Abstract
Ebolavirus is the pathogen for Ebola Hemorrhagic Fever (EHF). This disease exhibits a high fatality rate and has recently reached a historically epidemic proportion in West Africa. Out of the 5 known Ebolavirus species, only Reston ebolavirus has lost human pathogenicity, while retaining the ability to cause EHF in long-tailed macaque. Significant efforts have been spent to determine the three-dimensional (3D) structures of Ebolavirus proteins, to study their interaction with host proteins, and to identify the functional motifs in these viral proteins. Here, in light of these experimental results, we apply computational analysis to predict the 3D structures and functional sites for Ebolavirus protein domains with unknown structure, including a zinc-finger domain of VP30, the RNA-dependent RNA polymerase catalytic domain and a methyltransferase domain of protein L. In addition, we compare sequences of proteins that interact with Ebolavirus proteins from RESTV-resistant primates with those from RESTV-susceptible monkeys. The host proteins that interact with GP and VP35 show an elevated level of sequence divergence between the RESTV-resistant and RESTV-susceptible species, suggesting that they may be responsible for host specificity. Meanwhile, we detect variable positions in protein sequences that are likely associated with the loss of human pathogenicity in RESTV, map them onto the 3D structures and compare their positions to known functional sites. VP35 and VP30 are significantly enriched in these potential pathogenicity determinants and the clustering of such positions on the surfaces of VP35 and GP suggests possible uncharacterized interaction sites with host proteins that contribute to the virulence of Ebolavirus.
Keywords: comparative analysis, Ebolavirus, functional site prediction, Pathogenicity, structure prediction
Introduction
Zaire Ebolavirus, the pathogen for Ebola Hemorrhagic Fever (EHF) with a 25–90% fatality rate,1 continues to threaten people's lives. The current (2013 – Jun. 2015) West African outbreak of EHF has infected more than 27,000 people and caused 11,000 deaths.2 The genus Ebolavirus contains 5 known species: Bundibugyo (BDBV), Reston (RESTV), Sudan (SUDV), Taï Forest (TAFV) and Zaire ebolavirus (ZEBOV).3 The current outbreak is associated with ZEBOV.4 Four Ebolavirus species cause EHF in human, with the sole exception being RESTV.5 RESTV can cause EHF to long-tailed macaque (Macaca fascicularis). People who had contact with RESTV-infected monkeys tested positive for RESTV antibodies but did not develop symptoms associated with EHF.5
Ebolavirus belongs to the order Mononegavirales and the family Filoviridae.3 Its RNA genome encodes the following 7 protein products: Envelope glycoprotein (GP), Nucleoprotein (NP), RNA-dependent RNA polymerase L (L), Membrane-associated protein VP24 (VP24), Minor nucleoprotein VP30 (VP30), Polymerase cofactor VP35 (VP35), and Matrix protein VP40 (VP40). The GP transcript can be edited,6 and the gene product can be processed by host protease, giving rise to 4 alternative forms of gene products: GP1,2; GP1,2delta; sGP and ssGP. Host furin can cleave the longest product translated from edited GP mRNA and generate GP1,2, which consists of 2 peptide chains connected by a disulfide bond,7,8 GP1 and GP2. GP1,2 is assembled on the membrane of Ebolavirus and mediates cell entry. GP1,2delta is the processed product after removal of the C-terminal transmembrane region of GP1,2 by host ADAM17.9 Other products of the GP gene, sGP and ssGP are translated from the unedited mRNA and alternatively edited mRNA, respectively.10,11 These products share the N-terminal 295 residues with GP1,2, but differ in their short tails (69 and 3 residues, respectively). GP1,2delta, sGP and ssGP may prevent the neutralizing antibodies from binding GP1,2 on the virus surface, contributing to the immune evasion of the virus.12
In addition to serving as structural components, the Ebolavirus proteins play multiple roles in the virus life cycle. GP mediates cell entry13,14 and membrane fusion15,16 between the virus and the host cell. NP encapsidates the genome and protects it from nucleases.17,18 VP30 is a transcription anti-terminator19,20 and regulates the switch between transcription and replication.21,22 VP35 acts as a cofactor of the polymerase,23,24 and VP40 may also play a role in genome replication and transcription.25 VP24 and VP35 participate in viral nucleocapsid assembly,18 and VP40 is essential for virus budding and assembly.26-28 In addition, GP, VP24, VP30, VP35 and VP40 interact with multiple host proteins to complete the viral life cycle and to suppress the host immune response.
Three-dimensional (3D) structures are available for a number of Ebolavirus proteins. Interpreting available experimental data and sequence variation among Ebolavirus species in the context of the 3D structures not only allows researchers to understand detailed mechanisms for cell entry, virus assembly and immune suppression, but also provides promising leads for structure-based drug design. In the current study, we predict the 3D structure and functional sites for Ebolavirus protein domains that are not yet characterized. In addition, we compare sequences of Ebolavirus proteins' interacting partners from RESTV-resistant primates with those from RESTV-susceptible monkeys. Elevated sequence divergence for GP and VP35's interaction partners suggests that these 2 viral proteins may be responsible for host specificity in RESTV. Finally, we compare the protein sequences from different Ebolavirus species to detect positions that are conserved among human pathogenic species but different in non-pathogenic RESTV (RESTV-specific mutations). Mapping of these RESTV-specific mutations and known functional sites to the 3D structures reveals clusters of RESTV-specific mutations on the surfaces of GP, VP35 and VP24. These clusters do not overlap with the known functional sites and may suggest novel interaction sites with host proteins.
Materials and Methods
Sequence analysis of Ebolavirus proteins
The protein sequences of ZEBOV were downloaded from the UniProt database29 and submitted to the MESSA web server30 to predict the secondary structure,31,32 disordered regions,33-36 transmembrane helices,37-41 signal peptides,38,39,42 coiled coils43 and detect structure templates.44,45 The 3D structures are mostly known, except for protein L, the N-terminal zinc-finger domain of VP30 and the coiled-coil region of VP35. For proteins and domains without known structure, we considered putative structural templates detected by HHpred,45 iTASSER46,47 and known structures for proteins of similar function from other families of RNA virus in PDB and ECOD databases.48 Once a candidate structural template was detected, we further validated its relationship to the Ebolavirus protein by similarity in function, compatibility between the predicted secondary structure49 of the Ebolavirus protein and the 3D structure of the template, conservation of residues in the Ebolavirus protein that were aligned to the active sites of the template, and the consistency among multiple structural templates. Sequences of the structural templates and the ZEBOV protein were aligned by Promals3D50,51 and the alignments were manually adjusted to ensure that the corresponding secondary structure elements in different templates were aligned together. Based on these alignments and knowledge about functional sites in the template structures from literature, the active sites of the uncharacterized Ebolavirus domains were predicted.
Identification of positions associated with human pathogenicity
We downloaded protein sequences of 124 Ebolavirus samples from 5 Ebolavirus species4 at www.sciencemag.org/content/345/6202/1369/suppl/DC1, aligned them using MAFFT,52 and evaluated the similarity between amino acids at a certain position using BLOSUM62 scores.53 We considered a position in the sequence alignment to be associated with the loss of human pathogenicity if it satisfies the following 2 criteria. First, the similarity in amino acids at this position from pathogenic species is always higher than the similarity between RESTV and a pathogenic species. Second, the average similarity in amino acids at this position from 4 pathogenic species (BDBV, TAFV, SUDV and ZEBOV) is significantly (p-value < 0.05) higher than that between RESTV and pathogenic species. In order to calculate the p-value for each position, we obtained an estimate of the background distribution for the positional difference between the average sequence similarity within a group of any 4 Ebolavirus species (all possible combinations except the one with all 4 pathogenic species) and the average sequence similarity between a fifth species and those in the group. This distribution suggests that a difference larger than 2 is associated with p-value less than 0.05. Enrichment of these pathogenicity-associated positions in each protein was measured by a binomial test (p = total number of pathogenicity-associated positions/total length of all proteins, m = number of selected positions in this protein, N = length of this protein). These pathogenicity-associated positions and the functional sites reported in literature were further mapped to the known 3D structures of Ebolavirus proteins.
Results and Discussion
3D structure and functional sites prediction for Ebolavirus proteins
Domain diagrams of all the Ebolavirus proteins are shown in Figure 1. Variable positions among the different Ebolavirus species are marked as black lines above the domain diagram. The average protein sequence identity between different Ebolavirus species ranges from 60% to 80% (Table S1). Ebolavirus proteins contain a significant fraction (20%) of structurally disordered regions, and the fraction of variable positions in these regions is significantly higher (p < 0.01) than in the structurally ordered regions. The 3D structures of globular regions are mostly known (Table S2)54-71 except for the N-terminal zinc-finger domain of VP30, the coiled-coil domain of VP35, and protein L. Identification and analysis of structurally characterized homologs allowed us to predict the structure of the zinc-finger domain in VP30, the overall topology of NP, and the structure and catalytic sites for the catalytic domains of protein L.
Figure 1.
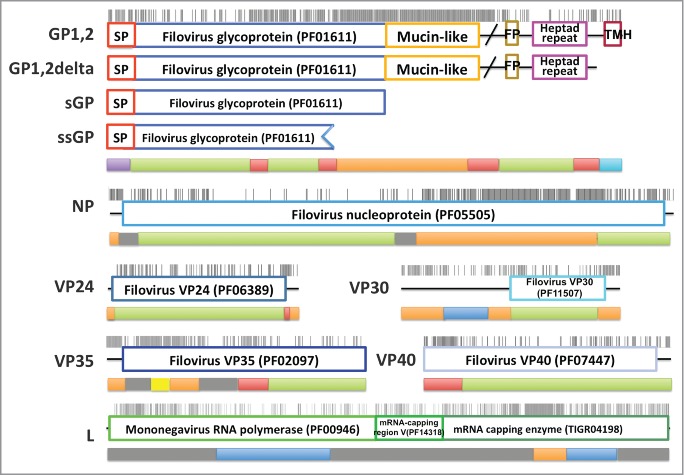
Domain diagrams for Ebolavirus proteins and coverage of the proteins by experimentally determined and predicted structures. The domains of each protein are represented by boxes on a thread and the positions that are variable among different Ebolavirus species are marked by black sticks above the domain diagrams. The band below is aligned to the domain diagram and the color of this band indicates the prediction status of the corresponding region. The color codes are: green, regions that are structurally characterized and adopt globular structure; red, regions that are experimentally determined but are intrinsically disordered; blue, regions with predicted 3D structure; yellow, coiled coil; cyan, transmembrane helix; purple, signal peptide; orange, predicted intrinsically disordered regions; gray, predicted regions that have a propensity to adopt global structure but 3D structure cannot be predicted;. Abbreviations: SP, signal peptide; FP, fusion peptide; TMH, transmembrane helix.
