Abstract
Objective
The aim of the current study was to evaluate the early experience of the application of transcatheter aortic valve implantation with the balloon-expandable system in China. The transcatheter aortic valve implantation technology has been widely used for patients with inoperable severe aortic stenosis in the developed world. The application of transcatheter aortic valve implantation is still in the early stages of testing in China, particularly for the balloon-expandable valve procedure.
Methods
This was a retrospective study. All patients undergoing transcatheter aortic valve implantation with balloon-expandable system in our hospital between 2011 and 2014 were included. Edwards SAPIEN XT Transcatheter Heart Valve was used. The improvement of valve and heart function was evaluated as well as 30-day mortality and major complications according to the VARC-2 definition.
Results
A total of 10 transcatheter aortic valve implantation procedures with the balloon-expandable system were performed in our hospital, of which 9 were transfemoral and 1 was transapical. The median age was 76 years, and the median STS score and Logistic EuroSCORE (%) were 8.9 and 16.2. The implantation was successfully conducted in all patients, only 2 patients had mild paravalvular leak. There was no second valve implantation. Moreover, no 30-day mortality or complications was reported. Following the transcatheter aortic valve implantation procedure, the heart and valve functions had improved significantly. During the follow-up period of 3-34 months, one patient died of lung cancer 13 months after the operation.
Conclusion
This early experience has provided preliminary evidence for the safety and efficacy of transcatheter aortic valve implantation procedure with the balloon-expandable system in the developing world with an increasing aging population.
Keywords: Aortic Valve Stenosis, Cardiac Catheterization, Heart Valve Diseases
Abstract
Objetivo:
O objetivo do presente estudo foi avaliar a experiência inicial da aplicação do implante percutâneo da válvula aórtica com o sistema balão-expansível na China. A tecnologia TAVI tem sido amplamente utilizada para pacientes com estenose aórtica grave inoperável no mundo desenvolvido. A aplicação de implante percutâneo da válvula aórtica está ainda nas etapas iniciais de teste na China, em particular o procedimento de válvula balão-expansível.
Métodos:
O estudo foi retrospectivo e todos os pacientes submetidos a implante percutâneo da válvula aórtica com sistema balão-expansível em nosso hospital entre 2011 e 2014 foram incluídos. Edwards SAPIEN XT Válvula Cardíaca Transcatheter foi usado. A melhoria do funcionamento da válvula e do coração foi avaliada, bem como mortalidade em 30 dias e as principais complicações de acordo com a definição VARC-2.
Resultados:
Um total de 10 procedimentos Tavi com o sistema balão-expansível foram realizados em nosso hospital, dos quais 9 foram transfemorais e 1 foi transapical. A idade média foi de 76 anos, e os STS mediana marcar e Logistic EuroSCORE (%) foram de 8,9 e 16,2. A implantação foi realizada com sucesso em todos os pacientes, apenas 2 pacientes tiveram vazamento paravalvar leve. Não houve um futuro implante valvar. Além disso, mortalidade em 30 dias ou complicações não foram relatadas. Seguindo o procedimento de implante percutâneo da válvula aórtica, as válvulas cardíacas e funções melhoraram significativamente. Durante o período de acompanhamento de 3-34 meses, um paciente morreu de câncer de pulmão 13 meses após a operação.
Conclusão:
Esta experiência inicial apresentou elementos de prova preliminar para a segurança e eficácia do procedimento implante percutâneo da válvula aórtica com o sistema balão-expansível no mundo em desenvolvimento com crescente envelhecimento da população.
| Abbreviations, acronyms & symbols | |
|---|---|
| AS | Aortic stenosis |
| EOAI | Effective disc mouth area index |
| LVEF | Left ventricular ejection fraction |
| NYHA | New York Heart Association |
| PARTNER | Placement of aortic transcatheter valves |
| SAVR | Surgical aortic valve replacements |
| SD | Standard deviation |
| TAVR | Transcatheter aortic valve replacement |
| TTE | Transthoracic echocardiography |
| TAVI | Transcatheter aortic valve implantation |
INTRODUCTION
During the past 50 years, the etiology of valvular heart diseases has changed greatly in developed countries, with an increase in non-rheumatic valvular heart diseases such as age-related calcific aortic stenosis (AS)[1,2]. AS is now considered one of the most common valvular diseases in the developed world. For instance, the year-round surgical aortic valve replacements (SAVR) quantity is estimated to be 67,500 in the United States[3]. In China, although limited data indicated that the prevalence of rheumatic heart disease was 10 times higher than developed countries in 2002[4], the rapid growth of an aging population also increases the number of vulnerable age-related AS.
