Abstract
Numerous studies have established that High Mobility Group A (HMGA) proteins play a pivotal role on the onset of human pituitary tumors. They are overexpressed in pituitary tumors, and, consistently, transgenic mice overexpressing either the Hmga1 or the Hmga2 gene develop pituitary tumors. In contrast with HMGA2, HMGA1 overexpression is not related to any rearrangement or amplification of the HMGA1 locus in these tumors. We have recently identified 2 HMGA1 pseudogenes, HMGA1P6 and HMGA1P7, acting as competitive endogenous RNA decoys for HMGA1 and other cancer related genes. Here, we show that HMGA1 pseudogene expression significantly correlates with HMGA1 mRNA levels in growth hormone and nonfunctioning pituitary adenomas likely inhibiting the repression of HMGA1 through microRNAs action. According to our functional studies, these HMGA1 pseudogenes enhance the proliferation and migration of the mouse pituitary tumor cell line, at least in part, through their upregulation. Our results point out that the overexpression of HMGA1P6 and HMGA1P7 could contribute to increase HMGA1 levels in human pituitary tumors, and then to pituitary tumorigenesis.
Keywords: HMGA1P6, HMGA1P7, HMGA1, ceRNA, miRNA
Introduction
The High Mobility Group A (HMGA) proteins are non-histone chromatinic proteins involved in transcriptional regulation of gene expression.1 The HMGA protein family consists of 3 proteins: HMGA1a, HMGA1b, and HMGA2 encoded by 2 different genes, with the HMGA1 proteins being products of the same gene generated through alternative splicing.1 HMGA overexpression is a feature of malignant neoplasias and its causal role in cell transformation and cancer progression is supported by many studies.1-3
We have already reported several evidences that HMGA proteins act as drivers of human pituitary tumors (PT),4-8 with both the HMGA proteins overexpressed. However, in these tumors, only the overexpression of HMGA2 is associated to gene rearrangement and amplification following trisomy of chromosome 12.4 Consistently, it is well known that transgenic mice overexpressing either hmga1 or hmga2 develop PT,5-7 and that the HMGA overexpression is associated to the downregulation of several miRNAs able to target both HMGA1 and HMGA2 mRNAs in PT (miR-15, miR-16, miR-23b, miR-26a, miR-34b, miR-130b, miR-196a2, miR-326, miR-432, miR-548c-3p, miR-570, miR-603, and Let-7a).9-11
We have recently identified 2 HMGA1 non-coding pseudogenes, HMGA1P6 and HMGA1P7, having conserved seed matches for miRNAs targeting the HMGA1 gene. The overexpression of HMGA1 pseudogene (HMGA1Ps) increases HMGA1 protein levels, working as competitive endogenous RNA (ceRNA), thereby inhibiting the suppression of HMGA1 protein synthesis by miRNAs.12,13 Since the HMGA1Ps untranslated regions (UTRs) contain also seed sequences for miRNAs able to target HMGA2, their overexpression leads also to increased HMGA2 protein levels. HMGA1Ps also show oncogenic activity by inhibiting apoptosis and increasing cell proliferation and migration.12,13
The aim of this study has been to investigate the expression and the role of HMGA1Ps in PT.
HMGA1andHMGA1Psexpression positively correlates in pituitary tumors
Briefly, we analyzed the expression of HMGA1Ps by qRT-PCR in a panel of 41 human PT, including 14 growth hormone (GH) tumors with acromegaly and 27 nonfunctioning pituitary adenomas (NFPA) or gonadotroph FSH-LH tumors detected by immunohistochemistry (IHC). As shown in Fig. 1 (Panels A and B), HMGA1P6 and HMGA1P7 were differently expressed with regard to the IHC type, when compared with the normal pituitary gland. To verify whether the 2 HMGA1Ps may also function as ceRNAs in the regulation of HMGA1 mRNA levels in PT, we analyzed HMGA1 expression in these same samples (Fig. 1C).
Figure 1.