The zinc-finger domain of VP30
The zinc-finger domain of VP30 binds zinc72 and contains a conserved C-x8-C-x4-C-x3-H motif. A search using the VP30 zinc finger motif (residues 70–95) as a query against the SUPERFAMILY73 database with HHpred web server (MSA generation method: HHblits, Maximal MSA Generation iterations: 3, Score secondary structure: yes, Alignment mode: local) reveals similarity (Probability: 52.4; Identity: 35%; E-value: 2.2) to the CCCH zinc finger superfamily (seed: SCOP domain d1m9oa_). Although this is not the best hit according to HHpred probability, it has the highest coverage and is the only one (probability cutoff: 20) that contains all the zinc-binding residues. In addition, a scan of PDB sequences with the conserved pattern C-x(8)-C-x(4)-C-x(3)-H using ScanProsite74 reveals exactly the same motif in CCCH zinc fingers (PDB id: 2d9n). All the CCCH zinc fingers belongs to one homologous group in the ECOD database,48 and this family contains the N-terminal domain of the transcription antiterminator M2-1 from another Mononegavirales, Pneumovirus (4C3B75 and 4CS7,76 alignment shown in Fig. S1). In addition to their common function, the C-terminal domain of M2-1 and Ebolavirus VP30 share the same topology (Fig. 2A, B). M2-1 uses a C-x7-C-x5-C-x3-H motif to bind zinc, which is connected to an α-helix at its C-terminus. The VP30 zinc-finger domain very likely adopts a similar structure (Fig. 2C, D), as supported by the presence of a similar C-x8-C-x4-C-x3-H motif and a predicted α-helix following the motif.
Figure 2.
Structure prediction for N-terminal domain of VP30. (A) 3D structure (PDB id: 2I8B) for VP30 C-terminal domain; (B) 3D structure (PDB ID: 4C3B) for Pneumovirus M2-1 C-terminal domain; (C) 3D structure (PDB ID: 4C3B) for Pneumovirus M2-1 N-terminal domain, which was used as template to predict the structure for the VP30 N-terminal domain; (D) structure model for the Ebolavirus VP30 N-terminal domain.
The N-terminal domain of NP
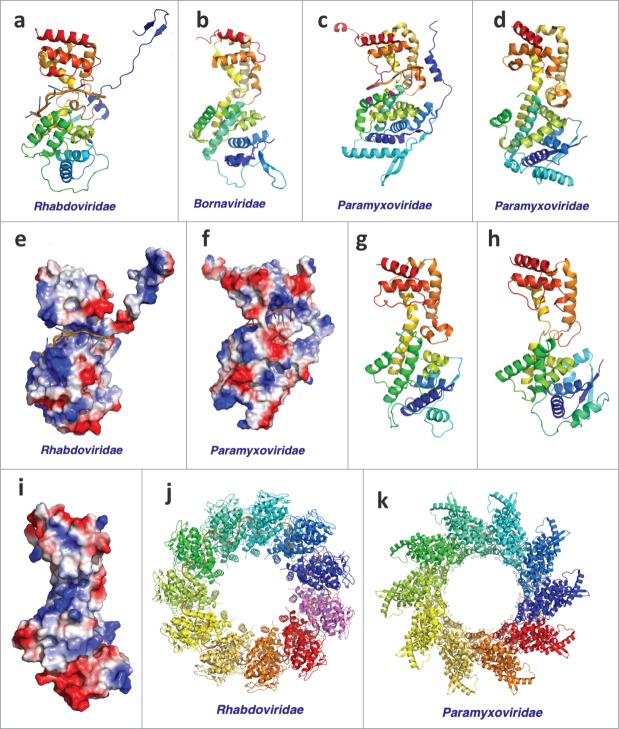
NP has 2 structural domains that are connected by a long disordered linker of about 240 amino acids. The C-terminal domain (PDB id: 4QAZ) is shared among Filoviridae and is involved in protein-protein interaction.54 The N-terminal domain is likely shared among Mononegavirales. Known 3D structures of NP from several virus families77-81 in this order possess the same topology (Fig. 3A–D). Structures of NP from Rhabdoviridae and Paramyxoviridae families are determined in complex with ssRNA (Fig. 3A, C), and they both clamp around the RNA using positively charged grooves (Fig. 3G, H) between the 2 subdomains after a remarkable conformational change compared to the RNA-free form (Fig. 3C, D). The RNA-bound NPs oligomerize to form a ring (Fig. 3I, J), but the oligmerization interfaces vary: Rhabdoviridae pack the ssRNA inside the ring formed by NPs while ssRNA binds on the outside of the NP oligomer in Paramyxoviridae.
Figure 3.
Structures of Mononegavirales Nucleoproteins. The virus family is labeled below. (A–D) Monomeric structures (PDB IDs: 2GIC, 1N93, 2WJ8, and 4CO6) of Nucleoproteins from Mononegavirales. The structures are colored in rainbow; (E, F) The electrostatic potential mapped onto the surface of Nucleoprotein structures (PDB ids: 2GIC and 2WJ8). Blue area corresponds to positively charged surface and the red area corresponds to negatively charged surface; (G) Structure model for the N-terminal domain of Ebolavirus NP; (H) Experimental structure of the N-terminal domain of Ebolavirus NP (PDB id: 4YPI); (I) The electrostatic potential mapped on to the surface of the experimental structure of the N-terminal domain of Ebolavirus NP; (J, K) Structure complex of RNA and Nucleoproteins from Rhabdoviridae and Paramyxoviridae (PDB ids: 2GIC and 2WJ8).
We predicted that the N-terminal domain of Ebolavirus NP adopts the same conserved topology as the other viral NPs and suggested that its structure is similar to the NP from Nipah virus (PDB id: 4CO6).81 The 3D structure of this domain was released while our manuscript was under review and supported our prediction (Fig. 3E, F). The available 3D structures for Ebolavirus NP71,82 were all determined in the absence of RNA. But its similarity to the NPs of other Mononegavirales and the presence of a positively charged groove between the 2 subdomains suggest a similar RNA binding mode.
The RNA-dependent RNA polymerase catalytic domain of protein L
Sequence analysis suggests that the N-terminal half of protein L functions as a RNA-dependent RNA polymerase (RdRP), and is responsible for both DNA replication and transcription. HHpred45 detects the Bunyavirus RdRP (PDB id: 5AMR83) as a structural template (Probability: 84%). The alignment between Ebolavirus RdRP and Bunyavirus RdRP includes both the catalytic domain and a helical bundle connected to its C-terminus. These two domains are conserved among known structures of RdRPs from RNA viruses84-88 (Fig. 4A–C). Known RdRPs from RNA viruses share the same topology except for Birnavirus RdRP, which has a circular permutation in the catalytic domain. This structural conservation of RdRPs across different groups of RNA virus suggests that the RdRP of Ebolavirus also adopts the same topology. Secondary structure prediction for the Ebolavirus RdRP is consistent with the topology adopted by most RNA viruses, but not with the circular permuted structure from Birnavirus (Fig. 4E).
Figure 4.
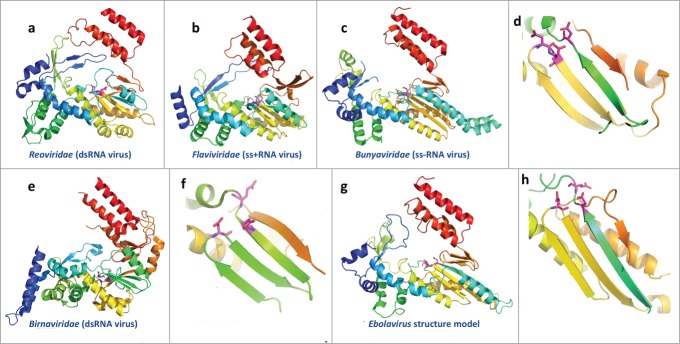
Structures of the catalytic domains of RNA-dependent RNA polymerases (RdRP) from RNA viruses and the structure model for Ebolavirus RdRP. The virus family is labeled below. The structures are colored in rainbow, with equivalent secondary structure elements from different structures colored similarly, except for the Birnaviridae RdRP, which has a circularly permutated topology. The functional sites used to coordinate Mg2+ are shown as sticks and colored in magenta. (A–C) Overall structure of the core domains of RdRPs from RNA viruses (PDB IDs: 2R7O, 1GX5, and 5AMR); (D) close up view of the classic arrangement of functional sites for the core domains of RdRPs from RNA viruses; (E–F) overall structure and close up view of the functional sites for the RdRP from Birnaviridae (dsRNA virus); PDB id: 2PGG; (G–H) structure model for the core domains of ZEBOV RdRP and close up view of the predicted active sites.
Multiple sequence alignment and 3D structures suggest a conserved catalytic mechanism of RdRP from RNA viruses. Two conserved Asp residues that are used to coordinate Magnesium ions in the catalytic site are in the same position in the 3D structures89 (Fig. 4). A sequence alignment of these RdRPs (Fig. S2) allows us to predict the catalytic sites for Ebolavirus RdRPs: D632D and D742. These two positions are conserved among close homologs of Ebolavirus RdRP detected by PSI-BLAST.90 The second conserved Asp residue immediately follows a conserved Gly residue, forming a GD motif. Another Asp residue after the GD motif also participates in coordinating Mg2+ in most of the templates (Fig. 4D). However, this residue is not conserved in Ebolavirus and Birnavirus RdRPs. Alternatively, Birnavirus RdRP has a Glu residue after the first conserved Asp (Fig. 4F), which is in the correct position to bind Mg2+. Similarly, a conserved Glu residue (634E) in the same position in the Ebolavirus RdRP may participate in Mg2+ binding, and the arrangement of these active site residues likely resembles that in Birnavirus RdRP.