Once AS becomes severe and symptomatic, the prognosis is poor with high mortality if left untreated[5,6]. A recent meta-analysis found 69% and 36% of increased risks of cardiovascular and consequential mortality in AS patients, respectively[7], might be partially explained by the selected high risk patients with older age and comorbities. Although the conventional SAVR has excellent outcomes[8-10], it has been reported that patients with severe symptomatic AS had higher mortality when treated by SAVR[11,12]. The emergence and rapid development of transcatheter aortic valve replacement (TAVR) indicated hope for those inoperable or high-risk patients[13,14]. Since 2007, more than 100,000 patients have been treated by TAVR worldwide[15], most of whom were from developed countries. Moreover, a recent meta-analysis estimated that approximately 290,000 elderly patients are TAVR candidates in European countries and North America[16]. Within the 2 widely used device types, the use of balloon-expandable valve (Cribier-Edward) has been shown to have higher success rate than self-expandable valve (CoreValve) in a multi-center study[17]. However, evidence from developing countries was scarce. For example, the initial experience of transcatheter aortic valve implantation (TAVI) was reported in Brazil[18,19], South Africa[20], and India[21], respectively. In mainland China, the use of TAVI did not start until the first successful procedure with self-expandable valve in 2010[22]. The use of balloon-expandable valve remains limited.
Since 2011, our hospital was the first to introduce TAVI with the balloon-expandable system in mainland China. The present study aimed to evaluate the early experience of TAVI procedure using balloon-expandable valve in mainland China, and to provide potential evidence for the application and generalization of this novel technology in the developing world with an increasing aging population.
METHODS
Patients
This was a retrospective study. All patients that underwent TAVI with the balloon-expandable system in our hospital between 2011 and 2014 were included. All patients were selected by a multidisciplinary core team after extensive screening, including transthoracic echocardiography, coronary arteriography, computed tomographic angiography and lung function examination to evaluate the severity of AS and the existence of any contraindications. Patients that met at least one of the following criteria were included: 1) severe AS with an aortic valve area < 1 cm2; 2) a New York Heart Association (NYHA) functional class II or higher; 3) a STS of 5%~15%; or 4) a Logistic EuroSCORE of 20% or higher. Exclusion criteria included bicuspid aortic valve, acute myocardial infarction, LVEF<20%, aortic valve ring> 25mm or <18mm, severe coronary artery diseases, severe aortic or mitral regurgitation, severe kidney dysfunction, or transient ischemic attack within 6 month. Eligible patients had aortic annulus diameters of 20-25 mm, as determined by the transesophageal echocardiography (TEE). A total of 10 patients (9 male) with NYHA functional class II or higher were included in the current study.
Ethics Statement
The current study was approved by the Research Ethics Committee of Shanghai Changhai Hospital (CHEC2011-099, 9/16/2011) and a waiver of informed consents was granted as the data were retrospectively reviewed and analyzed anonymously.
Device and procedure
Procedures were performed in hybrid operating room under intratracheal intubation anesthesia. Edwards SAPIEN XT Transcatheter Heart Valve (Edwards Lifescience Corp) was used for all patients (23 and 26 mm). Aortic valve multidetector computed tomography and aorta computed tomography angiography were done before the procedure to evaluate the calcification level. Biplanar TEE was used for real-time supervision during the TAVI procedure. The aortic annulus diameter, aortic valve area, regurgitation velocity, regurgitation gradient, and distance from coronary artery were reevaluated by TEE. A standard transfemoral retrograde approach was applied. In brief, a 18-F eSheath with the introducer (5Fr sheath) was inserted over a 0.035 guidewire into the femoral artery. The 5Fr sheath was advanced over an extra stiff 0.035-inch guide wire (Amplatz, Cook, Inc., Bloomington, IN) into the left ventricle. The 5Fr sheath was then retrieved and the eSheath was introduced to the aortic valve. Following the balloon aortic valvuloplasty under rapid ventricular pacing, the SAPIEN XT Transcatheter Heart Valve delivery system was inserted into the eSheath hub. After accurate positioning by aortic root angiograms and TEE guidance, the Transcatheter Heart Valve was deployed during rapid ventricular pacing (Figure 1). Thereafter, the delivery system was retrieved and the femoral access site was percutaneously closed (ProGlideTM, Abbott Vascular, Inc., Abbott Park, IL). For cases that failed to deliver the SAPIEN XT Transcatheter Heart Valve through the femoral access, a transapical approach was conducted through the left ventricular apex. The transpical approacch was previous planned since the patient had severe iliac artery calcification.
