Abstract
The Zuotin-related factor 1, ZRF1, has recently been identified as an epigenetic regulator of gene transcription in stem cells and cancer. During differentiation of human teratocarcinoma cells, ZRF1 promotes transcriptional induction of developmental genes that are repressed by Polycomb complexes. Importantly, ZRF1 has recently been shown to be required for both neural differentiation of embryonic stem cells (ESCs) and for maintenance of neural progenitor cell (NPC) identity. Moreover, a dual role has now emerged for ZRF1 in cancer: on the one hand, ZRF1 plays a crucial role in oncogene-induced senescence (OIS) by activating the INK4/ARF locus, thus working as a tumor suppressor; on the other hand, ZRF1 promotes leukemogenesis in acute myeloid leukemia (AML) in a Polycomb-independent fashion. Therefore, increasing evidence points to ZRF1 as a novel target for therapy of neurodegenerative diseases and cancer.
Keywords: cancer, cell fate, development, differentiation, epigenetics, polycomb, retinoic acid, senescence, stem cell, transcription, ZRF1
Abbreviations
- AML
acute myeloid leukemia
- ChIP
chromatin immunoprecipitation
- ESC
embryonic stem cells
- HDAC
histone deacetylase
- H2Aub1
mono-ubiquitinated histone H2A
- NPC
neural progenitor cells
- OIS
oncogene-induced senescence
- PRC1
polycomb repressive complex 1
- PRC2
polycomb repressive complex 2
- RA
retinoic acid
- RARa
retinoic acid receptor a
- UBD
ubiquitin binding domain
Introduction
Epigenetic regulatory mechanisms, such as DNA methylation and histone post-translational modifications, are essential for maintaining cell identity, and alterations in these processes are implicated in multiple diseases, including cancer.1 Histone modifications play a crucial role in regulating gene expression, both by directly controlling chromatin structure and by acting as docking sites for proteins involved in gene transcription.2 The Polycomb group of proteins are repressors of gene transcription that work in part by decorating histones with repressive marks (such as di- and tri-methylation of histone H3 and mono-ubiquitination of H2A), leading to chromatin compaction and transcription impairment.3 Organized in multiprotein complexes termed Polycomb repressive complexes 1 and 2 (PRC1 and PRC2), Polycomb proteins play a crucial role in several processes including cell proliferation, X chromosome inactivation, and senescence4 and are misregulated in several types of cancer.5,6 Importantly, PRC1 and PRC2 are master regulators of stem cell identity. In ESCs, PRC1 and PRC2 help to maintain cell identity by repressing developmental genes in all 3 germ layers (i.e. endoderm, mesoderm, and ectoderm). In line with this, depletion of Polycomb proteins impairs ESC differentiation2,4,7,8 and leads to embryonic lethality.8
The Zuotin-related factor 1, ZRF1 (also termed MPP11, MIDA1, and DNAJC2), has recently emerged as regulator of the expression of Polycomb targets in stem cells and cancer. Indeed, it was reported that ZRF1 promotes Polycomb displacement and transcriptional activation of Polycomb-repressed genes in specific conditions.9,10 In this review, recent reports elucidating the role of human/mouse ZRF1 in stem cells and cancer will be discussed.11-14 Notably, several evidences demonstrate that ZRF1 plays an important role in the control of the expression of master genes and signaling pathways governing stem cell identity and carcinogenesis.
The Domain Structure of ZRF1 Reflects its Diverse Functions Along Evolution
Several domains have been annotated in the ZRF1 protein (Fig. 1). The amino-terminal part of the protein, which is conserved from yeast to mammals, contains a DnaJ domain. The DnaJ domain mediates interactions with heat shock proteins and is mainly implicated in protein-chaperone functions associated with ribosomes. Consistently, the ZRF1 homolog in yeast, Zuotin (also called Zuo1), works as a molecular chaperone associated to ribosomes.15 Apart from its role as a chaperone, Zuotin can bind to nucleic acids through its DnaJ domain16,17 and has been suggested to have a role in transcriptional regulation.18,19
Figure 1.
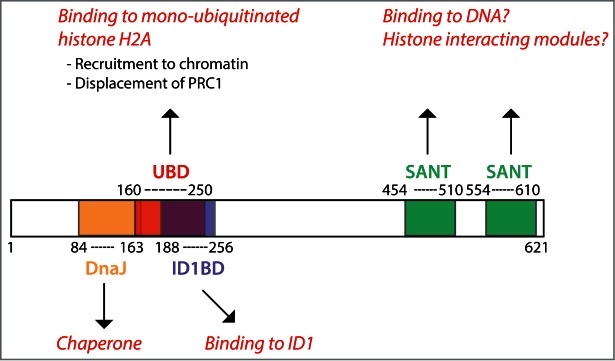
In its amino-terminus, ZRF1 contains a DnaJ domain that is mainly responsible for its role as a chaperone associated to ribosomes. A region slightly overlapping with the DnaJ domain and extending toward the carboxy-terminus contains the ubiquitin-binding domain (UBD), which is responsible for recognizing mono-ubiquitinated histone H2A (HA2ub1), an histone mark deposited by PRC1. Interestingly, the UBD overlaps with the ID1-binding domain, suggesting that ID1 binding impairs ZRF1 recognition of H2Aub1. The carboxy-terminus contains 2 SANT domains of yet unknown function.
