Abstract
MicroRNAs (miRNAs) are small noncoding RNAs that act as posttranscriptional regulators of gene expression, and are frequently altered in human neoplasias. Here, we have analyzed the miRNA expression profile of human gonadotroph adenomas versus normal pituitary tissue using a miRNACHIP microarray. We demonstrate that miRNA-410 is downregulated in gonadotroph adenomas when compared with normal pituitary gland. We validate CCNB1 as target of miRNA-410 since its overexpression reduces CCNB1 at protein and mRNA levels, decreasing cell proliferation. In conclusion, our study suggess that the downregulation of miRNA-410 plays a role in the behavior of gonadotroph tumors.
Keywords: cyclin B1, miRNA-410, pituitary adenomas
Introduction
Pituitary adenomas (PA) represent about 10% of all intracranial neoplasia with an estimated clinical prevalence in the general population of approximately 1/1000.1,2 Virtually all organs or tissues are affected directly or indirectly by the hormones secreted by the anterior pituitary.3,4 Pituitary adenomas result of a multistep process in which the multi-causal and inherited genetic predisposition, endocrine factors, and specific somatic mutations are factors that may contribute to their development.5,6 Gonadotroph adenomas constitute the vast majority of clinically non-functioning pituitary adenomas, which account for 15–22% of all pituitary adenomas overall1,2 and up to 57% of those developing in male patients.2 They are diagnosed primarily in middle-aged patients because of tumor mass effects. Due to the lack of specific pharmacological therapy, surgery remains the first line treatment in affected patients. As other pituitary adenomas, the gonadotroph adenomas are monoclonal in origin meaning that genetic alterations in a single cell confer proliferative capacity.
MicroRNAs (miRNAs) are small noncoding RNAs acting as posttranscriptional regulators of gene expression. They repress target gene expression by binding of the 3′ untranslated regions (UTRs) of target genes, through imperfect or perfect base pairing, resulting in translational repression or mRNA destabilization and degradation, respectively.7 Many studies have indicated that miRNAs are associated with cancer being involved in cell proliferation, invasion and metastases.8 Thus, miRNAs are likely to be useful as diagnostic and prognostic cancer biomarkers.9
Altered miRNA expression in pituitary adenomas has been already reported, suggesting a critical role in pituitary tumorigenesis. Indeed, Cheng et al. reported that inhibition of the expression of miRNAs, miR-150, miR-152, miR-191 and miR-192 (upregulated in pituitary adenomas) suppresses cell growth, suggesting that overexpression of these miRNAs may result in increased proliferation of pituitary cells.6,10 Moreover, we have previously shown alterations of miRNA expression in GH-secreting adenomas vs. normal pituitary gland. Most of the altered miRNAs had HMGA1 and/or HMGA2, and E2F1 as target genes11,12 which has been already proved to play a critical role in pituitary tumorigenesis. In fact, HMGA proteins are overexpressed in most of the human pituitary adenomas.13,14 Moreover transgenic mice overexpressing these genes developed mixed PRL/GH pituitary adenomas15 and increased E2F1 activity or expression can lead to pituitary tumorigenesis.16,17 Furthermore, Leone et. al. have demonstrated that miRNA-23b and miRNA-130b are downregulated in pituitary adenomas, and these miRNAs target genes such as HMGA2 and CCNA2, whose overexpression plays a critical role in pituitary tumorigenesis, suggesting a possible therapy of pituitary adenomas based on the restoration of downregulated miRNAs.18
In the present study we have analyzed the miRNA expression profile of 12 gonadotroph adenomas. We found 57 miRNAs downregulated and 44 upregulated with a fold change ≥2 in gonadotroph adenomas in comparison with the normal gland.
Then, we focused our attention on miR-410, one of the most downregulated miRNAs in our series, which was reported to behave as tumor suppressor gene in glioma targeting the MET gene.19 We demonstrated that CCNB1 gene, an important cell cycle regulator involved in pituitary tumor behavior, is a target of miRNA-410. Moreover, the restoration of miRNA-410 expression in pituitary adenoma cells resulted in the inhibition of cell proliferation.
Results
miRNA expression profile of gonadotroph pituitary adenomas
We have analyzed the miRNA expression profile of 12 gonadotroph adenomas vs. 3 normal pituitary glands using a miRNACHIP microarray.20 By applying biostatistical analyses we obtained a list of miRNAs differently expressed (p < 0.05) between gonadotroph adenomas and normal pituitary. As shown in Table 1, 57 miRNAs were downregulated and 44 were upregulated in the neoplastic tissues when compared with normal pituitary with a fold change ≥2. To validate the results obtained by miRNACHIP we evaluated the expression of 2 downregulated miRNAs, miR-432 and miR-410, and other 2 upregulated, miR-374b and miR-17, by qRT-PCR on 21 samples of gonadotroph adenomas, including those used for the microarray analysis (Fig. 1).