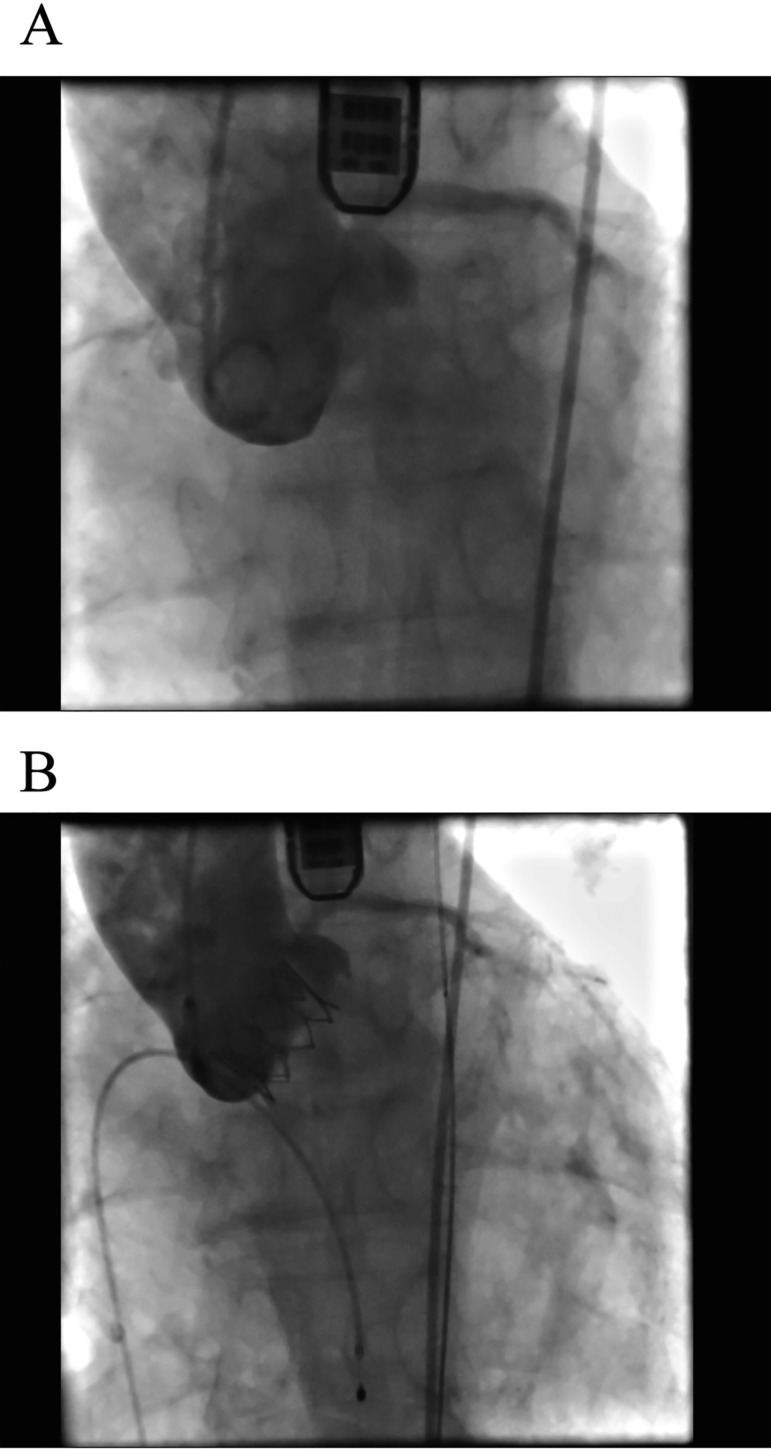
Fig. 1.
Angiography image before (A) and after (B) balloonexpandable valve implantation.