In higher eukaryotes, the protein structure of ZRF1 contains an additional C-terminal extension with 2 SANT domains (Fig. 1). SANT domains are similar to the Myb-DNA binding motif,20 which is required for Myb proto-oncogene to bind DNA. Indeed, it has been suggested that ZRF1 can directly bind to DNA.21,22 Importantly, SANT domains have been identified in multiple proteins associated to chromatin.20
ZRF1 has been shown to have important functions in several model organisms. In the green algae Volvox, the ZRF1 homolog GlsA is essential for asymmetric cell division and germ cell specification.23-25 In C. elegans, the ZRF1 homolog dnj-11 plays an important role in asymmetric cell division of neuroblasts by regulating the activity of the Snail-related factor26,27 and thus controlling the stress response.28
In mammals, ZRF1 (which is widely expressed both in the embryo and in the adult29) was recently found to play a crucial role in epigenetic regulation of gene expression as a chromatin-associated factor. The first indication that ZRF1 could work as chromatin-bound protein came from affinity purification assays designed to identify proteins capable of binding to mono-ubiquitinated histone H2A (H2Aub1), a repressive mark deposited by PRC1.10 In this study, a fraction of the amino-terminal region of ZRF1, annotated as ubiquitin-binding domain (UBD), was found to be sufficient for its binding to H2Aub1 (Fig. 1). The presence of 2 SANT domains further supports the role of ZRF1 as a chromatin-bound protein, although their specific functions remain to be addressed.
ZRF1 Regulates Gene Transcription as a Chromatin-Associated Factor
The identification of ZRF1 as a protein capable of recognizing H2Aub1 suggested a functional link between ZRF1 and Polycomb complexes. Indeed, in vitro data indicate that ZRF1 is able to displace PRC1 from chromatin by competing for the H2Aub1 mark.10 For in vivo studies, stem cell–like human teratocarcinoma NTera2 cells, clone D1 (NT2D1), were either grown in proliferating conditions or treated with retinoic acid (RA) to induce differentiation.30 Chromatin immunoprecipitation (ChIP)-on-Chip experiments demonstrated that, upon differentiation, ZRF1 is recruited to the promoters of developmental genes that were previously occupied by Polycomb in proliferating conditions, thus suggesting that ZRF1 and PRC1 also compete for H2Aub1 binding in vivo. Importantly, Polycomb occupancy on target genes remains high during differentiation in the absence of ZRF1, further demonstrating that ZRF1 plays a crucial role in displacing Polycomb complexes from chromatin and therefore in gene activation.10 Once bound to chromatin, ZRF1 promotes (at least in vitro) removal of the H2Aub1 mark together with the ubiquitin specific peptidase, USP21. In line with this, during differentiation, the induction of developmental genes regulated by Polycomb is impaired in the absence of ZRF1.10 Therefore, a dual function for H2Aub1 has been suggested: as repressor in basal conditions, and as docking site for ZRF1, which is necessary for transcriptional activation during differentiation (reviewed in Ref.9).
That ZRF1 has a crucial function in transcriptional activation of developmental genes is underscored by its activity as an essential regulator for neural commitment of ESCs11,12 (see below). In addition, ZRF1 controls oncogene-induced senescence in a Polycomb dependent manner,14 while it regulates leukemogenesis through a Polycomb independent mechanism13 (see below).
The Role of ZRF1 in Stem Cells
ZRF1 is required for neural differentiation of mouse ESCs
ZRF1 has been reported to be essential for neural differentiation of mouse ESCs.11,12 ESCs are self-renewing, pluripotent cells, capable of generating all cell types of the embryo in vivo and in vitro.31 In self-renewing mouse ESCs, ZRF1 binding to chromatin is impaired by its direct interaction with the inhibitor of differentiation 1 (ID1).12 ID1 belongs to the ID family of factors (namely ID1/2/3/4), which function as dominant negative proteins by sequestering their binding partner.32 In ESCs, ID1 controls the maintenance of the self-renewing state and inhibits neural differentiation. In line with this, over-expression of ID1 impairs ESC differentiation.33-36 Importantly, the region of ZRF1 that interacts with ID1 partially overlaps with UBD (the domain involved in H2Aub1 recognition),10 consistent with the low number of Zrf1 chromatin-binding sites observed in self-renewing ESCs. ID1 expression levels rapidly decrease in differentiation conditions, thereby allowing ZRF1 binding to chromatin.12 During differentiation, ZRF1 is specifically located on promoters of genes involved in the specification of neural progenitor cells (NPCs). Once bound to chromatin, ZRF1 drives the transcriptional activation of NPC markers by displacing Polycomb repressive complexes from chromatin, thus making ZRF1 essential for neural fate specification from ESCs in a Polycomb-dependent fashion (Fig. 2A). In line with this, ZRF1 overexpression rescues neural differentiation in ESCs stably overexpressing ID1. Moreover, ZRF1 depletion rescues the expression of Polycomb targets involved in neural specification, which are upregulated in Id1 knock-out ESCs. Therefore, ZRF1 is essential for neural fate specification by activating neural genes blocked by ID1 in ESCs (Fig. 2A).12
Figure 2.
