Abstract
Pseudoxanthoma elasticum (PXE) and generalized arterial calcification of infancy (GACI) are heritable ectopic mineralization disorders. Most cases of PXE and many cases of GACI harbor mutations in the ABCC6 gene. There is no effective treatment for these disorders. We explored the potential efficacy of bisphosphonates to prevent ectopic calcification caused by ABCC6 mutations by feeding Abcc6−/− mice with diet containing etidronate disodium (ETD) or alendronate sodium trihydrate (AST) in quantities corresponding to 1x, 5x, or 12x of the doses used to treat osteoporosis in humans. The mice were placed on diet at 4 weeks of age, and the degree of mineralization was assessed at 12 weeks by quantitation of the calcium deposits in the dermal sheath of vibrissae, a progressive biomarker of the mineralization, by computerized morphometry of histopathologic sections and by direct chemical assay of calcium. We found that ETD, but not AST, at the 12x dosage, significantly reduced mineralization, suggesting that selected bisphosphonates may be helpful for prevention of mineral deposits in PXE and GACI caused by mutations in the ABCC6 gene, when combined with careful monitoring of efficacy and potential side-effects.
Keywords: bisphosphonates, ectopic mineralization, generalized arterial calcification of infancy, pseudoxanthoma elasticum
Abbreviations
- PXE
pseudoxanthoma elasticum
- GACI
generalized arterial calcification of infancy
- ETD
etidronate disodium
- AST
alendronate sodium trihydrate
- KO
knock-out
- WT
wild-type
Introduction
Ectopic mineralization, characterized by deposition of calcium phosphate complexes in soft connective tissues, is associated with several diseases that affect the skin and vascular connective tissues and causes considerable morbidity and mortality. Ectopic mineralization can result from 2 types of independent processes: (a) Metastatic calcification, associated with elevated levels of calcium and/or phosphate, as exemplified by chronic renal failure; and (b) Dystrophic calcification, secondary to an insult to the tissue, as for example in autoimmune diseases and cancer. A number of factors, both genetic and environmental, have been identified contributing to the calcium phosphate deposition in these conditions (for reviews, see 1-3).
Particularly insightful information on the genetics of ectopic calcification has been obtained from studies of monogenic disorders manifesting with premature calcification of tissues, particularly the arterial blood vessels.4,5 The prototype of such conditions is pseudoxanthoma elasticum (PXE; OMIM264800), an autosomal recessive multi-system disorder characterized by calcium phosphate deposition in a number of tissues, with primary clinical manifestations in the skin, the eyes, and the cardiovascular system.6,7 The classic, late-onset and slowly progressive form of PXE is caused by mutations in the ABCC6 gene, which encodes a putative transmembrane transporter, ABCC6, expressed primarily in the liver and to a lesser extent in the kidneys, but not in the tissues directly affected by ectopic mineralization.8 Over 300 distinct loss-of-function mutations in the ABCC6 gene have been reported representing well over 1,000 mutant alleles in PXE.9 The precise function of ABCC6 under physiological homeostasis, and specifically, the nature of the transported molecule(s) are currently unknown.8 However, it has recently been suggested that functional ABCC6 is required for physiological release of ATP from cells, and loss-of-function mutations in this gene result in reduced extracellular ATP concentrations.10,11
Another ectopic mineralization disorder is generalized arterial calcification of infancy (GACI; OMIM20800), an autosomal recessive disorder usually diagnosed by prenatal ultrasound. In most cases the affected children die during the first 6 months of life from cardiovascular complications.12 GACI type 1, the classic form, is caused by mutations in the ENPP1 gene which encodes ectonucleotide pyrophosphatase phosphodiesterase (ENPP1). This enzyme hydrolyses ATP to AMP and inorganic pyrophosphate (PPi),13,14 the latter molecule being a powerful anti-mineralization factor under normal physiologic conditions. In the presence of loss-of-function mutations in the ENPP1 gene, the synthesis of PPi is reduced leading to reduced PPi/Pi ratio which allows deposition of calcium phosphate complexes to ensue.
Recent studies have demonstrated considerable, both genotypic and phenotypic overlap between PXE and GACI.12 Some patients with characteristic features of GACI, including extensive pre- and perinatal mineralization of the arterial blood vessels, have been shown to harbor mutations in the ABCC6 gene, a condition termed GACI type 2.15,16 Interestingly, many of the mutations in the ABCC6 gene found in patients with GACI are the same as those in patients with typical PXE with late-onset, slowly progressive disease. On the other hand, patients with evidence of vascular mineralization due to mutations in the ENPP1 gene have been shown to depict skin findings clinically and histopathologically similar to those found in PXE.17 Finally, there is a report of a family in which one of the brothers died early in life from extensive mineralization clinically diagnosed as GACI, while an older brother with mutations in the ABCC6 gene developed typical features of PXE later in life.18 The reasons for this sort of extensive phenotypic variability in patients with the same pathogenic mutations in the ABCC6 and ENPP1 genes are currently unknown, but genetic modifier genes, epigenetic modulation, and environmental as well as lifestyle variables have been suggested to be contributing factors.8
The patients with GACI type 1 have previously been shown to have a markedly reduced PPi/Pi ratio which would explain the severe mineralization of arterial blood vessels.13,14 Quite recently, patients with PXE with identified ABCC6 mutations, have also been reported to have reduced plasma PPi/Pi ratio,11 and fibroblasts from patients with PXE show altered PPi metabolism.19 These and related observations have led to the suggestion that administration of PPi to the patients could counteract the ectopic mineralization.20 Because PPi is relatively unstable in circulation and has a short half-life, administration of bisphosphonates, which are non-hydrolyzable PPi analogs, have been used to treat these patients with ectopic mineralization, including newborns or infants with GACI type 1 with ENPP1 mutations.21-25 Careful analysis of these studies suggests improvements in some cases, no change in others, and even development of serious side effects in some patients. At the same time, recent demonstration of reduced PPi level in PXE patients suggested that bisphosphonates might be useful to treat ectopic calcification in patients with PXE and GACI type 2 due to ABCC6 mutations.11
In this study, we report on the effects of bisphosphonate administration on ectopic mineralization in Abcc6−/− mice, a model for PXE caused by mutations in the ABCC6 gene.
