C, Mateos-Gómez PA, Palacio L, Zuniga S, Cruz JL, Almazán F, Enjuanes L. The polypyrimidine tract-binding protein affects coronavirus RNA accumulation levels and relocalizes viral RNAs to novel cytoplasmic domains different from replication-transcription sites. J Virol 2011; 85:5136-49; PMID:21411518; http://dx.doi.org/ 10.1128/JVI.00195-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iseni F, Garcin D, Nishio M, Kedersha N, Anderson P, Kolakofsky D. Sendai virus trailer RNA binds TIAR, a cellular protein involved in virus-induced apoptosis. EMBO J 2002; 21:5141-50; PMID:12356730; http://dx.doi.org/ 10.1093/emboj/cdf513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White JP, Lloyd RE. Poliovirus unlinks TIA1 aggregation and mRNA stress granule formation. J Virol 2011; 85:12442-54; PMID:21957303; http://dx.doi.org/ 10.1128/JVI.05888-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Álvarez E, Castelló A, Carrasco L, Izquierdo JM. Poliovirus 2A protease triggers a selective nucleo-cytoplasmic redistribution of splicing factors to regulate alternative pre-mRNA splicing. PLoS One 2013; 8:e73723; PMID:24066065; http://dx.doi.org/ 10.1371/journal.pone.0073723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li W, Li Y, Kedersha N, Anderson P, Emara M, Swiderek KM, Moreno GT, Brinton MA. Cell proteins TIA-1 and TIAR interact with the 3′ stem-loop of the West Nile virus complementary minus-strand RNA and facilitate virus replication. J Virol 2002; 76:11989-00; PMID:12414941; http://dx.doi.org/ 10.1128/JVI.76.23.11989-12000.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci 2007; 104:9041-46; PMID:17502609; http://dx.doi.org/ 10.1073/pnas.0703348104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garaigorta U, Heim MH, Boyd B, Wieland S, Chisari FV. Hepatitis C virus (HCV) induces formation of stress granules whose proteins regulate HCV RNA replication and virus assembly and egress. J Virol 2012; 86:11043-56; PMID:22855484; http://dx.doi.org/ 10.1128/JVI.07101-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi EY, Pintel D. Splicing of the large intron present in the nonstructural gene of minute virus of mice is governed by TIA-1/TIAR binding downstream of the nonconsensus donor. J Virol 2009; 83:6306-11; PMID:19339348; http://dx.doi.org/ 10.1128/JVI.00213-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.López-Urrutia E, Valdés J, Bonilla-Moreno R, Martínez-Salazar M, Martínez-García M, Berumen J, Villegas-Sepúlveda N. A few nucleotide polymorphisms are sufficient to recruit nuclear factors differentially to the intron 1 of HPV-16 intratypic variants. Virus Res 2012; 166:43-53; http://dx.doi.org/ 10.1016/j.virusres.2012.02.026 [DOI] [PubMed] [Google Scholar]
- 103.Tang H, Huang Y, Chen J, Yu C, Huang AL. Cellular protein TIA-1 regulates the expression of HBV surface antigen by binding the HBV posttranscriptional regulatory element. Intervirology 2008; 51:203-09; PMID:18753794; http://dx.doi.org/ 10.1159/000151632 [DOI] [PubMed] [Google Scholar]
- 104.Nanda S, Havert MB, Calderón GM, Thomson M, Jacobson C, Kastner D, Liang TJ. Hepatic transcriptome analysis of hepatitis C virus infection in chimpanzees defines unique gene expression patterns associated with viral clearance. PLoS One 2008; 3:e3442; PMID:18927617; http://dx.doi.org/ 10.1371/journal.pone.0003442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Phillips K, Kedersha N, Shen L, Blackshear PJ, Anderson P. Arthritis suppressor genes TIA-1 and TTP dampen the expression of tumor necrosis factor alpha, cyclooxygenase 2, and inflammatory arthritis. Proc Natl Acad Sci 2004; 101:2011-16; PMID:14769925; http://dx.doi.org/ 10.1073/pnas.0400148101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sugihara M, Tsutsumi A, Suzuki E, Wakamatsu E, Suzuki T, Ogishima H, Hayashi T, Chino Y, Ishii W, Mamura M, et al.. Effects of infliximab therapy on gene expression levels of tumor necrosis factor alpha, tristetraprolin, T cell intracellular antigen 1, and Hu antigen R in patients with rheumatoid arthritis. Arthritis Rheum 2007; 56:2160-69; PMID:17599736; http://dx.doi.org/ 10.1002/art.22724 [DOI] [PubMed] [Google Scholar]