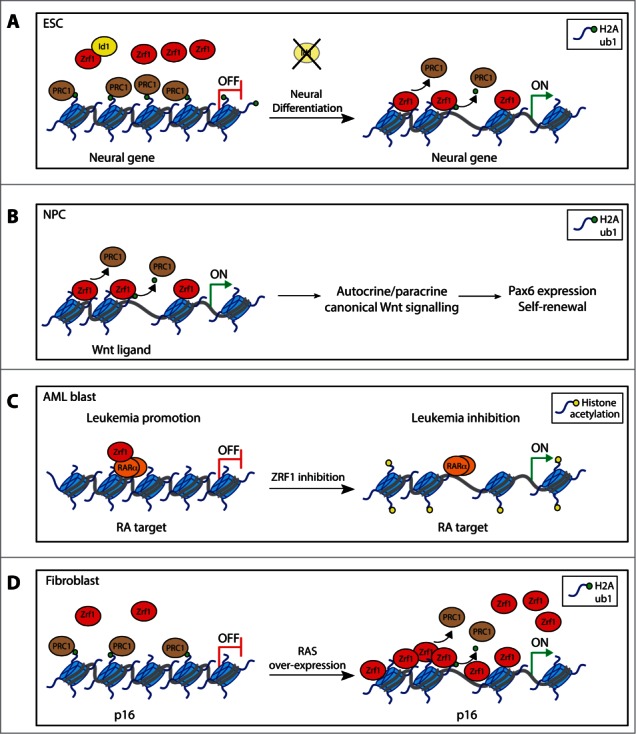
(A) In self-renewing ESCs, the direct interaction between ZRF1 and ID1 impairs ZRF1 binding to chromatin. During differentiation of ESCs, ZRF1 is increasingly localized to chromatin, consistent with decreased expression levels of ID1. Once recruited to chromatin, ZRF1 specifically induces the expression of neural genes involved in the specification of NPCs by displacing the Polycomb complexes. (B) In NPCs, ZRF1 regulates the expression of a specific subset of Wnt ligands in a Polycomb-dependent manner. ZRF1-regulated Wnt ligands activate an autocrine/paracrine Wnt signaling pathway, which in turn promotes Pax6 expression and sustains self-renewal of NPCs. (C) In AML cells, ZRF1 overexpression contributes to leukemia development by directly interacting with RARα to repress RA targets that are involved in the regulation of differentiation, proliferation, and apoptosis. Consequently, ZRF1 depletion leads to activation of these genes, resulting in inhibition of leukemogenesis. Gene reactivation by ZRF1 depletion is associated with increased histone acetylation. (D) Polycomb complexes repress the pro-senescence INK4/ARF locus. RAS overexpression induces ZRF1 up-regulation and thus its recruitment to the INK4/ARF locus. In this way, ZRF1 induces the expression of genes encoded by the IINK4/ARF locus, thus promoting OIS.
ZRF1 is essential for generating and maintaining NPCs of the embryonic cortex
In addition to its essential role for NPC-specification from ESCs (discussed above), the role of ZRF1 in maintaining NPC identity has also been elucidated.11 NPCs of the embryonic cortex (also termed as radial glial cells) are stem cells capable of self-renewal and differentiation into neurons and glial cells.37 Both in cell culture experiments and in vivo, ZRF1 depletion impairs NPC self-renewal and differentiation into neurons, thus making ZRF1 essential for maintenance of the identity of NPCs of the embryonic cortex.11 Consistently, the expression of the master regulator of NPCs, PAX6, is impaired in ZRF1-depleted NPCs. Moreover, ZRF1 induces the expression of a specific subset of Wnt ligand genes in a polycomb-dependent manner.11 In consequence of this, ZRF1 depletion impairs canonical Wnt signaling activity in NPCs. Of note, autocrine/paracrine canonical Wnt signaling generated by secreted Wnt ligands is known to control NPC identity.38-41 Importantly, bypassing ZRF1 regulation by treating ZRF1-depleted NPCs with recombinant Wnt ligands or with compounds that re-activate Wnt signaling, rescues Pax6 expression and the self-renewal capacity of NPCs.11 Therefore, this indicates that ZRF1 controls Pax6 expression and self-renewal of NPCs by regulating the canonical Wnt signaling pathway (Fig. 2B).
