Abstract
Quiescent cancer cells are resistant to cytotoxic agents which target only proliferating cancer cells. Time-lapse imaging demonstrated that tumor-targeting Salmonella typhimurium A1-R (A1-R) decoyed cancer cells in monolayer culture and in tumor spheres to cycle from G0/G1 to S/G2/M, as demonstrated by fluorescence ubiquitination-based cell cycle indicator (FUCCI) imaging. A1-R infection of FUCCI-expressing subcutaneous tumors growing in nude mice also decoyed quiescent cancer cells, which were the majority of the cells in the tumors, to cycle from G0/G1 to S/G2/M, thereby making them sensitive to cytotoxic agents. The combination of A1-R and cisplatinum or paclitaxel reduced tumor size compared with A1-R monotherapy or cisplatinum or paclitaxel alone. The results of this study demonstrate that A1-R can decoy quiescent cancer cells to cycle to S/G2/M and sensitize them to cytotoxic chemotherapy. These results suggest a new paradigm of bacterial-decoy chemotherapy of cancer.
Keywords: cell cycle, chemotherapy, decoy, FUCCI, GFP, RFP, imaging, S. typhimurium A1-R, tumor-targeting bacteria
Abbreviations
- FUCCI
fluorescence ubiquitination-based cell cycle indicator
- S. typhimurium
Salmonella typhimurium
Introduction
The phase of the cell cycle can determine whether a cancer cell can respond to a given drug. Monitoring of real-time cell cycle dynamics of cancer cells throughout a live tumor intravitally using a fluorescence ubiquitination-based cell cycle indicator (FUCCI),1 we previously demonstrated approximately 90% of cancer cells in the center and 80% of total cells of an established tumor are in G0/G1 phase. Longitudinal real-time imaging demonstrated that cytotoxic agents killed only proliferating cancer cells at the surface or near blood vessels and, in contrast, had little effect on quiescent cancer cells.2
With FUCCI imaging, we also previously observed that cancer cells in G0/G1 phase in Gelfoam histoculture migrated more rapidly and further than cancer cells in S/G2/M phases. Cancer cells ceased migrating when they entered S/G2/M phases and restarted migrating after cell division when the cells re-entered G0/G1. Migrating cancer cells also were resistant to cytotoxic chemotherapy, since they were preponderantly in G0/G1.3
The OBP-301 telomerase-dependent adenovirus decoyed quiescent cancer cells to S/G2/M phases where they became chemosensitive in tumors in vivo and tumor spheres in vivo, visualized with FUCCI imaging.4
Records for >200 y have documented cancer patients going into remission after a bacterial infection.5 In the late 19th century and early 20th century, William B. Coley at New York Cancer Hospital, the precursor of Sloan-Kettering Memorial Cancer Center, treated cancer patients with Streptococcus pyogenes.6
S. typhimurium, is a facultative anaerobe which confers important advantages, compared to obligate anaerobes, in that a facultative anaerobe can grow in the oxic viable region of tumors as well as necrotic regions.7 Attenuated auxotrophic mutants of S. typhimurium retained their tumor-targeting capabilities.8
In a Phase I clinical trial on patients with metastatic melanoma and renal carcinoma, the S. typhimurium strain tested (VNP20009), attenuated by msbB, amino-acid, and purI mutations, was safely administered to patients, but did not sufficiently colonize the patients’ tumors, perhaps because this strain was overattenuated.9
The S. typhimurium A1-R strain developed by our laboratory has high tumor colonization efficacy and antitumor efficacy. S. typhimurium A1-R is auxotrophic for Leu-Arg, which prevents it from mounting a continuous infection in normal tissues. S. typhimurium A1-R has no other apparent attenuating mutations in contrast to VNP20009 and, therefore, has very high tumor-targeting capability. S. typhimurium A1-R was able to eradicate primary and metastatic tumors as monotherapy in nude mouse models of prostate,10,11 breast,12 lung,13,14 pancreatic15,16 and ovarian17 cancers, as well as sarcoma18,19 and glioma,20 all of which are highly aggressive tumor models. S. typhimurium A1-R also targeted pancreatic cancer stem-like cells21 and pancreatic cancer patient-like orthotopic xenograft (PDOX) models.22
In the present report, we demonstrate that S. typhimurium A1-R can decoy quiescent G0/G1 cancer cells to cycle to S/G2/M and become chemosensitive.