Results
Treatment of Abcc6-/- mice with bisphosphonates
Bisphosphonates, a group of inorganic pyrophosphate analogs, demonstrate both anti-mineralization and anti-osteoclastic activities.26–28 The early bisphosphonates, such as ETD, favor anti-mineralization activity while they are relatively weak anti-osteoclastic molecules as compared to newer, more powerful structural analogs, such as AST. To test the potential efficacy of these bisphosphonates on the development of ectopic mineralization in the Abcc6−/− mice, an animal model of PXE, we developed a protocol wherein these mice were placed on bisphosphonate-containing diets at 4 weeks of age and followed for another 8 weeks. At this point, at the age of 12 weeks, the degree of mineralization in the dermal sheath of vibrissae, a biomarker reflecting the progression of the overall mineralization in these mice, was determined by 2 independent assays. First, muzzle skin containing the follicles of vibrissae, surrounded by the dermal connective tissue sheath, were biopsied and mineralization was detected by H&E and Alizarin red stains. Secondly, as an independent measure, calcium was extracted from a biopsy of the muzzle skin, and the calcium content was determined and expressed as μmole of Ca per gram of tissue.
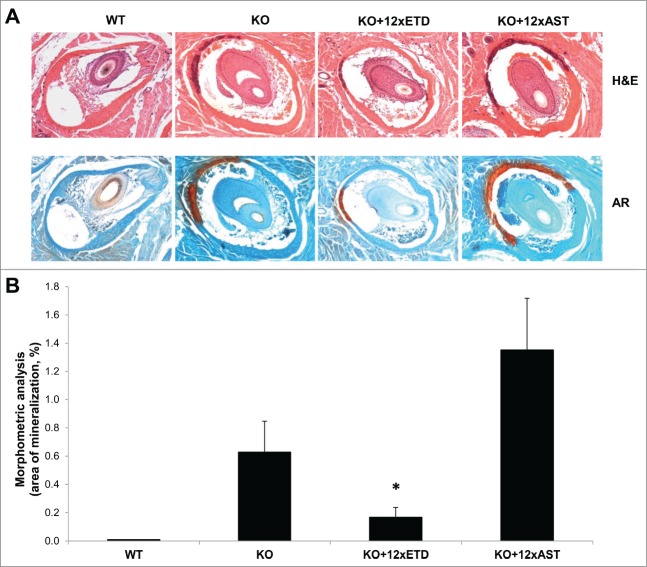
The Abcc6−/− knockout (KO) mice were fed with a diet mixed with ETD or AST in 3 different concentrations which were calculated to correspond to 1x, 5x, and 12x of the corresponding human dose used for treatment of osteoporosis, respectively. KO and wild-type (WT) littermates of the mice on bisphosphonate containing diet were kept on standard mouse diet and served as positive and negative controls of mineralization, respectively (for the experimental groups, see Table 1). Histopathology of the dermal sheath of vibrissae demonstrated extensive mineralization in KO mice on standard diet, while the WT mice had no evidence of mineralization (Fig. 1). No apparent difference in the degree of mineralization was noted in mice treated with 1x or 5xETD, but mice fed with diet containing 12xETD showed a markedly reduced amount of calcium depositions as judged by histopathology (Fig. 1A). Quantitation of mineralization by computerized morphometric analysis demonstrated that there was, on the average, a 73.3% reduction in the degree of mineralization in the mice treated with 12xETD compared to KO mice kept on standard diet (Fig. 1B). In contrast, treatment with diet containing 12xAST increased the mineral content by 53.5% as quantitated by computerized morphometric analysis (Fig. 1B).
Table 1.
Experimental groups of Abcc6+/+ and Abcc6−/− mice by genotype and diet*
| Group | Genotype | No. of mice examined | Diet |
|---|---|---|---|
| WT | Abcc6+/+ | 7 | Normal diet |
| KO | Abcc6−/− | 9 | Normal diet |
| KO+1xETD | Abcc6−/− | 7 | Normal diet + 1xETD |
| KO+5xETD | Abcc6−/− | 6 | Normal diet + 5xETD |
| KO+12xETD | Abcc6−/− | 8 | Normal diet + 12xETD |
| KO+1xAST | Abcc6−/− | 7 | Normal diet + 1xAST |
| KO+5xAST | Abcc6−/− | 7 | Normal diet + 5xAST |
| KO+12xAST | Abcc6−/− | 11 | Normal diet + 12xAST |
The mice were placed on bisphosphonate-containing diets at 4 weeks of age and followed for another 8 weeks. The mice were sacrificed at the age of 12 weeks and compared with control mice, either Abcc6+/+ (WT) or Abcc6−/− (KO), maintained on normal diet.
Figure 1.
