Abstract
In addition to governing mitotic progression, Plk1 also suppresses the activation of the G2 DNA damage checkpoint and promotes checkpoint recovery. Previous studies have shown that checkpoint activation after DNA damage requires inhibition of Plk1, but the underlying mechanism of Plk1 regulation was unknown. In this study we show that the specific phosphatase activity toward Plk1 Thr-210 in interphase Xenopus egg extracts is predominantly PP2A-dependent, and this phosphatase activity is upregulated by DNA damage. Consistently, PP2A associates with Plk1 and the association increases after DNA damage. We further revealed that B55α, a targeting subunit of PP2A and putative tumor suppressor, mediates PP2A/Plk1 association and Plk1 dephosphorylation. B55α and PP2A association is greatly strengthened after DNA damage in an ATM/ATR and checkpoint kinase-dependent manner. Collectively, we report a phosphatase-dependent mechanism that responds to DNA damage and regulates Plk1 and checkpoint recovery.
Keywords: B55α, checkpoint recovery, DNA damage, Plk1, PP2A
Introduction
Polo-like kinase 1 (Plk1, also known as Plx1 in Xenopus) is the best-studied member of the evolutionarily conserved polo-like kinase family. Plk1 contains a kinase domain at its N-terminus and a polo-box domain composed of 2 polo-box motifs at the C-terminus. The polo-box domain is believed to regulate the subcellular localization of Plk1 and mediate its association with specific substrate proteins. As the polo-box domain preferentially binds phosphorylated peptides, Plk1-mediated phosphorylation typically involves priming phosphorylation of the substrates by other protein kinases, such as cyclin-dependent kinase 1 (Cdk1).1-4 It has been shown that Plk1 plays critical roles in many aspects of cell division, including centrosome maturation and separation, mitotic entry, spindle and chromosome dynamics, and cytokinesis. The extensive involvement of Plk1 in mitosis is consistent with both the complex localization of Plk1 in the cell and the diverse substrates that are targeted by Plk1, including Cdc25, Mst2, Nek9, BubR1, Emi1, Cyclin B, NuMA, and PRC1. The critical role of Plk1 in mitotic regulation was also reflected by the fact that inhibition of Plk1 induced mitotic defects and cell death.1-3,5-7
Interestingly, in addition to regulation of cell division, Plk1 has also been shown to govern the cellular DNA damage response (DDR), an essential surveillance mechanism that prevents genomic instability and maintains cell homeostasis.5,8 As DNA damage is frequently induced by both endogenous and exogenous agents, all eukaryotic cells commit a great deal of resources to the DDR process. With more than 100 genes involved, the DDR encompasses complex network of signal transduction and a broad spectrum of enzymatic activities. In principle, DDR activation leads to DNA repair, and cell cycle arrest via the checkpoint mechanism. Upon completion of DNA repair, the cell will recover from the DDR and re-enter cell cycle progression, a process termed DNA damage checkpoint recovery, whereas failure in DNA repair leads to apoptosis or senescence.9,10 At the center of the DDR are the phosphoinositide 3 kinase-related kinases ATM and ATR. Activation of ATM/ATR by DNA damage results in phosphorylation of dozens of physiological substrates that control various pathways including DNA repair, checkpoint control, apoptosis and transcription.11 For example, ATM and ATR activate the checkpoint kinases Chk1 and Chk2, which phosphorylate Cdc25, leading to its proteolysis and nuclear export. Subsequently, loss of Cdc25-dependent dephosphorylation of Cdks at inhibitory residues prevents Cdk activation and cell cycle progression.9 Germline mutations in DDR genes, such as TP53, ATM, CHEK2, BRCA1, BRCA2, MRE11, RAD50, NBS1, MSH2, MLH1, FANCD2, often lead to cancer predisposition, indicating a critical role of the DDR in tumor suppression.12,13
Plk1 acts as an important regulator of the DDR by inhibiting DNA damage signaling and promoting checkpoint recovery. The role of Plk1 in checkpoint recovery likely differs from its role in unperturbed mitosis, as suggested by a few lines of evidence. First, a previous study showed that Plk1 was required for mitotic reentry following DNA damage and inactivation of ATM/ATR, whereas these cells were not dependent on Plk1 for mitotic entry in the absence of DNA damage.14 Second, detailed investigations revealed a number of DNA damage checkpoint factors, including claspin, 53BP1, and Chk2 as substrates of Plk1. Phosphorylation of these factors by Plk1 typically led to disruption of their functions.15-18 In addition to the function of Plk1 in checkpoint recovery, recent studies also discovered the molecular mechanism that leads to Plk1 activation. It has been shown that, during both checkpoint recovery and unperturbed mitosis, Aurora A kinase activates Plk1 by phosphorylating Plk1 at its T-loop activation site, Thr-210.19,20 The mechanism of Plk1 activation would, on the other hand, suggest that deactivation of Plk1 can be readily achieved through Thr-210 dephosphorylation. Though the detailed mechanism of Plk1 dephosphorylation is largely obscure, it has been shown that introduction of a phospho-mimetic form of Plk1 that cannot be dephosphorylated suppressed the activation of the DNA damage checkpoint.21
Dephosphorylation of Ser/Thr residues is catalyzed by a group of Ser/Thr phosphatases. In particular, protein phosphatases 1 and 2A (PP1 and PP2A) are the most abundant forms that together account for over 90% of the total cellular Ser/Thr activity. The specific action of PP1 and PP2A relies on a large array of targeting and regulatory subunits. The holoenzyme containing the catalytic and targeting subunits, and in the case of PP2A, an additional scaffold subunit, is believed to recognize and dephosphorylate specific substrates.22 With the existence of various targeting subunits that mediate the diverse function of PP1 and PP2A, it is not surprising that PP1 and PP2A have been shown to govern many important biological processes. In this study, we investigated whether and how DNA damage modulates Plk1 through protein phosphatases. Our study revealed that the phosphatase activity toward Plk1 Thr-210 increases after DNA damage. Dephosphorylation of Plk1 Thr-210 is predominantly mediated by PP2A, consistent with increased PP2A association with Plk1 after DNA damage. A specific targeting subunit of PP2A, B55α, mediates PP2A and Plk1 association, and is responsible for DNA damage-induced dephosphorylation of Plk1. B55α and PP2A association was greatly strengthened after DNA damage, in an ATM/ATR and checkpoint kinase-dependent manner. The characterization of B55α as a regulator of Plk1 and DNA damage checkpoint recovery may shed important light on the emerging role of B55α as a major tumor suppressor in various human cancers.
Results
DNA damage induces dephosphorylation of Plk1 Thr-210 in a PP2A-dependent manner
We incubated purified, active Xenopus Plk1 in interphase Xenopus egg extracts, and monitored its dephosphorylation at Thr-210, the well-characterized T-loop phosphorylation site required for Plk1 activation.19,20,23 As shown in Figure 1A, Plk1 was dephosphorylated in approximately 20 min, suggesting that the phosphatase activity that dephosphorylates Plk1 dominates the kinase activity that phosphorylates Plk1 in interphase egg extracts. To investigate the impact of DNA damage on Plk1 dephosphorylation, we supplemented the extract with dA-dT oligonucleotides, a well-defined method to induce the DDR.24 Interestingly, Plk1 was more efficiently dephosphorylated in extracts containing dA-dT (Fig. 1A). Dephosphorylation of Plk1 in extracts containing damaged DNA was correlated with a reduced kinase activity of Plk1 (Fig. 1B).