Outcome measurements
The indicators of valve and heart functions including: left ventricular ejection fraction (LVEF, %); aortic annulus diameter (mm); aortic valve area (cm2); effective disc mouth area index (EOAI) and jet velocity (cm/s) were assessed. In regards to the prognosis indicators, the operative (30-day) mortality and major complications including: cerebrovascular accident; arrhythmia; congestive heart failure; myocardial infarction; angina; paravalvular regurgitation; valve migration; valve infection; bleeding; pulmonary infection; urinary system infection; respiratory failure and dialysis-dependent renal failure were evaluated. Outcomes were also evaluated by the VARC II definitions.
Statistical analyses
Data were presented as median (IQR) for continuous variables, and numbers (%) for categorical variables. The comparison of valve and heart function before and after TVAR were analyzed by Wilcoxon signed-rank test or Fisher's Exact Test. A P value of P<0.05 was considered statistically significant.
The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in manuscript writing; or in the decision to submit the article for publication.
RESULTS
Between 2011 and 2014, a total of 10 patients (9 male) underwent TAVI procedures with the balloon-expandable system in our hospital, of which 9 were transfemoral and 1 was transapical. The baseline and procedure characteristics of patients were presented in Table 1. The median age was 76 (IQR: 75, 78) years. All patients had a NYHA functional class II or higher. Three patients (30%) had atrioventricular block. The median STS score and Logistic EuroSCORE (%) were 8.9 (IQR: 8.1, 11.0) and 16.2 (IQR: 15.5, 17.7), respectively.
Table 1.
Baseline and Procedure Characteristics (n=10).
| Patient Characteristics | |
|---|---|
| Baseline Characteristics | |
| Age (years)* | 76 (75,78) |
| Gender, male, n (%) | 9 (90.0) |
| Hypertension, yes, n (%) | 9 (90.0) |
| Diabetes, yes, n (%) | 2 (20.0) |
| Coronary artery stenosis, yes, n (%) | 3 (30.0) |
| Surgery history, yes, n (%) | 4 (40.0) |
| Other comorbidities, yes, n (%) | 2 (20.0) |
| Aortic calcification, yes, n (%) | |
| Mildly calcified | 6 (60.0) |
| Multiple spots | 3 (30.0) |
| Iliac artery stenosis | 1 (10.0) |
| Femoral artery diameter, yes, n (%) | |
| 7.0-8.0 mm | 4 (40.0) |
| 8.1-9.0 mm | 5 (50.0) |
| >9.0 mm | 1 (10.0) |
| NYHA functional class, yes, n (%) | |
| I | 0 |
| ≥ II | 10 (100) |
| Atrioventricular block, yes, n (%) | 3 (30.0) |
| STS score* | 8.9 (8.1, 11.0) |
| LVEF (%)* | 62.6 (55.0, 67.0) |
| TTE Aortic annulus diameter (mm)* | 23.0 (21.0, 24.0) |
| TTE Aortic valve area (cm2)* | 0.86 (0.82, 0.90) |
| EOAI* | 0.52 (0.49, 0.53) |
| Peak trans aortic gradient (mmHg)* | 104 (88, 117) |
| Jet velocity (cm/s)* | 510 (456,542) |
| Logistic EuroSCORE (%)* | 16.2 (15.5, 17.7) |
| Procedure Characteristics | |
| Procedure time (min)* | 200 (185, 255) |
| Blood products (ml)* | 200 (0, 800) |
| Length of stay (day)* | 7 (6, 8) |
| ICU time (day)* | 0 (0, 1) |
| Mild paravalvular leak, yes, n (%) | 2 (20.0) |
| Readmission, yes, n (%) | 0 (0) |
Data were presented as median (IQR) for continuous variables.
EOAI=effective disc mouth area index; NYHA=New York Heart Association; LVEF=left ventricular ejection fraction; TTE=transthoracic echocardiography
Following successful implantations with a median procedure time of 200 min, all patients had stable vital signs and were discharged from hospital 4 to 8 days after the TAVI procedure. Only 2 patients (20%) had mild paravalvular leak (Table 1). There was no second valve implantation, valve migration, or infection in any patient. In addition, there was no 30-day mortality or vascular complications according to the VARC-2 definition (Table 2). During the follow-up period of 3-34 months, 1 patient died of lung cancer at 13 months after the TAVI procedure.
Table 2.