Demonstration that ETD prevents ectopic mineralization in Abcc6−/− knock-out (KO) mice. (A) The Abcc6−/− mice develop ectopic mineralization of the dermal sheath of vibrissae when examined at 12 weeks of age by histopathology with Hematoxylin and Eosin (H&E, upper panel) and Alizarin Red (AR, lower panel) stains. Note that the corresponding wild-type (WT) mice have no evidence of mineralization. Feeding the mice with diet supplemented with 12xETD markedly reduced the mineral content of the dermal sheath of vibrissae, while there was no change in mice fed with 12xAST containing diet. (B) The degree of mineralization was quantitated by computerized morphometric analysis of the histopathologic sections, confirming the reduction in mice fed with 12xETD containing diet in comparison to KO mice (mean ± SE; n = 8–11; *, P < 0.05).
The results from histopathology combined with computerized morphometry were correlated with direct chemical assay of the calcium content in the muzzle skin. While the KO mice demonstrated statistically increased content of calcium over WT mice (P < 0.01), the mice treated with 1xETD or 5xETD containing diet did not differ from the KO mice (Fig. 2). However, calcium content in the skin of mice fed with 12xETD was reduced by 50.2% as compared to KO mice on standard diet (P < 0.01). Similar chemical assays of calcium showed no difference between the KO mice on standard diet as compared to those fed 1x, 5x, or 12xAST containing diet (Fig. 2). The supplementation of the diet with varying concentrations of ETD or AST did not alter the serum calcium or phosphate concentrations or change the Ca/Pi ratio (Table S1).
Figure 2.
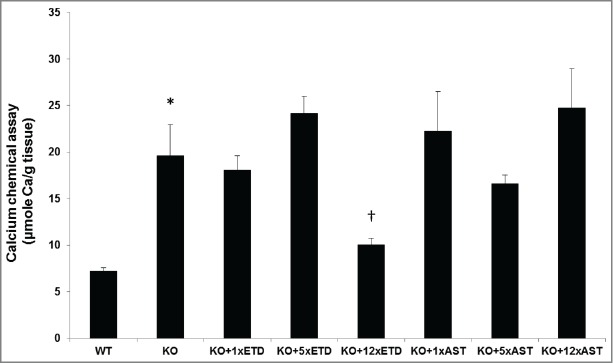
Quantitation of the ectopic mineralization by direct chemical assay of calcium in skin biopsies containing the dermal sheath of vibrissae. Note the significantly elevated calcium content in KO mice as compared to the WT mice (*, P < 0.01). Treatment of KO mice with diet supplemented with 12xETD resulted in significant reduction in the calcium content in comparison to the KO mice on control diet (†, P < 0.01). Feeding of mice with 1x or 5xETD containing diet, or with 1x, 5x or 12xAST containing diet did not significantly change the calcium content of the skin (mean + SE; n = 6–11).
Effects of bisphosphonates on bone morphology and mineralization
Because bisphosphonates have both anti-mineralization and anti-osteoclastic activities, and they have been used for treatment of bone disorders, such as osteoporosis, Paget disease, and bone metastases,26-28 we also evaluated the bones in mice treated with bisphosphonates. First, the total content of calcium and phosphorus in the left femur of each mouse was determined. As shown in Table 2, the calcium content in KO mice treated with diet supplemented with 12xETD was reduced, as expressed by milligram per gram of tissue, by comparison to KO mice on the standard diet. However, the 12xETD resulted in increased weight of the femurs, and consequently, the total content of Ca and P, expressed per femur, was not altered (Table 2).
Table 2.
Calcium and phosphorus content in the left femur of the mice*
| Group | Ca (mg/g) | P (mg/g) | Femur (mg) | Ca (mg) per femur | P (mg) per femur |
|---|---|---|---|---|---|
| WT | 130.5 ± 6.6 | 73.0 ± 2.8 | 71.3 ± 6.4 | 9.0 ± 0.6 | 5.2 ± 1.5 |
| KO | 123.7 ± 5.4 | 68.3 ± 2.5 | 78.5 ± 3.2 | 9.6 ± 0.3 | 5.3 ± 0.3 |
| KO+1xETD | 128.2 ± 4.1 | 59.3 ± 6.4 | 74.0 ± 3.4 | 9.4 ± 0.3 | 4.3 ± 0.4 |
| KO+5xETD | 140.1 ± 7.5 | 61.3 ± 2.5 | 75.4 ± 2.8 | 9.1 ± 0.3 | 4.0 ± 0.3 |
| KO+12xETD | 97.9 ± 6.22+ | 58.9 ± 2.6 | 97.7 ± 8.2+ | 9.3 ± 0.3 | 5.7 ± 0.5 |
| KO+1xAST | 125.8 ± 5.8 | 74.7 ± 2.2 | 74.1 ± 4.5 | 9.2 ± 0.4 | 5.5 ± 0.2 |
| KO+5xAST | 137.9 ± 4.8+ | 76.8 ± 2.9 | 73.2 ± 2.6 | 10.0 ± 0.1 | 5.6 ± 0.1 |
| KO+12xAST | 139.8 ± 3.02+ | 77.1 ± 2.0+ | 73.9 ± 2.1 | 10.3 ± 0.2 | 5.7 ± 0.3 |
Wild-type (WT) and Abcc6−/− (KO) mice at 4 weeks of age were placed on different diets for 8 weeks. Calcium and phosphorus contents of the left femur were determined at 12 weeks of age and expressed as mean ± SE. Statistical significance in comparison to the KO mice on control diet is indicated: + p < 0.05, 2+ p < 0.01.