Results and Discussion
S. typhimurium A1-R stimulates cell cycle transit of quiescent cancer cells in monolayer culture
Time-lapse imaging of S. typhimurium A1-R interacting with quiescent FUCCI-expressing MKN45 cancer cells in monolayer culture demonstrated that S. typhimurium A1-R targets quiescent cancer cells and induces their cell cycle transit from G0/G1 to S/G2/M phase (Fig. 1). Before S. typhimurium A1-R treatment, approximately 95% of the cancer cells were in G0/G1 (Fig. 1). After S. typhimurium A1-R treatment, the percentage of cancer cells in G0/G1 was reduced to less than 40% with approximately 60% in S/G2/M.
Figure 1.
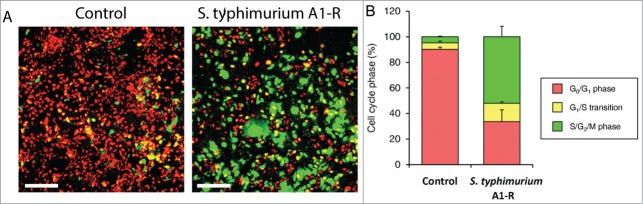
S. typhimurium A1-R stimulates cell cycle transit of quiescent cancer cells in monolayer culture. S. typhimurium A1-R targeted quiescent cancer cells and stimulates cell cycle transit from G0/G1 to S/G2/M phases. (A) Representative images of control cancer cells and cancer cells treated with S. typhimurium A1-R. (B) Histogram shows cell cycle distribution in control and S. typhimuriam A1-R-treated cultures. Scale bar: 500 μm.
S. typhimurium A1-R stimulates cell cycle transit in quiescent tumor spheres
Time-lapse imaging of quiescent FUCCI-expressing MKN45 tumor spheres on agar demonstrated that S. typhimurium A1-R targeted quiescent tumor spheres and stimulated cell cycle transit, of the cancer cells within the spheres, from G0/G1 to S/G2/M phases (Fig. 2). Before S. typhimurium A1-R treatment, approximately 95% of the cancer cells were in G0/G1. After S. typhimurium A1-R treatment, approximately 30% of the cancer cells were in G0/G1 and 70% in S/G2/M (Fig. 2).
Figure 2.
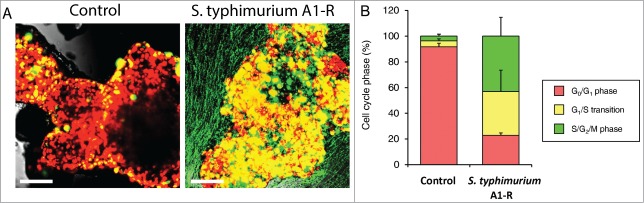
S. typhimurium A1-R stimulates cell cycle transit in quiescent tumor spheres in vitro. S. typhimurium A1-R stimulated cell cycle transit from G0/G1 to S/G2/M phase. (A) Representative images of control tumor spheres and tumor spheres treated with S. typhimurium A1-R. (B) Histogram shows cell cycle distribution in control and S. typhimurium A1-R-treated tumor spheres. Scale bar: 500 μm.
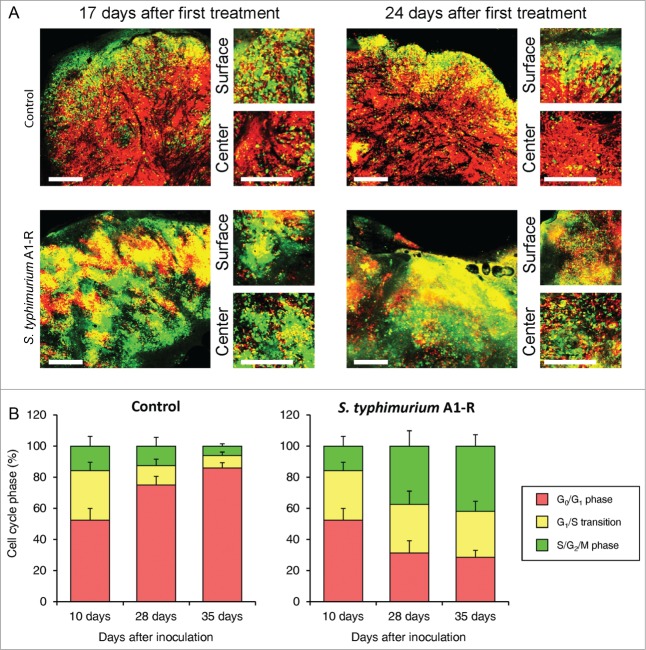
S. typhimurium A1-R mobilizes the cell cycle transit of quiescent cancer cells in tumors in vivo
Before S. typhimurium A1-R treatment, FUCCI-expressing MKN45 tumors had approximately 95% of the cancer cells in G0/G1 after 35 d growth in nude mice. Thirty-five d after treatment with S. typhimurium A1-R, approximately 30% of the cancer cells were in G0/G1 and 70% in S/G2/M (Fig. 3).