Role of ZRF1 in Cancer
A link between ZRF1 and cancer was initially suggested by the observation that the region of chromosome 7 where the ZRF1 gene is located (namely, 7q22–31) is commonly altered in several types of human cancers, including breast, prostate, pancreatic, ovarian, gastric, colon, germ cell, glioblastoma, and head and neck malignancies.42 In line with this, ZRF1 was proposed to have an oncogenic role in head and neck squamous cell carcinoma (HNSCC).42 Recent findings regarding the function of ZRF1 in acute myeloid leukemia (AML) and in oncogene-induced senescence (OIS) suggest that ZRF1 can either work as tumor suppressor or induce carcinogenesis, depending on the cellular context.13,14 One attractive hypothesis is that ZRF1 induces senescence in pre-malignant and benign lesions in tissues, whereas it promotes cancer progression in malignant cells, in which senescence-linked pathways are often reduced or completely abolished.
ZRF1 promotes leukemogenesis in AML
Several studies have shown that ZRF1 is overexpressed in leukemia, and specifically, in acute and chronic myeloid leukemia (AML and CML) and in B-cell chronic lymphocytic leukemia (CLL).43-47 In line with this, ZRF1 overexpression, a pro-leukemogenic role for ZRF1 has been recently described in AML.13 In proliferating AML cells, ZRF1 positively regulates proliferation, impairs apoptosis, and blocks differentiation. Consistently, ZRF1 depletion leads to a decrease in cell growth that results in a strong inhibition of leukemogenic potential in vivo, upon cell transplantation in mouse xenograft models.13
The differentiating agent retinoic acid (RA) induces differentiation of leukemic blasts and is currently being used to treat certain types of AMLs.48,49 Interestingly, ZRF1 directly interacts with the RA receptor α, RARα, and binds to RA-target genes, suggesting that RARα can recruit ZRF1 to chromatin.13 Consistently, genome-wide expression profiles indicate that ZRF1 depletion affects nearly half of the RA-regulated transcriptome. Moreover, as previously showed for RARα,49,50 ZRF1 has a dual role in transcriptional regulation, working mainly as a transcriptional repressor in the absence of RA and predominantly as a transcriptional activator in the presence of RA. In untreated AML cells, ZRF1 represses RA target genes that regulate proliferation, apoptosis, and differentiation, thus contributing to leukemia development. Consequently, ZRF1 depletion leads to a reactivation of these genes, resulting in an inhibition of leukemogenesis (Fig. 2C).
Supporting the functional cooperation between ZRF1 and the RA pathway, combining ZRF1 depletion and RA treatment has a synergistic effect in leukemia suppression in vivo.13 Therefore, ZRF1 inhibition, alone or in combination with RA treatment, represents a novel potential strategy for AML treatment.
Role of ZRF1 in oncogene-induced senescence
In mouse embryonic fibroblasts (MEFs) and in human fetal lung fibroblasts (IMR-90), overexpression of the RAS oncogene leads to the upregulation of ZRF1 expression at both mRNA and protein levels.14 Whereas the INK4/ARF locus (which encodes for the senescence effectors p15, p16, and p19) is repressed by Polycomb complexes in normal conditions,51 it is induced by RAS expression within a process known as oncogene-induced senescence (OIS).52 Upon RAS overexpression, ZRF1 is recruited to the INK4/ARF locus while Polycomb occupancy decreases, suggesting a Polycomb-dependent role for ZRF1 in OIS (Fig. 2D). Moreover, ZRF1 overexpression is sufficient to induce p15 and p16 expression in human and mouse fibroblasts in a RAS-independent manner. Consistently, ZRF1 depletion impairs upregulation of p16 and induces neoplastic growth of RAS overexpressing-fibroblasts, thus leading to the bypass of OIS.14
Future Perspectives
ZRF1 plays a crucial role in several processes, including stem cell maintenance, cell-fate decisions, differentiation, senescence, and carcinogenesis. In all these processes, ZRF1 regulates gene transcription as a chromatin-associated factor. At the molecular level, it has been reported that ZRF1 regulates the expression of the master neural developmental factor Pax6 and the pro-senescence INK4/ARF locus and is involved in regulating signaling pathways, such as Wnt, RA, and RAS.10-14 However, there are still several points to be addressed.
The specificity of ZRF1 binding to chromatin loci
ZRF1 is bound to chromatin in specific loci and in specific time frames. Addressing how ZRF1 specifically selects its target genes is a key point to be addressed. Several hypotheses can be proposed. First, specific protein interactions induced by different cellular contexts could drive ZRF1 binding to chromatin. Second, post-translational modifications of ZRF1 induced by different signaling pathways (such as RA in AML, RAS in OIS, and fibroblast growth factor in NPCs) could influence ZRF1 binding to target genes. Interestingly, a genome-wide phosphoproteomic study suggested that ZRF1 is phosphorylated during RA-induced differentiation in mouse P19 cells.53 Third, recognition of other histone modifications or of specific DNA sequences through the SANT domains could determine binding specificity. Therefore, proteomic analysis of ZRF1 modifications and interactors, as well as further analyses of chromatin modifications that decorate ZRF1 targets genes, could shed new light on the specificity of ZRF1 function.