Secondly, morphometric microarchitecture of the bones in KO mice on standard diet or those supplemented either with 12xETD or 12xAST was evaluated by μCT followed by 3D reconstruction and segmentation. In agreement with previous publications,29,30 there were significant differences between the male and female mice on bone microarchitecture (Fig. 3). Sex-matched comparisons between KO mice and those fed with 12xETD or 12xAST revealed differences that have previously been attributed to bisphosphonates (Table 3). Specifically, distal femoral bone volume fraction was increased by bisphosphonate treatment. Trabecular number in 12xETD and 12xAST treated Abcc6−/− mice increased significantly, whereas trabecular thickness was unchanged. Interestingly, trabecular separation was significantly less in 12xETD and 12xAST treated Abcc6−/− mice. The connectivity density was significantly higher in sex-matched mice when fed with either 12xETD or 12xAST (Table 3). Collectively, the effects of bisphosphonate on bone microarchitecture in Abcc6−/− mice are consistent with previous publications.
Figure 3.
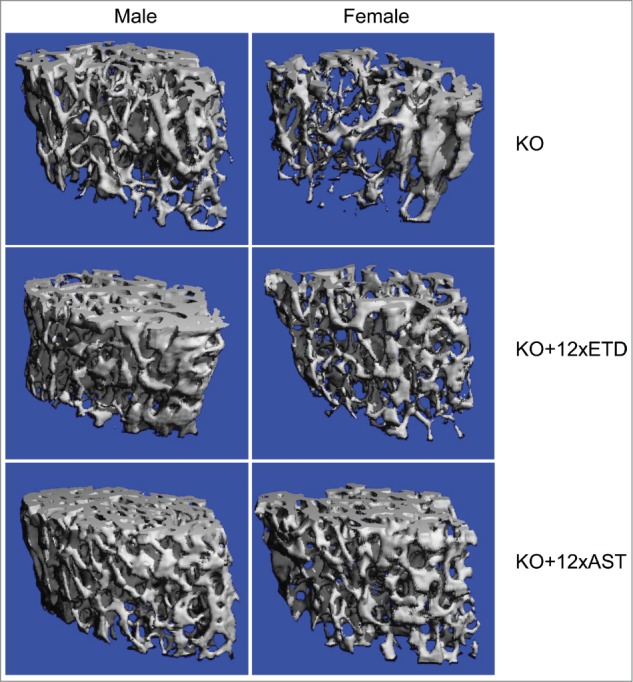
Bone microarchitecture in male and female Abcc6−/− mice on control diet (KO) or treated with diet containing 12xETD or 12xAST. Note the distinct difference between male and female mice on control diet, assessed by μCT scan (top panels). Treatment with bisphosphonates caused definitive changes (middle and bottom panels), as quantitatively detailed in Table 3. Three males and 3 females were examined in each group.
Table 3.
Trabecular bone phenotypes by microCT of the right distal femur of the mice*
| Group | Sex | BV/TV (%) | Tb.Th (μm) | Tb.N (1/mm) | Tb.Sp (μm) | SMI | Conn.D (TV/mm3) |
|---|---|---|---|---|---|---|---|
| KO | M | 19.4 ± 1.4 | 49.6 ± 1.8 | 5.5 ± 0.2 | 171.7 ± 6.4 | 1.7 ± 0.2 | 199.6 ± 8.6 |
| KO | F | 7.2 ± 1.4 | 39.7 ± 2.3 | 4.1 ± 0.1 | 242.4 ± 6.3 | 3.1 ± 0.2 | 63.5 ± 9.3 |
| KO+12xETD | M | 24.1 ± 2.6 | 49.3 ± 2.5 | 6.3 ± 0.2+ | 146.3 ± 5.7+ | 1.3 ± 0.3 | 277.5 ± 15.9+ |
| KO+12xETD | F | 11.0 ± 0.4+ | 40.8 ± 0.4 | 4.6 ± 0.1+ | 213.5 ± 2.3+ | 2.4 ± 0.1+ | 142.5 ± 8.1+ |
| KO+12xAST | M | 20.1 ± 3.5 | 47.2 ± 3.8 | 5.8 ± 0.2 | 164.6 ± 5.6 | 1.6 ± 0.3 | 227.8 ± 14.8 |
| KO+12xAST | F | 14.1 ± 2.0+ | 43.3 ± 1.3 | 4.9 ± 0.2+ | 198.2 ± 11.4+ | 2.1 ± 0.2+ | 199.5 ± 27.2+ |
Abcc6−/− mice (KO) at 4 weeks of age were placed on different diets for 8 weeks. Values are expressed as mean ± SE (3 mice each gender per group). Statistical significance in comparison to the KO mice of the same sex on control diet is indicated: + p < 0.05. BV/TV, relative bone volume (%); Tb.Th, trabecular thickness (μm); Tb.N, trabecular number (1/mm); Tb.Sp, trabecular separation (marrow thickness, μm); SMI, structure model index; Conn.D, connectivity density.
