The development of distant metastases in vital organs is the most deadly aspect of cancer. This is mainly due to most metastatic cancers being inoperable, and treatment options for the later stages of the disease mainly aim at prolonging life rather than curing the patients. Thus, we need a better understanding of cancer progression in order to develop better treatment strategies for these patients. Metastasis is, however, a complex multi-step process that requires a yet unknown number of cellular changes. The most fundamental steps include a local invasion, intravasation, survival in the circulation, extravasation, and finally colonization and proliferation at the new site. Characterization of the features distinguishing cancer cells with metastatic capabilities from those without may enable the discovery of new diagnostic and prognostic biomarkers as well as therapeutic targets. Unfortunately, this task is not straightforward (Fig. 1). We believe that comparisons of primary tumor tissues with matched surgically resected metastases are instrumental for this endeavor, however, comparisons with relevant cancer cells without metastatic capabilities are of equal importance.
Figure 1.
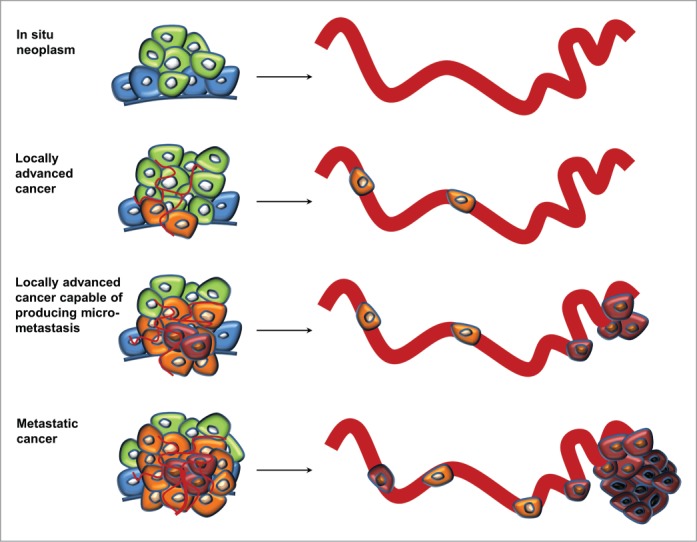
Simplified model of somatic evolution during cancer progression. Cancer cells are characterized by molecular alterations (indicated as color changes of the cells) leading to increased proliferation, such as inactivation of growth-suppressing- and pro-apoptotic genes, as well as activation of growth promoting oncogenes. The pace of the evolution is fuelled by defects in DNA repair genes or modifiers of the epigenome. These alterations may be regarded as indirect drivers of metastasis, and only additional alterations directly linked to the multi-step process of metastasis may distinguish cancer cells with metastatic capabilities from those without. As these alterations often precede the presence of detectable metastases, their characterization is not straightforward.
In our recent study1 of the engulfment and cell motility 3 (ELMO3) gene in primary tumors with matched surgically resected brain- and adrenal gland metastases from non-small cell lung cancer (NSCLC) patients we put great effort into assembling a control cohort of NSCLC patients without metastases. To account for the potential risk of these patients having occult metastasis, we only selected patients with at least five years of recurrence-free survival following surgery. Importantly, the control cohort and the cohort with distant metastases were matched for relevant confounders such as age, gender, histology, T-stage, smoking status, and the proportion of tumor cells within the tissue blocks. Since the specimens were formalin-fixed and paraffin-embedded (FFPE) we initially performed a study aiming at developing and validating a complete workflow for expression studies using FFPE tissues.2 When studying ELMO3 expression within the NSCLC cohorts we found significantly higher mRNA levels within the primary tumors from the patients with metastases compared to the primary tumors from metastasis-free patients. Moreover, when comparing tumor tissue with normal lung tissue ELMO3 was found to be overexpressed, and the highest gene expression levels were found in the metastatic lesions.