Table 1.
miRNA expression profile of Gonadotroph adenomas vs. normal pituitary
| miRNAs downregulated in Gonadotroph adenomas vs. normal pituitary |
miRNAs upregulated in Gonadotroph adenomas vs. normal pituitary |
||
|---|---|---|---|
| Fold-change | UniqueID | Fold-change | UniqueID |
| 0.054 | hsa-miR-539 | 2.08 | hsa-miR-135b |
| 0.059 | hsa-miR-154* | 2.18 | hsa-miR-128a |
| 0.077 | hsa-miR-410 | 2.2 | hsa-miR-601 |
| 0.081 | hsa-miR-411* | 2.21 | hsa-let-7e* |
| 0.087 | hsa-miR-376a | 2.24 | hsa-miR-25 |
| 0.087 | hsa-miR-495 | 2.25 | hsa-miR-181a |
| 0.093 | hsa-miR-148a | 2.31 | hsa-miR-100 |
| 0.1 | hsa-miR-432 | 2.36 | hsa-miR-17 |
| 0.13 | hsa-miR-801 | 2.36 | hsa-miR-532-3p |
| 0.14 | hsa-miR-560 | 2.51 | hsa-miR-181c |
| 0.15 | hsa-miR-134 | 2.58 | hsa-mir-571 |
| 0.19 | hsa-miR-433 | 2.69 | hsa-mir-196a-1 |
| 0.19 | hsa-miR-661 | 2.72 | hsa-miR-30e* |
| 0.21 | hsa-miR-708* | 2.91 | hsa-mir-135a-2 |
| 0.21 | hsa-miR-645 | 2.92 | hsa-miR-151-3p |
| 0.21 | hsa-miR-656 | 2.94 | hsa-miR-99b |
| 0.21 | hsa-miR-607 | 3.08 | hsa-miR-192 |
| 0.22 | hsa-miR-24 | 3.14 | hsa-miR-30a* |
| 0.22 | hsa-miR-369-3p | 3.16 | hsa-miR-181d |
| 0.22 | hsa-miR-452 | 3.28 | hsa-miR-378 |
| 0.23 | hsa-miR-376b | 3.33 | hsa-miR-532-5p |
| 0.23 | hsa-miR-663 | 3.36 | hsa-miR-34b |
| 0.24 | hsa-miR-214* | 3.4 | hsa-miR-454 |
| 0.25 | hsa-miR-96* | 3.43 | hsa-miR-125a-5p |
| 0.25 | hsa-mir-566 | 3.48 | hsa-miR-340 |
| 0.26 | hsa-mir-640 | 3.49 | hsa-miR-502-5p |
| 0.27 | hsa-miR-323-5p | 3.64 | hsa-miR-182 |
| 0.28 | hsa-miR-494 | 3.65 | hsa-miR-374b |
| 0.28 | hsa-mir-636 | 3.7 | hsa-mir-376b |
| 0.29 | hsa-miR-486-5p | 4.04 | hsa-miR-374a |
| 0.29 | hsa-miR-127-3p | 4.19 | hsa-miR-628-3p |
| 0.3 | hsa-miR-570 | 4.6 | hsa-miR-345 |
| 0.31 | hsa-miR-431 | 4.65 | hsa-miR-616* |
| 0.32 | hsa-mir-365-1 | 4.67 | hsa-miR-423-3p |
| 0.34 | hsa-miR-147b | 4.74 | hsa-miR-660 |
| 0.34 | hsa-miR-638 | 4.85 | hsa-miR-122* |
| 0.34 | hsa-mir-619 | 5.07 | hsa-miR-215 |
| 0.35 | hsa-miR-380 | 5.69 | hsa-miR-28-5p |
| 0.36 | hsa-mir-801 | 5.74 | hsa-miR-183 |
| 0.36 | hsa-mir-212 | 6.12 | hsa-miR-625* |
| 0.37 | hsa-miR-193a-5p | 6.54 | hsa-miR-196b |
| 0.4 | hsa-miR-566 | 7.97 | hsa-miR-582-5p |
| 0.42 | hsa-miR-30c-2* | 10.97 | hsa-miR-140-3p |
| 0.42 | hsa-miR-671-5p | 15.53 | hsa-miR-137 |
| 0.44 | hsa-miR-608 | ||
| 0.44 | hsa-miR-636 | ||
| 0.45 | hsa-miR-579 | ||
| 0.45 | hsa-mir-600 | ||
| 0.45 | hsa-mir-133b | ||
| 0.45 | hsa-miR-92b* | ||
| 0.46 | hsa-mir-345 | ||
| 0.47 | hsa-miR-596 | ||
| 0.5 | hsa-miR-654-3p | ||
| 0.52 | hsa-miR-483-3p | ||
| 0.53 | hsa-miR-632 | ||
| 0.54 | hsa-miR-372 | ||
| 0.57 | hsa-miR-557 | ||
Figure 1.
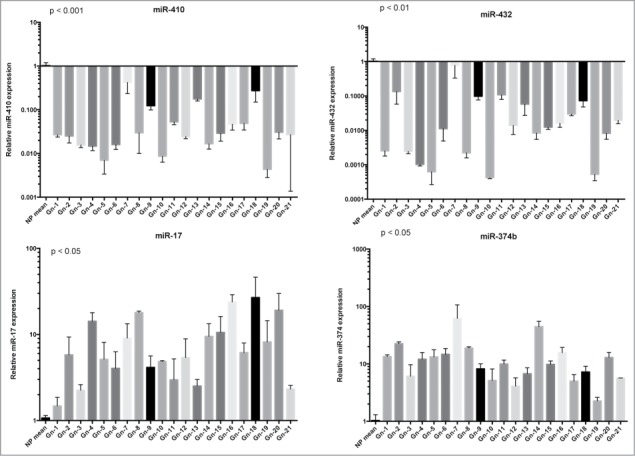
Validation of miRNA microarray data by qRT–PCR. qRT-PCR analysis of miR-410, miR-432, miR-17 and miR-374 expression in gonadotroph pituitary adenomas. The relative expression values indicate the relative change in the expression levels between 21 gonadotroph adenoma vs. the mean of tree normal pituitary samples. Each bar represents the mean value ± S.D. from independent experiments performed in triplicate. Asterisks indicate statistical significance compared with control cells.