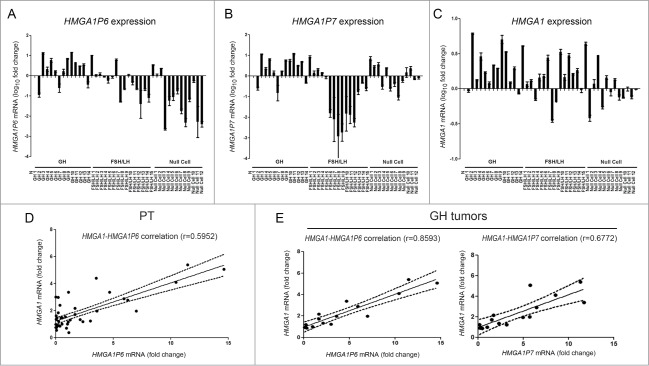
HMGA1 and HMGA1Ps expression positively correlates in pituitary tumors. (A) HMGA1P6, (B) HMGA1P7 and (C) HMGA1 qRT-PCR analysis in normal pituitary gland (N), GH, FSH/LH and null cell tumors. (D, E) The obtained values were then combined for correlation analysis. (D) Linear regression of HMGA1 versus HMGA1P6 in the whole series of PT. (E) Linear regressions of HMGA1 vs. HMGA1P6 (left panel) and HMGA1 (right panel) versus HMGA1P7 in GH tumors.
As indicated in Figure 1, Panel D, a significant linear correlation was found between HMGA1 and HMGA1P6 expression (r = 0.5952, P < 0.0001), suggesting that these genes are co-regulated, while there is no correlation between HMGA1 and HMGA1P7 expression in the whole tumor series. However, further analysis of HMGA1 and HMGA1Ps co-regulation disclosed some differences according to tumor type. Indeed, in the subset of GH tumors, HMGA1 strongly correlated with HMGA1P6 expression (r = 0.8593, P < 0 .0001), and also with HMGA1P7 expression (r = 0.6772, P < 0.0001) (Fig. 1E). Taken together, these data strongly support the hypothesis that HMGA1Ps could act as ceRNAs in PT and represent a novel potential mechanism of HMGA1 upregulation in these tumors, in particular in GH tumors with acromegaly.
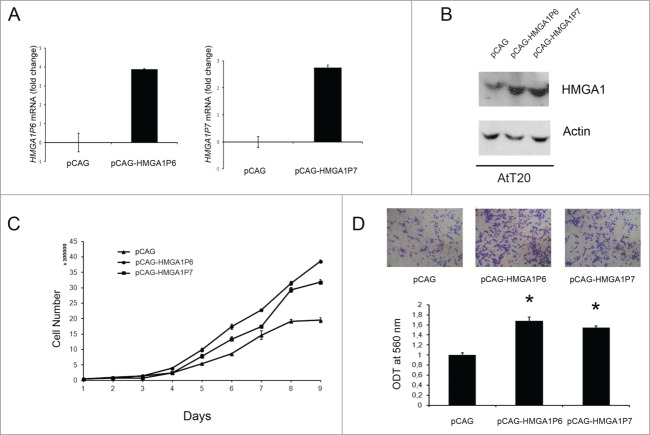
HMGA1Ps expression increases AtT20 cell proliferation and migration acting as ceRNAs for HMGA1
Subsequently, we examined the ability of HMGA1P6 and HMGA1P7 to function as a decoy for HMGA1-targeting miRNAs in the pituitary cell line AtT20. Figure 2, Panel A, confirms successful transfection of both genes. As shown in Figure 2, Panel B, overexpression of either HMGA1P6 or HMGA1P7 was associated with HMGA1 protein overexpression. As expected from our previously results,12 overexpression of HMGA1P6 or HMGA1P7 reduced the effects exerted by miRNA on the HMGA1 levels (Fig. 2B). Then, to evaluate the functional consequences of HMGA1P6 and HMGA1P7 overexpression in PT, we investigated their role in cellular proliferation, and migration in AtT20 cells. To this aim, AtT20 cells were transfected with the HMGA1P6, HMGA1P7, and the control vector, and counted each day for 9 d. Figure 2C, shows that the growth rate of AtT20 following the transfection of the HMGA1Ps was higher compared with the cells transfected with the control vector. Finally, we carried out cell migration assays in the same HMGA1Ps overexpressing cells especially considering that HMGA1 promotes cell migration.1 As expected from the increased HMGA1 protein levels in the pseudogene-transfected cells,12 cell migration was significantly higher in AtT20 cells overexpressing HMGA1P6 or HMGA1P7 than in control cells (Fig. 2D).
Figure 2.