The methyltransferase domain of protein L for mRNA capping
Addition of a 7-methylguanosine cap to the 5′ end of mRNA is essential for its subsequent translation and stability in eukaryotic cells.91 The C-terminal half of protein L is responsible for mRNA capping, and it contains an S-adenosyl-L-methionine-dependent methyltransferase domain that likely works in this process. HHpred detects several structural templates (Fig. 5) for this domain with probabilities above 95%. A sequence alignment between the Ebolavirus methyltransferase domain and the detected templates (Fig. S3) reveals that 3 residues, K1816, D1927, and K1962, are aligned to the conserved catalytic residues in the templates.92 In addition, the “GEGAGA” motif at positions 1836–1841 of Ebolavirus protein L is aligned to the conserved S-adenosyl-L-methionine-binding motif in the templates. This motif is also conserved in sequences from Filoviridae, suggesting a similar function in co-factor binding.
Figure 5.
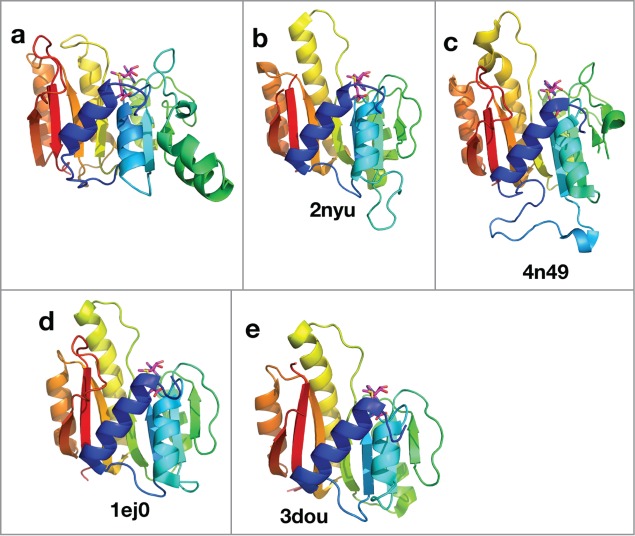
Structural model and templates for the mRNA capping methyltransferase domain in Ebolavirus protein L. The structures are colored in rainbow. Equivalent secondary structure elements from different structures are colored in the same color. The co-factor, S-adenosyl-L-methionine, is shown as sticks. (A) structure model for the mRNA capping methyltransferase domain of ZEBOV; (B–E) experimental structures of other methyltransferase domains.
Interaction between Ebolavirus proteins and host proteins
RESTV causes EHF symptoms to Asian cynomolgus monkeys (Macaca fascicularis), but not human and African green monkeys (Chlorocebus aethiops).5 This difference in susceptibility between closely related hosts is likely due to the sequence divergence in the host proteins that interact with virus proteins. Therefore, comparing the interacting partners of virus proteins from different hosts may provide insight into how host specificity is determined and further suggest the mechanism for RESTV's loss of human pathogenicity. The known interacting partners in the host for each Ebolavirus protein are summarized in Table 1.
Table 1.
Host proteins that functionally interact with Ebolavirus proteins, and their divergence level between RESTV-susceptible and RESTV-resistant species
| Name | Host protein | Functional implication | Chlorocebus vs Macaca | Homo vs Macaca |
|---|---|---|---|---|
| GP | NPC113,14 | Receptor for the virus | 6 (99.5%) | 28 (97.8%) |
| GP | TIM-199 | 11 (96.5%)*** | 56 (80.2%)*** | |
| GP | CD209 100,101 | Facilitate cell entry in specific cell types | 15 (95.9%)*** | 31 (92.1%)*** |
| GP | CLEC4M 100,101 | Not available in Macaca and Chlorocebus | ||
| GP | CLEC10A93 | 5 (98.4%) | 42 (86.7%) | |
| GP | FOLR1102 | 3 (98.8%) | 8 (96.9%) | |
| GP | FURIN7,8 | Process GP to GP1,2 | 1 (99.9%) | 9 (98.9%) |
| GP | CTSB103 | Process GP1,2 and initiate membrane fusion | 2 (99.4%) | 10 (97.0%) |
| GP | CTSL103 | 3 (99.1%) | 14 (95.8%) | |
| GP | ADAM179 | Process GP1,2 to GP1,2delta | 1 (99.9%) | 3 (99.6%) |
| GP | Dynamin (multiple)104 | Activates endothelial cells, reduces their barrier function | 0∼1 (99.9˜100%) | 2∼6 (99.3˜99.8%) |
| GP | ITGAV104 | 2 (99.8%) | 9 (99.1%) | |
| VP24 | STAT161 | Inhibit JAK-STAT pathway for interferon sensing | 0 (100%) | 5 (99.3%) |
| VP24 | KPNA556 | 0 (100%) | 2 (99.6%) | |
| VP24 | MAPK14 (p38)105 | Prevent phosphorylation and inhibit interferon sensing | 0 (100%) | 1 (99.6%) |
| VP30 | PPP1C21 | Dephosphorylate VP30, control replication-transcription switch | 0 (100%) | 0 (100%) |
| VP30 | PPP2C21 | 0 (100%) | 0 (100%) | |
| VP30 | Dicer106 | Antagonize RNAi machinery that could target viral RNA | 5 (99.7%) | 10 (99.5%) |
| VP30 | TRBP106 | 2 (99.5%) | 3 (99.2%) | |
| VP35 | Dicer106 | Antagonize RNAi machinery that could target viral RNA | 5 (99.7%) | 10 (99.5%) |
| VP35 | TRBP106 | 2 (99.5%) | 3 (99.2%) | |
| VP35 | ILF3 (DRBP76)107 | Inhibit the effect of interferon | 0 (100%) | 3 (99.7%) |
| VP35 | IKBKϵ108 | Block phosphorylation of IRF-3 by TBK-1 and IKBKϵ, inhibiting interferon production | 4 (99.4%) | 15 (97.9%) |
| VP35 | TBK-1108 | 2 (99.7%) | 8 (98.9%) | |
| VP35 | IRF-3108 | 2 (99.5%) | 17 (96.0%) | |
| VP35 | PACT109 | Inhibit its role as RIG-I activator | 0 (100%) | 0 (100%) |
| VP35 | PKR110 | Inhibit the effect of interferon | 42 (92.4%)*** | 110 (80%)*** |
| VP35 | UBE2I111 | Use SUMO E2 enzyme (UBE21) and E3 ligase (PIAS1) to modify IRF7 and inhibit its function | 0 (100%) | 0 (100%) |
| VP35 | PIAS1111 | 0 (100%) | 0 (100%) | |
| VP35 | IRF-7111 | 9 (98.2%) | 35 (92.9%) | |
| VP35 | DLC8112 | May regulate viral life cycle | 1 (98.9%) | 0 (100%) |
| VP40 | Sec24C113 | Virus utilize COPII vesicular transport system for life cycle | 7 (99.4%) | 24 (97.8%) |
| VP40 | TSG10128 | Virus uses multi-vesicular body biogenesis pathway for budding | 0 (100%) | 0 (100%) |
| VP40 | ABL1114 | ABL1 controls budding/release by phosphorylating VP40 | 5 (99.6%) | 13 (98.8%) |
| VP40 | NEDD4115 | NEDD4 facilitates budding by adding ubiquitin to VP40 | 5 (99.5%) | 28 (97.8%) |
| VP40 | Tubulin (multiple)116 | Virus utilize host cytoskeleton in its life cycle | 0 (100%) | 0 (100%) |
| VP40 | Actin (1 and 2)117 | 0 (100%) | 0 (100%) | |
| VP40 | IQGAP1118 | 2 (99.9%) | 9 (99.4%) |
significantly (p<0.05) elevated divergence level.
The known host proteins that interact with VP24, VP30, and VP40 are highly similar between the RESTV-resistant (Chlorocebus and human) and RESTV-susceptible species (Macaca), suggesting that they may not be responsible for RESTV's loss of human pathogenicity. In contrast, 7 most divergent host partners interact either with GP or VP35. Three of them (marked in Table 1) show significantly (P < 0.05) elevated divergence between the susceptible and resistant species, including Hepatitis A virus cellular receptor 1 (TIM-1) and pathogen-recognition receptor CD209 that interact with GP and facilitates cell entry, as well as the interferon-induced, dsRNA-activated kinase PKR that is inhibited by VP35.
The elevated divergence level for interacting partners of GP and VP35 in the host suggests that VP35 and GP may play important roles in determining host specificity. This is consistent with some indirect experimental data. RESTV GP pseudotyped viruses show significantly lower ability to infect human cells and to damage human endothelial cells than that of ZEBOV GP pseudotyped viruses.93 In addition, RESTV GP shows lower ability to deplete T cells and to down-regulate interferon-stimulated gene expression compared to ZEBOV GP.94,95 Meanwhile, ZEBOV VP35 shows stronger Interferon inhibition than RESTV VP35 in human cells.68 However, direct studies of all RESTV proteins' effect in cells of both RESTV-susceptible and RESTV-resistant species are needed to prove our hypothesis.
Interpreting residues associated with RESTV's loss of human pathogenicity in the context of 3D structure and known functional sites
We consider positions that are associated with the loss of pathogenicity in RESTV as those that are always and significantly more similar among pathogenic species (BDBV, TAFV, SUDV and ZEBOV) than between RESTV and the pathogenic species. We referred to them as “RESTV-specific mutations.” We identified 215 such positions (Table 2), and VP30 and VP35 are significantly enriched in such mutations.