The VARC-2 outcomes in the 30-day follow up period.
| Outcomes | Number (n=10) |
|---|---|
| All-cause mortality | 0 |
| Cardiac mortality | 0 |
| Stroke | 0 |
| Life-threatening bleeding | 0 |
| Acute kidney injury, stage 2 or 3 | 0 |
| Coronary artery obstruction | 0 |
| Major vascular complication | 0 |
| Valve-related dysfunction requiring repeat procedure | 0 |
Following the TAVI procedure, the valve functions were significantly improved (Table 3). The median aortic annulus diameter, aortic valve area and EOAI were significantly increased from 23.0 (IQR: 21.0, 24.0) mm to 26.0 (IQR: 23.0, 26.0) mm, 0.86 (IQR: 0.82, 0.90) cm2 to 1.78 (IQR: 1.72, 1.80) cm2 and 0.52 (IQR: 0.49, 0.53) to 1.06 (IQR: 1.01, 1.12), respectively (all P<0.05). In addition, the median jet velocity decreased from 510 (IQR: 456, 542) to 205 (IQR: 186, 236) cm/s (P<0.001). Meanwhile, the NYHA functional class of 8 patients (80%) had improved to class I within 30 days in the postoperative period.
Table 3.
Comparison of valve and heart function before and after TAVI.
| Baseline Characteristics | Before TAVI | After TAVI | P value |
|---|---|---|---|
| LVEF(%) | 62.6 (55.0, 67.0) | 64.5 (61.0, 67.0) | 0.43 |
| TTE Aortic annulus diameter (mm) | 23.0 (21.0, 24.0) | 26.0 (23.0, 26.0) | 0.021 |
| TTE Aortic valve area (cm2) | 0.86 (0.82, 0.90) | 1.78 (1.72, 1.80) | <0.001 |
| EOAI | 0.52 (0.49, 0.53) | 1.06 (1.01, 1.12) | <0.001 |
| Jet velocity (cm/s) | 510 (456, 542) | 205 (186, 236) | <0.001 |
| NYHA functional class ≥ II, yes, n (%) | 10 (100) | 2 (20) | <0.001 |
Data were presented as median (IQR) or n (%).
EOAI=effective disc mouth area index; NYHA=New York Heart Association; LVEF=left ventricular ejection fraction; TTE=transthoracic echocardiography
DISCUSSION
According to the China Report of the Development on Aging Cause, the percentage of the aging population in China was 14.8% (more than 0.2 billion) in 2013[23]. The rapid growth of an aging population has resulted in a significant challenge in defending age-related chronic diseases, such as calcific AS. Due to poor prognosis after the manifestation of cardiovascular symptoms, safe and effective medical procedures are in urgent need to treat AS, particularly for those elderly inoperable patients. Our hospital was the first to introduce TAVI with the balloon-expandable system in mainland China. Our early experiences with favorable improvement of heart and valve function has provided preliminary evidence for the safety and efficacy of the TAVI procedure in severe AS patients in mainland China.
Although conventional SAVR has excellent outcomes[8-10], the mortality and morbidity rates remain high in patients at extreme high-risk or inoperable patients with severe AS[11,12]. The emergence of TAVR technology offers a novel, less-invasive approach with a success procedure rate of over 93%[24-26]. The advantages of TAVR procedure have been evaluated thoroughly in developed counties, especially from large-scale studies of registry data from US and European countries including more than 10,000 patients[27-29]. Moreover, according to the findings from the Placement of Aortic Transcatheter Valves (PARTNER) B trial, inoperable patients that underwent TAVR had significantly lower 1-year mortality rate compared with those patients that underwent standard therapy (30.7% vs. 50.7%, respectively). Among the survivors, the 1-year rate of cardiac symptoms was also lower in the TAVR group compared with the standard therapy group (25.2% vs 58.0%, respectively)[30]. In the PARTNER A trial, the TAVR and SAVR procedure had comparable mortality and symptom improvement for high-risk surgical candidates during the 2-year follow-up period[31,32]. However, evidence from developing countries including China remains limited.
Mortality, as well as major complications such as cerebrovascular accident, paravalvular regurgitation, and vascular events, are concerning implications for the use of the TAVR procedure. In the TAVR group of the PARTNER A trial, the 30-day mortality, major cerebrovascular accident, and major vascular complications were 3.4%, 3.8% and 11.0%, respectively[31], whilst the all-cause mortality 2 years after the TAVR procedure was 33.9%[32]. Moreover, among all eligible TAVR cases utilizing the Sapien Transcatheter Heart Valve from November 2011 to May 2013 in the United States, the in-hospital mortality and cerebrovascular accident rates were 5.5% and 2.0%, respectively[33]. In contrast, in the present study, there were zero cases of mortality and major complications in the 30-day follow-up period and only one patient died of lung cancer 13 months after the TAVR procedure during the follow-up period. Although a decisive conclusion of low mortality and complication rate could not be made based on the findings of the current study, the extensive screening and careful evaluation for all patients by a multidisciplinary core team before TAVR procedure may have contributed to the higher success rate and better prognosis. In addition, in terms of the incorporation of TAVR in clinical practice, functional improvement would provide valuable information. A systematic review of current reports revealed consistent benefits of TAVR by the improvement in NYHA functional class[34]. In the current study, improvement in heart and valve function after TAVI was also observed. Studies with a larger number of cases and a longer follow-up period are required to validate the findings of the current study.