HMGA1Ps expression increases AtT20 cell proliferation and migration acting as ceRNAs for HMGA1. (A) qRT-PCR analysis of HMGAP6 and HMGA1P7 mRNA levels in AtT20 cells transfected with the empty, HMGA1P6 or HMGA1P7 vectors. (B) Western blot analysis of HMGA1 protein levels from the same samples shown in A. (C) AtT20 cell proliferation in HMGA1P6- and HMGA1P7-transfected cells. (D) Cell migration assays of AtT20 cells transfected with HMGA1P6, HMGA1P7 or with a control vector (Upper panel). Migrated cells were quantified and expressed as mean ± SD *, P < 0.05 (t test) (Lower panel).
Discussion
The critical role of High Mobility Group A proteins on the onset of human PT has been widely accepted. In fact, the overexpression of both HMGA genes in PT has been reported by numerous studies,4-8 and consistently the development of GH-PRL tumors is a feature of transgenic mice overexpressing either the Hmga1 or the Hmga2 gene.5, 6
The results reported here indicate that HMGA1Ps, which act as miRNA sponges for HMGA1 genes, also contribute to pituitary tumorigenesis by enhancing pituitary cell proliferation and migration.
Since no rearrangement or amplification of the HMGA1 locus have been detected in PT, where HMGA1 is overexpressed,4-8 HMGA1Ps overexpression contributes to high HMGA1 protein levels detected in PT together with tumor downregulation of miRNAs targeting HMGA1 (miR-15, miR-16, miR-23b, miR-26a, miR-34b, miR-130b, miR-196a2, miR-326, miR-432, miR-548c-3p, miR-570, miR-603, and Let-7a).9-11
Indeed, we found a direct correlation between HMGA1 and HMGA1Ps expression in a series of human PT, in particular in the somatotroph ones. Then, functional assays revealed that HMGA1P6 and HMGA1P7 increase cell proliferation and migration in pituitary cell line AtT20. This is in agreement with effects of HMGA1, which accelerates the G1-S transition by increasing E2F1 activity, and enhances cell migration in pituitary cell lines.
Noteworthy, HMGA1P6, HMGA1P7, as well as HMGA1 3' UTR are potential ceRNAs for other cancer-related genes such as High Mobility Group A2 (HMGA2) and Vascular Endothelial Growth Factor (VEGF) which may further contribute to pituitary tumorigenesis.
In conclusion, the results reported here clearly evidence that HMGA1 pseudogene overexpression contributes to pituitary tumor behavior, thereby disclosing an additional mechanism accounting for the high expression of HMGA1 (and likely HMGA2) in PT. Consequently, these results further support the perspective of an innovative molecular therapy of PT by restoring the expression of miRNAs able to target the HMGA genes and/or blocking that of the HMGA1 pseudogenes.
Methods
Cell culture and transfections
AtT20 cells were maintained in DMEM supplemented with 10% foetal calf serum (Euroclone; Milan, Italy), glutamine and antibiotics. Cells were repeatedly tested with MycoAlert (Lonza; Slough, UK) to ensure that cells were not infected with mycoplasma. Cells were transfected using Lipofectamine plus reagent (Life Technologies Italia; Monza, Italy) according to the manufacturer's instructions. The transfected cells were selected in a medium containing geneticin (Sigma; St. Louis, USA). For each transfection, several geneticin-resistant mass cell populations were isolated and expanded for additional analysis. Transfection efficiency was established for each experiment by evaluating GFP expression. HMGA1P6 and HMGA1P7 expression vectors have been previously described.12
Tissue collection and RNA isolation
Surgical samples of PT were obtained from patients operated in 2 centers: Lyon, France (29 tumors - n°1 to n°29) and at the Neuromed Institute, Pozzilli, Italy (12 tumors - n°30 to n°41). Among these, 14 presented with acromegaly and 27 with clinically non-functioning tumors, respectively. According to diagnostic immunohistochemistry with pituitary hormones, immunostaining for GH was confirmed in tumors from acromegalic patients whereas the large majority of clinically non-functioning tumors showed some degree of immunopositivity for FSH and/or LH and the few immunonegative samples were assimilated to gonadotroph tumors. For each tumor, fragments were fixed in the Bouin-Holland fluid or formol and embedded in paraffin for pathological diagnosis, including IHC. Other fragments were immediately frozen and stored at −80°C. We declare that informed consent for the scientific use of biological material was obtained from all patients.