Discussion
PXE, caused in its classic form by mutations in the ABCC6 gene, is characterized by late-onset and slow, yet progressive mineralization associated with life-long development of complications in the skin, eyes and the cardiovascular system.6-8 GACI, a severe disorder of extensive vascular mineralization, usually lethal within the first few months of life. The classic form, GACI type 1, is caused by mutations in the ENPP1 gene.12-14 There are, however, patients with GACI who also develop PXE-like cutaneous findings, while a number of patients with GACI type 2 have recently been shown to harbor mutations in the ABCC6 gene.15,16 Thus, there is considerable genotypic and phenotypic overlap between these 2 conditions. The ENPP1 gene encodes an enzyme, ENPP1, which converts ATP to AMP and PPi. Consequently, patients with loss-of-function mutations in ENPP1 demonstrate low levels of PPi, and similar findings have been made in mice with absent or reduced ENPP1 activity.31,32 The low PPi/Pi ratio then allows ectopic mineralization to ensue.
The ABCC6 gene encodes ABCC6, also known as multidrug resistance associated protein 6, a putative efflux transporter with high level of expression in the liver.33,34 The precise physiologic function of ABCC6 is currently unknown, and specifically, the endogenous substrate(s) transported by this protein remain to be identified. There is currently no effective treatment for the systemic manifestations of PXE or GACI.8
A limited number of case studies have reported on attempts to treat patients with GACI with oral and intravenous bisphosphonates.21–25 There is no consensus, however, about the efficacy of bisphosphonates on the ectopic mineralization in these disorders. Some studies report apparent improvement, in some cases there has been very little, if any, effect, and in others bisphosphonate treatment has been accompanied by severe side effects. These discrepancies may reflect in part differences in the types and doses of bisphosphonates used and the route of administration. For example, oral bisphosphonates are poorly absorbed, with less than 1% of the enteral dose absorbed when taken after fasting.26 Hence, in a young infant, who is feeding frequently, enteral absorption of bisphosphonates may be even less. Moreover, first generation bisphosphonates, such as ETD, are less potent inhibitors of osteoclasts than currently used third generation bisphosphonates, such as AST and pamidronate. Hence, on a molar basis, greater amounts of ETD are typically administered than other bisphosphonates, with increased delivery of the non-hydrolyzable pyrophosphate analog. Although the use of intravenous bisphosphonates offers the advantage of greater delivery of drug compared to oral administration, third generation bisphosphonates are typically administered in relatively small doses due to their greater anti-osteoclastic activity compared to ETD, hence very little pyrophosphate analog is actually needed for efficacy.
Bisphosphonates are structural analogs of PPi with replacement of the oxygen linkage to phosphonate (PO3) groups by a carbon making the molecule more stable.28 In addition, there are side groups (R1, R2) which determine the pharmacologic properties, the mode of action, and the potency of bisphosphonates. There are 2 classes of bisphosphonates, viz., those containing nitrogen in the side chain and non-N containing forms. The initially synthesized non-N containing bisphosphonates, such as ETD, are relatively weak and have both anti-mineralization and anti-osteoclastic activities. The N-containing bisphosphonates, such as AST, are hundreds or thousands times more potent acting on bone metabolism as inhibitors of prenylation and they favor anti-osteoclastic activities.26-28
To clarify the effects of bisphosphonates in patients with ABCC6 mutations, we undertook this study treating Abcc6−/− mice, a genetically controlled model of ectopic mineralization, with 2 bisphosphonates, ETD and AST, in a controlled environment. In our study, we demonstrated that ETD, but not AST, inhibits the ectopic mineralization in Abcc6−/− mice, as monitored by calcium phosphate content of dermal sheath of vibrissae, an early biomarker of the overall mineralization in these mice. Progressive mineralization of the dermal sheath of vibrissae has been shown to reflect the mineralization in internal organs, including arterial blood vessles, in these mice.35,36 However, significant level of inhibition with ETD was obtained only at doses that were about 12 times higher than those used for treatment of patients with bone disorders which might indicate poor absorption of the drug or a need for very high concentration of the bisphosphonate. By contrast, AST which was used at lower molar doses than ETD due to its ∼1,000 times higher anti-osteoclastic activity did not prevent the ectopic mineralization, and in fact it increased the mineral content in the dermal sheath of vibrissae. These results suggest that the anti-mineralization effect results not from the biological anti-osteoclastic activity of the bisphosphonate but rather from the delivery of a sufficient number of PPi-analog molecules to exert physiochemical inhibition of calcification. Nevertheless, these observations suggest that ETD, in sufficient doses, might well be beneficial for treatment of ectopic mineralization in selected patients with GACI and PXE. It should be noted, however, that administration of ETD also affected the mineralization and microarchitecture of femur, as determined by direct calcium assay and by morphometric analysis of the bone microarchitecture using μCT. Thus, long-term administration of ETD, for example for treatment of PXE, should be accompanied by regular assessment of bone density and mineralization.
In summary, irrespective of the underlying mechanisms that lead from mutations in the ABCC6 gene to ectopic mineralization in patients with PXE or GACI, bisphosphonates in appropriate doses, particularly those that favor the anti-mineralization activity, might be helpful for prevention of the development of complications of these diseases. However, careful monitoring of the side effects, including alterations in the bone microarchitecture, is required to ensure safety of this approach.
Materials and Methods
Mice
The Abcc6tm1JfK mouse was developed by targeted ablation of the Abcc6 gene (this mouse is referred to as Abcc6−/−).35 Abcc6 wild-type (Abcc6+/+) and knockout (Abcc6−/−) mice were made congenic by backcrossing heterozygous (Abcc6+/−) mice on C57BL/6J background for 10 backcrosses. Mice were fed a standard rodent diet (Lab Diet 5010; PMI Nutrition, Brentwood, MO, USA) and had free access to water. Mice were maintained under standard conditions at the Animal Facility of Thomas Jefferson University. All protocols were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Proper handling and care were practiced according to the animal welfare policies of the United States Public Health Service.