Because ELMO3 has a CpG island in its promoter region and methylation of CpG islands have been associated with transcriptional silencing of the corresponding genes, we went on to semi-quantitatively investigate the methylation levels of ELMO3. High methylation levels were observed in all normal lung tissue samples and most primary tumors from patients without metastases, whereas significantly lower levels of methylation were observed within the primary tumors from patients with metastases as well as within the metastases. Notably these changes would not have been observed by the widely used conventional methylation-specific PCR (MSP) technique, as it is purely qualitative. As expected we also observed a highly significant correlation between lower methylation levels and increased expression. Moreover, the methylation levels in the primary tumors were strongly associated with the methylation levels within the matched metastases, though a tendency toward even lower methylation levels in the metastatic lesions were observed.
Because ELMO3 is significantly higher expressed in tumors from patients with distant metastases compared to tumors from patients without, we believe that ELMO3 could be a direct driver of cancer metastasis. In addition, the higher expression levels and the lower methylation levels observed within the metastases compared to the primary tumors may suggest that not all of the primary cancer cells have this molecular alteration, and thus may not be able to metastasize.
However, in order to classify a gene as a direct driver of cancer metastasis, functional studies should support its involvement in at least one of the steps within the multi-step process of metastasis. In mammalian cells ELMO proteins have been shown to play important roles in cytoskeleton rearrangements during phagocytic clearance of apoptotic cells and cell migration and the formation of an ELMO/DOCK180 complex seems to be vital for Rac-induced cell polarization and migration.3 While ELMO3 has been less well studied in cancer, it has been shown that ELMO1 is involved in migration and invasion of breast cancer cells, but not required for cell division.4 We hypothesize that ELMO3 is silenced by promoter methylation in normal lung epithelial cells as they do not need to migrate, while cancer cells acquire this ability by demethylating the ELMO3 promoter. However, evidence from experimental models of metastasis would be required to establish if ELMO3 is a direct driver of cancer metastasis.
An interesting aspect of our ELMO3 study is that the gene becomes hypomethylated during cancer progression. Since most CpG islands are unmethylated in normal tissues, the most common gene-associated methylation change observed in cancer is hypermethylation resulting in gene silencing. This has encouraged the development of demethylating agents, such as decitabine and azacitidine, which has been approved for treatment of myelodysplastic syndrome and acute myeloid leukemia with low blast counts.5 These drugs are also likely to be approved for treatment of solid tumors, alone or in combination with other drugs, as several clinical trials with promising results have been reported, including a study of NSCLC.6 Therefore, we believe that clinical trials investigating demethylating drugs should monitor the methylation levels of proto-oncogenes or oncomiRs observed to undergo demethylation during cancer progression, such as ELMO3 and miR135b,7 respectively, since these events could potentially lead to relapse or unwanted side-effects.
References
- 1. Soes S, et al. Oncoscience 2014; 1:367-74.25594031 [Google Scholar]
- 2. Soes S, et al. Lung Cancer 2013; 81:180-6; PMID:23643276; http://dx.doi.org/ 10.1016/j.lungcan.2013.04.007 [DOI] [PubMed] [Google Scholar]
- 3. Patel M, et al. Small GTPases 2011; 2:268-75; PMID:22292130; http://dx.doi.org/ 10.4161/sgtp.2.5.17716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li H, et al. Nat Commun 2013; 4:1706; PMID:23591873; http://dx.doi.org/ 10.1038/ncomms2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Treppendahl MB, et al. J Clin Invest 2014; 124:47-55; PMID:24382389; http://dx.doi.org/ 10.1172/JCI69737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juergens RA, et al. Cancer Disc 2011; 1:598-607; PMID:22586682; http://dx.doi.org/ 10.1158/2159-8290.CD-11-0214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin CW, et al. Nat Commun 2013; 4:1877; PMID:23695671; http://dx.doi.org/ 10.1038/ncomms2876 [DOI] [PubMed] [Google Scholar]