Figure 1.
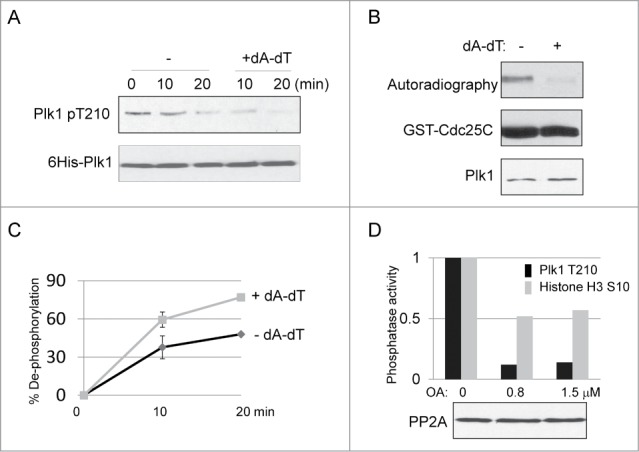
DNA damage-induced dephosphorylation of Plk1 Thr-210. (A) Active 6His-Plk1 was added into interphase egg extracts with or without supplementation of dA-dT (20 ng/μl), Plk1 dephosphorylation was analyzed by immunoblotting using phospho-Plk Thr-210 and His-tag antibodies. (B) Plk1 immunoprecipitation (IP)-kinase assay was performed using GST-Cdc25C as substrate, as described in Materials and Methods. Plk1 was incubated in and immunoprecipitated from extracts treated with or without dA-dT (for 10 min, as in panel A). The autoradiographic image, and immunoblots of GST and Plk1 are shown. (C) As described in Materials and Methods, the phosphatase assay was performed using Plk1 Thr-210 peptide as substrate, dephosphorylation was measured at the indicated time points in extracts with or without dA-dT. (D) The phosphatase activity toward Plk1 Thr-210 and Histone H3 Ser-10 was measured as in panel C. Okadaic acid (OA) was added into the extract as indicated. The level of PP2A in these extracts was measured by immunoblotting.
Furthermore, we adopted from Morchida et al.25 an in-extract phosphatase assay to specifically measure the phosphatase activity toward Plk1 Thr-210. We first expressed a short motif surrounding Thr-210, and then phosphorylated the recombinant protein in vitro using Aurora A and32P-labeled ATP. The specificity of the phosphorylation was confirmed using a phospho-deficient mutant (Plk1 Thr210 ->Ala, data not shown). We then eluted the phosphorylated substrate and added it into extracts, in which during dephosphorylation it released free32P-ATP. Extracts were then treated with trichloroacetic acid to precipitate proteins, and the supernatant was evaluated for the release of free32P-ATP in proportion to the total radioactivity. As shown in Figure 1C, extracts with DNA damage consistently exhibited higher phosphatase activity toward Plk1 Thr-210, as compared to those without DNA damage. The phosphatase activity toward Plk1 Thr-210 was likely PP2A-dependent, as it was inhibited by okadaic acid (OA) (Fig. 1D), at concentrations sufficient to inhibit PP2A, but not PP1.25 The protein level of PP2A remained unchanged upon treatment with OA. As a negative control, we included in the experiment histone H3 Ser-10 (Fig. 1D), which was shown to be dephosphorylated by PP1.26
PP2A association with Plk1 increases after DNA damage
Consistent with the above results showing PP2A-dependent dephosphorylation of Plk1 at Thr-210, we observed protein association between PP2A and Plk1. As shown in Figure 2A, Plk1 was found in the PP2A immunoprecipitate isolated from interphase egg extracts. Interestingly, a higher level of Plk1 was recovered by PP2A immunoprecipitation from extracts supplemented with dA-dT, indicating that the association between PP2A and Plk1 increases after DNA damage (Fig. 2A). To verify these findings, an alternative pull-down assay was performed using microcystin beads that bind both PP1 and PP2A. As expected, microcystin beads pulled down a portion of Plk1 from interphase egg extracts (Fig. 2B). More Plk1 was bound to microcystin beads in extracts containing dA-dT, confirming an increased association between Plk1 and microcystin-bound phosphatases after DNA damage (Fig. 2B). Furthermore, addition of OA can disrupt the binding of PP2A, but not PP1, to microcystin beads (Fig. 2B, and25,27). As in Figure 2B, Plk1 was not detected in microcystin pull-down prepared from extracts containing both dA-dT and OA, confirming a dependence on PP2A, but not PP1.
Figure 2.
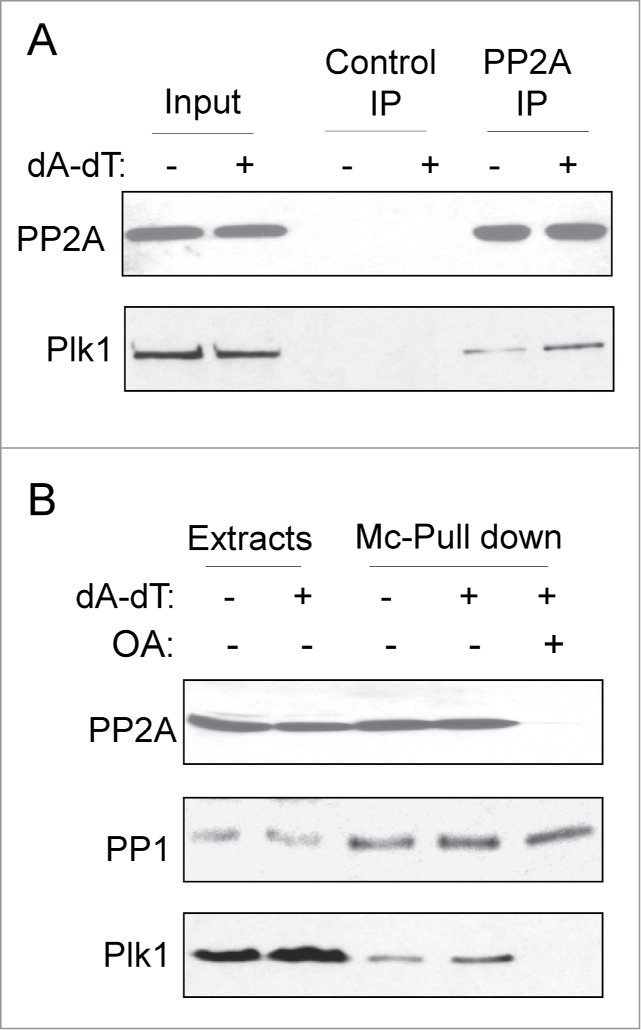
PP2A association with Plk1. (A) As described in Materials and Methods, PP2A or control immunoprecipitation (IP) was performed in interphase egg extracts with or without DNA damage treatment (dA-dT). The input and IP products were analyzed by immunoblotting for PP2A and Plk1. (B) Microcystin-beads pull down was performed in extracts with or without dA-dT. OA was added as indicated to compete off PP2A-binding to Microcystin-beads. The input extracts and pull-down products were analyzed by immunoblotting for PP1, PP2A and Plk1.