- 107.Cok SJ, Acton SJ, Morrison AR. The proximal region of the 3′-untranslated region of cyclooxygenase-2 is recognized by a multimeric protein complex containing HuR, TIA-1, TIAR, and the heterogeneous nuclear ribonucleoprotein U. J Biol Chem 2003; 278:36157-62; PMID:12855701; http://dx.doi.org/ 10.1074/jbc.M302547200 [DOI] [PubMed] [Google Scholar]
- 108.Katsanou V, Papadaki O, Milatos S, Blackshear PJ, Anderson P, Kollias G, Kontoyiannis DL. HuR as a negative posttranscriptional modulator in inflammation. Mol Cell 2005; 19:777-89; PMID:16168373; http://dx.doi.org/ 10.1016/j.molcel.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 109.Karalok HM, Aydin E, Saglam O, Torun A, Guzeloglu-Kayisli O, Lalioti MD, Kristiansson H, Duke CM, Choe G, Flannery C, et al.. mRNA-Binding Protein TIA-1 Reduces Cytokine Expression in Human Endometrial Stromal Cells and Is Down-Regulated in Ectopic Endometrium. J Clin Endocrinol Metab 2014; 99:E2610-19; PMID:25140393; http://dx.doi.org/ 10.1210/jc.2013-3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lipman ML, Stevens AC, Strom TB. Heightened intragraft CTL gene expression in acutely rejecting renal allografts. J Immunol 1994; 152:5120-27; PMID:8176228 [PubMed] [Google Scholar]
- 111.Olive C, Cheung C, Falk MC. Apoptosis and expression of cytotoxic T lymphocyte effector molecules in renal allografts. Transpl Immunol 1999; 7:27-36; PMID:10375075; http://dx.doi.org/ 10.1016/S0966-3274(99)80016-1 [DOI] [PubMed] [Google Scholar]
- 112.Augustin M, Karttunen TJ, Kokkonen J. TIA1 and mast cell tryptase in food allergy of children: increase of intraepithelial lymphocytes expressing TIA1 associates with allergy. J Pediatr Gastroenterol Nutr 2001; 32:11-18; PMID:11176318; http://dx.doi.org/ 10.1097/00005176-200101000-00008 [DOI] [PubMed] [Google Scholar]
- 113.Mitomi H, Ohkura Y, Yokoyama K, Sada M, Kobayashi K, Tanabe S, Fukui N, Kanazawa H, Kishimoto I, Saigenji K. Contribution of TIA-1+ and granzyme B+ cytotoxic T lymphocytes to cryptal apoptosis and ulceration in active inflammatory bowel disease. Pathol Res Pract 2007; 203:717-23; PMID:17869012; http://dx.doi.org/ 10.1016/j.prp.2007.06.007 [DOI] [PubMed] [Google Scholar]
- 114.Xu JL, Nagasaka T, Nakashima N. Involvement of cytotoxic granules in the apoptosis of aplastic anaemia. Br J Haematol 2003; 120:850-52; PMID:12614221; http://dx.doi.org/ 10.1046/j.1365-2141.2003.04147.x [DOI] [PubMed] [Google Scholar]
- 115.Corduan A, Plé H, Laffont B, Wallon T, Plante I, Landry P, Provost P. Dissociation of SERPINE1 mRNA from the translational repressor proteins Ago2 and TIA-1 upon platelet activation. Thromb Haemost 2015; 113:1046-59; PMID:25673011; http://dx.doi.org/ 10.1160/TH14-07-0622 [DOI] [PubMed] [Google Scholar]
- 116.Vermeer MH, van Doorn R, Dukers D, Bekkenk MW, Meijer CJ, Willemze R. CD8+ T cells in cutaneous T-cell lymphoma: expression of cytotoxic proteins, Fas Ligand, and killing inhibitory receptors and their relationship with clinical behavior. J Clin Oncol 2001; 19:4322-29; PMID:11731515 [DOI] [PubMed] [Google Scholar]
- 117.Zhang L, Conejo-García JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al.. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003; 348:203-13; PMID:12529460; http://dx.doi.org/ 10.1056/NEJMoa020177 [DOI] [PubMed] [Google Scholar]
- 118.Pages F, Berger A, Camus M, Sánchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al.. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 2005; 353:2654-66; PMID:16371631; http://dx.doi.org/ 10.1056/NEJMoa051424 [DOI] [PubMed] [Google Scholar]