Figure 3.
S. typhimurium A1-R mobilizes the cell cycle transit of quiescent cancer cells in tumors in vivo. (A) Representative images of cross sections of FUCCI-expressing MKN45 tumor xenografts treated with S. typhimurium A1-R or untreated control. (B) Histograms show the cell cycle phase distribution of FUCCI-expressing cells within the tumor treated with S. typhimurium A1-R or untreated control. Scale bars: 500 μm.
S. typhimurium-decoyed tumors became sensitive to chemotherapy
FUCCI-expressing MKN45 cells were injected subcutaneously into the left flanks of mice. When the subcutaneous tumors reached approximately 8 mm in diameter (tumor volume, 300 mm3), mice were administered S. typhimurium A1-R (iv) alone, or in combination with cisplatinum (4 mg/kg) or in combination with paclitaxel (5 mg/kg, ip) for 5 cycles every 3 d S. typhimurium A1-R sensitized the tumors to chemotherapy due to cell-cycle decoy of the cancer cells within the tumor (Fig. 4). Cisplatinum or paclitaxel alone had only modest growth inhibition on the MKN45 tumor. S. typhimurium had a larger growth inhibition effect than the chemotherapy drugs. The greatest effect was the combination of by S. typhimurium A1-R with either of the chemotherapy drugs (Fig. 4).
Figure 4.
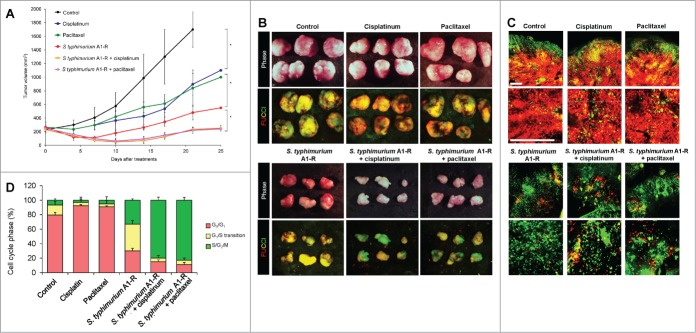
S. typhimurium A1-R-decoyed tumors became sensitive to chemotherapy. FUCCI-expressing MKN45 cells (5 × 106 cells/mouse) were injected subcutaneously into the left flank of nude mouse. When the tumors reached approximately 8 mm in diameter (tumor volume, 300 mm3), mice were administered iv S. typhimurium A1-R alone, or with cisplatinum (4 mg/kg ip) or paclitaxel (5 mg/kg ip) for 5 cycles every 3 d. (A) Growth curves of tumors derived from FUCCI-expressing MKN45 cells after treatment with chemotherapy, S. typhimurium A1-R or in combination with S. typhimurium A1-R and chemotherapy. The difference between control and cisplatinum-treated: P < 0.01; the difference between control and paclitaxel-treated: P < 0.05; the difference between control and S. typhimurium A1-R: P < 0.05; the difference between control and the combination of S. typhimurium A1-R and cisplatinum: P < 0.01; the difference between control and the combination of S. typhimurium A1-R and paclitaxel: P < 0.01. (B) Macroscopic photographs of FUCCI-expressing tumors, untreated control, S. typhimurium A1-R-treated, cisplatinum-treated, paclitaxel-treated, or treated with the combination of S. typhimurium A1-R and either cisplatinum or paclitaxel (right). Scale bars, 10 mm. (C) Representative images of cross-sections of FUCCI-expressing MKN45 subcutaneous tumors, untreated control: S. typhimurium A1-R-treated, cisplatinum-treated, paclitaxel-treated, or treated with the combination of S. typhimurium A1-R and either cisplainum or paclitaxel. (D) Histogram shows cell cycle phase of FUCCI-expressing MKN45 subcutaneous tumors, including untreated control, S. typhimurium A1-R-treated, cisplatinum-treated, paclitaxel-treated, or treated with the combination of S. typhimurium A1-R and either cisplatinum or paclitaxel. Scale bars: 500 μm.