Table 2.
Positions in ZEBOV that are likely associated with the loss of human pathogenicity by RESTV
| Name | UniProt ID | Length | P-value | Mutations associated with the loss of human pathogenicity |
|---|---|---|---|---|
| GP | Q05320 | 676 | 0.457 | F31I, Q44K, V45A, E156N, S196A, L199A, S210T, Y261R, T269S, T283P, S307H, T335P, E337T, H339N, E345T, H354L, E359T, A361E, A427M, G488K, R498K, R500K, N514D, D607S, K622E, I627K, Q638H, D642L, W644L, T659I |
| L | Q05318 | 2212 | 0.690 | V66T, E93T, Q109H, N120A, V128T, E130I, F132T, L146V, L179F, N201T, T202I, A221S, Q223L, H227Q, V229L, P262V, V263D, S274L, L283V, Y312F, A326S, T330D, S343Y, E350D, T361S, L365F, I402N, Q447H, P450S, D465N, R654H, E689S, S847A, S868A, F896Y, L925F, A954S, S995T, T1024N, R1073K, A1119S, Q1149P, S1154L, P1163A, K1171D, D1189S, A1214S, R1217K, D1237E, Q1253N, Y1322L, R1354K, T1366A, I1408M, S1436N, K1461Q, S1473C, L1488Y, S1506A, A1538S, V1562L, E1564S, T1571K, Q1608I, H1619L, L1624Y, C1628S, D1744G, E1752P, S1769G, Q1782L, R1792H, W1822L, V1850T, R1916N, K1938Q, E1941R, V1955Y, Q2024G, P2038V, S2077T, K2078G, R2079L, E2098D, Q2105L, Q2108E, Y2131F, L2157V, R2168H, R2175K, L2177F, M2186L, L2203F |
| NP | P18272 | 739 | 0.587 | R4G, T15G, S30T, R39K, I52M, R105K, M137L, F212Y, K274R, S279A, K373R, K374R, A411L, K416N, Y421Q, D426E, D435N, Q442L, D443E, T453I, V458A, D492E, Q507S, S511I, N551R, T563S, E633L, S647K, A705R, T714Y, D716N |
| VP24 | Q05322 | 251 | 0.932 | T131S, N132T, M136L, Q139R, T226A, S248L |
| VP30 | Q05323 | 288 | 0.010 | G20P, V25S, Y39R, T52N, V53L, T63I, E93D, T96N, R98H, K107R, S111I, L116S, N117Q, A120S, Q135S, T150I, Q157R, R196H, E205D, R262A, S268Q |
| VP35 | Q05127 | 340 | 0.019 | T5L, L25T, S26T, E48D, D76E, C79Y, N80V, E85K, S92M, V97T, Q98S, S106A, A154S, T159V, E160D, G167K, S174A, I258T, E269D, A290V, A291P, V314A, Q329K |
| VP40 | Q05128 | 326 | 0.786 | M14N, T46V, P85T, A128I, G201N, F209L, Q245P, H269Q, T277Q, V323H, E325D |
P-value: binomial test for enrichment of residues that may be associated with RESTV's loss of human pathogenicity in each protein.
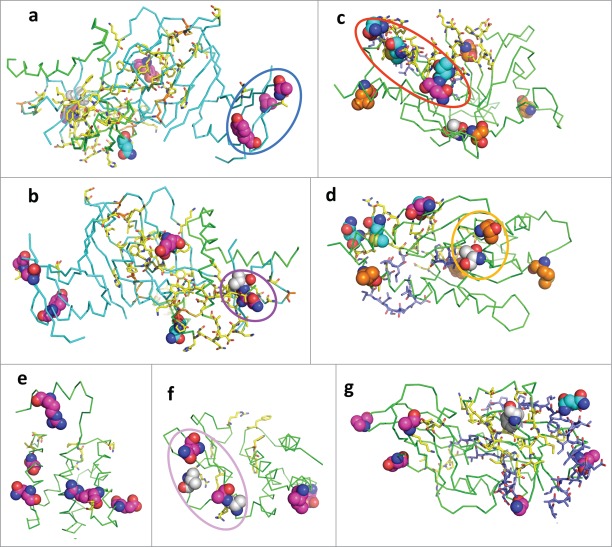
43 of the RESTV-specific mutations can be mapped to known 3D structures of Ebolavirus proteins. None of them overlap with functional sites that are proved to be crucial by mutagenesis and 6 of them overlap with interaction surfaces (summarized in Table 3) on these structures. They may affect the binding affinities but would not likely abolish the interactions. One loop (129–141) of VP24 at the boundary of the interacting surface between VP24 and KPNA550 contains 4 RESTV-specific mutations (T131S, N132T, M136L, Q139R, within the red circle in Fig. 6C). These mutations may affect the binding affinity between RESTV and KPNA5 in RESTV, weakening the immune suppression by RESTV. One mutation to GP (N514D) is at the boundary of its interacting surface with neutralizing antibodies from a human survivor55 and this mutation may affect the efficiency of the ZEBOV antibodies to antagonize RESTV.
Table 3.
Experimentally characterized functional sites in Ebolavirus proteins
| Name | Residues | Function | Experimental evidence |
|---|---|---|---|
| GP | 40 | Glycosylated by host | N40D loss ability to infect119 |
| GP | 41–43, 503–511, 513, 514 | Interact with antibody | On the interacting surface with neutralizing antibody 55 |
| GP | 51, 68, 86, 99, 109,111, 113, 122, 139, 154, 159, 161, 162, 171, 176, 183–185 | Maintain the hydrophobic core structure | W86A, Y99A, Y109A, H139A, H154A, F159A, L161A, Y162A, Y171A, F176A, F183A reduce expression, reduce viral incorporation and abolish infectivity; L111A, I113A, L122A reduce viral incorporation and abolish infectivity; L51A, L68A, L184A, I185A abolish infectivity120 |
| GP | 53, 108, 121, 135, 147, 511, 556, 601, 608, 609 | Disulfide bond | C53G, C108A, C121G, C135S, C147S, C511G, C556S, C601S, C608G, C609G reduce expression and abolish infectivity119 |
| GP | 55, 85, 103, 117, 178 | Hydrophilic to maintain the structure | E85A, E103A, E178A reduce expression; E85A, E103A, D117A, E178A reduce viral incorporation; D55A, E103A, D117A, E178A loss ability to infect120 |
| GP | 529, 531–533, 535–537 | Fusion peptide | I529A, W531R, W531A, I532R, P533R, F535R, G536R, G536A, P537R loss ability to infect15 |
| GP | 57, 63, 64, 88, 95, 170 | Cell entry | L63K, L63A reduce expression; L57A, L57F, L57I, L57K, L63K, L63A, L63F, R64E, R64A, F88E, F88A, K95E, K95A, I170A, I170E loss ability to infect120 |
| VP24 | 96–98, 106–121 | Interact with STAT1 | Show reduced hydrogen exchange rate upon binding61 |
| VP24 | 113, 115, 117, 121, 124, 125, 128–131, 134–141, 184–186, 201–205, 218 | Interact with KPNA5 | On the interacting surface in crystal structure with KPNA5 (PDB id: 4U2X)56 |
| VP24 | 50, 71, 147, 187 | Adapt to new host | T50I mouse adaptation98; M71I, L147P, and T187I guinea pig adaptation97 |
| VP30 | 179, 180, 183, 197 | Activate transcription | Mutation to Ala reduces interaction with nucleocapsid; K180A, K183A, E197A block transcription activation59 |
| VP30 | 143, 146 | Phosphorylation | T143A, T143D, T146A, T146D inhibit transcription21 |
| VP35 | 239, 240, 309, 312, 319, 322, 339 | Bind dsRNA | K309A, K319A reduce dsRNA binding; F239A, H240A, R312A, R322A, K339A abolishes dsRNA binding70 |
| VP35 | 239, 240, 309, 312, 319, 322, 339 | IRF-3 inhibition | K309A, K319A reduce IRF-3 inhibition; F239A, H240A, R312A, R322A, K339A greatly reduce IRF-3 inhibition70 |
| VP35 | 235, 240 | Polymerase cofactor | F235A, H240A impair replication of mini-genome24 |
| VP35 | 312, 322, 339 | Bind DRPB76 | Mutation to alanine reduce ability to bind DRPB76107 |
| VP35 | 309, 312 | Inhibit RNAi | K309A and R312A lost the inhibition effect121 |
| VP35 | 305, 309, 312 | Inhibit PKR | Mutant any 2 to alanine abolish the inhibition122 |
| VP40 | 303–308 | Interact with Sec24C | 303–306A and 305–308A cannot interact with Sec24C, and reduce virus-like particles113 |
| VP40 | 51–54, 96–101, 212–214, 286–291, 303–308, 314–316 | Release of virus-like particles | 51–52A, 53–54A, deletion of 96–101, K212A, L213A, R214A, 286–288A, 289–291A, 303–306A, 305–308A reduce the release of virus-like particles113,123,124 |
| VP40 | 127, 129, 130, 283, 286, 293, 295, 298, 309–317 | Membrane localization | K127A, T129A, N130A, P283L, P286L, I293A, L295A, V298A and deletion of 309–317 reduce membrane localization27, 125 |
| VP40 | 226–255 | Interaction with microtubules | Deletion of 226–240 or 241–255 abolish ability to protect microtubules from depolymerization116 |
| VP40 | 213, 293, 295, 298 | Penetrate membrane | Mutation to alanine reduces membrane localization126 |
Figure 6.