B55α associates with Plk1
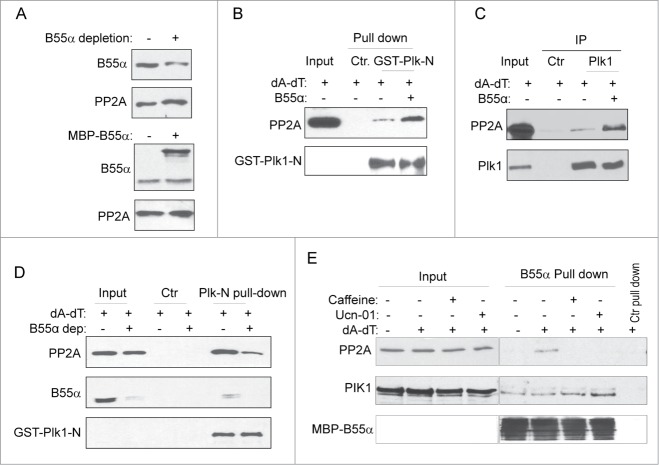
The specificity of PP2A is conferred through its various targeting subunits, or B subunits. Among several B, B′, and B″ family members, we observed protein association between B55α (also known as Ppp2r2a) and Plk1 (Fig. 3A). The specificity of B55α and Plk1 association was underscored by the fact that B55β, another member of the B family sharing 86% sequence identify with B55α, did not exhibit a detectable association with Plk1 (Fig. 3A). The B55α and Plk1 association was confirmed at the endogenous level by reciprocal immunoprecipitation using either Plk1 or B55α antibody (Fig. 3B and C). It has been shown that, in many cases, Plk1 associates with substrates and functional partners via its C-terminal polo-box domain. However, the N-terminus of Plk1 that contains the kinase domain was sufficient in binding B55α and PP2A (Fig. 3D).
Figure 3.
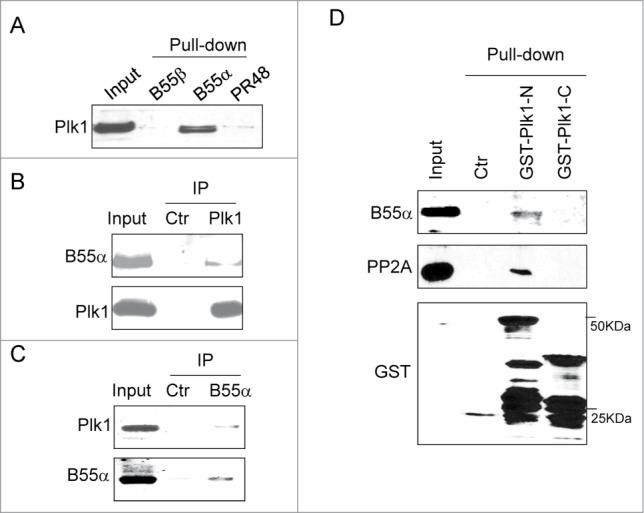
B55α association with Plk1. (A) The pull-down assay was performed in Xenopus egg extracts as described in Materials and Methods using purified B55α and other PP2A subunits. Immunoblotting of Plk1 was shown. (B) Immunoprecipitation was performed in in Xenopus egg extracts as described in Materials and Methods using Plk1 antibody. Immunoblotting of B55α and Plk1 was shown. (C) Immunoprecipitation was performed in in Xenopus egg extracts using B55α antibody. Immunoblotting of B55α and Plk1 was shown. (D) N-terminal (aa 1–380) or C-terminal (aa 380–598) Xenopus Plk1 was expressed, purified, and used for pull down assays, as described in Materials and Methods. Input and pull-down products were analyzed by immunoblotting for B55α, PP2A and GST.
B55α mediates Plk1 association with PP2A
With the discovery of Plk1/B55α association, we speculated that B55α may mediate the association between PP2A and Plk1. To reveal a detailed role of B55α using both loss-of-function and gain-of-function approaches, we altered the protein level of B55α in extracts by immunodepletion using a specific antibody or by supplementation of purified, recombinant B55α (Fig. 4A). Interestingly, we found that the addition of B55α in interphase egg extracts led to increased association of PP2A with Plk1 N-terminus (Fig. 4B). Similarly, immunoprecipitation of Plk1 recovered more PP2A with the addition of B55α (Fig. 4C). Conversely, a partial depletion of endogenous B55α resulted in a reduced level of PP2A that was associated with Plk1 N-terminus (Fig. 4D). Collectively, these results demonstrated the role of B55α in mediating Plk1 and PP2A association.
Figure 4.
B55α mediates the association between Plk1 with PP2A. (A) B55α depletion (upper panel) and supplementation (lower panel). Immunodepletion of B55α was performed as described in Materials and Methods, the mock or B55α-depleted extract was analyzed by immunoblotting for B55α and PP2A (upper panel). MBP-B55α was cloned, expressed, and purified as described in Materials and Methods. Extracts added with MBP-B55α or control buffer were analyzed by immunoblotting for B55α and PP2A (lower panel). (B) As in Figure 3D, pull-down with Plk1 N-terminus was performed in Xenopus egg extracts treated with dA-dT and B55α, as indicated. Input extract and pull-down products were analyzed by immunoblotting for PP2A and GST. The control pull-down was performed using glutathione beads. (C) Immunoprecipation of Plk1 was performed from extracts supplemented with dA-dT and B55α. Input extracts and IP products were analyzed by immunoblotting for PP2A and Plk1. (D) Plk1-N Pull down assay was performed in extracts with mock or B55α depletion as in Figure 3D. Input extracts, control and Plk1-N pull down products were analyzed by immunoblotting for PP2A, B55α, and GST. (E) MBP-B55α pull-down was performed in Xenopus egg extracts treated with dA-dT, caffeine (2.5 mM) and Ucn-01 (100 nM), as indicated. Input extract and pull-down products were analyzed by immunoblotting for PP2A, Plk1, and MBP.
Increased B55α-PP2A association after DNA damage
We showed that DNA damage led to an increased association between PP2A and Plk1, which finding was in line with the elevated phosphatase activity toward Plk1 Thr-210. We then asked if the association of B55α with Plk1 or PP2A changed after DNA damage. We did not observe an increase in Plk1/B55α association after the addition of dA-dT in egg extracts (Fig. 4E). However, our results revealed an induced association between B55α and PP2A after DNA damage (Fig. 4E). The B55α and PP2A association was disrupted upon treatment with caffeine, an inhibitor of ATM/ATR, or Ucn-01, an inhibitor of Chk1/Chk2 (Fig. 4E,28). This result suggested that checkpoint signaling through these DNA damage kinases modulates B55α/PP2A association. As B55α mediates Plk1 and PP2A association, this mode of regulation would account for the increased association between Plk1 and PP2A, and the elevated phosphatase activity toward Plk1.