Currently, the TAVR approach has been widely utilized in developed countries. The application of TAVR is, however still in the very early stages in developing countries such as China, due to high-demand technology and expensive therapeutic fees. Whilst previous reports have shown favorable outcomes of the TAVR procedure in inoperable or high-risk patients with severe AS, the generalization of TAVR in routine therapy remains complex, including the accessibility of a facility for this procedure in a clinical center, the experience of the operator and the core team, the selection and evaluation of high-risk patients, the procedure performance, and perioperative and postoperative care. Furthermore, whilst rapid incorporation of the TAVR procedure in clinical treatment is progressing, technical challenges remain[15]. In the PARTNER A trial, among patients that have undergone TAVR, there was increased paravalvular regurgitation and major vascular complications than those patients treated by SAVR[31,32]. In the PARTNER B trial, there was also a higher incidence of major cerebrovascular accident and major vascular events in the TAVR group compared with the standard therapy group[30]. In contrast, the considerably advanced technology in the SAVR procedure has greatly improved the surgical results in high-risk patients[35]. To avoid the misuse of TAVR, the ACCF/AATS/SCAI/STS expert consensus published in 2012, has provided standards for applying TAVR in the clinical practice in the United States[5]. However, whether these standards derived from Western populations, can be applied or not in China, remain unknown. Moreover, whether the commercially available Transcatheter Heart Valve designed for Western patients will fit Chinese patients needs to be elucidated in large multicenter studies with longer follow-up duration.
Strength and Limitations
To the best of our knowledge, the current study was the first attempt to evaluate the TAVI approach with the balloon-expandable system in mainland China. This study contains several limitations. The number of patients that underwent the TAVI procedure with the balloon-expandable system was very small, due to the demanding technology and high expenditure of this procedure. Secondly, the current study was a single center study. Due to the complexity of incorporating TAVI in clinical practice, the results of the current study may not be generalized to other centers in China.
CONCLUSION
In conclusion, the current study has provided an evaluation of early experience in the application of TAVI procedure with the balloon-expandable system in mainland China. Further clinical evidence and longer follow-up duration are required to further explore the clinical value and general possibility of introducing TAVI procedure in the developing world with an increasing aging population.
| Authors’ roles & responsibilities | |
|---|---|
| QL | Analysis and/or interpretation of data; statistical analysis; final manuscript approval; implementation of projects and or experiments; manuscript writing or critical review of its content |
| YP | Final manuscript approval; implementation of projects and/ or experiments; manuscript writing or critical review of its content |
| HW | Final manuscript approval; implementation of projects and/ or experiments; manuscript writing or critical review of its content |
| ZW | Final manuscript approval; implementation of projects and/ or experiments; manuscript writing or critical review of its content |
| JZ | Final manuscript approval; study design; implementation of projects and/or experiments; manuscript writing or critical review of its content |
ACKNOWLEDGEMENTS
This study was supported by the 1255 Discipline Construction Program of the Shanghai Changhai Hospital (grant CH12550300), and the Health Care Program of the General Logistics Department of the Chinese People's Liberation Army (grant 13BJZ30). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the manuscript writing; or in the decision to submit the article for publication. All authors confirmed that we had full control of the design and methods of the study, the data analysis and production of the written report.
Funding Statement
This study was supported by the 1255 Discipline Construction Program of the Shanghai Changhai Hospital (grant CH12550300), and the Health Care Program of the General Logistics Department of the Chinese People’s Liberation Army (grant 13BJZ30). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the manuscript writing; or in the decision to submit the article for publication.
Footnotes
This study was carried out at Shanghai Changhai Hospital, China.
Financial support: This study was supported by the 1255 Discipline Construction Program of the Shanghai Changhai Hospital (grant CH12550300), and the Health Care Program of the General Logistics Department of the Chinese People’s Liberation Army (grant 13BJZ30). The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the manuscript writing; or in the decision to submit the article for publication.