Mapping of RESTV-specific residues, functional sites and interaction surfaces to known 3D structures of Ebolavirus proteins. The structure is shown in ribbon; the functional sites are shown as sticks; and positions with RESTV-specific mutations and alternate host (rodent) adaptation residues are shown as spheres. Carbon atoms of the functional sites and sites with RESTV-specific mutations are colored to show the property of that residue: RESTV-specific surface residues are in magenta; RESTV-specific buried residues are in white; RESTV-specific residues that belong to interaction surfaces are in cyan; known functional sites are in yellow; disulfide bonded and alternate host (rodent) adaptation residues are in orange; predicted functional residues are in blue. Other atoms are colored as follows: oxygen (red); nitrogen (blue) and sulfur (orange). Circles highlight clusters of RESTV-specific residues that are discussed in the text. (A, B) GP (PDB id: 3CSY); (C, D) VP24 (PDB id: 4U2X); (E) VP30 (PDB id: 2I8B); (F) VP35 (PDB id: 3L26); (G) VP40 (PDB id: 1ES6).
Mapping of the RESTV-specific mutations to the 3D structures revealed a couple of mutation clusters in GP and VP35, which may be related to RESTV's difference in pathogenicity (Fig. 6). A first cluster is in the C-terminal subdomain of GP. The cluster consists of 3 mutations on the surface: Y261R, T269S, and S307H (inside the blue circle in Fig. 6A). The functional role of this subdomain is not clear, and the cell entry of ZEBOV is mostly mediated by the interaction between N-terminal 150 residues of GP and cell receptors like NPC1 and TIM-1. One possibility is that it may interact with other host proteins, such as lectins that facilitate the infection of Ebolavirus. In contrast, another cluster of mutations (Q44K, and V45A, inside the magenta circle in Fig. 6B) may affect the interaction between GP and the cell receptors. Even more, the mutation E156N is close to functional sites that are shown by mutagenesis to be important for maintaining the infectivity of ZEBOV. Therefore, these RESTV-specific mutations of GP may cause a significantly lower infectivity in RESTV and contribute to the loss of human pathogenicity.
RESTV-specific mutations (A290V, A291P, V314A, and Q329K) in VP35 form a cluster (inside the pink circle in Fig. 6F) on the opposite side of the dsRNA-binding surface of VP35. Host immune suppression by VP35 is mainly related to its interaction with dsRNA, but the loss of dsRNA-binding ability does not completely abolish VP35-mediated immune suppression.96 This observation indicates the existence of other mechanisms for immune suppression by VP35, where the surface enriched in RESTV-specific mutations may play a role. One RESTV-specific mutation (T226A) is adjacent to the position in VP24 that is mutated (T50I) during adaptation to mice97,98 (orange circles in Fig. 6D). This adaptation site is not close to any known functional sites, but the clustering of the adaptation site and RESTV-specific mutation suggests the possibility that they are at the interface of some uncharacterized interaction with other host proteins.
Funding
This work was supported in part by the National Institutes of Health (GM094575 to NVG) and the Welch Foundation (I-1505 to NVG).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Drs. R. Dustin Schaeffer and Lisa N. Kinch for critical reading of the manuscript. Qian Cong is a Howard Hughes Medical Institute International Student Research fellow.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1.Organization WH. 2014. Ebola virus disease Fact Sheets: http://www.who.int/mediacentre/factsheets/fs103/en/ [Google Scholar]
- 2.Organization WH. 2015. Ebola data and statistics (published on 18 February 2015): http://apps.who.int/gho/data/view.ebola-sitrep.ebola-summary-20150218?lang=en [Google Scholar]
- 3.Kuhn JH, Becker S, Ebihara H, Geisbert TW, Johnson KM, Kawaoka Y, Lipkin WI, Negredo AI, Netesov SV, Nichol ST, Palacios G, et al.. Proposal for a revised taxonomy of the family Filoviridae: classification, names of taxa and viruses, and virus abbreviations. Arch Virol 2010; 155:2083-103; PMID:21046175; http://dx.doi.org/ 10.1007/s00705-010-0814-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gire SK, Goba A, Andersen KG, Sealfon RS, Park DJ, Kanneh L, Jalloh S, Momoh M, Fullah M, Dudas G, et al.. Genomic surveillance elucidates Ebola virus origin and transmission during the 2014 outbreak. Science 2014; 345:1369-72; PMID:25214632; http://dx.doi.org/ 10.1126/science.1259657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morikawa S, Saijo M, Kurane I. Current knowledge on lower virulence of Reston Ebola virus (in French: Connaissances actuelles sur la moindre virulence du virus Ebola Reston). Compar Immunol Microbiol Infect Dis 2007; 30:391-8; PMID:17610952; http://dx.doi.org/ 10.1016/j.cimid.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez A, Trappier SG, Mahy BW, Peters CJ, Nichol ST. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc Natl Acad Sci U S A 1996. 93:3602-7; PMID:8622982; http://dx.doi.org/ 10.1073/pnas.93.8.3602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volchkov VE, Feldmann H, Volchkova VA, Klenk HD. Processing of the Ebola virus glycoprotein by the proprotein convertase furin. Proc Natl Acad Sci U S A 1998; 95:5762-7; PMID:9576958; http://dx.doi.org/ 10.1073/pnas.95.10.5762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wool-Lewis RJ, Bates P. Endoproteolytic processing of the ebola virus envelope glycoprotein: cleavage is not required for function. J Virol 1999. 73:1419-26; PMID:9882347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolnik O, Volchkova V, Garten W, Carbonnelle C, Becker S, Kahnt J, Stroher U, Klenk HD, Volchkov V. Ectodomain shedding of the glycoprotein GP of Ebola virus. EMBO J 2004; 23:2175-84; PMID:15103332; http://dx.doi.org/ 10.1038/sj.emboj.7600219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehedi M, Falzarano D, Seebach J, Hu X, Carpenter MS, Schnittler HJ, Feldmann H. A new Ebola virus nonstructural glycoprotein expressed through RNA editing. J Virol 2011; 85:5406-14; PMID:21411529; http://dx.doi.org/ 10.1128/JVI.02190-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Volchkova VA, Feldmann H, Klenk HD, Volchkov VE. The nonstructural small glycoprotein sGP of Ebola virus is secreted as an antiparallel-orientated homodimer. Virology 1998; 250:408-14; PMID:9792851; http://dx.doi.org/ 10.1006/viro.1998.9389 [DOI] [PubMed] [Google Scholar]
- 12.Mohamadzadeh M, Chen L, Schmaljohn AL. How Ebola and Marburg viruses battle the immune system. Nat Rev Immunol 2007; 7:556-67; PMID:17589545 [DOI] [PubMed] [Google Scholar]
- 13.Carette JE, Raaben M, Wong AC, Herbert AS, Obernosterer G, Mulherkar N, Kuehne AI, Kranzusch PJ, Griffin AM, Ruthel G, et al.. Ebola virus entry requires the cholesterol transporter Niemann-Pick C1. Nature 2011; 477:340-3; PMID:21866103; http://dx.doi.org/ 10.1038/nature10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EH, Obernosterer G, Raaben M, Herbert AS, Deffieu MS, Krishnan A, Ndungo E, Sandesara RG, Carette JE, Kuehne AI, et al.. Ebola virus entry requires the host-programmed recognition of an intracellular receptor. EMBO J 2012; 31:1947-60; PMID:22395071; http://dx.doi.org/ 10.1038/emboj.2012.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito H, Watanabe S, Sanchez A, Whitt MA, Kawaoka Y. Mutational analysis of the putative fusion domain of Ebola virus glycoprotein. J Virol 1999; 73:8907-12; PMID:10482652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomara MJ, Mora P, Mingarro I, Nieva JL. Roles of a conserved proline in the internal fusion peptide of Ebola glycoprotein. FEBS Lett 2004; 569:261-6; PMID:15225645; http://dx.doi.org/ 10.1016/j.febslet.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Watanabe S, Noda T, Kawaoka Y. Functional mapping of the nucleoprotein of Ebola virus. J Virol 2006; 80:3743-51; PMID:16571791; http://dx.doi.org/ 10.1128/JVI.80.8.3743-3751.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Y, Xu L, Sun Y, Nabel GJ. The assembly of Ebola virus nucleocapsid requires virion-associated proteins 35 and 24 and posttranslational modification of nucleoprotein. Mol Cell 2002; 10:307-16; PMID:12191476; http://dx.doi.org/ 10.1016/S1097-2765(02)00588-9 [DOI] [PubMed] [Google Scholar]
- 19.Weik M, Modrof J, Klenk HD, Becker S, Muhlberger E. Ebola virus VP30-mediated transcription is regulated by RNA secondary structure formation. J Virol 2002; 76:8532-9; PMID:12163572; http://dx.doi.org/ 10.1128/JVI.76.17.8532-8539.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez MJ, Biedenkopf N, Volchkova V, Hartlieb B, Alazard-Dany N, Reynard O, Becker S, Volchkov V. Role of Ebola virus VP30 in transcription reinitiation. J Virol 2008; 82:12569-73; PMID:18829754; http://dx.doi.org/ 10.1128/JVI.01395-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilinykh PA, Tigabu B, Ivanov A, Ammosova T, Obukhov Y, Garron T, Kumari N, Kovalskyy D, Platonov MO, Naumchik VS, et al.. Role of protein phosphatase 1 in dephosphorylation of Ebola virus VP30 protein and its targeting for the inhibition of viral transcription. J Biol Chem 2014; 289:22723-38; PMID:24936058; http://dx.doi.org/ 10.1074/jbc.M114.575050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biedenkopf N, Hartlieb B, Hoenen T, Becker S. Phosphorylation of Ebola virus VP30 influences the composition of the viral nucleocapsid complex: impact on viral transcription and replication. J Biol Chem 2013; 288:11165-74; PMID:23493393; http://dx.doi.org/ 10.1074/jbc.M113.461285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muhlberger E, Weik M, Volchkov VE, Klenk HD, Becker S. Comparison of the transcription and replication strategies of marburg virus and Ebola virus by using artificial replication systems. J Virol 1999; 73:2333-42; PMID:9971816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prins KC, Binning JM, Shabman RS, Leung DW, Amarasinghe GK, Basler CF. Basic residues within the ebolavirus VP35 protein are required for its viral polymerase cofactor function. J Virol 2010; 84:10581-91; PMID:20686031; http://dx.doi.org/ 10.1128/JVI.00925-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoenen T, Jung S, Herwig A, Groseth A, Becker S. Both matrix proteins of Ebola virus contribute to the regulation of viral genome replication and transcription. Virology 2010; 403:56-66; PMID:20444481; http://dx.doi.org/ 10.1016/j.virol.2010.04.002 [DOI] [PubMed] [Google Scholar]