Specific Polycomb-dependent activity of ZRF1 in stem cells
During differentiation of ESCs, ZRF1 specifically regulates the expression of master neural genes in a Polycomb-dependent manner.11,12 Consistently, ZRF1 depletion impairs neural differentiation but does not affect differentiation toward mesendodermal lineages.11 In contrast to the broad role of Polycomb complexes as regulators of ESC differentiation into all 3 germ layers,8 ZRF1 function in ESCs is restricted to NPC specification.11 ChIP sequencing analysis revealed that about 30% of ZRF1 targets in NPCs overlap with Polycomb targets in ESCs, with these common targets representing about 10% of all Polycomb targets in ESCs. As expected, Gene Ontology analysis revealed that these common genes are involved in neurogenesis.11 However, a significant fraction of Polycomb target genes involved in neurogenesis is not bound by ZRF1 in NPCs, indicating that: (1) ZRF1 specifically regulates a subset of genes involved in neurogenesis; and (2) Polycomb possesses a ZRF1-independent function in neurogenesis. Therefore, further investigation of ZRF1 function might unveil novel Polycomb functions in stem cells and development. As discussed above, deciphering how ZRF1 is specifically recruited to neural genes will help to understand this process.
In NPCs, ZRF1 regulates Wnt signaling activity by inducing the expression of a specific subset of Wnt ligand genes in a Polycomb-dependent manner.11 Interestingly, ZRF1-bound Wnt ligands are up-regulated in NPCs as compared to ESCs, suggesting that they exert a unique function in NPCs. Therefore, further investigation of the role of ZRF1 might unveil novel cell-specific functions of Polycomb targets.
Function of ZRF1 as a transcriptional activator and repressor
Whether the effect of ZRF1 as a transcriptional activator is only due to the removal of H2Aub1 mark or also involves direct transcriptional activation needs to be addressed. In this regard, increasing evidence (Demajo et al., unpublished) suggests that ZRF1 might interact with 2 different complexes that are known to participate in transcriptional activation: the FACT complex, which works as a histone chaperone to facilitate nucleosome remodeling during transcriptional elongation,54 and the MLL complex, which trimethylates H3K4, a mark associated with transcriptional initiation.3 Understanding whether ZRF1 functionally cooperates with the FACT complex and/or the MLL complex will help to answer the question of how it functions as a transcriptional activator.
In AML cells, ZRF1 can not only activate but also repress transcription.13 Specifically, ZRF1 represses RA target genes by regulating basal histone acetylation levels. Interestingly, RARα-mediated repression also involves the regulation of histone acetylation, and the histone deacetylases (HDACs) are fundamental components of the RARα-associated corepressor complexes.49 Further investigation is required to understand to which extent ZRF1 is involved in this complex regulatory mechanism. Additionally, the molecular mechanisms that determines whether ZRF1 works as a transcriptional activator or repressor in different genes and cellular contexts also need to be elucidated.
Downstream targets of ZRF1
A more extensive knowledge about the downstream targets of ZRF1 could help to develop novel therapeutic strategies in cancer and disease. In AML, RA and ZRF1 cooperate to regulate gene transcription.13 Identification of key common targets of RA and ZRF1 that promote leukemogenesis could lead to the development of new therapeutic strategies in AML. ChIP sequencing of ZRF1 and RARα will represent an important initial step in revealing these targets.
In NPCs, ZRF1 regulates the Wnt signaling activity by sustaining the expression of Wnt ligand genes.11 Interestingly, alterations of the Wnt signaling pathway have been reported in several neurodegenerative diseases and cancers related to neural lineages.38,39 One key target of the ZRF1-regulated Wnt ligands is the master transcription factor Pax6. Identification of additional targets of the ZRF1-regulated Wnt ligands in NPCs might open new horizons in therapy of neural cancer and neural diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We are grateful to Dr. Veronica A. Raker for critical reading of the manuscript and to members of the Di Croce laboratory for helpful discussions.
Funding
This work was supported by grants from the Spanish “Ministerio de Educación y Ciencia” (SAF2013–48926-P), AGAUR, AICR (10–0177), from the Fundación Vencer El Cáncer (VEC), and the European Commission's 7th Framework Program 4DCellFate grant number 277899 to L.DC.