B55α mediates DNA damage-induced dephosphorylation of Plk1 Thr-210
It has been well-demonstrated that the phosphatase holoenzymes containing targeting subunits act in a substrate-specific fashion.22 To directly examine the role of B55α/PP2A in the dephosphorylation of Plk1 Thr-210, we isolated recombinant B55α from interphase egg extracts, and measured the co-purified phosphatase activity toward Plk1 Thr-210. The B55α complex was able to dephosphorylate active Plk1 in vitro (Fig. 5A). Furthermore, we incubated the B55α complex with CSF extract containing Plk1 Thr-210 and other mitotic phosphorylation, and observed efficient dephosphorylation of Plk1 Thr-210, but not H3 Ser-10 (Fig. 5B). To further reveal the role of B55α in mediating Plk1 dephosphorylation in extracts. Active Plk1 was incubated in interphase egg extracts with or without immunodepletion of B55α (Fig. 5C), and assessed for its dephosphorylation. As expected, immunodepletion of B55α caused a delay in the dephosphorylation of Plk1 Thr-210 in extracts (Fig. 5D, Plk1 was efficiently dephosphorylated within 10–20 min in the mock-depleted, but not B55α-depleted extract). Add-back of recombinant B55α restored Plk1 dephosphorylation (Fig. 5D).
Figure 5.
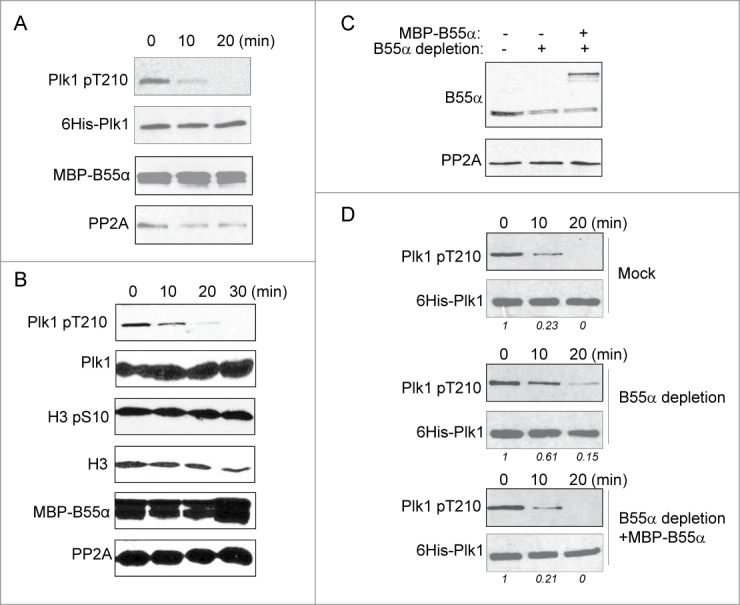
B55α mediates DNA damage-induced dephosphorylation of Plk1 Thr-210. (A) In vitro phosphatase assay was performed using purified, active His-Plk1 as substrate. Recombinant B55α incubated in, and then re-isolated from interphase Xenopus egg extracts with dA-dT was added to the reaction. The reaction was analyzed at the indicated time points by immunoblotting using phospho-Plk1 Thr-210, His-tag, MBP-tag and PP2A antibodies. (B) MBP-B55α beads were prepared as in panel A, and incubated with 1:5 diluted CSF extract. The reaction was analyzed at the indicated time points by immunoblotting for phospho-Plk1 Thr-210, Plk1, phospho-histone H3 Ser-10, H3, MBP-tag and PP2A. (C) Immunodepletion and supplementation of B55α was performed in interphase Xenopus egg extracts as in Figure 4A. These extracts were analyzed by immunoblotting for B55α and PP2A. (D) Purified, active His-Plk1 was added into extracts prepared as in panel C. The extract samples were analyzed by immunoblotting using phospho-Plk1 Thr-210 and His-tag antibodies. The band intensity was measured using NIH Image-J software and the phospho-Plk1/His-Plk1 ratio is shown.
B55α regulates DNA damage checkpoint recovery through Plk1
It has been shown that Plk1 negatively regulates the DNA damage checkpoint and is thereby required for checkpoint recovery.14 As we characterized B55α as a regulator of Plk1 that directs an elevated phosphatase activity toward Plk1 in response to DNA damage, we speculated that B55α may control checkpoint recovery via modulation of Plk1. As in our previous studies,29,30 checkpoint recovery was initiated by removing dA-dT from the extract, which was then monitored for phosphorylation of Chk1 as a marker of checkpoint signaling, and phosphorylation of Cdc27 and Cdc25 as markers of mitotic entry (Fig. 6A). Interestingly, supplementation of B55α in interphase egg extracts prevented Plk1 phosphorylation, and suppressed checkpoint recovery, as judged by the sustained Chk1 and Cdc25 phosphorylation and abrogated Cdc27 phosphorylation (Fig. 6A). Moreover, the effect of B55α on checkpoint recovery was rescued by the co-addition of a phospho-mimetic form of Plk1, confirming that B55α controls checkpoint recovery via regulation of Plk1 (Fig. 6B). We then examined the association of PP2A/B55α with Plk1 to reveal more insights into the role of this phosphatase complex in regulation of Plk1 during checkpoint recovery. We have shown that the association of PP2A with B55α and Plk1 increased after DNA damage. Likewise, during the initial stage (time 0) of checkpoint recovery, Plk1 associated with PP2A, to a much greater extent compared to that in the control interphase extract (Fig. 6C). Consistently, an elevated association between PP2A and B55α was also observed in the recovery extract at time 0 (Fig. 6D). Interestingly, as the extracts underwent recovery, the PP2A/B55α association was reduced, presumably to allow the phosphorylation and activation of Plk1 (Fig. 6D).
Figure 6.
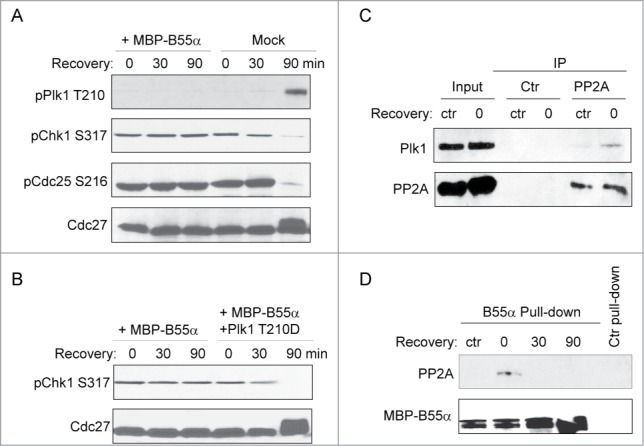
B55α regulates DNA damage checkpoint recovery through Plk1. (A) Checkpoint recovery was performed in Xenopus egg extracts as described in Materials and Methods, with or without supplementation of B55α. Extract samples were analyzed at the indicated time points for Plk1 Thr-210, Chk1 Ser-317, Cdc25 Ser-216, and Cdc27. (B) Checkpoint recovery was performed in Xenopus egg extracts with supplementation of B55α and Plk1 as indicated. Extract samples were analyzed at the indicated time points for Chk1 Ser-317 and Cdc27. (C) PP2A IP, or control beads IP, was performed in the control interphase extract (ctr), or recovery extract at time 0 (0). The input extracts and the IP products were analyzed by immunoblotting for Plk1 and PP2A. (D) MBP-B55α pull-down was performed in the control interphase extract (ctr), or recovery extract at the indicated time points. The pull-down levels of PP2A and MBP are shown. The control pull-down was performed using amylose beads.