- 26.Han Z, Boshra H, Sunyer JO, Zwiers SH, Paragas J, Harty RN. Biochemical and functional characterization of the Ebola virus VP24 protein: implications for a role in virus assembly and budding. J Virol 2003; 77:1793-800; PMID:12525613; http://dx.doi.org/ 10.1128/JVI.77.3.1793-1800.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panchal RG, Ruthel G, Kenny TA, Kallstrom GH, Lane D, Badie SS, Li L, Bavari S, Aman MJ. In vivo oligomerization and raft localization of Ebola virus protein VP40 during vesicular budding. Proc Natl Acad Sci U S A 2003; 100:15936-41; PMID:14673115; http://dx.doi.org/ 10.1073/pnas.2533915100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin-Serrano J, Zang T, Bieniasz PD. HIV-1 and Ebola virus encode small peptide motifs that recruit Tsg101 to sites of particle assembly to facilitate egress. Nat Med 2001; 7:1313-9; PMID:11726971; http://dx.doi.org/ 10.1038/nm1201-1313 [DOI] [PubMed] [Google Scholar]
- 29.UniProt C. UniProt: a hub for protein information. Nucleic Acids Res 2015; 43:D204-212; PMID:25348405; http://dx.doi.org/ 10.1093/nar/gku989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cong Q, Grishin NV. MESSA: MEta-Server for protein Sequence Analysis. BMC Biol 2012; 10:82; PMID:23031578; http://dx.doi.org/ 10.1186/1741-7007-10-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pollastri G, Przybylski D, Rost B, Baldi P. Improving the prediction of protein secondary structure in three and eight classes using recurrent neural networks and profiles. Proteins 2002; 47:228-35; PMID:11933069; http://dx.doi.org/ 10.1002/prot.10082 [DOI] [PubMed] [Google Scholar]
- 32.Jones DT. Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 1999; 292:195-202; PMID:10493868; http://dx.doi.org/ 10.1006/jmbi.1999.3091 [DOI] [PubMed] [Google Scholar]
- 33.Cheng J, Sweredoski M, Baldi P. Accurate prediction of protein disordered regions by mining protein structure data. Data Mining Knowl Discov 2005; 11:213-22; http://dx.doi.org/ 10.1007/s10618-005-0001-y [DOI] [Google Scholar]
- 34.Linding R, Jensen LJ, Diella F, Bork P, Gibson TJ, Russell RB. Protein disorder prediction: implications for structural proteomics. Structure 2003; 11:1453-9; PMID:14604535; http://dx.doi.org/ 10.1016/j.str.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 35.Ward JJ, Sodhi JS, McGuffin LJ, Buxton BF, Jones DT. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J Mol Biol 2004; 337:635-45; PMID:15019783; http://dx.doi.org/ 10.1016/j.jmb.2004.02.002 [DOI] [PubMed] [Google Scholar]
- 36.Lobanov MY, Galzitskaya OV. 2011. The Ising model for prediction of disordered residues from protein sequence alone. Phys Biol 8:035004; PMID:21572175; http://dx.doi.org/ 10.1088/1478-3975/8/3/035004 [DOI] [PubMed] [Google Scholar]
- 37.Tusnady GE, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol 1998; 283:489-506; PMID:9769220; http://dx.doi.org/ 10.1006/jmbi.1998.2107 [DOI] [PubMed] [Google Scholar]
- 38.Jones DT. Improving the accuracy of transmembrane protein topology prediction using evolutionary information. Bioinformatics 2007; 23:538-44; PMID:17237066; http://dx.doi.org/ 10.1093/bioinformatics/btl677 [DOI] [PubMed] [Google Scholar]
- 39.Kall L, Krogh A, Sonnhammer EL. A combined transmembrane topol signal peptide prediction method. J Mol Biol 2004; 338:1027-36; PMID:15111065; http://dx.doi.org/ 10.1016/j.jmb.2004.03.016 [DOI] [PubMed] [Google Scholar]
- 40.Krogh A, Larsson B, von Heijne G, Sonnhammer EL. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J Mol Biol 2001; 305:567-80; PMID:11152613; http://dx.doi.org/ 10.1006/jmbi.2000.4315 [DOI] [PubMed] [Google Scholar]
- 41.von Heijne G. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J Mol Biol 1992; 225:487-94; PMID:1593632; http://dx.doi.org/ 10.1016/0022-2836(92)90934-C [DOI] [PubMed] [Google Scholar]
- 42.Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 2004; 340:783-95; PMID:15223320; http://dx.doi.org/ 10.1016/j.jmb.2004.05.028 [DOI] [PubMed] [Google Scholar]
- 43.Lupas A, Van Dyke M, Stock J. Predicting coiled coils from protein sequences. Science 1991; 252:1162-4; PMID:2031185; http://dx.doi.org/ 10.1126/science.252.5009.1162 [DOI] [PubMed] [Google Scholar]
- 44.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403-10; PMID:2231712; http://dx.doi.org/ 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 45.Remmert M, Biegert A, Hauser A, Soding J. HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nat Methods 2012; 9:173-5; http://dx.doi.org/ 10.1038/nmeth.1818 [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC Bioinfor 2008; 9:40; PMID:18215316; http://dx.doi.org/ 10.1186/1471-2105-9-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protocols 2010; 5:725-38; PMID:20360767; http://dx.doi.org/ 10.1038/nprot.2010.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng H, Schaeffer RD, Liao Y, Kinch LN, Pei J, Shi S, Kim BH, Grishin NV. ECOD: an evolutionary classification of protein domains. PLoS Comput Biol 2014; 10:e1003926; PMID:25474468; http://dx.doi.org/ 10.1371/journal.pcbi.1003926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole C, Barber JD, Barton GJ. Jpred 3 secondary structure prediction server. Nucleic Acids Res 2008; 36:W197-201; PMID:18463136; http://dx.doi.org/ 10.1093/nar/gkn238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pei J, Kim BH, Grishin NV. PROMALS3D: a tool for multiple protein sequence and structure alignments. Nucleic Acids Res 2008; 36:2295-300; PMID:18287115; http://dx.doi.org/ 10.1093/nar/gkn072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei J, Grishin NV. PROMALS3D: multiple protein sequence alignment enhanced with evolutionary and three-dimensional structural information. Methods Mol Biol 2014; 1079:263-71; PMID:24170408; http://dx.doi.org/ 10.1007/978-1-62703-646-7_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katoh K, Standley DM. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol biol Evolut 2013; 30:772-80; PMID:23329690; http://dx.doi.org/ 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henikoff S, Henikoff JG. Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A 1992; 89:10915-9; PMID:1438297; http://dx.doi.org/ 10.1073/pnas.89.22.10915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dziubanska PJ, Derewenda U, Ellena JF, Engel DA, Derewenda ZS. The structure of the C-terminal domain of the Zaire ebolavirus nucleoprotein. Acta crystallographica. Sec D Biol Bryst 2014; 70:2420-9; http://dx.doi.org/ 10.1107/S1399004714014710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature 2008; 454:177-82; PMID:18615077; http://dx.doi.org/ 10.1038/nature07082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu W, Edwards MR, Borek DM, Feagins AR, Mittal A, Alinger JB, Berry KN, Yen B, Hamilton J, Brett TJ, et al.. Ebola virus VP24 targets a unique NLS binding site on karyopherin α 5 to selectively compete with nuclear import of phosphorylated STAT1. Cell Host Microb 2014; 16:187-200; PMID:25121748; http://dx.doi.org/ 10.1016/j.chom.2014.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Edwards MR, Johnson B, Mire CE, Xu W, Shabman RS, Speller LN, Leung DW, Geisbert TW, Amarasinghe GK, Basler CF. The Marburg virus VP24 protein interacts with Keap1 to activate the cytoprotective antioxidant response pathway. Cell Rep 2014; 6:1017-25; PMID:24630991; http://dx.doi.org/ 10.1016/j.celrep.2014.01.