References
- 1. Leeb M, Wutz A. Establishment of epigenetic patterns in development. Chromosoma 2012; 121:251-62; PMID:22427185; http://dx.doi.org/ 10.1007/s00412-012-0365-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Croce L, Helin K. Transcriptional regulation by Polycomb group proteins. Nat Struct Mol Biol 2013; 20:1147-55; PMID:24096405; http://dx.doi.org/ 10.1038/nsmb.2669 [DOI] [PubMed] [Google Scholar]
- 3. Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007; 128:735-45; PMID:17320510; http://dx.doi.org/ 10.1016/j.cell.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 4. Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell 2010; 7:299-313; PMID:20804967; http://dx.doi.org/ 10.1016/j.stem.2010.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Richly H, Aloia L, Di Croce L. Roles of the Polycomb group proteins in stem cells and cancer. Cell Death Dis 2011; 2:e204; PMID:21881606; http://dx.doi.org/ 10.1038/cddis.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piunti A, Pasini D. Epigenetic factors in cancer development: polycomb group proteins. Future Oncol 2012; 7:57-75; http://dx.doi.org/ 10.2217/fon.10.157 [DOI] [PubMed] [Google Scholar]
- 7. Schuettengruber B, Cavalli G. Recruitment of polycomb group complexes and their role in the dynamic regulation of cell fate choice. Development 2009; 136:3531-42; PMID:19820181; http://dx.doi.org/ 10.1242/dev.033902 [DOI] [PubMed] [Google Scholar]
- 8. Aloia L, Di Stefano B, Di Croce L. Polycomb complexes in stem cells and embryonic development. Development 2013; 140:2525-34; PMID: 23715546; http://dx.doi.org/21311219 10.1242/dev.091553 [DOI] [PubMed] [Google Scholar]
- 9. Richly H, Di Croce L. The flip side of the coin: role of ZRF1 and histone H2A ubiquitination in transcriptional activation. Cell Cycle 2011; 10:745-50; PMID:21311219; http://dx.doi.org/ 10.4161/cc.10.5.14795 [DOI] [PubMed] [Google Scholar]
- 10. Richly H, Rocha-Viegas L, Ribeiro JD, Demajo S, Gundem G, Lopez-Bigas N, Nakagawa T, Rospert S, Ito T, Di Croce L. Transcriptional activation of polycomb-repressed genes by ZRF1. Nature 2010; 468:1124-8; PMID:21179169; http://dx.doi.org/ 10.1038/nature09574 [DOI] [PubMed] [Google Scholar]
- 11. Aloia L, Di Stefano B, Sessa A, Morey L, Santanach A, Gutierrez A, Cozzuto L, Benitah SA, Graf T, Broccoli V, et al. Zrf1 is required to establish and maintain neural progenitor identity. Genes Dev 2014; 28:182-97; http://dx.doi.org/ 10.1101/gad.228510.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aloia L, Gutierrez A, Caballero J, Di Croce L. Direct interaction between ID1 and ZRF1 controls neural differentiation of embryonic stem cells. Embo Reports 2015; 16(1):63-70; PMID:25361733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demajo S, Uribesalgo I, Gutierrez A, Ballare C, Capdevila S, Roth M, Zuber J, Martin-Caballero J, Di Croce L. ZRF1 controls the retinoic acid pathway and regulates leukemogenic potential in acute myeloid leukemia. Oncogene 2013; 33(48):5501-10; PMID:24292673 [DOI] [PubMed] [Google Scholar]
- 14. Ribeiro JD, Morey L, Mas A, Gutierrez A, Luis NM, Mejetta S, Richly H, Benitah SA, Keyes WM, Di Croce L. ZRF1 controls oncogene-induced senescence through the INK4-ARF locus. Oncogene 2013; 32:2161-8; PMID:22733129; http://dx.doi.org/ 10.1038/onc.2012.241 [DOI] [PubMed] [Google Scholar]
- 15. Yan W, Schilke B, Pfund C, Walter W, Kim S, Craig EA. Zuotin, a ribosome-associated DnaJ molecular chaperone. EMBO J 1998; 17:4809-17; PMID:9707440; http://dx.doi.org/ 10.1093/emboj/17.16.4809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang S, Lockshin C, Herbert A, Winter E, Rich A. Zuotin, a putative Z-DNA binding protein in Saccharomyces cerevisiae. EMBO J 1992; 11:3787-96; PMID:1396572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wilhelm ML, Reinbolt J, Gangloff J, Dirheimer G, Wilhelm FX. Transfer RNA binding protein in the nucleus of Saccharomyces cerevisiae. FEBS Lett 1994; 349:260-4; PMID:8050578; http://dx.doi.org/ 10.1016/0014-5793(94)00683-0 [DOI] [PubMed] [Google Scholar]
- 18. Eisenman HC, Craig EA. Activation of pleiotropic drug resistance by the J-protein and Hsp70-related proteins, Zuo1 and Ssz1. Mol Microbiol 2004; 53:335-44; PMID:15225326; http://dx.doi.org/ 10.1111/j.1365-2958.2004.04134.x [DOI] [PubMed] [Google Scholar]