Experimental design and diets
Mice were placed on specific diets at 4 weeks of age and maintained on the same diet for an additional 8 weeks. The groups of mice are characterized by genotype and diet (Table 1). Abcc6+/+ and Abcc6-/- mice were fed either a control diet 5010 (PMI Nutrition International, Brentwood, MO) (n = 7–9 per group) or the same diet supplemented with either etidronate disodium (ETD) or alendronate sodium trihydrate (AST), 6–11 mice per group. ETD treatment consisted of 3 groups: Abcc6−/− mice were fed with a dose calculated to be equivalent to that used for treatment of humans for osteoporosis (20 mg/kg/day orally), i.e., 8 mg etidronate disodium (Alfa Aesar, Ward Hill, MA) per 100 g of diet (1xETD), or 5- and 12-fold greater doses (5xETD and 12xETD), respectively. AST treatment also consisted of 3 groups:
Abcc6−/− mice were fed with a dose equivalent to human (0.5 mg/kg/day orally), which received 0.2 mg alendronate sodium trihydrate (Alfa Aesar) per 100 g of diet (1xAST), and 5- and 12-fold increase of the human equivalent dose (5xAST and 12xAST). All mice were euthanized at 12 weeks of age.
Histopathological analysis
Biopsies from muzzle skin containing vibrissae were fixed in 10% phosphate-buffered formalin and embedded in paraffin. The tissues were sectioned (6 μm thick), placed onto slides, and stained with Hematoxylin and Eosin (H&E) and Alizarin Red (AR) using standard procedures. Slides were examined under light microscopy for mineralization.
Quantitation of tissue mineralization by computerized morphometric analysis
Computerized morphometric analysis of mineralization was performed on H&E-stained sections with a Nikon (Tokyo, Japan) Te2000 microscope and an AutoQuant imaging system (AutoQuant Imaging, Watervliet, NY). Oblique sections of vibrissae in the muzzle skin, including lower infundibulum above the bulb, were obtained. These sections allowed visualization of the blood filled sinuses surrounded by the dermal connective tissue sheath, and the area of mineralization in the dermal sheath was expressed as a percentage of the total area of vibrissae per mouse. The values from each mouse represent analysis of 10 follicles on the average in one section, and the average percentage of mineralization was determined for each group (n = 6–11). All images were analyzed with Image-Pro Plus software version 6.1 (Media Cybernetics, Rockville, MD).
Chemical quantitation of calcium and phosphate in the skin, bone and serum
To quantify the mineral deposition in vibrissae, muzzle skin was harvested and decalcified with 0.15 mol/L HCl for 48 hours at room temperature. Right femurs were also harvested and decalcified with 1 mol/L HCl for 2 weeks at room temperature. Solubilized calcium was then determined by colorimetric analysis using the ơ-cresolphthalein complexone method [calcium (CPC) LiquiColor; Stanbio Laboratory, Boerne, TX]. Phosphate in the bone extract was also measured by the Malachite Green Phosphate Assay Kit (Bioassay Systems, Hayward, CA), and the values were normalized to tissue weight. Serum calcium and phosphate concentrations were determined with the same assays.
Microcomputed tomography
Microarchitecture of the distal trabecular bone of the right femur was analyzed. A 1.25 mm-thick region located proximal to the distal growth plate of femur was scanned at a 10.5 μm resolution using the micro-CT (μCT) system (vivaCT40; Scanco Medical AG, Bassersdorf, Switzerland). The microstructural parameters were obtained through 3-dimensional (3D) reconstruction and segmentation (using a Gaussian filter and a global threshold of 2272 Hounsfield units) in the manufacturer-provided software.
Statistical analysis
We performed comparisons between different groups of mice using the 2-sided Kruskal–Wallis nonparametric test. The justification of using nonparametric tests is as follows: First, the sample size in each group is less than 30, therefore, we cannot use the rule-of-thumb assumption with regard to the Central Limit Theorem that the sampling distribution is normally distributed. Secondly, tests of normality were performed on all data, indicating that the sample distributions do not fit a normal distribution, with very few exceptions. Therefore, nonparametric tests are most appropriate. All statistical computations were completed using SPSS version 15.0 software (SPSS Inc., Chicago, IL).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors thank Joshua Kingman, Dian Wang and Jocelyn Andrel Sendecki for technical assistance. Carol Kelly helped in manuscript preparation.