- 119.Milne K, Kobel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009, 4:e6412; PMID:19641607; http://dx.doi.org/ 10.1371/journal.pone.0006412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zlobec I, Karamitopoulou E, Terracciano L, Piscuoglio S, Iezzi G, Muraro MG, Spagnoli G, Baker K, Tzankov A, Lugli A. TIA-1 cytotoxic granule-associated RNA binding protein improves the prognostic performance of CD8 in mismatch repair-proficient colorectal cancer. PLoS One 2010; 5:e14282; PMID:21179245; http://dx.doi.org/ 10.1371/journal.pone.0014282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.González-Pérez A, Pérez-Llamas C, Deu-Pons J, Tamborero D, Schroeder MP, Jene-Sanz A, Santos A, López-Bigas N. IntOGen-mutations identifies cancer drivers across tumor types. Nat Methods 2013; 10:1081-82; http://dx.doi.org/ 10.1038/nmeth.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang C, Romaniuk PJ. The ratio of +/-KTS splice variants of the Wilms' tumour suppressor protein WT1 mRNA is determined by an intronic enhancer. Biochem Cell Biol 2008; 86:312-321; PMID:18756326 [DOI] [PubMed] [Google Scholar]
- 123.Xu H, Sun H, Zhang H, Liu J, Fan F, Li Y, Ning X, Sun Y, Dai S, Liu B, et al.. An shRNA based genetic screen identified SESN2 as a potential pumor suppressor in lung cancer via suppression of Akt-mTOR-p70S6K signaling. PLoS One 2015; 10:e0124033; PMID:25962159; http://dx.doi.org/ 10.1371/journal.pone.0124033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lal A, Abdelmohsen K, Pullmann R, Kawai T, Galban S, Yang X, Brewer G, Gorospe M. Posttranscriptional derepression of GADD45alpha by genotoxic stress. Mol Cell 2006; 22:117-28; PMID:16600875 [DOI] [PubMed] [Google Scholar]
- 125.Barohn RJ, Amato AA, Griggs RC. Overview of distal myopathies: from the clinical to the molecular. Neuromuscul Disord 1998; 8:309-16; PMID:9673984 [DOI] [PubMed] [Google Scholar]
- 126.Udd B. Distal myopathies–new genetic entities expand diagnostic challenge. Neuromuscul Disord 2012; 22:5-12; PMID:22197426 [DOI] [PubMed] [Google Scholar]
- 127.Welander L. Myopathia distalis tarda hereditaria; 249 examined cases in 72 pedigrees. Acta Med Scand Suppl 1951; 265:1-124; PMID:14894174 [PubMed] [Google Scholar]
- 128.Masuda K, Marasa B, Martindale JL, Halushka MK, Gorospe M. Tissue- and age-dependent expression of RNA-binding proteins that influence mRNA turnover and translation. Aging 2009; 1:681-98; PMID:20157551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lindberg C, Borg K, Edström L, Hedström A, Oldfors A. Inclusion body myositis and Welander distal myopathy: a clinical, neurophysiological and morphological comparison. J Neurol Sci 1991; 103:76-81; PMID:1650819 [DOI] [PubMed] [Google Scholar]
- 130.von Tell D, Somer H, Udd B, Edström L, Borg K, Ahlberg G. Welander distal myopathy outside the Swedish population: phenotype and genotype. Neuromuscul Disord 2002; 12:544-47; PMID:12117477 [DOI] [PubMed] [Google Scholar]
- 131.Ahlberg G, von Tell D, Borg K, Edström L, Anvret M. Genetic linkage of Welander distal myopathy to chromosome 2p13. Ann Neurol 1999; 46:399-04; PMID:10482271 [DOI] [PubMed] [Google Scholar]
- 132.von Tell D, Bruder CE, Anderson LV, Anvret M, Ahlberg G. Refined mapping of the Welander distal myopathy region on chromosome 2p13 positions the new candidate region telomeric of the DYSF locus. Neurogenetics 2003; 4:173-77; PMID:12836053; http://dx.doi.org/ 10.1007/s10048-003-0154-z [DOI] [PubMed] [Google Scholar]
- 133.Klar J, Sobol M, Melberg A, Mäbert K, Ameur A, Johansson AC, Feuk L, Entesarian M, Orlén H, Casar-Borota O, et al.. Welander distal myopathy caused by an ancient founder mutation in TIA1 associated with perturbed splicing. Hum Mutat 2013; 34:572-77; PMID:23348830 [DOI] [PubMed] [Google Scholar]
- 134.Hackman P, Sarparanta J, Lehtinen S, Vihola A, Evilä A, Jonson PH, Luque H, Kere J, Screen M, Chinnery PF, et al.. Welander distal myopathy is caused by a mutation in the RNA-binding protein TIA1. Ann Neurol 2013; 73:500-09; PMID:23401021 [DOI] [PubMed] [Google Scholar]