FUCCI cell cycle imaging showed that in tumors treated with cisplatinum or paclitaxel, the percentage of cancer cells in G0/G1 increased to over 95% from approximately 80% before treatment. In contrast, S. typhimurium treatment reduced the percentage of cancer cells in G0/G1 to approximately 30%. The combination of S. typhimurium A1-R and chemotherapy decreased the percentage of cancer cells in G0/G1 to 15% or less. The percentage of S/G2/M cells in tumors treated in combination with S. typhimurium A1-R and either cisplatin or paclitaxel approached 90% (Fig. 4).
FUCCI imaging demonstrated that the combination of S. typhimurium A1-R decoy therapy and chemotherapy can effectively kill quiescent cancer cells that are resistant to conventional chemotherapy. The combination of S. typhimurium A1-R and either cisplatinum or paclitaxel decoyed almost all the cancer cells to cycle, greatly enhancing their sensitivity.
We previously compared the cell cycle dynamics of invading and non-invading cancer cells in 3-dimensional Gelfoam histoculture, where cancer cells have in vivo-like behavior. We demonstrated with FUCCI imaging that cancer cells in G0/G1 phase can migrate faster and further than cancer cells in S/G2/M phases. When cancer cells in G0/G1 cycled into S/G2/M phases, they ceased movement and then only restarted migration after re-entry into G0/G1 phase after cell division. Chemotherapy had little effect on G0/G1 invading cancer cells. Decoy chemotherapy may also be useful to target invasive cancer cells, which may otherwise be highly chemoresistant.3
We previously showed with FUCCI imaging that the vast majority of cancer cells in a tumor was in G0/G1. We demonstrated that cytotoxic chemotherapy kills only cancer cells in S/G2/M phases, which are in a minority in an established tumor, and had little effect on cancer cells in G0/G1 phase. Moreover, we showed the efficacy of chemotherapy depends not on tumor size, but the cell cycle phase of each cancer cell, which depends on the location in the tumor. We spatially and temporally demonstrated the cell cycle dynamics of individual cancer cells during tumor growth before, as well as during and after treatment with cytotoxic agents, within the same tumors. Our results explained why temporary regression may be often seen in the clinic after chemotherapy, as the drugs are effective only on cells in the outer layer of the tumor or near blood vessels, where cancer cells proliferate. Recurrence takes place when some of the quiescent cells re-enter the cell cycle as they replace the cycling cells killed by chemotherapy at the surface or near blood vessels.2
We previously demonstrated, using FUCCI imaging, that a genetically-engineered telomerase-specific adenovirus, OBP-301, could decoy the cell cycle of cancer cells in tumor spheres and tumors thereby sensitizing them to chemotherapy.4
The present study demonstrated that S. typhimurium A1-R can decoy the cell-cycle transit of quiescent cancer cells and sensitize the cancer cells to chemotherapy.
Previously developed concepts and strategies of highly selective tumor-targeting23-34 can take advantage of spatial–temporal cell cycle imaging of a tumor described in the present report.
Future studies will focus on optimizing decoy chemotherapy with S. typhimurium A1-R and to screen for other decoy agents. Decoy chemotherapy is a promising approach to overcome the problem that the majority of cancer cells in most tumors are quiescent and are thereby chemoresistant.