Funding
This study was supported by NIH/NIAMS grants R01 AR055225 (JU) and K01 AR064766 (QL). The authors acknowledge Penn Center for Musculoskeletal Disorders, supported by NIH/NIAMS P30 AR050950, for assistance in analysis of bone morphometry.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, Flores FR, Callister TQ, Raggi P, Berman DS. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol 2007; 49:1860-70; PMID:17481445; http://dx.doi.org/ 10.1016/j.jacc.2006.10.079 [DOI] [PubMed] [Google Scholar]
- 2. Chander S, Gordon P. Soft tissue and subcutaneous calcification in connective tissue diseases. Curr Opin Rheumatol 2012; 24:158-64; PMID:22227955; http://dx.doi.org/ 10.1097/BOR.0b013e32834ff5cd [DOI] [PubMed] [Google Scholar]
- 3. Sprecher E. Familial tumoral calcinosis: from characterization of a rare phenotype to the pathogenesis of ectopic calcification. J Invest Dermatol 2010; 130:652-60; PMID:19865099; http://dx.doi.org/ 10.1038/jid.2009.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Q, Uitto J. Mineralization/anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol 2013; 183:10-8; PMID:23665350; http://dx.doi.org/ 10.1016/j.ajpath.2013.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rutsch F, Nitschke Y, Terkeltaub R. Genetics in arterial calcification: pieces of a puzzle and cogs in a wheel. Circ Res 2011; 109:578-92; PMID:21852556; http://dx.doi.org/ 10.1161/CIRCRESAHA.111.247965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neldner KH. Pseudoxanthoma elasticum. Clin Dermatol 1988; 6:1-159; PMID:3359381; http://dx.doi.org/ 10.1016/0738-081X(88)90003-X [DOI] [PubMed] [Google Scholar]
- 7. Uitto J, Li Q, Jiang Q. Pseudoxanthoma elasticum: molecular genetics and putative pathomechanisms. J Invest Dermatol 2010; 130:661-70; PMID:20032990; http://dx.doi.org/ 10.1038/jid.2009.411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Uitto J, Varadi A, Bercovitch L, Terry PF, Terry SF. Pseudoxanthoma elasticum: progress in research toward treatment: summary of the 2012 PXE International Research Meeting. J Invest Dermatol 2013; 133:1444-9; PMID:23673496; http://dx.doi.org/ 10.1038/jid.2013.20 [DOI] [PubMed] [Google Scholar]
- 9. Terry S, Hefferson T. LOVD Gene Homepage. 2013. Available from: www.ncbi.nlm.nih.gov/lovd/home.php?select_db=ABCC6 [Google Scholar]
- 10. Jansen RS, Kucukosmanoglu A, de Haas M, Sapthu S, Otero JA, Hegman IE, Bergen AA, Gorgels TG, Borst P, van de Wetering K. ABCC6 prevents ectopic mineralization seen in pseudoxanthoma elasticum by inducing cellular nucleotide release. Proc Nat Acad Sci USA 2013; 110:20206-11; PMID:24277820; http://dx.doi.org/ 10.1073/pnas.1319582110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jansen RS, Duijst S, Mahakena S, Sommer D, Szeri F, Varadi A, Plomp A, Bergen AA, Oude Elferink RP, Borst P, et al. ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 2014; 34:1985-9; http://dx.doi.org/ 10.1161/ATVBAHA.114.304017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nitschke Y, Rutsch F. Generalized arterial calcification of infancy and pseudoxanthoma elasticum: two sides of the same coin. Front Genet 2012; 3:302; PMID:23269929; http://dx.doi.org/ 10.3389/fgene.2012.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rutsch F, Ruf N, Vaingankar S, Toliat MR, Suk A, Hohne W, Schauer G, Lehmann M, Roscioli T, Schnabel D, et al. Mutations in ENPP1 are associated with 'idiopathic' infantile arterial calcification. Nature Genet 2003; 34:379-81; PMID:12881724; http://dx.doi.org/ 10.1038/ng1221 [DOI] [PubMed] [Google Scholar]
- 14. Ruf N, Uhlenberg B, Terkeltaub R, Nurnberg P, Rutsch F. The mutational spectrum of ENPP1 as arising after the analysis of 23 unrelated patients with generalized arterial calcification of infancy (GACI). Hum Mutat 2005; 25:98; PMID:15605415; http://dx.doi.org/ 10.1002/humu.9297 [DOI] [PubMed] [Google Scholar]
- 15. Nitschke Y, Baujat G, Botschen U, Wittkampf T, du Moulin M, Stella J, Le Merrer M, Guest G, Lambot K, Tazarourte-Pinturier MF, et al. Generalized arterial calcification of infancy and pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 2012; 90:25-39; PMID:22209248; http://dx.doi.org/ 10.1016/j.ajhg.2011.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Q, Brodsky JL, Conlin L, Pawel B, Glatz A, Gafni RI, Schurgers LJ, Uitto J, Hakonarson H, Deardoff MA, et al. Mutations in the ABCC6 gene as a cause of generalized arterial calcification of infancy - genotypic overlap with pseudoxanthoma elasticum. J Invest Dermatol 2014; 134:658-65; PMID:24008425; http://dx.doi.org/ 10.1038/jid.2013.370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Q, Schumacher W, Siegel D, Jablonski D, Uitto J. Cutaneous features of pseudoxanthoma elasticum in a patient with generalized arterial calcification of infancy due to a homozygous missense mutation in the ENPP1 gene. Br J Dermatol 2012; 166:1107-11; PMID:22229486; http://dx.doi.org/ 10.1111/j.1365-2133.2012.10811.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Le Boulanger G, Labreze C, Croue A, Schurgers LJ, Chassaing N, Wittkampf T, Rutsch F, Martin L. An unusual severe vascular case of pseudoxanthoma elasticum presenting as generalized arterial calcification of infancy. Am J Med Genet A 2010; 152A:118-23; PMID:20034067; http://dx.doi.org/ 10.1002/ajmg.a.33162 [DOI] [PubMed] [Google Scholar]