- 19. Yoshida M, Inoue T, Shoji W, Ikawa S, Obinata M. Reporter gene stimulation by MIDA1 through its DnaJ homology region. Biochem Biophys Res Commun 2004; 324:326-32; PMID:15465022; http://dx.doi.org/ 10.1016/j.bbrc.2004.09.059 [DOI] [PubMed] [Google Scholar]
- 20. Boyer LA, Latek RR, Peterson CL. The SANT domain: a unique histone-tail-binding module? Nat Rev Mol Cell Biol 2004; 5:158-63; PMID:15040448; http://dx.doi.org/ 10.1038/nrm1314 [DOI] [PubMed] [Google Scholar]
- 21. Inoue T, Shoji W, Obinata M. MIDA1 is a sequence specific DNA binding protein with novel DNA binding properties. Genes Cells 2000; 5:699-709; PMID:10971652; http://dx.doi.org/ 10.1046/j.1365-2443.2000.00362.x [DOI] [PubMed] [Google Scholar]
- 22. Inoue T, Shoji W, Obinata M. MIDA1, an Id-associating protein, has two distinct DNA binding activities that are converted by the association with Id1: a novel function of Id protein. Biochem Biophys Res Commun 1999; 266:147-51; PMID:10581180; http://dx.doi.org/ 10.1006/bbrc.1999.1779 [DOI] [PubMed] [Google Scholar]
- 23. Miller SM, Kirk DL. glsA, a Volvox gene required for asymmetric division and germ cell specification, encodes a chaperone-like protein. Development 1999; 126:649-58; PMID:9895313 [DOI] [PubMed] [Google Scholar]
- 24. Cheng Q, Pappas V, Hallmann A, Miller SM. Hsp70A and GlsA interact as partner chaperones to regulate asymmetric division in Volvox. Dev Biol 2005; 286:537-48; PMID:16168403; http://dx.doi.org/ 10.1016/j.ydbio.2005.08.028 [DOI] [PubMed] [Google Scholar]
- 25. Pappas V, Miller SM. Functional analysis of the Volvox carteri asymmetric division protein GlsA. Mech Dev 2009; 126:842-51; PMID:19646527; http://dx.doi.org/ 10.1016/j.mod.2009.07.007 [DOI] [PubMed] [Google Scholar]
- 26. Hatzold J, Conradt B. Control of apoptosis by asymmetric cell division. PLoS Biol 2008; 6:e84; PMID:18399720; http://dx.doi.org/ 10.1371/journal.pbio.0060084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan B, Memar N, Gallinger J, Conradt B. Coordination of cell proliferation and cell fate determination by CES-1 snail. PLoS Genet 2013; 9:e1003884; PMID:24204299; http://dx.doi.org/ 10.1371/journal.pgen.1003884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahu SN, Lewis J, Patel I, Bozdag S, Lee JH, Sprando R, Cinar HN. Genomic analysis of stress response against arsenic in Caenorhabditis elegans. PLoS One 2013; 8:e66431; PMID:23894281; http://dx.doi.org/ 10.1371/journal.pone.0066431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, Zhang J, Soden R, Hayakawa M, Kreiman G, et al. A gene atlas of the mouse and human protein-encoding transcriptomes. Proc Natl Acad Sci U S A 2004; 101:6062-7; PMID:15075390; http://dx.doi.org/ 10.1073/pnas.0400782101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol 1984; 103:285-93; PMID:6144603; http://dx.doi.org/ 10.1016/0012-1606(84)90316-6 [DOI] [PubMed] [Google Scholar]
- 31. Boiani M, Scholer HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol 2005; 6:872-84; PMID:16227977; http://dx.doi.org/ 10.1038/nrm1744 [DOI] [PubMed] [Google Scholar]
- 32. Ruzinova MB, Benezra R. Id proteins in development, cell cycle and cancer. Trends Cell Biol 2003; 13:410-8; PMID:12888293; http://dx.doi.org/ 10.1016/S0962-8924(03)00147-8 [DOI] [PubMed] [Google Scholar]
- 33. Romero-Lanman EE, Pavlovic S, Amlani B, Chin Y, Benezra R. Id1 maintains embryonic stem cell self-renewal by up-regulation of Nanog and repression of Brachyury expression. Stem Cells Dev 2012; 21:384-93; PMID:22013995; http://dx.doi.org/ 10.1089/scd.2011.0428 [DOI] [PubMed] [Google Scholar]
- 34. Ying QL, Nichols J, Chambers I, Smith A. BMP induction of Id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with STAT3. Cell 2003; 115:281-92; PMID:14636556; http://dx.doi.org/ 10.1016/S0092-8674(03)00847-X [DOI] [PubMed] [Google Scholar]
- 35. Davies OR, Lin CY, Radzisheuskaya A, Zhou X, Taube J, Blin G, Waterhouse A, Smith AJ, Lowell S. Tcf15 primes pluripotent cells for differentiation. Cell Rep 2013; 3:472-84; PMID:23395635; http://dx.doi.org/ 10.1016/j.celrep.2013.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malaguti M, Nistor PA, Blin G, Pegg A, Zhou X, Lowell S. Bone morphogenic protein signalling suppresses differentiation of pluripotent cells by maintaining expression of E-Cadherin. Elife 2013; 2:e01197; PMID:24347544; http://dx.doi.org/ 10.7554/eLife.01197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gage FH, Temple S. Neural stem cells: generating and regenerating the brain. Neuron 2013; 80:588-601; PMID:24183012; http://dx.doi.org/ 10.1016/j.neuron.2013.10.037 [DOI] [PubMed] [Google Scholar]