Materials and Methods
FUCCI (Fluorescence ubiquitination cell cycle indicater)
The FUCCI probe was generated by fusing mKO2 (monomeric kusabira orange2) and mAG (monomeric azami green) to the ubiquitination domains of human Cdt1 and geminin, respectively. These 2 chimeric proteins, mKO2-hCdt1and mAG-hGem, accumulate reciprocally in the nuclei of transfected cells during the cell cycle, labeling the nuclei of G1 phase cells orange and nuclei of cells in S/G2/M phase green.1 Plasmids expressing mKO2-hCdt1 (green fluorescent protein) or mAG-hGem (orange fluorescent protein) were obtained from the Medical and Biological Laboratory. Plasmids expressing mKO2-hCdt1 were transfected into MKN45 cells using Lipofectamine™ LTX (Invitrogen). The cells were incubated for 48 h after transfection and were then trypsinized and seeded in 96-well plates at a density of 10 cells/well. In the first step, cells were sorted into green (S, G2, and M phase) cells using a FACSAria cell sorter (Becton Dickinson). The first-step-sorted green-fluorescent cells were then re-transfected with mAG-hGem (orange) and then sorted by orange fluorescence.4
Cells
MKN45 is a radio-resistant poorly differentiated stomach adenocarcinoma cell line derived from a liver metastasis of a patient.4
Animal experiments
Athymic nu/nu nude mice (AntiCancer, Inc.) were maintained in a barrier facility under HEPA filtration and fed with autoclaved laboratory rodent diet (Teklad LM-485; Harlan). All animal studies were conducted in accordance with the principles and procedures outlined in the National Institute of Health Guide for the Care and Use of Animals under Assurance Number A3873–1.
Tumor model
All animal procedures were performed under anesthesia using s.c. administration of a ketamine mixture (10 μl ketamine HCl, 7.6 μl xylazine, 2.4 μl acepromazine maleate, and 10 μl PBS) (Henry-Schein). FUCCI-expressing MKN45 cells were harvested from monolayer culture by brief trypsinization. Single-cell suspensions were prepared at a final concentration of 5 × 106 cells and injected subcutaneously in the left flank of nude mice.
Decoy chemotherapy
When the tumors reached approximately 8 mm in diameter (tumor volume, 300 mm3), mice were administered iv S. typhimurium A1-R, alone or in combination with cisplatinum (4 mg/kg ip) or paclitaxel (5 mg/kg ip) for 5 cycles every 3 d.
Statistical analysis
Data are shown as means ± SD. For comparison between 2 groups, significant differences were determined using the Student t test. For comparison of more than 2 groups, statistical significance was determined with a one-way ANOVA followed by a Bonferroni multiple-group comparison test. P < 0.05 was considered significant.
Competing Financial Interests
Y.Z. and M.Z. are employees of AntiCancer Inc. SY, YH, SM, FU, HK, and RMH are or were unsalaried associates of AntiCancer Inc. There are no other competing financial interests.
Authors Contributions
SY and RMH conceived the idea for this project. SY and RMH designed all experiments and wrote the manuscript. SY, YZ, YH, SM, and FU performed all experiments. HK, HT, MZ, MB, and TF provided crucial ideas and helped with data interpretation. YZ and HT provided special technical assistance.
Funding
This work was supported in part by National Cancer Institute grant CA132971.
Dedication
This paper is dedicated to the memory of AR Moossa, MD.