Discussion
Given the critical function of Plk1 in regulation of cell division and the DNA damage checkpoint, the fine modulation of Plk1 activity is undoubtedly a critical task. In particular, previous studies have shown that Plk1 inhibition was necessary for the activation of the DNA damage checkpoint, whereas sustained activation of Plk1 overrode the DNA damage checkpoint, leading to premature mitotic entry in the presence of DNA damage.14 As Plk1 is activated via phosphorylation at the Thr-210 site within its activation loop, it is plausible that Thr-210 dephosphorylation constitutes an effective mechanism to switch off Plk1 activity. A few previous studies shed some light on how Plk1 can be dephosphorylated and deactivated by either PP1 or PP2A.31-34 Interestingly, it was suggested that mitotic DNA damage can lead to dephosphorylation of mitotically active Plk1 in a PP2A-dependent manner,31 a conclusion that was, however, not supported by another report that found no dephosphorylation of Plk1 at Thr-210 or Ser-137 in response to mitotic DNA damage.32 More importantly, activation of the DNA damage checkpoint in interphase prevents cells from entering mitosis, but the above studies did not address how Plk1 is regulated prior to mitotic entry. Plk1 can also be regulated by PP1, mediated by myosin phosphatase-targeting subunit 1 (MYPT). MYPT/PP1 dephosphorylates Plk1 and antagonizes the function of Plk1 in centrosome maturation and other aspects of mitosis.34 A more recent study further suggested that MYPT/PP1-dependent regulation of Plk1 may play a role in G2 phase or early M-phase before nuclear membrane breakdown. In that study, Kachaner et al.35 showed that cytoplasmic optineurin is phosphorylated by Plk1 and thereby shuttled to the nucleus. Once in the nucleus, optineurin promotes dephosphorylation of nuclear Plk1 by the MYPT complex.
To delineate the specific phosphatase activity that acts on Plk1 Thr-210 in interphase, and investigate whether such activity is modulated by DNA damage, we adopted an in-extract phosphatase assay using radioactively phosphorylated Plk1 Thr-210 motif as substrate. This method measures specific dephosphorylation of a given phospho-residue, while minimizing the influence of counteracting protein kinases as rephosphorylation of the substrate in extracts would utilize predominantly endogenous ATP. Our results clarified that dephosphorylation of Plk1 in interphase egg extracts was mediated by PP2A, but not PP1, and that the phosphatase activity was upregulated in response to DNA damage. This conclusion was further supported by the fact that Plk1 binds PP2A but not PP1, and Plk1/PP2A association increased after DNA damage. Importantly, our study identified B55α as the specific targeting subunit mediating PP2A-dependent regulation of Plk1 after DNA damage. B55α binds Plk1 and mediates its association with PP2A. Unlike some other targeting subunits of PP2A, B55α exhibits minimal association with the catalytic subunit PP2A, whereas DNA damage-induced checkpoint signaling strengthened B55α-PP2A association. This interesting feature of B55α-PP2A association explained the increased phosphatase activity toward Plk1 Thr-210 in response to DNA damage, and provided new evidence in support of an important and highly regulated role of protein phosphatase in the DDR.36 Our results further demonstrated that B55α regulates DNA damage checkpoint recovery through Plk1. Thus, the study discovered a detailed mechanism that accounts for phosphatase-dependent regulation of Plk1. It should be noted that other PP1 and PP2A subunits may also be involved in regulation of Plk1, and future studies are needed to clarify whether and how multiple phosphatases govern Plk1 in a manner specific to subcellular localization and biological processes.
The classic model of the G2/M DNA damage checkpoint emphasizes the ATM/ATR-Chk1/Chk2-Cdc25 axis that prevents Cdk1 activation.37 However, other mitotic kinases, particularly Plk1 and Aurora A, also play important roles in regulation of mitotic entry. It is thus plausible that the DNA damage checkpoint directly inhibits non-Cdk kinases to reinforce cell cycle arrest. In fact, the critical nature of DNA damage-induced inhibition of Plk1 and Aurora A has been illustrated in previous studies as constitutively active Plk1 or Aurora A was sufficient to suppress the DNA damage checkpoint and promote premature checkpoint recovery.21,38,39 Therefore, to fully understand how the DNA damage checkpoint prevents mitosis, it is important to investigate whether and how DNA damage employs alterative mechanisms to regulate Plk1 and Aurora A in addition to the conventional Chk1/2-Cdc25-Cdk1 pathway. The present study revealed important insights into this question: through ATM/ATR and checkpoint kinases, DNA damage strengthens the incorporation of PP2A catalytic subunit into the B55α/Plk1 complex, leading to increased dephosphorylation and deactivation of Plk1. In theory, a similar phosphatase-dependent regulatory mechanism may also account for inhibition of Aurora A after DNA damage, given that activation of Aurora A is dependent on its T-loop phosphorylation at Thr-288. A previous study reported that DNA damage inhibited Aurora A in a manner that appeared independent of Cdk1. Intriguingly, phosphorylation of Aurora A at Ser-342, a regulatory site, was suggested to be involved.38
B55α belongs to the B family of PP2A targeting subunits, and, like many other phosphatase subunits, is poorly studied. Several genomic studies in human cancers suggested that B55α functions as a tumor suppressor. For example, one study showed depletion of B55α gene accounts for 67% of all prostate cancer cases.40 Moreover, a large-scale genomic and transcriptomic analysis of 2,000 breast tumors identified B55α as one of the most commonly silenced genes in breast cancer with a comparable or higher mutation rate than CDKN2, PTEN, etc.41 The same study also reported that the subgroup of breast cancer patients with loss of B55α suffered poor treatment outcome and survival.41 Others linked B55α to childhood teratoma,42 prostate cancer,43 colorectal cancer,44 lung cancer,45 and leukemia.46 In this study we characterized B55α as a regulator of Plk1 and the DNA damage checkpoint, which finding may mechanistically explain how B55α functions as an important tumor suppressor. After all, it has been well established that dysregulation of Plk1 contributes to cancer progression and resistance, and the DNA damage checkpoint pathway constitutes an important anti-cancer barrier. A recent finding also showed that B55α negatively regulates ATM,47 which is not in apparent agreement with the role of B55α as a tumor suppressor. However, it is plausible that B55α may play a rather complex role in the DDR by targeting multiple DDR factors. In addition to tumor suppression, the DNA damage pathway is also a determinant of the outcome of cancer therapy using radiation and other DNA damaging agents. Therefore, detailed investigations on the function and regulation of B55α in the DDR process may reveal new insights into how cancer cells escape from cellular surveillance and survive cancer therapy.