Subsequently, we decided to focus our attention on miR-410, which is one of the most downregulated miRNAs. First of all, in order to investigate whether miR-410 downregulation was a general event in pituitary tumors, we examined its expression in 12 PRL and 12 GH-secreting adenoma samples by qRT-PCR. As shown in Figure 2, miR-410 was found to be upregulated in almost all prolactinomas as well as in 6 out of the 12 GH-secreting adenomas. This finding suggests that a reduced miR-410 expression seems to be restricted to gonadotroph adenomas.
Figure 2.
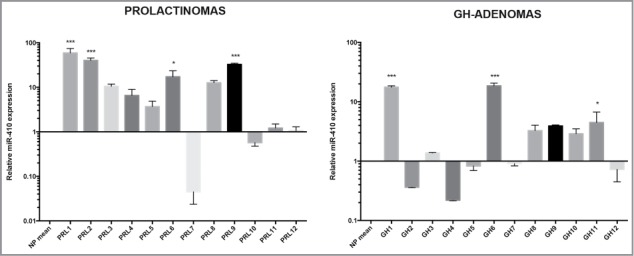
miR-410 expression in Prolactinomas and GH-secreting adenomas: qRT-PCR analysis of miR-410 in 12 samples of Prolactinomas and 12 GH-secreting adenomas compared with tree normal pituitary samples. The asterisks indicate statistical significance compared with control cells.
CCNB1 is target of miR-410
To understand the role of miRNA-410 in the pituitary tumorigenesis, we search for the potential targets of this miRNA using bioinformatic tools (MiRwalk, miRanda and TargetScan). Interestingly, among the candidate targets of miR-410 we found several genes coding for proteins involved in the cell cycle regulation such as cyclin A, cyclin B1, cyclin D and CDKs. We decided to focus our attention on CCNB1 gene coding for the cyclin B1 protein since it is a regulatory protein essential for the cell cycle regulatory machinery and is directly implicated in the G2/M transition,21 and it is well known that the cell cycle dysregulation plays a critical role in the development of pituitary adenomas.22
In Figure 3A we report the miRNA-targeting sites of miRNA-410 on CCNB1 3′UTR (www.targetscan.org). To validate the influence of the miRNA-410 on CCNB1 expression, we analyzed, by western blot, the CCNB1 protein levels in HEK-293 cells, 48 and 72 hours after transfection with miRNA-410 or scrambled oligonucleotide. As shown in Figure 3B, CCNB1 protein levels were significantly decreased when compared to the scrambled-transfected cells. Interestingly, this reduction in CCNB1 protein levels was associated to a decrease in the CCNB1 specific mRNA levels, both at 48 and 72 hours, suggesting that miRNA-410 regulates CCNB1 protein expression, at least in part, through CCNB1 mRNA degradation (Fig. 3C).
Figure 3.
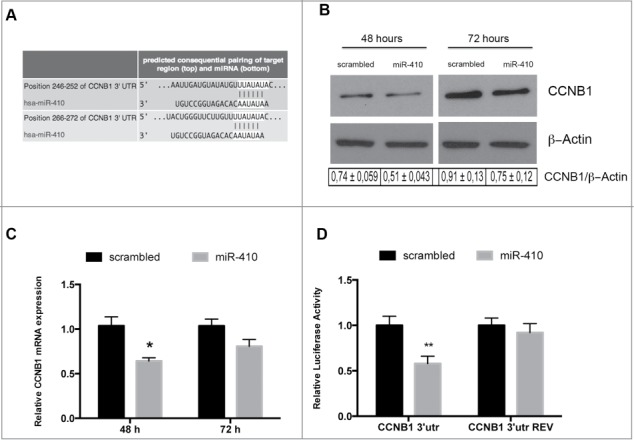
miR-410 targets CCNB1. (A) Representation of human CCNB1 3′-UTR and the relative position of the predicted miRNA-binding sites (http://www.targetscan.org) (B) Western blots analysis of the CCNB1 protein expression level in HEK-293 cells transfected with miR-410 or a scrambled oligonucleotide and collected at 48 and 72 hours after transfection. β-actin expression was used for normalization control. The densitometric analysis was performed using ImageJ software and normalizing to β-actin. (C) qRT–PCR analysis of CCNB1 mRNA in HEK-293 cells transfected and collected at the indicated times. Expression values indicate the relative change in CCNB1 mRNA expression levels between miRNA-treated or scrambled oligonucleotide-treated, normalized with G6PD, assuming that value of the scrambled oligonucleotide-treated sample was equal to 1. The error bars represent the mean value ± S.D. *p<0,05 compared to scrambled oligonucleotideo-transfected cells using Student t-test analysis (D) Relative luciferase activity in HEK-293 cells transiently transfected with Luc-CCNB1–3′UTR or with Luc-CCNB1–3′UTR-REV along with miRNA-410 or scrambled oligonucleotide. The relative activity of firefly luciferase expression was standardized to a transfection control, using Renilla luciferase. The scale bars represent the mean ± S.D. of at least three independent experiments performed in triplicate. **p < 0.01 compared to scrambled oligonucleotide-transfected cells.
In order to verify that a direct interaction between this miRNA and the CCNB1 mRNA is responsible for the decrease in CCNB1 protein levels, we inserted the 3′UTR of CCNB1, downstream the luciferase ORF, in sense and antisense orientation (CCNB1–3′UTR and CCNB1–3′UTR REV). The luciferase activity of the CCNB1–3′UTR was significantly reduced after transfection with miRNA-410, compared with the scrambled oligonucleotide, while the luciferase activity of CCNB1–3′UTR REV construct did not decrease. This result demonstrates that the inhibition of CCNB1 protein expression by miR-410 is dependent on its direct binding to the CCNB1 gene 3′UTR (Fig. 3D).
miRNA-410 inhibits cell proliferation
To examine the role of miRNA-410 downregulation in pituitary cell behavior we analyzed its effect on cell proliferation, performing a cell growth curve in HEK-293 cells transiently transfected with miRNA-410 or scrambled oligonucleotide. Because miRNA-410 targeting sites on CCNB1 are not conserved in rat or mouse, and, since human pituitary cell lines are not available, the effects of miRNA were not analyzed in a pituitary adenoma system. As shown in Figure 4A a significant decrease of cell number was observed 72 and 96 hours after transfection with miRNA-410 when compared with control cells. The same result was obtained when HEK-293 cells were stably transfected with a pMIRNA expression vector carrying miR-410 precursor, compared with the empty vector ones (Fig. 4B).
