Abstract
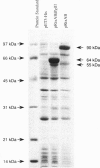
Some strains of Bradyrhizobium japonicum produce rhizobitoxine, a phytotoxin that causes foliar chlorosis on susceptible host plants. We have previously obtained Tn5-induced rhizobitoxine null mutants of B. japonicum. DNA sequence analysis of the region surrounding two Tn5 insertions identifies two overlapping open reading frames. The first open reading frame (rtxA) predicts a 54-kDa protein for which the N-terminal 280 residues have sequence similarity to serine: pyruvate aminotransferase. The sequence homology to aminotransferase is consistent with the involvement of this gene in serinol production, a likely intermediate in rhizobitoxine biosynthesis. Previously, a mutant in this open reading frame was shown not to make serinol. The predicted amino acid sequence of the second open reading frame (rtxB) has similarity to yeast O-acetylhomoserine sulfhydrolase. This enzyme function is similar to that required for dihydrorhizobitoxine synthase. The DNA sequence shows that the rtxB open reading frame overlaps rtxA, suggesting that expression of rtxB requires a -1 translational frameshift. Protein expression experiments demonstrate production of an RtxAB fusion protein. The ability of the overlapping rtxA and rtxB sequences to promote a translational frameshift was confirmed in a heterologous expression system. In Escherichia coli, this frameshift appears to be unusually efficient, occurring at a frequency of 80-90%.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Atkins J. F., Weiss R. B., Gesteland R. F. Ribosome gymnastics--degree of difficulty 9.5, style 10.0. Cell. 1990 Aug 10;62(3):413–423. doi: 10.1016/0092-8674(90)90007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babczinski P., Matern U., Strobel G. A. Serinol phosphate as an intermediate in serinol formation in sugarcane. Plant Physiol. 1978 Jan;61(1):46–49. doi: 10.1104/pp.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condron B. G., Atkins J. F., Gesteland R. F. Frameshifting in gene 10 of bacteriophage T7. J Bacteriol. 1991 Nov;173(21):6998–7003. doi: 10.1128/jb.173.21.6998-7003.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craigen W. J., Cook R. G., Tate W. P., Caskey C. T. Bacterial peptide chain release factors: conserved primary structure and possible frameshift regulation of release factor 2. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3616–3620. doi: 10.1073/pnas.82.11.3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas J. M., Prère M. F., Fayet O., Salvignol I., Galas D., Zerbib D., Chandler M. Translational control of transposition activity of the bacterial insertion sequence IS1. EMBO J. 1991 Mar;10(3):705–712. doi: 10.1002/j.1460-2075.1991.tb08000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins D. G., Bleasby A. J., Fuchs R. CLUSTAL V: improved software for multiple sequence alignment. Comput Appl Biosci. 1992 Apr;8(2):189–191. doi: 10.1093/bioinformatics/8.2.189. [DOI] [PubMed] [Google Scholar]
- Jacks T., Madhani H. D., Masiarz F. R., Varmus H. E. Signals for ribosomal frameshifting in the Rous sarcoma virus gag-pol region. Cell. 1988 Nov 4;55(3):447–458. doi: 10.1016/0092-8674(88)90031-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerjan P., Cherest H., Surdin-Kerjan Y. Nucleotide sequence of the Saccharomyces cerevisiae MET25 gene. Nucleic Acids Res. 1986 Oct 24;14(20):7861–7871. doi: 10.1093/nar/14.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Marck C. 'DNA Strider': a 'C' program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988 Mar 11;16(5):1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. A., Hartwig U. A., Joseph C. M., Phillips D. A. A Chalcone and Two Related Flavonoids Released from Alfalfa Roots Induce nod Genes of Rhizobium meliloti. Plant Physiol. 1989 Nov;91(3):842–847. doi: 10.1104/pp.91.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H. M., Long S. R., Ruvkun G. B., Brown S. E., Ausubel F. M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J Bacteriol. 1982 Jan;149(1):114–122. doi: 10.1128/jb.149.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama K., Berstein G., Oda T., Ichiyama A. Cloning and nucleotide sequence of cDNA encoding human liver serine-pyruvate aminotransferase. Eur J Biochem. 1990 Nov 26;194(1):9–18. doi: 10.1111/j.1432-1033.1990.tb19420.x. [DOI] [PubMed] [Google Scholar]
- Oda T., Funai T., Ichiyama A. Generation from a single gene of two mRNAs that encode the mitochondrial and peroxisomal serine:pyruvate aminotransferase of rat liver. J Biol Chem. 1990 May 5;265(13):7513–7519. [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Rhizobial-Induced Chlorosis in Soybeans: Isolation, Production in Nodules, and Varietal Specificity of the Toxin. Plant Physiol. 1965 Sep;40(5):927–930. doi: 10.1104/pp.40.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin N. T., Chamorro M., Varmus H. E. Human immunodeficiency virus type 1 gag-pol frameshifting is dependent on downstream mRNA secondary structure: demonstration by expression in vivo. J Virol. 1992 Aug;66(8):5147–5151. doi: 10.1128/jvi.66.8.5147-5151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Ruan X., Peters N. K. Isolation and characterization of rhizobitoxine mutants of Bradyrhizobium japonicum. J Bacteriol. 1992 Jun;174(11):3467–3473. doi: 10.1128/jb.174.11.3467-3473.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan X., Peters N. K. Rapid and sensitive assay for the phytotoxin rhizobitoxine. Appl Environ Microbiol. 1991 Jul;57(7):2097–2100. doi: 10.1128/aem.57.7.2097-2100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y., Ohtsubo E. Frameshifting is required for production of the transposase encoded by insertion sequence 1. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4609–4613. doi: 10.1073/pnas.86.12.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R., O'Connell M., Labes M., Pühler A. Plasmid vectors for the genetic analysis and manipulation of rhizobia and other gram-negative bacteria. Methods Enzymol. 1986;118:640–659. doi: 10.1016/0076-6879(86)18106-7. [DOI] [PubMed] [Google Scholar]
- Stanley J., Brown G. G., Verma D. P. Slow-growing Rhizobium japonicum comprises two highly divergent symbiotic types. J Bacteriol. 1985 Jul;163(1):148–154. doi: 10.1128/jb.163.1.148-154.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., ap Rhys C., Berman M. L., Hampar B., Jackson D., Silhavy T. J., Weisemann J., Zweig M. Open reading frame expression vectors: a general method for antigen production in Escherichia coli using protein fusions to beta-galactosidase. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4432–4436. doi: 10.1073/pnas.80.14.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]