Materials and Methods
Immunoblotting, immunoprecipitation, and immunodepletion
Immunoblotting, immunoprecipitation, and immunodepletion were performed in Xenopus egg extracts as previously described.30 For immunoblotting, samples were harvested in Laemmli sample buffer (Bio-Rad, Hercules, CA), resolved by SDS-PAGE, and then electrotransferred to PVDF membranes (Millipore, Billerica, MA). Membranes were blocked with 5% nonfat dry milk in 1× TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) for 1 hr, incubated with primary antibodies for 2 hr, with horseradish peroxidase (HRP)-conjugated secondary antibodies (Sigma) for 1 hr, and then detected using an Enhanced Chemiluminescence (ECL) substrate kit (Pierce). For immunoprecipitation, anti-mouse or anti-rabbit magnetic beads (New England Biolabs) were conjugated to specific primary antibodies, and then mixed with egg extracts. After 30 min incubation, the beads were removed with a magnet and washed 3 times in a washing buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM DTT, and 0.5% Tween 20) before elution with 2× Laemmli sample buffer and analysis by immunoblotting. For imunodepletion, anti-mouse or -rabbit magnetic beads were conjugated to the specific antibodies, washed, and then added into Xenopus egg extracts as described above. After incubation for 30 min, the beads were removed with a magnet and the remaining extracts collected for experiments.
Reagents
Commercial antibodies used in this study include: Cdc27 antibody purchased from BD Transduction Laboratories (San Jose, CA); PP1 and PP2A antibodies purchased from Bethyl Labs (Montgomery, TX); His-tag and B55α antibodies from Genetex (Irvine, CA); phospho-Plk1, phospho-Cdc25, and phospho-Chk1 antibodies from Cell Signaling Technology (Beverly, MA); MBP antibody from New England Biolabs (Ipswich, MA); and GST antibody from Sigma (St. Louis, MO). Rabbit polyclonal antibodies to Xenopus B55α was generated against the N-terminal sequence of Pnuts. Xenopus Plk1 antibody was provided by Dr. James Maller (University of Colorado, Denver). Purified Xenopus Plk1, as utilized in our previous study,30 was obtained from Drs. Frank Eckerdt (Northwestern University) and Junjun Liu (California State Polytechnic University).48,49
Phosphatase and kinase assays
The phosphatase assay was performed in Xenopus egg extracts as described in a previous study.25 Briefly, the substrate, GST-Plx1-T210, was phosphorylated by activated Aurora A in kinase buffer (20 mM HEPES pH 7.5, 2 mM DTT, 10 mM MgCl2, 0.1 mM EGTA, 100 μM cold ATP, and 2 μCi [γ-32P]ATP) by Incubating for 20 min @ 30°C. The phosphorylated substrate was added to the extract with or without additives as noted and incubated at room temp for the desired amount of time. At each time point a portion of the reaction was removed and mixed with 30% trichloroacetic acid at a ratio of 1:5 to precipitate the proteins. The precipitant was removed by centrifugation at high speed for 10 min and the supernatant containing the free [γ-32P]ATP was transferred to tube containing scintillation liquid and the radioactivity was read by a Beckman LS 6500 multi-purpose scintillation counter. To represent the total radioactivity in each reaction, the same volume removed at each time-point was transferred directly to the scintillation liquid. The phosphatase activity was calculated as a percentage by dividing the dpm of [γ-32P]ATP released into the trichloroacetic acid by the dpm of the total radioactivity in the reaction. For the Plk1 kinase assay, Plk1 and Cdc25C (provided by Dr. Jim Maller) were incubated in in a final volume of 30 μl of kinase buffer (20 mM HEPES pH 7.5, 2 mM DTT, 10 mM MgCl2, 0.1 mM EGTA, 100 μM cold ATP, 2 μCi [γ-32P] ATP), incubated for 20 min at 30°C. The kinase reaction was stopped by boiling in 2× Laemmli buffer.
Protein purification and pull-down
The Xenopus B55α gene was cloned from a Xenopus oocyte cDNA library as previously described.50 The following targeting sequences were used for amplification of B55α (ATGGAGGGAGCTAGTG; CTAATTGACTCGGTCC). The gene was then inserted into the pMAL-parallel II plasmid with an N-terminal MBP tag. Vectors that express the N or C-terminus of Xneopus Plk1 were provided by Dr. James Maller (University of Colorado, Denver). These proteins were then expressed in BL21 bacterial cells, and purified with glutathione or amylose beads. For re-isolation of MBP- or GST-tagged proteins from Xenopus egg extracts, proteins bound to either amylose or glutathione beads were added to egg extract and incubated at room temperature. The beads were separated from the extract with low speed centrifugation and washed 3 times, and then resolved by SDS-PAGE and analyzed by immunoblotting.
Xenopus egg extracts
Cytostatic factor (CSF) extracts were freshly prepared as previously described.30 Eggs were dejellied with 2% cysteine in 1× XB (1 M KCl, 10 mM MgCl2, 100 mM HEPES pH 7.7, and 500 mM sucrose), washed 4 times with 1× XB, and then once with 1× MEB (1 M KCl, 11 mM MgCl2, 100 mM HEPES pH 7.7, 500 mM sucrose, and 5 mM EGTA, pH 7.7). Eggs were packed in centrifuge tubes with low speed centrifugation then crushed by centrifugation at 10,000 g at 4°C for 10 min. The cytoplasmic layer was further separated by centrifugation at 10,000 g for 15 min at 4°C. The checkpoint recovery assay was performed as described in previous studies.29,30 Briefly, biotinylated dA-dT oligos were pre-bound to M-280 streptavidin Dynabeads (Invitrogen) following the standard protocol provided by the manufacturer, and the beads were then added to the extracts to produce a final concentration of 20 ug/ml dA-dT. After 30 min, the beads were removed with a magnet to initiate checkpoint recovery.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgment
We thank Drs. James Maller (University of Colorado Denver), Satoru Mochida (Kumamoto University, Japan), Veerle Janssens (KU Leuven, Belgium), Frank Eckerdt (Northwestern University) and Junjun Liu (California State Polytechnic University) for reagents.
Funding
This work was supported by National Institutes of Health grant 1R01CA172574 to A.P.