RNA extraction and quantitative reverse transcription PCR
Total RNA was extracted from tissues with Trizol (Life Technologies Italia; Monza, Italy) according to the manufacturer's instructions. For mRNA detection, total RNA was reverse transcribed by using the QuantiTect Reverse Transcription Kit (Qiagen; Valencia, USA), and then Real-time PCR was performed by using Power SYBR Green PCR Master Mix (Applied Biosystems-Life Technologies Italia; Monza, Italy) and the following primers:
HMGA1-Fw 5′-aaggggcagacccaaaaa-3′
HMGA1-Rev 5′-tccagtcccagaaggaagc-3′
HMGA1P6-Fw 5′-gcagacccacaaaactgga-3′
HMGA1P6-Rev 5′-gagcaaagctgtcccatcc-3′
HMGA1P7-Fw 5′-gctccttctcggctcctc-3′
HMGA1P7-Rev 5′-gcttgggcctcttttatgg-3′
G6PD-Fw 5′-acagagtgagcccttcttcaa-3′
G6PD-Rev 5′-ataggagttgcgggcaaag-3′
The 2−ΔΔCt formula was used to calculate the differential gene expression.
Protein extraction, western blotting and antibodies
Protein extraction and Western blotting were performed as before described.14 Antibodies against the HMGA1 proteins are described elsewhere.15 Blots were visualized by using the Western blotting detection reagents (Thermo Scientific, Waltham, USA).
Cell migration assay
Cell migration experiments were performed as previously described.16
Statistical analysis
Data were analyzed using a 2-sided unpaired Student's t test (GraphPad Prism, GraphPad Software, Inc.). Values of P < 0.05 were considered statistically significant. Regression analyses and correlation coefficients were generated using GraphPad Prism, GraphPad Software, Inc.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Mario Berardone for the artwork. We are grateful to E. Jouhanneau, V. Esposito and F. Giangaspero for tumors collection.
Funding
This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro-AIRC (IG 11477), P.O.R. Campania FSE 2007-2013 - Progetto CREMe - CUP B25B09000050007, “Progetto di Interesse strategico Invecchiamento (PNR-CNR Aging Program) PNR-CNR 2012–2014”, Progetto PON01-02782 “Nuove strategie nanotecnologiche per la messa a punto di farmaci e presidi diagnostici diretti verso cellule cancerose circolanti”, CNR Epigenomics Flagship Project “EPIGEN”.
References
- 1.Fusco A, Fedele M. Roles of the HMGA proteins in cancer. Nat Rev Cancer 2007; 7: 899-910; PMID:18004397; http://dx.doi.org/ 10.1038/nrc2271 [DOI] [PubMed] [Google Scholar]
- 2.Pegoraro S, Ros G, Piazza S, Sommaggio R, Ciani Y, Rosato A, Sgarra R, Del Sal G, Manfioletti G. HMGA1 promotes metastatic processes in basal-like breast cancer regulating EMT and stemness. Oncotarget 2013; 4: 1293-308; PMID:23945276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puca F, Colamaio M, Federico A, Gemei M, Tosti N, Bastos AU, Del Vecchio L, Pece S, Battista S, Fusco A. HMGA1 silencing restores normal stem cell characteristics in colon cancer stem cells by increasing p53 levels. Oncotarget 2014; 5: 3234-45; PMID:24833610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pierantoni GM, Finelli P, Valtorta E, Giardino D, Rodeschini O, Esposito F, Losa M, Fusco A, Larizza L. High-mobility group A2 gene expression is frequently induced in non-functioning pituitary adenomas (NFPAs), even in the absence of chromosome 12 polysomy. Endocr Relat Cancer 2005; 12: 867-74; PMID:16322327; http://dx.doi.org/ 10.1677/erc.1.01049 [DOI] [PubMed] [Google Scholar]
- 5.Fedele M, Pierantoni GM, Visone R, Fusco A. Critical role of the HMGA2 gene in pituitary adenomas. Cell Cycle 2006; 5: 2045-8; PMID:16969098; http://dx.doi.org/ 10.4161/cc.5.18.3211 [DOI] [PubMed] [Google Scholar]