Figure 4.
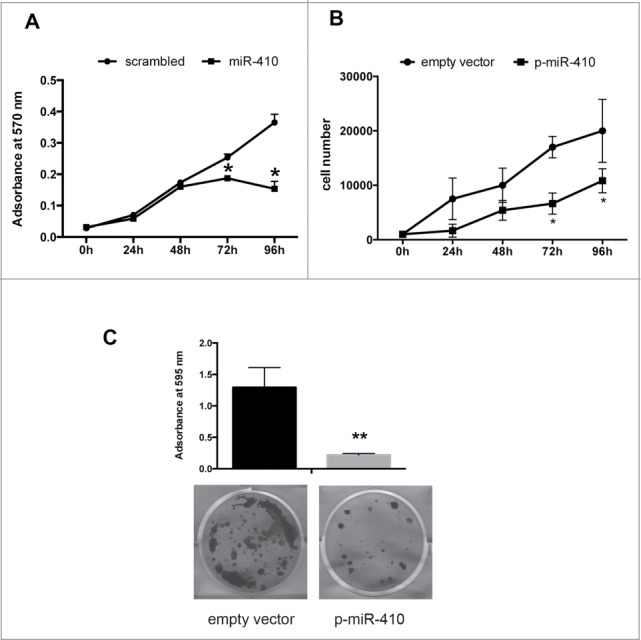
miR-410 inhibits cell proliferation. Cell growth curve of HEK-293 cells transiently transfected with miR-410 or scrambled oligonucleotide (A) and stably transfected with a pMIRNA expression vector carrying miR-410 precursor or with empty vector (B) and determined with an MTT assay. Cell growth was evaluated at 0, 24, 48, 72 and 96 h after plating and adsorbance was measured at 570 nm. The results were shown as mean ± SD of at least 3 independent experiments performed in triplicate. (C) Colony-forming assay performed on HEK-293 cells transfected with a p-miR-410 expression vector or with the empty vector.
Next, we performed a colony forming assay on HEK-293 cells after transfection with p-miR-410 expression vector. As shown in Figure 4C, cells transfected with p-miR-410 generated a significant lower number of colonies in comparison with the backbone vector-transfected cells, thus confirming the negative role of miR-410 in cell growth regulation.
CCNB1 mRNA overexpression is associated with miR-410 downregulation in human gonadotroph adenomas
To better evaluate the role of miR-410 on its target gene also in vivo, we analyzed the CCNB1 mRNA expression in gonadotroph adenoma samples by qRT-PCR. As shown in Figure 5, we found that CCNB1 mRNA is overexpressed in all samples, compared with normal pituitary, suggesting an opposite behavior between the expression of miR-410 and its target gene. In this Figure the Spearman's correlation value between CCNB1 and miR-410 expression is also reported.
Figure 5.
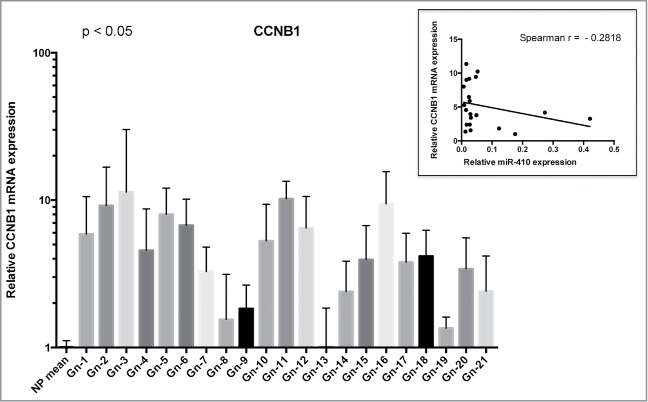
CCNB1 expression in gonadotroph pituitary tumors correlates with mir-410 downregulation. qRT–PCR analysis of CCNB1 mRNA levels in the same gonadotroph tumors of Figure 1 compared with normal pituitary tissue (3 samples). The bars indicate the relative change in the expression levels between adenomas and normal pituitary samples, assuming that the mean value of normal pituitary tissue is equal to 1. The scale bars represent the mean ± SD. The Spearman correlation is shown in the panel above indicating an inverse behavior between the expression of CCNB1 and miR-410.