References
- 1. Eckerdt F, Yuan JP, Strebhardt K. Polo-like kinases and oncogenesis. Oncogene 2005; 24:267-76; PMID:15640842; http://dx.doi.org/ 10.1038/sj.onc.1208273 [DOI] [PubMed] [Google Scholar]
- 2. Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol 2009; 185:193-202; PMID:19364923; http://dx.doi.org/ 10.1083/jcb.200812045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ma HT, Poon RYC. How protein kinases co-ordinate mitosis in animal cells. Biochem J 2011; M435:17-31; http://dx.doi.org/ 10.1042/BJ20100284 [DOI] [PubMed] [Google Scholar]
- 4. Liu JJ, Maller JL. Xenopus Polo-like kinase Plx1: a multifunctional mitotic kinase. Oncogene 2005; 24:238-47; PMID:15640839; http://dx.doi.org/ 10.1038/sj.onc.1208220 [DOI] [PubMed] [Google Scholar]
- 5. Lens SM, Voest EE, Medema RH. Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat Rev Cancer 2010; 10:825-41; PMID:21102634; http://dx.doi.org/ 10.1038/nrc2964 [DOI] [PubMed] [Google Scholar]
- 6. Janssen A, Medema RH. Mitosis as an anti-cancer target. Oncogene 2011; 30:2799-809; PMID:21339734; http://dx.doi.org/ 10.1038/onc.2011.30 [DOI] [PubMed] [Google Scholar]
- 7. Taylor S, Peters JM. Polo and Aurora kinases: lessons derived from chemical biology. Curr Opin Cell Biol 2008; 20:77-84; PMID:18249108; http://dx.doi.org/ 10.1016/j.ceb.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 8. Takaki T, Trenz K, Costanzo V, Petronczkil M. Polo-like kinase 1 reaches beyond mitosis-cytokinesis, DNA damage response, and development. Curr Opin Cell Biol 2008; 20:650-60; PMID:19000759; http://dx.doi.org/ 10.1016/j.ceb.2008.10.005 [DOI] [PubMed] [Google Scholar]
- 9. Zhou BBS, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature 2000; 408:433-9; PMID:11100718; http://dx.doi.org/ 10.1038/35044005 [DOI] [PubMed] [Google Scholar]
- 10. Bartek J, Lukas J. DNA damage checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 2007; 19:238-45; PMID:17303408; http://dx.doi.org/ 10.1016/j.ceb.2007.02.009 [DOI] [PubMed] [Google Scholar]
- 11. Shiloh Y. ATM and related protein kinases: Safeguarding genome integrity. Nat Rev Cancer 2003; 3:155-68; PMID:12612651; http://dx.doi.org/ 10.1038/nrc1011 [DOI] [PubMed] [Google Scholar]
- 12. Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 2001; 27:247-54; PMID:11242102; http://dx.doi.org/ 10.1038/85798 [DOI] [PubMed] [Google Scholar]
- 13. Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev 2004; 14:11-6; PMID:15108799; http://dx.doi.org/ 10.1016/j.gde.2003.12.003 [DOI] [PubMed] [Google Scholar]
- 14. van Vugt MATM, Bras A, Medema RH. Polo-like kinase-1 controls recovery from a G2 DNA damage-induced arrest in mammalian cells. Mol cell 2004; 15:799-11; PMID:15350223; http://dx.doi.org/ 10.1016/j.molcel.2004.07.015 [DOI] [PubMed] [Google Scholar]
- 15. Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Adaptation of a DNA replication checkpoint response depends upon inactivation of Claspin by the Polo-like kinase. Cell 2004; 117, 575-88; PMID:15163406; http://dx.doi.org/ 10.1016/S0092-8674(04)00417-9 [DOI] [PubMed] [Google Scholar]
- 16. Mamely I, van Vugt MA, Smits VA, Semple JI, Lemmens B, Perrakis A, Medema RH, Freire R. Polo-like kinase-1 controls proteasome-dependent degradation of claspin during checkpoint recovery. Curr Biol 2006; 16:1950-5; PMID:16934469; http://dx.doi.org/ 10.1016/j.cub.2006.08.026 [DOI] [PubMed] [Google Scholar]
- 17. Peschiaroli A, Dorrello NV, Guardavaccaro D, Venere M, Halazonetis T, Sherman NE, Pagano M. SCF beta TrCP-mediated degradation of claspin regulates recovery from the DNA replication checkpoint response. Mol Cell 2006; 23:319-29; PMID:16885022; http://dx.doi.org/ 10.1016/j.molcel.2006.06.013 [DOI] [PubMed] [Google Scholar]
- 18. van Vugt MATM, Gardino AK, Linding R, Ostheimer GJ, Reinhardt HC, Ong SE, Tan CS, Miao H, Keezer SM, Li J, et al. . A mitotic phosphorylation feedback network connects Cdk1, Plk1, 53BP1, and Chk2 to inactivate the G2/M DNA damage checkpoint. Plos Biol 2010; 8:e1000287; PMID:20126263; http://dx.doi.org/ 10.1371/journal.pbio.1000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seki A, Coppinger JA, Jang CY, Yates JR, Fang G. Bora and the kinase Aurora a cooperatively activate the kinase Plk1 and control mitotic entry. Science 2008; 320:1655-8; PMID:18566290; http://dx.doi.org/ 10.1126/science.1157425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macurek L, Lindqvist A, Lim D, Lampson MA, Klompmaker R, Freire R, Clouin C, Taylor SS, Yaffe MB, Medema RH. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature 2008; 455:119-23; PMID:18615013; http://dx.doi.org/ 10.1038/nature07185 [DOI] [PubMed] [Google Scholar]
- 21. Smits VAJ, Klompmaker R, Arnaud L, Rijksen G, Nigg EA, Medema RH. Polo-like kinase-1 is a target of the DNA damage checkpoint. Nat Cell biol 2000; 2:672-6; PMID:10980711; http://dx.doi.org/ 10.1038/35023629 [DOI] [PubMed] [Google Scholar]
- 22. Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 2009; 33, 537-45; PMID:19285938; http://dx.doi.org/ 10.1016/j.molcel.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 23. Lee KS, Erikson RL. Plk is a functional homolog of Saccharomyces cerevisiae Cdc5, and elevated Plk activity induces multiple septation structures. Mol Cell Biol 1997; 17:3408-17; PMID:9154840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kumagai A, Guo ZJ, Emami KH, Wang SX, Dunphy WG. The Xenopus Chk1 protein kinase mediates a caffeine-sensitive pathway of checkpoint control in cell-free extracts. J Cell Biol 1998; 142:1559-69; PMID:9744884; http://dx.doi.org/ 10.1083/jcb.142.6.1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mochida S, Ikeo S, Gannon J, Hunt T. Regulated activity of PP2A-B55 delta is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. Embo J 2009; 28:2777-85; PMID:19696736; http://dx.doi.org/ 10.1038/emboj.2009.238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vagnarelli P, Ribeiro S, Sennels L, Sanchez-Pulido L, de Lima Alves F, Verheyen T, Kelly DA, Ponting CP, Rappsilber J, Earnshaw WC. Repo-Man coordinates chromosomal reorganization with nuclear envelope reassembly during mitotic exit. Dev Cell 2011; 21:328-42; PMID:21820363; http://dx.doi.org/ 10.1016/j.devcel.2011.