- 38. Clevers H, Nusse R. Wntbeta-catenin signaling and disease. Cell 2012; 149:1192-205; PMID:22682243; http://dx.doi.org/ 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 39. Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 2004; 20:781-810; PMID:15473860; http://dx.doi.org/ 10.1146/annurev.cellbio.20.010403.113126 [DOI] [PubMed] [Google Scholar]
- 40. Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 2002; 297:365-9; PMID:12130776; http://dx.doi.org/ 10.1126/science.1074192 [DOI] [PubMed] [Google Scholar]
- 41. Kalani MY, Cheshier SH, Cord BJ, Bababeygy SR, Vogel H, Weissman IL, Palmer TD, Nusse R. Wnt-mediated self-renewal of neural stemprogenitor cells. Proc Natl Acad Sci U S A 2008; 105:16970-5; PMID:18957545; http://dx.doi.org/ 10.1073/pnas.0808616105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Resto VA, Caballero OL, Buta MR, Westra WH, Wu L, Westendorf JM, Jen J, Hieter P, Sidransky D. A putative oncogenic role for MPP11 in head and neck squamous cell cancer. Cancer Res 2000; 60:5529-35; PMID:11034098 [PubMed] [Google Scholar]
- 43. Siegel S, Wirth S, Schweizer M, Schmitz N, Zeis M. M-phase phosphoprotein 11 is a highly immunogenic tumor antigen in patients with acute myeloid leukemia. Acta Haematol 2012; 127:193-7; PMID:22433847; http://dx.doi.org/ 10.1159/000335133 [DOI] [PubMed] [Google Scholar]
- 44. Schmitt M, Li L, Giannopoulos K, Chen J, Brunner C, Barth T, Schmitt A, Wiesneth M, Dohner K, Dohner H, et al. Chronic myeloid leukemia cells express tumor-associated antigens eliciting specific CD8+ T-cell responses and are lacking costimulatory molecules. Exp Hematol 2006; 34:1709-19; PMID:17157168; http://dx.doi.org/ 10.1016/j.exphem.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 45. Greiner J, Ringhoffer M, Taniguchi M, Li L, Schmitt A, Shiku H, Dohner H, Schmitt M. mRNA expression of leukemia-associated antigens in patients with acute myeloid leukemia for the development of specific immunotherapies. Int J Cancer 2004; 108:704-11; PMID:14696097; http://dx.doi.org/ 10.1002/ijc.11623 [DOI] [PubMed] [Google Scholar]
- 46. Greiner J, Ringhoffer M, Taniguchi M, Hauser T, Schmitt A, Dohner H, Schmitt M. Characterization of several leukemia-associated antigens inducing humoral immune responses in acute and chronic myeloid leukemia. Int J Cancer 2003; 106:224-31; PMID:12800198; http://dx.doi.org/ 10.1002/ijc.11200 [DOI] [PubMed] [Google Scholar]
- 47. Giannopoulos K, Li L, Bojarska-Junak A, Rolinski J, Dmoszynska A, Hus I, Greiner J, Renner C, Dohner H, Schmitt M. Expression of RHAMMCD168 and other tumor-associated antigens in patients with B-cell chronic lymphocytic leukemia. Int J Oncol 2006; 29:95-103; PMID:16773189 [PubMed] [Google Scholar]
- 48. Tallman MS, Andersen JW, Schiffer CA, Appelbaum FR, Feusner JH, Ogden A, Shepherd L, Willman C, Bloomfield CD, Rowe JM, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med 1997; 337:1021-8; PMID:9321529; http://dx.doi.org/ 10.1056/NEJM199710093371501 [DOI] [PubMed] [Google Scholar]
- 49. Altucci L, Gronemeyer H. The promise of retinoids to fight against cancer. Nat Rev Cancer 2001; 1:181-93; PMID:11902573; http://dx.doi.org/ 10.1038/35106036 [DOI] [PubMed] [Google Scholar]
- 50. Oren T, Sher JA, Evans T. Hematopoiesis and retinoids: development and disease. Leuk Lymphoma 2003; 44:1881-91; PMID:14738139; http://dx.doi.org/ 10.1080/1042819031000116661 [DOI] [PubMed] [Google Scholar]
- 51. Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Monch K, Minucci S, Porse BT, Marine JC, et al. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev 2007; 21:525-30; PMID:17344414; http://dx.doi.org/ 10.1101/gad.415507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mason DX, Jackson TJ, Lin AW. Molecular signature of oncogenic ras-induced senescence. Oncogene 2004; 23:9238-46; PMID:15489886 [DOI] [PubMed] [Google Scholar]
- 53. Smith JC, Duchesne MA, Tozzi P, Ethier M, Figeys D. A differential phosphoproteomic analysis of retinoic acid-treated P19 cells. J Proteome Res 2007; 6:3174-86; PMID:17622165; http://dx.doi.org/ 10.1021/pr070122r [DOI] [PubMed] [Google Scholar]
- 54. Reinberg D, Sims RJ, 3rd. de FACTo nucleosome dynamics. J Biol Chem 2006; 281:23297-301; PMID:16766522; http://dx.doi.org/ 10.1074/jbc.R600007200 [DOI] [PubMed] [Google Scholar]