- 6.Fedele M, Visone R, De Martino I, Troncone G, Palmieri D, Battista S, Ciarmiello A, Pallante P, Arra C, Melillo RM, et al.. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell 2006; 9: 459-71; PMID:16766265; http://dx.doi.org/ 10.1016/j.ccr.2006.04.024 [DOI] [PubMed] [Google Scholar]
- 7.Fedele M, Fusco A. Role of the high mobility group A proteins in the regulation of pituitary cell cycle. J Mol Endocrinol 2010; 44: 309-18; PMID:20219853; http://dx.doi.org/ 10.1677/JME-09-0178 [DOI] [PubMed] [Google Scholar]
- 8.Palmieri D, Valentino T, De Martino I, Esposito F, Cappabianca P, Wierinckx A, Vitiello M, Lombardi G, Colao A, Trouillas J, et al.. PIT1 upregulation by HMGA proteins has a role in pituitary tumorigenesis. Endocr Relat Cancer 2012; 19: 123-35; PMID:22199144; http://dx.doi.org/ 10.1530/ERC-11-0135 [DOI] [PubMed] [Google Scholar]
- 9.Palmieri D, D'Angelo D, Valentino T, De Martino I, Ferraro A, Wierinckx A, Fedele M, Trouillas J, Fusco A. Downregulation of HMGA-targeting microRNAs has a critical role in human pituitary tumorigenesis. Oncogene 2012; 31: 3857-65; PMID:22139073; http://dx.doi.org/ 10.1038/onc.2011.557 [DOI] [PubMed] [Google Scholar]
- 10.D'Angelo D, Palmieri D, Mussnich P, Roche M, Wierinckx A, Raverot G, Fedele M, Croce CM, Trouillas J, Fusco A. Altered microRNA expression profile in human pituitary GH adenomas: down-regulation of miRNA targeting HMGA1, HMGA2, and E2F1. J Clin Endocrinol Metab 2012; 97: E1128-38; PMID:22564666; http://dx.doi.org/ 10.1210/jc.2011-3482 [DOI] [PubMed] [Google Scholar]
- 11.Leone V, Langella C, D'Angelo D, Mussnich P, Wierinckx A, Terracciano L, Raverot G, Lachuer J, Rotondi S, Jaffrain-Rea ML, et al.. Mir-23b and miR-130b expression is downregulated in pituitary adenomas. Mol Cell Endocrinol 2014; 390: 1-7; PMID:24681352; http://dx.doi.org/ 10.1016/j.mce.2014.03.002 [DOI] [PubMed] [Google Scholar]
- 12.Esposito F, De Martino M, Petti MG, Forzati F, Tornincasa M, Federico A, Arra C, Pierantoni GM, Fusco A. HMGA1 pseudogenes as candidate proto-oncogenic competitive endogenous RNAs. Oncotarget 2014; 5: 8341-54; PMID:25268743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito F, De Martino M, Forzati F, Fusco A. HMGA1-pseudogene overexpression contributes to cancer progression. Cell Cycle 2014; 13: 3636-9; PMID:25483074; http://dx.doi.org/ 10.4161/15384101.2014.974440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez Hoyos J, Ferraro A, Sacchetti S, Keller S, De Martino I, Borbone E, Pallante P, Fedele M, Montanaro D, Esposito F, et al.. HAND1 gene expression is negatively regulated by the High Mobility Group A1 proteins and is drastically reduced in human thyroid carcinomas. Oncogene 2009; 28: 876-85; PMID:19060921; http://dx.doi.org/ 10.1038/onc.2008.438 [DOI] [PubMed] [Google Scholar]
- 15.Piscuoglio S, Zlobec I, Pallante P, Sepe R, Esposito F, Zimmermann A, Diamantis I, Terracciano L, Fusco A, Karamitopoulou E. HMGA1 and HMGA2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology 2012; 60: 397-404; PMID:22276603; http://dx.doi.org/ 10.1111/j.1365-2559.2011.04121.x [DOI] [PubMed] [Google Scholar]
- 16.Borbone E, Troncone G, Ferraro A, Jasencakova Z, Stojic L, Esposito F, Horning N, Fusco A, Orlando V. Enhancer of zeste homolog 2 overexpression has a role in the development of anaplastic thyroid carcinomas. J Clin Endocrinol Metab 2011; 96: 1029-38; PMID:21289264; http://dx.doi.org/ 10.1210/jc.2010-1784 [DOI] [PubMed] [Google Scholar]