Discussion
In this study we first analyzed the miRNA expression profile of 12 gonadotroph adenomas vs. 3 normal pituitary glands. We found that 44 miRNAs were upregulated and 57 downregulated in the gonadotroph samples, with a fold-change higher than 2. Then, representative miRNAs have been validated by qRT-PCR in a higher number (21 samples) of gonadotroph adenomas. Among the upregulated miRNAs we found the miRNA-17 that has been previously demonstrated to target the p21 protein. Then, its upregulation would decrease the p21 WAF1/CIP1 (also known as cyclin-dependent kinase inhibitor 1) protein level, thereby enhancing the G1-S transition. Indeed, p21 protein binds to and inhibits the activity of cyclin-CDK2, -CDK1 and CDK4/6 complexes and, then, functions as a modulator-regulator of cell cycle progression at G1 and S phase. On the other hand previous studies have evidenced a role of the decrease in p21 (CIP1) levels in pituitary tumorigenesis, since it has been reported that p21 is able to restrain pituitary tumor growth also activating senescence program.23
Among the downregulated miRNAs we focused our attention on the miR-410 that was one of the most downregulated miRNAs. Interestingly, it results upregulated in almost all prolactinomas and in half of the all GH-secreting adenomas analyzed, suggesting that the downregulation of miR-410 is specific of gonadotroph adenomas. In agreement with our data, miR-410 was previously found downregulated in NFPAs (nonfunctioning pituitary adenomas) adenomas, within the DLK1-MEG3 locus, which contains one of the largest miRNA clusters in humans, and its expression has been correlated with MEG3 (maternally expressed gene 3), a maternally expressed noncoding RNA frequently lost in clinically NFPAs of gonadotroph origin.24
Moreover, other previous studies have evidenced a tumor suppressor activity for this miRNA. Indeed, miR-410 has been found downregulated gliomas19 and gastric carcinomas 25 where lower miRNA-410 expression levels significantly correlated with the presence of lymph-node metastasis. Consistently, maintenance of miR-410 expression is associated with a better overall survival in advanced serous ovarian cancer.26 The miR-410 oncosupressor activity appears due to its ability to negatively regulate MET and MDM2 protein levels. More recently, miR-410 tumor suppressor activity has been confirmed in pancreatic cancer where miR-410 expression decreases cell growth, migration, invasion and angiogenesis via the downregulation of AGTR1.27
Then, the next step of our work has been to identify candidate target genes that could account for the role of miR-410 downregulation in pituitary tumors. Among the potential targets we focused on the CCNB1 gene. Indeed, we previously demonstrated that another member of cyclin B family, CCNB2, was increased in pituitary adenomas with respect to normal pituitary14 and that its expression was mainly regulated by the HMGA1 and HMGA2 proteins whose overexpression represents an important event in pituitary adenomas as also demonstrated by the development of GH/PRL adenomas in transgenic mice overexpressing these genes.15 Moreover, cyclin B1 regulates the G2/M transition in the cell cycle, and its appropriate regulation is essential for mitosis initiation.28 Therefore, a deregulated expression of CCNB1 could play a critical role in pituitary tumor development, since the regulation of the cell cycle is a complex network that includes many regulatory molecules, and the deregulation of the cell cycle and its effectors is the key mechanism in pituitary tumors. In fact, it has been demonstrated that several cell cycle regulators such as Rb 1, E2F1, p16, p21, p27 and different cyclins can play a crucial role in pituitary tumors.29,30 Moreover, recent findings have reported an aberrant expression of cyclin B1 in several malignant cancers, including breast,21 squamous cell esophageal,31 non small cell lung,32 gastric33 and hepatocellular carcinomas.34
We have validated CCNB1 as target of miR-410 since its overexpression reduces CCNB1 mRNA and protein levels, suggesting that miRNA-410 acts also enhancing CCNB1 mRNA degradation. Then, functional studies seem to confirm a critical role of miR-410 downregulation in tumor progression since its overexpression leads to a reduced cell growth.
The analysis of 17 gonadotroph adenomas revealed an opposite behavior between miR-410 and CCNB1 expression even though the correlation was slightly statistically significant. However, this is not surprising, since the expression level of a protein gene is mainly depending on the transcription factors, gene amplification and only partially by miRNA and other non coding RNAs, then a complete statistical significance is not expected.
It is worth to note that among the potential targets, assessed by bioinformatic analysis, but not validated yet, there are cyclin A, cyclin D and CDKs that have a critical role in cell cycle regulation. Therefore, miR-410 downregulation might contribute to the generation or progression of pituitary adenomas leading to incresead cyclin A and D protein levels affecting then the G1-S phase of the cell cycle.
Interestingly, in contrast with the reduced expression of miR-410 in gonadotroph tumors and several human carcinomas, the upregulation miRNA-410 has been found in almost PRL adenomas tested and in some GH-secreting adenomas, and it was already reported in esophageal carcinomas.35 This result is reasonable since it has been frequently demonstrated that miRNA may have oncogenic or tumor suppressor activity depending on the cell context.36
In our previous works we have identified other miRNAs that are downregulated in gonadotroph adenomas starting from the analysis of GH-secreting adenomas. Some of these have been confirmed by the analysis of the profile of gonadotroph adenomas. Studies performed by other groups revealed different dysregulated miRNAs. This is not surprising since the differences in sample preparation, platform for miRNA screening and normal pituitaries used as controls might account for these discrepancies.
In conclusion, our study indicates that the deregulated expression of miRNAs has a role in the development of pituitary tumors and proposes that the downregulation of miR-410 may be involved in the pathogenesis of the gonadotroph adenomas, suggesting the perspective of using the restoration of miR-410 expression as tool for the therapy of such tumors.
Methods
Tissue collection and RNA isolation
We obtained the gonadotroph and the GH-secreting adenomas with acromegaly from patients operated by E Jouanneau, Service de Neurochirurgie U300, Groupement Hospitalier Est, Hospices Civils de Lyon. Some fragments of each tumor were frozen in liquid nitrogen and stored at −80°C until RNA extraction. Total RNA extraction was performed with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions
RNA of 12 PRL tumors was extracted from formalin-fixed paraffin-embedded (FFPE) tissue of patients operated at Department of Neurosurgery, Ospedale Cannizzaro, Catania, Italy. The miRNeasy FFPE Kit (Qiagen) was used to purify miRNAs extracted from FFPE tissues, according to the manufacturer's instructions.