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kimberlin CR, Bornholdt ZA, Li S, Woods VL Jr., MacRae IJ, Saphire EO. Ebolavirus VP35 uses a bimodal strategy to bind dsRNA for innate immune suppression. Proc Natl Acad Sci U S A 2010; 107:314-9; PMID:20018665; http://dx.doi.org/ 10.1073/pnas.0910547107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hartlieb B, Muziol T, Weissenhorn W, Becker S. Crystal structure of the C-terminal domain of Ebola virus VP30 reveals a role in transcription and nucleocapsid association. Proc Natl Acad Sci U S A 2007; 104:624-9; PMID:17202263; http://dx.doi.org/ 10.1073/pnas.0606730104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bale S, Julien JP, Bornholdt ZA, Krois AS, Wilson IA, Saphire EO. Ebolavirus VP35 coats the backbone of double-stranded RNA for interferon antagonism. J Virol 2013; 87:10385-8; PMID:23824825; http://dx.doi.org/ 10.1128/JVI.01452-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang AP, Bornholdt ZA, Liu T, Abelson DM, Lee DE, Li S, Woods VL Jr., Saphire EO. The ebola virus interferon antagonist VP24 directly binds STAT1 and has a novel, pyramidal fold. PLoS Pathog 2012; 8:e1002550; PMID:22383882; http://dx.doi.org/ 10.1371/journal.ppat.1002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bale S, Dias JM, Fusco ML, Hashiguchi T, Wong AC, Liu T, Keuhne AI, Li S, Woods VL Jr., Chandran K, et al.. Structural basis for differential neutralization of ebolaviruses. Viruses 2012; 4:447-70; PMID:22590681; http://dx.doi.org/ 10.3390/v4040447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dias JM, Kuehne AI, Abelson DM, Bale S, Wong AC, Halfmann P, Muhammad MA, Fusco ML, Zak SE, Kang E, et al.. A shared structural solution for neutralizing ebolaviruses. Nat Struct Mol Biol 2011; 18:1424-7; PMID:22101933; http://dx.doi.org/ 10.1038/nsmb.2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Binning JM, Wang T, Luthra P, Shabman RS, Borek DM, Liu G, Xu W, Leung DW, Basler CF, Amarasinghe GK. Development of RNA aptamers targeting Ebola virus VP35. Biochemistry 2013; 52:8406-19; PMID:24067086; http://dx.doi.org/ 10.1021/bi400704d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malashkevich VN, Schneider BJ, McNally ML, Milhollen MA, Pang JX, Kim PS. Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution. Proc Natl Acad Sci U S A 1999; 96:2662-7; PMID:10077567; http://dx.doi.org/ 10.1073/pnas.96.6.2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bornholdt ZA, Noda T, Abelson DM, Halfmann P, Wood MR, Kawaoka Y, Saphire EO. Structural rearrangement of ebola virus VP40 begets multiple functions in the virus life cycle. Cell 2013; 154:763-74; PMID:23953110; http://dx.doi.org/ 10.1016/j.cell.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prins KC, Delpeut S, Leung DW, Reynard O, Volchkova VA, Reid SP, Ramanan P, Cardenas WB, Amarasinghe GK, Volchkov VE, et al.. Mutations abrogating VP35 interaction with double-stranded RNA render Ebola virus avirulent in guinea pigs. J Virol 2010; 84:3004-15; PMID:20071589; http://dx.doi.org/ 10.1128/JVI.02459-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leung DW, Shabman RS, Farahbakhsh M, Prins KC, Borek DM, Wang T, Muhlberger E, Basler CF, Amarasinghe GK. Structural and functional characterization of Reston Ebola virus VP35 interferon inhibitory domain. J Mol Biol 2010; 399:347-57; PMID:20399790; http://dx.doi.org/ 10.1016/j.jmb.2010.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leung DW, Ginder ND, Fulton DB, Nix J, Basler CF, Honzatko RB, Amarasinghe GK. Structure of the Ebola VP35 interferon inhibitory domain. Proc Natl Acad Sci U S A 2009; 106:411-6; PMID:19122151; http://dx.doi.org/ 10.1073/pnas.0807854106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leung DW, Prins KC, Borek DM, Farahbakhsh M, Tufariello JM, Ramanan P, Nix JC, Helgeson LA, Otwinowski Z, Honzatko RB, et al.. Structural basis for dsRNA recognition and interferon antagonism by Ebola VP35. Nat Struct Mol Biol 2010; 17:165-72; PMID:20081868; http://dx.doi.org/ 10.1038/nsmb.1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Leung DW, Borek D, Luthra P, Binning JM, Anantpadma M, Liu G, Harvey IB, Su Z, Endlich-Frazier A, Pan J, et al.. An Intrinsically Disordered Peptide from Ebola Virus VP35 Controls Viral RNA Synthesis by Modulating Nucleoprotein-RNA Interactions. Cell Rep 2015; 11:376-89; PMID:25865894; http://dx.doi.org/ 10.1016/j.celrep.2015.03.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Modrof J, Becker S, Muhlberger E. Ebola virus transcription activator VP30 is a zinc-binding protein. J Virol 2003; 77:3334-8; PMID:12584359; http://dx.doi.org/ 10.1128/JVI.77.5.3334-3338.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gough J, Karplus K, Hughey R, Chothia C. Assignment of homology to genome sequences using a library of hidden Markov models that represent all proteins of known structure. J Mol Biol 2001; 313:903-19; PMID:11697912; http://dx.doi.org/ 10.1006/jmbi.2001.5080 [DOI] [PubMed] [Google Scholar]
- 74.de Castro E, Sigrist CJ, Gattiker A, Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A, Hulo N. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res 2006; 34:W362-5; PMID:16845026; http://dx.doi.org/ 10.1093/nar/gkl124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanner SJ, Ariza A, Richard CA, Kyle HF, Dods RL, Blondot ML, Wu W, Trincao J, Trinh CH, Hiscox JA, et al.. Crystal structure of the essential transcription antiterminator M2-1 protein of human respiratory syncytial virus and implications of its phosphorylation. Proc Natl Acad Sci U S A 2014; 111:1580-5; PMID:24434552; http://dx.doi.org/ 10.1073/pnas.1317262111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leyrat C, Renner M, Harlos K, Huiskonen JT, Grimes JM. Drastic changes in conformational dynamics of the antiterminator M2-1 regulate transcription efficiency in Pneumovirinae. eLife 2014; 3:e02674; PMID:24842877; http://dx.doi.org/ 10.7554/eLife.02674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rudolph MG, Kraus I, Dickmanns A, Eickmann M, Garten W, Ficner R. Crystal structure of the borna disease virus nucleoprotein. Structure 2003; 11:1219-26; PMID:14527390; http://dx.doi.org/ 10.1016/j.str.2003.08.011 [DOI] [PubMed] [Google Scholar]
- 78.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science 2006; 313:357-60; PMID:16778022; http://dx.doi.org/ 10.1126/science.1126953 [DOI] [PubMed] [Google Scholar]
- 79.Albertini AA, Wernimont AK, Muziol T, Ravelli RB, Clapier CR, Schoehn G, Weissenhorn W, Ruigrok RW. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science 2006; 313:360-3; PMID:16778023; http://dx.doi.org/ 10.1126/science.1125280 [DOI] [PubMed] [Google Scholar]
- 80.Tawar RG, Duquerroy S, Vonrhein C, Varela PF, Damier-Piolle L, Castagne N, MacLellan K, Bedouelle H, Bricogne G, Bhella D, et al.. Crystal structure of a nucleocapsid-like nucleoprotein-RNA complex of respiratory syncytial virus. Science 2009; 326:1279-83; PMID:19965480; http://dx.doi.org/ 10.1126/science.1177634 [DOI] [PubMed] [Google Scholar]
- 81.Yabukarski F, Lawrence P, Tarbouriech N, Bourhis JM, Delaforge E, Jensen MR, Ruigrok RW, Blackledge M, Volchkov V, Jamin M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat Struct Mol Biol 2014; 21:754-9; PMID:25108352; http://dx.doi.org/ 10.1038/nsmb.2868 [DOI] [PubMed] [Google Scholar]
- 82.Dong S, Yang P, Li G, Liu B, Wang W, Liu X, Xia B, Yang C, Lou Z, Guo Y, et al.. Insight into the Ebola virus nucleocapsid assembly mechanism: crystal structure of Ebola virus nucleoprotein core domain at 1.8 A resolution. Protein cell 2015; 6:351-62; PMID:25910597; http://dx.doi.org/ 10.1007/s13238-015-0163-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gerlach P, Malet H, Cusack S, Reguera J. Structural Insights into Bunyavirus Replication and Its Regulation by the vRNA Promoter. Cell 2015; 161:1267-79; PMID:26004069; http://dx.doi.org/ 10.1016/j.cell.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu X, McDonald SM, Tortorici MA, Tao YJ, Vasquez-Del Carpio R, Nibert ML, Patton JT, Harrison SC. Mechanism for coordinated RNA packaging and genome replication by rotavirus polymerase VP1. Structure 2008; 16:1678-88; PMID:19000820; http://dx.doi.org/ 10.1016/j.str.2008.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tao Y, Farsetta DL, Nibert ML, Harrison SC. RNA synthesis in a cage–structural studies of reovirus polymerase lambda3. Cell 2002; 111:733-45; PMID:12464184; http://dx.doi.org/ 10.1016/S0092-8674(02)01110-8 [DOI] [PubMed] [Google Scholar]
- 86.Pan J, Vakharia VN, Tao YJ. The structure of a birnavirus polymerase reveals a distinct active site topology. Proc Natl Acad Sci U S A 2007; 104:7385-90; PMID:17456597; http://dx.doi.org/ 10.1073/pnas.0611599104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bressanelli S, Tomei L, Rey FA, De Francesco R. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J Virol 2002; 76:3482-92; PMID:11884572; http://dx.doi.org/ 10.1128/JVI.76.7.3482-3492.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ferrer-Orta C, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J Biol Chem 2004; 279:47212-21; PMID:15294895; http://dx.doi.org/ 10.1074/jbc.M405465200 [DOI] [PubMed] [Google Scholar]
- 89.te Velthuis AJ. Common and unique features of viral RNA-dependent polymerases. Cell Mol Life Sci 2014; 71:4403-20; PMID:25080879; http://dx.doi.org/ 10.1007/s00018-014-1695-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 1997; 25:3389-402; PMID:9254694; http://dx.doi.org/ 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouvet M, Ferron F, Imbert I, Gluais L, Selisko B, Coutard B, Canard B, Decroly E. [Capping strategies in RNA viruses]. Med Sci 2012; 28:423-9; PMID:22549871; http://dx.doi.org/ 10.1051/medsci/2012284021 [DOI] [PubMed] [Google Scholar]
- 92.Smietanski M, Werner M, Purta E, Kaminska KH, Stepinski J, Darzynkiewicz E, Nowotny M, Bujnicki JM. Structural analysis of human 2′-O-ribose methyltransferases involved in mRNA cap structure formation. Nat Commun 2014; 5:3004; PMID:24402442; http://dx.doi.org/ 10.1038/ncomms4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takada A, Fujioka K, Tsuiji M, Morikawa A, Higashi N, Ebihara H, Kobasa D, Feldmann H, Irimura T, Kawaoka Y. Human macrophage C-type lectin specific for galactose and N-acetylgalactosamine promotes filovirus entry. J Virol 2004; 78:2943-7; PMID:14990712; http://dx.doi.org/ 10.1128/JVI.78.6.2943-2947.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yaddanapudi K, Palacios G, Towner JS, Chen I, Sariol CA, Nichol ST, Lipkin WI. Implication of a retrovirus-like glycoprotein peptide in the immunopathogenesis of Ebola and Marburg viruses. FASEB J 2006; 20:2519-30; PMID:17023517; http://dx.doi.org/ 10.1096/fj.06-6151com [DOI] [PubMed] [Google Scholar]