- 19. Dabisch-Ruthe M, Kuzaj P, Gotting C, Knabbe C, Hendig D. Pyrophosphates as a major inhibitor of matrix calcification in Pseudoxanthoma elasticum. J Dermatol Sci 2014; 75:109-20; PMID:24907773; http://dx.doi.org/ 10.1016/j.jdermsci.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 20. O'Neill WC, Lomashvili KA, Malluche HH, Faugere MC, Riser BL. Treatment with pyrophosphate inhibits uremic vascular calcification. Kidney Int 2011; 79:512-7; PMID:21124302; http://dx.doi.org/ 10.1038/ki.2010.461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramjan KA, Roscioli T, Rutsch F, Sillence D, Munns CF. Generalized arterial calcification of infancy: treatment with bisphosphonates. Nat Clin Pract Endocrinol Metab 2009; 5:167-72; PMID:19229237; http://dx.doi.org/ 10.1038/ncpendmet1067 [DOI] [PubMed] [Google Scholar]
- 22. Rutsch F, Boyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, Weissen-Plenz G, Fischer RJ, Mughal Z, Gregory JW, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet 2008; 1:133-40; PMID:20016754; http://dx.doi.org/ 10.1161/CIRCGENETICS.108.797704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Edouard T, Chabot G, Miro J, Buhas DC, Nitschke Y, Lapierre C, Rutsch F, Alos N. Efficacy and safety of 2-year etidronate treatment in a child with generalized arterial calcification of infancy. Eur J Pediatr 2011; 170:1585-90; PMID:21932012; http://dx.doi.org/ 10.1007/s00431-011-1572-9 [DOI] [PubMed] [Google Scholar]
- 24. Galletti S, Nitschke Y, Malavolti AM, Aquilano G, Faldella G, Corvaglia L, Rutsch F. Generalized Arterial Calcification of Infancy: Fatal Clinical Course Associated with a Novel Mutation in ENPP1. JIMD Rep 2011; 1:23-7; PMID:23430823; http://dx.doi.org/ 10.1007/8904_2011_11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Otero JE, Gottesman GS, McAlister WH, Mumm S, Madson KL, Kiffer-Moreira T, Sheen C, Millan JL, Ericson KL, Whyte MP. Severe skeletal toxicity from protracted etidronate therapy for generalized arterial calcification of infancy. J Bone Miner Res 2013; 28:419-30; PMID:22972716; http://dx.doi.org/ 10.1002/jbmr.1752 [DOI] [PubMed] [Google Scholar]
- 26. Russell RG. Bisphosphonates: from bench to bedside. Ann N Y Acad Sci 2006; 1068:367-401; PMID:16831938; http://dx.doi.org/ 10.1196/annals.1346.041 [DOI] [PubMed] [Google Scholar]
- 27. Uludag H. Bisphosphonates as a foundation of drug delivery to bone. Curr Pharm Des 2002; 8:1929-44; PMID:12171528; http://dx.doi.org/ 10.2174/1381612023393585 [DOI] [PubMed] [Google Scholar]
- 28. Rodan GA, Fleisch HA. Bisphosphonates: mechanisms of action. J Clin Invest 1996; 97:2692-6; PMID:8675678; http://dx.doi.org/ 10.1172/JCI118722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pennypacker B, Shea M, Liu Q, Masarachia P, Saftig P, Rodan S, Rodan G, Kimmel D. Bone density, strength, and formation in adult cathepsin K (-/-) mice. Bone 2009; 44:199-207; PMID:18845279; http://dx.doi.org/ 10.1016/j.bone.2008.08.130 [DOI] [PubMed] [Google Scholar]
- 30. Bouleftour W, Boudiffa M, Wade-Gueye NM, Bouet G, Cardelli M, Laroche N, Vanden-Bossche A, Thomas M, Bonnelye E, Aubin JE, et al. Skeletal development of mice lacking bone sialoprotein (BSP)–impairment of long bone growth and progressive establishment of high trabecular bone mass. PLoS One 2014; 9:e95144; PMID:24816232; http://dx.doi.org/ 10.1371/journal.pone.0095144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sali A, Favaloro JM, Terkeltaub R, Goding JW. Germline deletion of the nucleoside triphosphate pyrophosphohydrolase (NTPPPH) plasma cell membrane glycoprotein-1 (PC-1) produces abnormal calcification of periarticular tissues. In: Vanduffel L LR, ed. Ecto-ATPases and related ectoenzymes. Maastricht, The Netherlands: Shaker Publishing, 1999:267-82. [Google Scholar]
- 32. Li Q, Guo H, Chou DW, Berndt A, Sundberg JP, Uitto J. Mutant Enpp1asj mouse as a model for generalized arterial calcification of infancy. Dis Model Mech 2013; 6:1227-35; PMID:23798568; http://dx.doi.org/ 10.1242/dmm.012765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Varadi A, Szabo Z, Pomozi V, de Boussac H, Fulop K, Aranyi T. ABCC6 as a target in pseudoxanthoma elasticum. Curr Drug Targets 2011; 12:671-82; PMID:21039331; http://dx.doi.org/ 10.2174/138945011795378612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Belinsky MG, Kruh GD. MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer 1999; 80:1342-9; PMID:10424734; http://dx.doi.org/ 10.1038/sj.bjc.6690527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Klement JF, Matsuzaki Y, Jiang QJ, Terlizzi J, Choi HY, Fujimoto N, Li K, Pulkkinen L, Birk DE, Sundberg JP, et al. Targeted ablation of the Abcc6 gene results in ectopic mineralization of connective tissues. Mol Cell Biol 2005; 25:8299-310; PMID:16135817; http://dx.doi.org/ 10.1128/MCB.25.18.8299-8310.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jiang Q, Li Q, Uitto J. Aberrant mineralization of connective tissues in a mouse model of pseudoxanthoma elasticum: Systemic and local regulatory factors. J Invest Dermatol 2007; 127:1392-402; PMID:17273159; http://dx.doi.org/ 10.1038/sj.jid.5700729 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.