RNAs from human normal pituitary glands obtained from autopsies of 2 females and one male, aged between 40–50 years, deprived of endocrine diseases, were used as control. We declare that informed consent for the scientific use of biological material was obtained from all patients.
miRNACHIP microarray
The microarray analysis, RNA labeling, and hybridization on miRNA microarray chip, were performed as previously described.20 The miRNAs differentially expressed in pituitary adenoma tissue samples were analyzed using Student's t test procedure. The results of the data present in the microarray were analyzed also by significance analysis of microarray test. Each sample was analyzed in triplicate for miRNA expression profile.
Bioinformatic prediction of miRNA target genes
For the identification of genes potentially targeted by the analyzed miRNAs we used different on-line available tools such as TargetScan (www.targetscan.org), MiRwalk (www.umm.uni-heidelberg.de/apps/zmf/mirwalk) and miRanda (www.microrna.org) at their latest release.
Reverse transcription and quantitative real-time (qRT)-PCR
For qRT-PCR assay the total RNA was extracted from cells or tissues using TRIzol. Reverse transcription was performed with miScript system kit (QIAGEN, Valencia, CA), according to manufacturer's instructions. To calculate the relative expression levels we used the 2−ΔΔCT method.37 Primers for small nucleolar RNA (U44) were used for normalization. The primers used to amplify the above mentioned gene are:
CCNB1 F: ACATGGTGCACTTTCCTCCT
CCNB1 R: AGGTAATGTTGTAGAGTTGGTGTCC
Cell lines and transfection
Human Embryonic Kidney HEK-293 cells were cultured in DMEM containing 10% fetal bovine serum, 1% glutamine and 1% penicillin/streptomycin (Life Technologies, Monza, Italy), and were incubated at 37°C in a 5% CO2 atmosphere. Cell transfections of miRNA oligonucleotides were performed with 50 nmol/ml pre-miRNA precursors or control no-targeting scrambled oligonucleotides (Ambion), using siPORT neoFX Transfection Agent (Ambion).
Plasmids and constructs
The region 87–587 bp of the CCNB1–3′-UTR (full length 612 bp), including binding sites for miR-410, was cloned both in sense and in antisense orientation into the pmirGLO Dual-Luciferase Expression Vector (Promega) at the XbaI site immediately downstream from the stop codon of the luciferase gene.
Luciferase target assay
Cells were co-transfected with the modified firefly luciferase vectors and the miRNA oligonucleotides. Firefly and renilla luciferase activities were measured 48 hours after transfection with the dual-luciferase reporter system (Promega, Madison, WI). Firefly activity was normalized to renilla activity as control of transfection efficiency.
Protein extraction, protein gel blotting and antibodies
For protein extraction, cells were lysed in lysis buffer containing 1% NP-40, 1 mM EDTA, 50 mM Tris-HCl (pH 7.5) and 150 mM NaCl, supplemented with complete protease inhibitors mixture (Roche, Monza, Italy). Protein concentration was determined by the Bradford assay (Bio-Rad) using bovine serum albumin as standard.
Total proteins were separated on a 12% SDS-PAGE and transferred to nitrocellulose membranes (GE Healthcare, Milano, Italy). Membranes were blocked with 5% non-fat dry milk. After blotting, membranes were incubated with primary antibodies against CCNB1 (sc-245, Santa Cruz) and β-actin (sc-1615, Santa Cruz). The reaction was detected with a Western blotting detection system (Thermo Scientific, Rockville, IL).
Growth curve assay
Cells were transfected with 50 nmol/ml pre-miR miRNA precursor or scrambled oligonucleotide using siPORT neoFX, and with p-miR-410 expression vector or with the backbone vector, and plated in 24-well plates. Cell growth was assessed using an MTT (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) assay, at 0, 24, 48, 72 and 96 h after plating.
Colony-forming assay
HEK-293 cells were transfected with p-miR-410 expression vector or with the backbone vector (OriGene, Rockville, MD). The transfected cells were selected by using 1 μg/ml neomycin diluted in the medium used for culture. After 10 days, the cells were fixed and stained with 0.1% crystal violet in 20% methanol and colonies were evaluated.
Statistical analysis
Student's t-test and ANOVA test were used to determine the significance for the quantitative experiments. All error bars represent the standard deviation (SD) of the average. Statistical significance for all the tests, assessed by calculating the p-value, was <0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants from AIRC (IG 11477) and CNR Epigenomics Flagship Project “EPIGEN.” Paula Müssnich is recipient of a fellowship from CAPES Foundation, Ministry of Education of Brazil, Brasilia DF 70040-020, Brazil (Proc. n° BEX 9772-13-8).