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li HH, Cai X, Shouse GP, Piluso LG, Liu X. A specific PP2A regulatory subunit, B56gamma, mediates DNA damage-induced dephosphorylation of p53 at Thr55. EMBO j 2007; 26:402-11; PMID:17245430; http://dx.doi.org/ 10.1038/sj.emboj.7601519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peng A, Lewellyn AL, Maller JL. Undamaged DNA transmits and enhances DNA damage checkpoint signals in early embryos. Mol Cell Biol 2007; 27:6852-62; PMID:17664286; http://dx.doi.org/ 10.1128/MCB.00195-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Peng AM, Yamamoto TM, Goldberg ML, Maller JL. A novel role for greatwall kinase in recovery from DNA damage. Cell Cycle 2010; 9:4364-9; PMID:20980823; http://dx.doi.org/ 10.4161/cc.9.21.13632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peng A, Wang L, Fisher LA. Greatwall and Polo-like kinase 1 coordinate to promote checkpoint recovery. J Biol Chem 2011; 286:28996-9004; PMID:21708943; http://dx.doi.org/ 10.1074/jbc.M111.257121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jang YJ, Ji JH, Choi YC, Ryu CJ, Ko SY. Regulation of Polo-like kinase 1 by DNA damage in mitosis. Inhibition of mitotic PLK-1 by protein phosphatase 2A. J Biol Chem 2007; 282:2473-82; PMID:17121863; http://dx.doi.org/ 10.1074/jbc.M605480200 [DOI] [PubMed] [Google Scholar]
- 32. Tsvetkov L, Stern DF. Phosphorylation of Plk1 at S137 and T210 is inhibited in response to DNA damage. Cell Cycle 2005; 4:166-71; PMID:15611664; http://dx.doi.org/ 10.4161/cc.4.1.1348 [DOI] [PubMed] [Google Scholar]
- 33. Lee HJ, Hwang HI, Jang YJ. Mitotic DNA damage response: polo-like kinase-1 is dephosphorylated through ATM-Chk1 pathway. Cell Cycle 2010; 9:2389-98; PMID:20581453; http://dx.doi.org/ 10.4161/cc.9.12.11904 [DOI] [PubMed] [Google Scholar]
- 34. Yamashiro S, Yamakita Y, Totsukawa G, Goto H, Kaibuchi K, Ito M, Hartshorne DJ, Matsumura F. Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1. Dev Cell 2008; 14:787-97; PMID:18477460; http://dx.doi.org/ 10.1016/j.devcel.2008.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kachaner D, Filipe J, Laplantine E, Bauch A, Bennett KL, Superti-Furga G, Israël A, Weil R. Plk1-dependent phosphorylation of optineurin provides a negative feedback mechanism for mitotic progression. Mol Cell 2012; 45, 553-66; PMID:22365832; http://dx.doi.org/ 10.1016/j.molcel.2011.12.030 [DOI] [PubMed] [Google Scholar]
- 36. Peng A, Maller JL. Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene 2010; 29:5977-88; PMID:20838380; http://dx.doi.org/ 10.1038/onc.2010.371 [DOI] [PubMed] [Google Scholar]
- 37. Melo J, Toczyski D. A unified view of the DNA-damage checkpoint. Curr opin cell biol 2002; 14:237-45; http://dx.doi.org/ 10.1016/S0955-0674(02)00312-5 [DOI] [PubMed] [Google Scholar]
- 38. Krystyniak A, Garcia-Echeverria C, Prigent C, Ferrari S. Inhibition of Aurora A in response to DNA damage. Oncogene 2006; 25:338-48; PMID:16158051 [DOI] [PubMed] [Google Scholar]
- 39. Marumoto T, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, Sasayama T, Kuninaka S, Mimori T, Tamaki N, et al. . Roles of aurora-A kinase in mitotic entry and G2 checkpoint in mammalian cells. Genes Cell 2002; 7:1173-82; PMID:12390251; http://dx.doi.org/ 10.1046/j.1365-2443.2002.00592.x [DOI] [PubMed] [Google Scholar]
- 40. Cheng Y, Hirota T, Morisaki T, Kunitoku N, Zhang D, Ichikawa Y, Sasayama T, Kuninaka S, Mimori T, Tamaki N. Evaluation of PPP2R2A as a prostate cancer susceptibility gene: a comprehensive germline and somatic study. Cancer Genet 2011; 204:375-81; PMID:21872824; http://dx.doi.org/ 10.1016/j.cancergen.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, Speed D, Lynch AG, Samarajiwa S, Yuan Y, et al. . The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 2012; 486:346-52; PMID:22522925; http://dx.doi.org/ 10.1038/nature10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jin Y, Mertens F, Kullendorff CM, Panagopoulos I. Fusion of the tumor-suppressor gene CHEK2 and the gene for the regulatory subunit B of protein phosphatase 2 PPP2R2A in childhood teratoma. Neoplasia 2006; 8:413-8; PMID:16790090; http://dx.doi.org/ 10.1593/neo.06139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu W, Xie CC, Zhu Y, Li T, Sun J, Cheng Y, Ewing CM, Dalrymple S, Turner AR, Sun J, et al. . Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 2008; 10:897-907; PMID:18670647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cristobal I, Manso R, Rincón R, Caramés C, Senin C, Borrero A, Martínez-Useros J, Rodriguez M, Zazo S, Aguilera O, et al. . PP2A inhibition is a common event in colorectal cancer and its restoration using FTY720 shows promising therapeutic potential. Mol Cancer Ther 2014; 13:938-47; PMID:24448818; http://dx.doi.org/ 10.1158/1535-7163.MCT-13-0150 [DOI] [PubMed] [Google Scholar]
- 45. Shen S, Yue H, Li Y, Qin J, Li K, Liu Y, Wang J. Upregulation of miR-136 in human non-small cell lung cancer cells promotes Erk1/2 activation by targeting PPP2R2A. Tumour Biol 2014; 35:631-40; PMID:23959478; http://dx.doi.org/ 10.1007/s13277-013-1087-2 [DOI] [PubMed] [Google Scholar]
- 46. Mosca L, Musto P, Todoerti K, Barbieri M, Agnelli L, Fabris S, Tuana G, Lionetti M, Bonaparte E, Sirchia SM, et al. . Genome-wide analysis of primary plasma cell leukemia identifies recurrent imbalances associated with changes in transcriptional profiles. AmJ Hematol 2013; 88:16-23; PMID:23044976; http://dx.doi.org/ 10.1002/ajh.23339 [DOI] [PubMed] [Google Scholar]
- 47. Kalev P, Simicek M, Vazquez I, Munck S, Chen L, Soin T, Danda N, Chen W, Sablina A. Loss of PPP2R2A inhibits homologous recombination DNA repair and predicts tumor sensitivity to PARP inhibition. Cancer Res 2012; 72:6414-24; PMID:23087057; http://dx.doi.org/ 10.1158/0008-5472.CAN-12-1667 [DOI] [PubMed] [Google Scholar]
- 48. Liu J, Maller JL. Calcium elevation at fertilization coordinates phosphorylation of XErp1/Emi2 by Plx1 and CaMK II to release metaphase arrest by cytostatic factor. Curr Biol 2005; 15:1458-68; PMID:16040245; http://dx.doi.org/ 10.1016/j.cub.2005.07.030 [DOI] [PubMed] [Google Scholar]
- 49. Eckerdt F, Pascreau G, Phistry M, Lewellyn AL, DePaoli-Roach AA, Maller JL. Phosphorylation of TPX2 by Plx1 enhances activation of Aurora A. Cell Cycle 2009; 8, 2413-9; PMID:19556869; http://dx.doi.org/ 10.4161/cc.8.15.9086 [DOI] [PubMed] [Google Scholar]
- 50. Peng AM, Lewellyn AL, Schiemann WP, Maller JL. Repo-man controls a protein phosphatase 1-dependent threshold for DNA damage checkpoint activation. Curr Biol 2010; 20, 387-96; PMID:20188555; http://dx.doi.org/ 10.1016/j.cub.2010.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]