References
- 1. Sakaue-Sawano A, Kurokawa H, Morimura T, Hanyu A, Hama H, Osawa H, Kashiwagi S, Fukami K, Miyata T, Miyoshi H, et al. . Visualizing spatiotemporal dynamics of multicellular cell cycle progression. Cell 2008; 132:487-98; PMID:18267078; http://dx.doi.org/ 10.1016/j.cell.2007.12.033 [DOI] [PubMed] [Google Scholar]
- 2. Yano S, Zhang Y, Miwa S, Tome Y, Hiroshima Y, Uehara F, Yamamoto M, Suetsugu A, Kishimoto H, Tazawa H, et al. . Spatial-temporal FUCCI imaging of each cell in a tumor demonstrates locational dependence of cell cycle dynamics and chemoresponsiveness. Cell Cycle 2014; 13:2110-9; PMID:24811200; http://dx.doi.org/ 10.4161/cc.29156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yano S, Miwa S, Mii S, Hiroshima Y, Uehara F, Yamamoto M, Kishimoto H, Tazawa H, Bouvet M, Fujiwara T, Hoffman RM. Invading cancer cells are predominantly in G0/G1 resulting in chemoresistance demonstrated by real-time FUCCI imaging. Cell Cycle 2014; 13:953-60; PMID:24552821; http://dx.doi.org/ 10.4161/cc.27818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yano S, Tazawa H, Hashimoto Y, Shirakawa Y, Kuroda S, Nishizaki M, Kishimoto H, Uno F, Nagasaka T, Urata Y, et al. . A genetically engineered oncolytic adenovirus decoys and lethally traps quiescent cancer stem-like cells into S/G2/M phases. Clin Cancer Res 2013; 19:6495-505; PMID:24081978; http://dx.doi.org/ 10.1158/1078-0432.CCR-13-0742 [DOI] [PubMed] [Google Scholar]
- 5. William Coley Available from: http://en.wikipedia.org/wiki/William_Coley [Last accessed 12 December 2013]. [Google Scholar]
- 6. Hoffman RM, Zhao M. Methods for the development of tumor-targeting bacteria. Expert Opin Drug Discov 2014; 9:741-50; PMID:24949888; http://dx.doi.org/ 10.1517/17460441.2014.916270 [DOI] [PubMed] [Google Scholar]
- 7. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol 2003; 4:548-56; PMID:12965276; http://dx.doi.org/ 10.1016/S1470-2045(03)01194-X [DOI] [PubMed] [Google Scholar]
- 8. Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res 1997; 57:4537-44; PMID:9377566 [PubMed] [Google Scholar]
- 9. Toso JF, Gill VJ, Hwu P, Marincola FM, Restifo NP, Schwartzentruber DJ, Sherry RM, Topalian SL, Yang JC, Stock F, et al. . Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol 2002; 20:142-52; PMID:11773163; http://dx.doi.org/ 10.1200/JCO.20.1.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao M, Yang M, Li X-M, Jiang P, Baranov E, Li S, Xu M, Penman S, Hoffman RM. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci USA 2005; 102:755-60; PMID:15644448; http://dx.doi.org/ 10.1073/pnas.0408422102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao M, Geller J, Ma H, Yang M, Penman S, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci USA 2007; 104:10170-4; PMID:17548809; http://dx.doi.org/ 10.1073/pnas.0703867104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao M, Yang M, Ma H, Li X, Tan X, Li S, Yang Z, Hoffman RM. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res 2006; 66:7647-52; PMID:16885365; http://dx.doi.org/ 10.1158/0008-5472.CAN-06-0716 [DOI] [PubMed] [Google Scholar]
- 13. Uchugonova A, Zhao M, Zhang Y, Weinigel M, König K, Hoffman RM. Cancer-cell killing by engineered Salmonella imaged by multiphoton tomography in live mice. Anticancer Res 2012; 32:4331-8; PMID:23060555 [PubMed] [Google Scholar]
- 14. Liu F, Zhang L, Hoffman RM, Zhao M. Vessel destruction by tumor-targeting Salmonella typhimurium A1-R is enhanced by high tumor vascularity. Cell Cycle 2010; 9:4518-24; PMID:21135579; http://dx.doi.org/ 10.4161/cc.9.22.13744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nagakura C, Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Bouvet M, Hoffman RM. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res 2009; 29:1873-8; PMID:19528442 [PubMed] [Google Scholar]
- 16. Yam C, Zhao M, Hayashi K, Ma H, Kishimoto H, McElroy M, Bouvet M, Hoffman RM. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium inhibits liver metastasis in a mouse model of pancreatic cancer. J Surg Res 2010;164:248-55; PMID:19766244; http://dx.doi.org/ 10.1016/j.jss.2009.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Matsumoto Y, Miwa S, Zhang Y, Hiroshima Y, Yano S, Uehara F, Yamamoto M, Toneri M, Bouvet M, Matsubara H, et al. . Efficacy of tumor-targeting Salmonella typhimurium A1-R on nude mouse models of metastatic and disseminated human ovarian cancer. J Cell Biochem 2014; 115:1996-2003. [DOI] [PubMed] [Google Scholar]