References
- 1.Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab 2006; 91:4769-75. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16968795; PMID:16968795; http://dx.doi.org/ 10.1210/jc.2006-1668 [DOI] [PubMed] [Google Scholar]
- 2.Fernandez A, Karavitaki N, Wass JAH. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol 2010; 72:377-82; PMID:19650784; http://dx.doi.org/ 10.1111/j.1365-2265.2009.03667.x [DOI] [PubMed] [Google Scholar]
- 3.Asa SL, Ezzat S. The pathogenesis of pituitary tumors. Annu Rev Pathol 2009; 4:97-126. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19400692; PMID:19400692; http://dx.doi.org/ 10.1146/annurev.pathol.4.110807.092259 [DOI] [PubMed] [Google Scholar]
- 4.Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. The prevalence of pituitary adenomas: a systematic review. Cancer 2004; 101:613-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15274075; PMID:15274075; http://dx.doi.org/ 10.1002/cncr.20412 [DOI] [PubMed] [Google Scholar]
- 5.Asa SL, Ezzat S. The pathogenesis of pituitary tumours. Nat Rev Cancer 2002; 2:836-49. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12415254; PMID:12415254; http://dx.doi.org/ 10.1038/nrc926 [DOI] [PubMed] [Google Scholar]
- 6.Jaffrain-Rea ML, Rotondi S, Alesse E. New insights in the pathogenesis of pituitary tumours In: Monica F. Ed. Hot Topics in Endocrine and Endocrine-Related Diseases, 1st Ed. InTech 2013: 27-84. [Google Scholar]
- 7.Bartel DP, Lee R, Feinbaum R. MicroRNAs: genomics, biogenesis, mechanism, and function genomics: the miRNA genes. Cell 2004; 116:281-97; PMID:14744438 [DOI] [PubMed] [Google Scholar]
- 8.Bottoni A, Zatelli MC, Ferracin M, Tagliati F, Piccin D, Vignali C, Calin GA, Negrini M, Croce CM, Uberti ECD. Identification of differentially expressed microRNAs by microarray: a possible role for microRNA genes in pituitary adenomas. J Cell Physiol 2007; 377:370-7; PMID:17111382; http://dx.doi.org/ 10.1002/jcp.20832 [DOI] [PubMed] [Google Scholar]
- 9.Fabbri M, Croce CM, Calin GA. MicroRNAs. Cancer J; 14:1-6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18303474 [DOI] [PubMed] [Google Scholar]
- 10.Cheng AM, Byrom MW, Shelton J, Ford LP. Antisense inhibition of human miRNAs and indications for an involvement of miRNA in cell growth and apoptosis. Nucleic Acids Res 2005; 33:1290-7. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=552951&tool=pmcentrez&rendertype=abstract; PMID:15741182; http://dx.doi.org/ 10.1093/nar/gki200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Angelo D, Palmieri D, Mussnich P, Roche M, Wierinckx A, Raverot G, Fedele M, Croce CM, Trouillas J, Fusco A. Altered microRNA expression profile in human pituitary GH adenomas: down-regulation of miRNA targeting HMGA1, HMGA2, and E2F1. J Clin Endocrinol Metab 2012; 97:E1128-38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22564666; PMID:22564666; http://dx.doi.org/ 10.1210/jc.2011-3482 [DOI] [PubMed] [Google Scholar]
- 12.Palmieri D, D'Angelo D, Valentino T, De Martino I, Ferraro A, Wierinckx A, Fedele M, Trouillas J, Fusco A. Downregulation of HMGA-targeting microRNAs has a critical role in human pituitary tumorigenesis. Oncogene 2011; 1-9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22139073; PMID:22139073; http://dx.doi.org/ 10.1038/onc.2011.557 [DOI] [PubMed] [Google Scholar]
- 13.Finelli P, Pierantoni GM, Giardino D, Losa M, Rodeschini O, Fedele M, Valtorta E, Mortini P, Croce CM, Larizza L, et al.. The high mobility group A2 gene is amplified and overexpressed in human prolactinomas. Cancer Res 2002; 62:2398-405. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11956103; PMID:11956103 [PubMed] [Google Scholar]
- 14.De Martino I, Visone R, Wierinckx A, Palmieri D, Ferraro A, Cappabianca P, Chiappetta G, Forzati F, Lombardi G, Colao A, et al.. HMGA proteins up-regulate CCNB2 gene in mouse and human pituitary adenomas. Cancer Res 2009; 69:1844-50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19223528; PMID:19223528 [DOI] [PubMed] [Google Scholar]
- 15.Fedele M, Pentimalli F, Baldassarre G, Battista S, Klein-Szanto AJP, Kenyon L, Visone R, De Martino I, Ciarmiello A, Arra C, et al.. Transgenic mice overexpressing the wild-type form of the HMGA1 gene develop mixed growth hormone/prolactin cell pituitary adenomas and natural killer cell lymphomas. Oncogene 2005; 24:3427-35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15735694; PMID:15735694; http://dx.doi.org/ 10.1038/sj.onc.1208501 [DOI] [PubMed] [Google Scholar]
- 16.Fedele M, Visone R, De Martino I, Troncone G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C, Melillo RM, et al.. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell 2006; 9:459-71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16766265; PMID:16766265; http://dx.doi.org/ 10.1016/j.ccr.2006.04.024 [DOI] [PubMed] [Google Scholar]
- 17.Yamasaki L, Bronson R, Williams BO, Dyson NJ, Harlow E, Jacks T. Loss of E2F-1 reduces tumorigenesis and extends the lifespan of Rb1(+/−)mice. Nat Genet 1998; 18:360-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9537419; PMID:9537419; http://dx.doi.org/ 10.1038/ng0498-360 [DOI] [PubMed] [Google Scholar]
- 18.Leone V, Langella C, D'Angelo D, Mussnich P, Wierinckx A, Terracciano L, Raverot G, Lachuer J, Rotondi S, Jaffrain-Rea M-L, et al.. miR-23b and miR-130b expression is downregulated in pituitary adenomas. Mol Cell Endocrinol 2014; 390:1-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23603256; PMID:24681352 [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Zhang J, Feng Y, Li R, Sun X, Du W, Piao X, Wang H, Yang D, Sun Y, et al.. MiR-410 regulates MET to influence the proliferation and invasion of glioma. Int J Biochem Cell Biol 2012; 44:1711-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22750473; PMID:22750473 [DOI] [PubMed] [Google Scholar]