REFERENCES
- 1.Boudoulas H. Etiology of valvular heart disease. Expert Rev Cardiovasc Ther. 2003;1(4):523–532. doi: 10.1586/14779072.1.4.523. [DOI] [PubMed] [Google Scholar]
- 2.Jang SY, Ju EY, Seo SR, Choi JY, Park SJ, Kim DK, et al. Changes in the etiology of valvular heart disease in the rapidly aging Korean population. Int J Cardiol. 2014;174(2):355–359. doi: 10.1016/j.ijcard.2014.04.112. [DOI] [PubMed] [Google Scholar]
- 3.Clark MA, Duhay FG, Thompson AK, Keyes MJ, Svensson LG, Bonow RO, et al. Clinical and economic outcomes after surgical aortic valve replacement in Medicare patients. Risk Manag Healthc Policy. 2012;5:117–126. doi: 10.2147/RMHP.S34587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhimin W, Yubao Z, Lei S, Xianliang Z, Wei Z, Li S, et al. Prevalence of chronic rheumatic heart disease in Chinese adults. Int J Cardiol. 2006;107(3):356–359. doi: 10.1016/j.ijcard.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Holmes DR, Jr, Mack MJ, Kaul S, Agnihotri A, Alexander KP, Bailey SR, et al. 2012 ACCF/AATS/SCAI/STS expert consensus document on transcatheter aortic valve replacement. J Am Coll Cardiol. 2012;59(13):1200–1254. doi: 10.1016/j.jacc.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Dor I, Pichard AD, Gonzalez MA, Weissman G, Li Y, Goldstein SA, et al. Correlates and causes of death in patients with severe symptomatic aortic stenosis who are not eligible to participate in a clinical trial of transcatheter aortic valve implantation. Circulation. 2010;122(11) Suppl:S37–S42. doi: 10.1161/CIRCULATIONAHA.109.926873. [DOI] [PubMed] [Google Scholar]
- 7.Coffey S, Cox B, Williams MJ. The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. Pt AJ Am Coll Cardiol. 2014;63(25):2852–2861. doi: 10.1016/j.jacc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 8.Schwarz F, Baumann P, Manthey J, Hoffmann M, Schuler G, Mehmel HC, et al. The effect of aortic valve replacement on survival. Circulation. 1982;66(5):1105–1110. doi: 10.1161/01.cir.66.5.1105. [DOI] [PubMed] [Google Scholar]
- 9.Murphy ES, Lawson RM, Starr A, Rahimtoola SH. Severe aortic stenosis in patients 60 years of age or older: left ventricular function and 10-year survival after valve replacement. Pt 2Circulation. 1981;64(2):II184–II188. [PubMed] [Google Scholar]
- 10.Pei HJ, Wu YJ, Yang YJ, Xu B, Chen JL, Qiao SB, et al. Current treatment status in patients with severe aortic valve stenosis and outcome of long term follow-up at advanced age: a Chinese single center study. Chin Med J (Engl) 2011;124(18):2879–2882. [PubMed] [Google Scholar]
- 11.Bakaeen FG, Chu D, Huh J, Carabello BA. Is an age of 80 years or greater an important predictor of short-term outcomes of isolated aortic valve replacement in veterans? Ann Thorac Surg. 2010;90(3):769–774. doi: 10.1016/j.athoracsur.2010.04.066. [DOI] [PubMed] [Google Scholar]
- 12.Thourani VH, Ailawadi G, Szeto WY, Dewey TM, Guyton RA, Mack MJ, et al. Outcomes of surgical aortic valve replacement in high-risk patients: a multiinstitutional study. Ann Thorac Surg. 2011;91(1):49–55. doi: 10.1016/j.athoracsur.2010.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Andersen HR, Knudsen LL, Hasenkam JM. Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. Eur Heart J. 1992;13(5):704–708. doi: 10.1093/oxfordjournals.eurheartj.a060238. [DOI] [PubMed] [Google Scholar]
- 14.Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002;106(24):3006–3008. doi: 10.1161/01.cir.0000047200.36165.b8. [DOI] [PubMed] [Google Scholar]
- 15.Leon MB, Gada H, Fontana GP. Challenges and future opportunities for transcatheter aortic valve therapy. Prog Cardiovasc Dis. 2014;56(6):635–645. doi: 10.1016/j.pcad.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Osnabrugge RL, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, LeReun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol. 2013;62(11):1002–1012. doi: 10.1016/j.jacc.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Abdel-Wahab M, Mehilli J, Frerker C, Neumann FJ, Kurz T, Tölg R, et al. CHOICE investigators Comparison of balloon-expandable vs self-expandable valves in patients undergoing transcatheter aortic valve replacement: the CHOICE randomized clinical trial. JAMA. 2014;311(15):1503–1514. doi: 10.1001/jama.2014.3316. [DOI] [PubMed] [Google Scholar]