- 95.Kash JC, Muhlberger E, Carter V, Grosch M, Perwitasari O, Proll SC, Thomas MJ, Weber F, Klenk HD, Katze MG. Global suppression of the host antiviral response by Ebola- and Marburgviruses: increased antagonism of the type I interferon response is associated with enhanced virulence. J Virol 2006; 80:3009-20; PMID:16501110; http://dx.doi.org/ 10.1128/JVI.80.6.3009-3020.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cardenas WB, Loo YM, Gale M Jr., Hartman AL, Kimberlin CR, Martinez-Sobrido L, Saphire EO, Basler CF. Ebola virus VP35 protein binds double-stranded RNA and inhibits α/β interferon production induced by RIG-I signaling. J Virol 2006; 80:5168-78; PMID:16698997; http://dx.doi.org/ 10.1128/JVI.02199-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Volchkov VE, Chepurnov AA, Volchkova VA, Ternovoj VA, Klenk HD. Molecular characterization of guinea pig-adapted variants of Ebola virus. Virol 2000; 277:147-55; PMID:11062045; http://dx.doi.org/ 10.1006/viro.2000.0572 [DOI] [PubMed] [Google Scholar]
- 98.Ebihara H, Takada A, Kobasa D, Jones S, Neumann G, Theriault S, Bray M, Feldmann H, Kawaoka Y. Molecular determinants of Ebola virus virulence in mice. PLoS Pathog 2006; 2:e73; http://dx.doi.org/ 10.1371/journal.ppat.0020073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, et al.. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci U S A 2011; 108:8426-31; PMID:21536871; http://dx.doi.org/ 10.1073/pnas.1019030108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Alvarez CP, Lasala F, Carrillo J, Muniz O, Corbi AL, Delgado R. C-type lectins DC-SIGN and L-SIGN mediate cellular entry by Ebola virus in cis and in trans. J Virol 2002; 76:6841-4; PMID:12050398; http://dx.doi.org/ 10.1128/JVI.76.13.6841-6844.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, et al.. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virol 2003; 305:115-23; PMID:12504546; http://dx.doi.org/ 10.1006/viro.2002.1730 [DOI] [PubMed] [Google Scholar]
- 102.Chan SY, Empig CJ, Welte FJ, Speck RF, Schmaljohn A, Kreisberg JF, Goldsmith MA. Folate receptor-α is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 2001; 106:117-26; PMID:11461707; http://dx.doi.org/ 10.1016/S0092-8674(01)00418-4 [DOI] [PubMed] [Google Scholar]
- 103.Chandran K, Sullivan NJ, Felbor U, Whelan SP, Cunningham JM. Endosomal proteolysis of the Ebola virus glycoprotein is necessary for infection. Science 2005; 308:1643-5; PMID:15831716; http://dx.doi.org/ 10.1126/science.1110656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sullivan NJ, Peterson M, Yang ZY, Kong WP, Duckers H, Nabel E, Nabel GJ. Ebola virus glycoprotein toxicity is mediated by a dynamin-dependent protein-trafficking pathway. J Virol 2005; 79:547-53; PMID:15596847; http://dx.doi.org/ 10.1128/JVI.79.1.547-553.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Halfmann P, Neumann G, Kawaoka Y. The Ebolavirus VP24 protein blocks phosphorylation of p38 mitogen-activated protein kinase. J Infect Dis 2011; 204 Suppl 3:S953-6; PMID:21987775; http://dx.doi.org/ 10.1093/infdis/jir325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fabozzi G, Nabel CS, Dolan MA, Sullivan NJ. Ebolavirus proteins suppress the effects of small interfering RNA by direct interaction with the mammalian RNA interference pathway. J Virol 2011; 85:2512-23; PMID:21228243; http://dx.doi.org/ 10.1128/JVI.01160-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shabman RS, Leung DW, Johnson J, Glennon N, Gulcicek EE, Stone KL, Leung L, Hensley L, Amarasinghe GK, Basler CF. DRBP76 associates with Ebola virus VP35 and suppresses viral polymerase function. J Infect Dis 2011; 204 Suppl 3:S911-8; PMID:21987769; http://dx.doi.org/ 10.1093/infdis/jir343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prins KC, Cardenas WB, Basler CF. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol 2009; 83:3069-77; PMID:19153231; http://dx.doi.org/ 10.1128/JVI.01875-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Luthra P, Ramanan P, Mire CE, Weisend C, Tsuda Y, Yen B, Liu G, Leung DW, Geisbert TW, Ebihara H, et al.. Mutual antagonism between the Ebola virus VP35 protein and the RIG-I activator PACT determines infection outcome. Cell Host Microbe 2013; 14:74-84; PMID:23870315; http://dx.doi.org/ 10.1016/j.chom.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feng Z, Cerveny M, Yan Z, He B. The VP35 protein of Ebola virus inhibits the antiviral effect mediated by double-stranded RNA-dependent protein kinase PKR. J Virol 2007; 81:182-92; PMID:17065211; http://dx.doi.org/ 10.1128/JVI.01006-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chang TH, Kubota T, Matsuoka M, Jones S, Bradfute SB, Bray M, Ozato K. Ebola Zaire virus blocks type I interferon production by exploiting the host SUMO modification machinery. PLoS Pathog 2009; 5:e1000493; PMID:19557165; http://dx.doi.org/ 10.1371/journal.ppat.1000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kubota T, Matsuoka M, Chang TH, Bray M, Jones S, Tashiro M, Kato A, Ozato K. Ebolavirus VP35 interacts with the cytoplasmic dynein light chain 8. J Virol 2009; 83:6952-6; PMID:19403681; http://dx.doi.org/ 10.1128/JVI.00480-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yamayoshi S, Noda T, Ebihara H, Goto H, Morikawa Y, Lukashevich IS, Neumann G, Feldmann H, Kawaoka Y. Ebola virus matrix protein VP40 uses the COPII transport system for its intracellular transport. Cell Host Microbe 2008; 3:168-77; PMID:18329616; http://dx.doi.org/ 10.1016/j.chom.2008.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garcia M, Cooper A, Shi W, Bornmann W, Carrion R, Kalman D, Nabel GJ. Productive replication of Ebola virus is regulated by the c-Abl1 tyrosine kinase. Sci Transl Med 2012; 4:123ra124; http://dx.doi.org/ 10.1126/scitranslmed.3003500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Harty RN, Brown ME, Wang G, Huibregtse J, Hayes FP. A PPxY motif within the VP40 protein of Ebola virus interacts physically and functionally with a ubiquitin ligase: implications for filovirus budding. Proc Natl Acad Sci U S A 2000; 97:13871-6; PMID:11095724; http://dx.doi.org/ 10.1073/pnas.250277297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ruthel G, Demmin GL, Kallstrom G, Javid MP, Badie SS, Will AB, Nelle T, Schokman R, Nguyen TL, Carra JH, et al.. Association of ebola virus matrix protein VP40 with microtubules. J Virol 2005; 79:4709-19; PMID:15795257; http://dx.doi.org/ 10.1128/JVI.79.8.4709-4719.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Han Z, Harty RN. Packaging of actin into Ebola virus VLPs. Virol J 2005; 2:92; PMID:16367999; http://dx.doi.org/ 10.1186/1743-422X-2-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu J, Qu Y, Liu Y, Jambusaria R, Han Z, Ruthel G, Freedman BD, Harty RN. Host IQGAP1 and Ebola virus VP40 interactions facilitate virus-like particle egress. J Virol 2013; 87:7777-80; PMID:23637409; http://dx.doi.org/ 10.1128/JVI.00470-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeffers SA, Sanders DA, Sanchez A. Covalent modifications of the ebola virus glycoprotein. J Virol 2002; 76:12463-72; PMID:12438572; http://dx.doi.org/ 10.1128/JVI.76.24.12463-12472.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manicassamy B, Wang J, Jiang H, Rong L. Comprehensive analysis of ebola virus GP1 in viral entry. J Virol 2005; 79:4793-805; PMID:15795265; http://dx.doi.org/ 10.1128/JVI.79.8.4793-4805.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haasnoot J, de Vries W, Geutjes EJ, Prins M, de Haan P, Berkhout B. The Ebola virus VP35 protein is a suppressor of RNA silencing. PLoS Pathog 2007; 3:e86; PMID:17590081; http://dx.doi.org/ 10.1371/journal.ppat.0030086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schumann M, Gantke T, Muhlberger E. Ebola virus VP35 antagonizes PKR activity through its C-terminal interferon inhibitory domain. J Virol 2009; 83:8993-7; PMID:19515768; http://dx.doi.org/ 10.1128/JVI.00523-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McCarthy SE, Johnson RF, Zhang YA, Sunyer JO, Harty RN. Role for amino acids 212KLR214 of Ebola virus VP40 in assembly and budding. J Virol 2007; 81:11452-60; PMID:17699576; http://dx.doi.org/ 10.1128/JVI.00853-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamayoshi S, Kawaoka Y. Mapping of a region of Ebola virus VP40 that is important in the production of virus-like particles. J Infect Dis 2007; 196 Suppl 2:S291-5; PMID:17940963; http://dx.doi.org/ 10.1086/520595 [DOI] [PubMed] [Google Scholar]
- 125.Adu-Gyamfi E, Soni SP, Jee CS, Digman MA, Gratton E, Stahelin RV. A loop region in the N-terminal domain of Ebola virus VP40 is important in viral assembly, budding, and egress. Viruses 2014; 6:3837-54; PMID:25330123; http://dx.doi.org/ 10.3390/v6103837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Adu-Gyamfi E, Soni SP, Xue Y, Digman MA, Gratton E, Stahelin RV. The Ebola virus matrix protein penetrates into the plasma membrane: a key step in viral protein 40 (VP40) oligomerization and viral egress. J Biol Chem 2013; 288:5779-89; PMID:23297401; http://dx.doi.org/ 10.1074/jbc.M112.443960 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.