- 20.Liu C-G, Calin GA, Meloon B, Gamliel N, Sevignani C, Ferracin M, Dumitru CD, Shimizu M, Zupo S, Dono M, et al.. An oligonucleotide microchip for genome-wide microRNA profiling in human and mouse tissues. Proc Natl Acad Sci U S A 2004; 101:9740-4. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=470744&tool=pmcentrez&rendertype=abstract; PMID:15210942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niméus-Malmström E, Koliadi A, Ahlin C, Holmqvist M, Holmberg L, Amini R-M, Jirström K, Wärnberg F, Blomqvist C, Fernö M, et al.. Cyclin B1 is a prognostic proliferation marker with a high reproducibility in a population-based lymph node negative breast cancer cohort. Int J Cancer 2010; 127:961-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19957331 [DOI] [PubMed] [Google Scholar]
- 22.Fedele M, Palmieri D, Fusco A. HMGA2: a pituitary tumour subtype-specific oncogene? Mol Cell Endocrinol 2010; 326:19-24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20347930; PMID:20347930 [DOI] [PubMed] [Google Scholar]
- 23.Chesnokova V, Zonis S, Kovacs K, Ben-Shlomo A, Wawrowsky K, Bannykh S, Melmed S. p21(Cip1) restrains pituitary tumor growth. Proc Natl Acad Sci U S A 2008; 105:17498-503. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2577704&tool=pmcentrez&rendertype=abstract; PMID:18981426; http://dx.doi.org/ 10.1073/pnas.0804810105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheunsuchon P, Zhou Y, Zhang X, Lee H, Chen W, Nakayama Y, Rice KA, Tessa Hedley-Whyte E, Swearingen B, Klibanski A. Silencing of the imprinted DLK1-MEG3 locus in human clinically nonfunctioning pituitary adenomas. Am J Pathol 2011; 179:2120-30; PMID:21871428; http://dx.doi.org/ 10.1016/j.ajpath.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen J, Niu W, Zhou M, Zhang H, Ma J, Wang L, Zhang H. MicroRNA-410 suppresses migration and invasion by targeting MDM2 in gastric cancer. PLoS One 2014; 9:e104510. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4138091&tool=pmcentrez&rendertype=abstract; PMID:25136862; http://dx.doi.org/ 10.1371/journal.pone.0104510 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Shih KK, Qin L-X, Tanner EJ, Zhou Q, Bisogna M, Dao F, Olvera N, Viale A, Barakat RR, Levine DA. A microRNA survival signature (MiSS) for advanced ovarian cancer. Gynecol Oncol 2011; 121:444-50. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21354599; PMID:21354599; http://dx.doi.org/ 10.1016/j.ygyno.2011.01.025 [DOI] [PubMed] [Google Scholar]
- 27.Guo R, Gu J, Zhang Z, Wang Y, Gu C. MicroRNA-410 functions as a tumor suppressor by targeting angiotensin II type 1 receptor in pancreatic cancer. IUBMB Life 2015; 67:42-53; http://doi.wiley.com/10.1002/iub.1342 [DOI] [PubMed] [Google Scholar]
- 28.Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci U S A 1999; 96:2147-52. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=26751&tool=pmcentrez&rendertype=abstract; PMID:10051609; http://dx.doi.org/ 10.1073/pnas.96.5.2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrell WE. Epigenetic mechanisms of tumorigenesis. Horm Metab Res 2005; 37:361-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16001328; PMID:16001328; http://dx.doi.org/ 10.1055/s-2005-870153 [DOI] [PubMed] [Google Scholar]
- 30.Melmed S. Pathogenesis of pituitary tumors. Nat Rev Endocrinol 2011; 7:257-66. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21423242; PMID:21423242; http://dx.doi.org/ 10.1038/nrendo.2011.40 [DOI] [PubMed] [Google Scholar]
- 31.Murakami H, Furihata M, Ohtsuki Y, Ogoshi S. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch 1999; 434:153-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10071250; PMID:10071250; http://dx.doi.org/ 10.1007/s004280050319 [DOI] [PubMed] [Google Scholar]
- 32.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, Mao L. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res 2000; 60:4000-4. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10945597; PMID:10945597 [PubMed] [Google Scholar]
- 33.Begnami MD, Fregnani JHTG, Nonogaki S, Soares FA. Evaluation of cell cycle protein expression in gastric cancer: cyclin B1 expression and its prognostic implication. Hum Pathol 2010; 41:1120-7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20334896; PMID:20334896; http://dx.doi.org/ 10.1016/j.humpath.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 34.Calvisi DF, Simile MM, Ladu S, Frau M, Evert M, Tomasi ML, Demartis MI, Daino L, Seddaiu MA, Brozzetti S, et al.. Activation of v-Myb avian myeloblastosis viral oncogene homolog-like2 (MYBL2)-LIN9 complex contributes to human hepatocarcinogenesis and identifies a subset of hepatocellular carcinoma with mutant p53. Hepatology 2011; 53:1226-36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21480327; PMID:21480327; http://dx.doi.org/ 10.1002/hep.24174 [DOI] [PubMed] [Google Scholar]
- 35.Zhao B-S, Liu S-G, Wang T-Y, Ji Y-H, Qi B, Tao Y-P, Li H-C, Wu X-N. Screening of microRNA in patients with esophageal cancer at same tumor node metastasis stage with different prognoses. Asian Pac J Cancer Prev 2013; 14:139-43. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23534712; PMID:23534712 [DOI] [PubMed] [Google Scholar]
- 36.Schickel R, Boyerinas B, Park S-M, Peter ME. MicroRNAs: key players in the immune system, differentiation, tumorigenesis and cell death. Oncogene 2008; 27:5959-74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18836476; PMID:18836476; http://dx.doi.org/ 10.1038/onc.2008.274 [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11846609; PMID:11846609; http://dx.doi.org/ 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]