- 18.Pontes JC, Duarte JJ, Silva AD, Gardenal N, Dias AM, Benfatti RA, et al. Initial and pioneer experience of transcatheter aortic valve implantation (Inovare) through femoral or iliac artery. Rev Bras Cir Cardiovasc. 2013;28(2):208–216. doi: 10.5935/1678-9741.20130030. [DOI] [PubMed] [Google Scholar]
- 19.Gaia DF, Palma JH, Ferreira CB, Souza JA, Agreli G, Guilhen JC, et al. Transapical aortic valve implantation: results of a Brazilian prosthesis. Rev Bras Cir Cardiovasc. 2010;25(3):293–302. doi: 10.1590/s0102-76382010000300004. [DOI] [PubMed] [Google Scholar]
- 20.Mabin TA, Condolfi P. An analysis of real-world cost-effectiveness of TAVI in South Africa. Cardiovasc J Afr. 2014;25(1):21–26. doi: 10.5830/CVJA-2013-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maqbool S, Kumar V, Rastogi V, Seth A. Transcatheter aortic valve implantation under conscious sedation - the first Indian experience. Indian Heart J. 2014;66(2):208–210. doi: 10.1016/j.ihj.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge J, Zhou D, Pan W, Wang Z, Ge L, Pan C, et al. Initial experience of percutaneous aortic valve replacement in China. Chin J Intervent Cardiol. 2010;8:243–256. [Google Scholar]
- 23.Wu Y. China report of the development on aging cause (2013) Beijing: Social Sciences Academic Press; 2013. [Google Scholar]
- 24.Grube E, Naber C, Abizaid A, Sousa E, Mendiz O, Lemos P, et al. Feasibility of transcatheter aortic valve implantation without balloon pre-dilation: a pilot study. JACC Cardiovasc Interv. 2011;4(7):751–757. doi: 10.1016/j.jcin.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, et al. Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: a European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve. Circulation. 2010;122(1):62–69. doi: 10.1161/CIRCULATIONAHA.109.907402. [DOI] [PubMed] [Google Scholar]
- 26.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, et al. Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol. 2010;55(11):1080–1090. doi: 10.1016/j.jacc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Holmes DR, Jr, Brennan JM, Rumsfeld JS, Dai D, O'Brien SM, Vemulapalli S, et al. STS/ACC TVT Registry Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA. 2015;313(10):1019–1028. doi: 10.1001/jama.2015.1474. [DOI] [PubMed] [Google Scholar]
- 28.Walther T, Hamm CW, Schuler G, Berkowitsch A, Kötting J, Mangner N, et al. GARY Executive Board Perioperative results and complications in 15,964 transcatheter aortic valve replacements: prospective data from the GARY Registry. J Am Coll Cardiol. 2015;65(20):2173–2180. doi: 10.1016/j.jacc.2015.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, et al. Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: the U.K. Transcatheter Aortic Valve Implantation Registry. JACC Cardiovasc Interv. 2015;8(5):645–653. doi: 10.1016/j.jcin.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 31.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. PARTNER Trial Investigators Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 32.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. PARTNER Trial Investigators Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366(18):1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 33.Mack MJ, Brennan JM, Brindis R, Carroll J, Edwards F, Grover F, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310(19):2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 34.Kim CA, Rasania SP, Afilalo J, Popma JJ, Lipsitz LA, Kim DH. Functional status and quality of life after transcatheter aortic valve replacement: a systematic review. Ann Intern Med. 2014;160(4):243–254. doi: 10.7326/M13-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bajona P, Suri RM, Greason KL, Schaff HV. Outcomes of surgical aortic valve replacement: the benchmark for percutaneous therapies. Prog Cardiovasc Dis. 2014;56(6):619–624. doi: 10.1016/j.pcad.2014.02.004. [DOI] [PubMed] [Google Scholar]