- 18. Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Hoffman RM. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem 2009;106:992-8; PMID:19199339; http://dx.doi.org/ 10.1002/jcb.22078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi K, Zhao M, Yamauchi K, Yamamoto N, Tsuchiya H, Tomita K, Kishimoto H, Bouvet M, Hoffman RM. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle 2009; 8:870-5; PMID:19221501; http://dx.doi.org/ 10.4161/cc.8.6.7891 [DOI] [PubMed] [Google Scholar]
- 20. Kimura H, Zhang L, Zhao M, Hayashi K, Tsuchiya H, Tomita K, Bouvet M, Wessels J, Hoffman RM. Targeted therapy of spinal cord glioma with a genetically-modified Salmonella typhimurium. Cell Prolif 2010; 43:41-8; PMID:19922490; http://dx.doi.org/ 10.1111/j.1365-2184.2009.00652.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hiroshima Y, Zhao M, Zhang Y, Maawy A, Hassanein MK, Uehara F, Miwa S, Yano S, Momiyama M, Suetsugu A, et al. . Comparison of efficacy of Salmonella typhimurium A1-R and chemotherapy on stem-like and non-stem human pancreatic cancer cells. Cell Cycle 2013; 12:2774-80; PMID:23966167; http://dx.doi.org/ 10.4161/cc.25872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiroshima Y, Zhao M, Maawy A, Zhang Y, Katz MH, Fleming JB, Uehara F, Miwa S, Yano S, Momiyama M, et al. . Efficacy of Salmonella typhimurium A1-R versus chemotherapy on a pancreatic cancer patient-derived orthotopic xenograft (PDOX). J Cell Biochem 2014; 115:1254-61; PMID:24435915; http://dx.doi.org/ 10.1002/jcb.24769 [DOI] [PubMed] [Google Scholar]
- 23. Blagosklonny MV. How cancer could be cured by 2015. Cell Cycle 2005; 4:269-78; PMID:15655345 [PubMed] [Google Scholar]
- 24. Blagosklonny MV. Tissue-selective therapy of cancer. Br J Cancer 2003; 89:1147-51; PMID:14520435; http://dx.doi.org/ 10.1038/sj.bjc.6601256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blagosklonny MV. Matching targets for selective cancer therapy. Drug Discov Today 2003; 8:1104-7; PMID:14678733; http://dx.doi.org/ 10.1016/S1359-6446(03)02806-X [DOI] [PubMed] [Google Scholar]
- 26. Blagosklonny MV. “Targeting the absence” and therapeutic engineering for cancer therapy. Cell Cycle 2008; 7:1307-12; PMID:18487952; http://dx.doi.org/ 10.4161/cc.7.10.6250 [DOI] [PubMed] [Google Scholar]
- 27. Blagosklonny MV. Teratogens as anti-cancer drugs. Cell Cycle 2005; 4:1518-21; PMID:16258270; http://dx.doi.org/ 10.4161/cc.4.11.2208 [DOI] [PubMed] [Google Scholar]
- 28. Blagosklonny MV. Treatment with inhibitors of caspases, that are substrates of drug transporters, selectively permits chemotherapy-induced apoptosis in multidrug-resistant cells but protects normal cells. Leukemia 2001; 15:936-41; PMID:11417480; http://dx.doi.org/ 10.1038/sj.leu.2402127 [DOI] [PubMed] [Google Scholar]
- 29. Blagosklonny MV. Target for cancer therapy: proliferating cells or stem cells. Leukemia 2006; 20:385-91; PMID:16357832; http://dx.doi.org/ 10.1038/sj.leu.2404075 [DOI] [PubMed] [Google Scholar]
- 30. Blagosklonny MV. Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biol Ther 2007; 6:1684-90; PMID:18344680; http://dx.doi.org/ 10.4161/cbt.6.11.5167 [DOI] [PubMed] [Google Scholar]
- 31. Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011; 2:222-33; PMID:21447859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rao B, van Leeuwen IM, Higgins M, Campbel J, Thompson AM, Lane DP, Lain S. Evaluation of an Actinomycin D/VX-680 aurora kinase inhibitor combination in p53-based cyclotherapy. Oncotarget 2010; 1:639-50; PMID:21317459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Blagosklonny MV. NCI's provocative questions on cancer: some answers to ignite discussion. Oncotarget 2011; 2:1352-67; PMID:22267462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Blagosklonny MV. Antagonistic drug combinations that select against drug resistance: from bacteria to cancer. Cancer Biol Ther 2007; 6:1013-4; PMID:17646740; http://dx.doi.org/ 10.4161/cbt.6.7.4340 [DOI] [PubMed] [Google Scholar]