Abstract
Although most animal cells contain centrosomes, consisting of a pair of centrioles, their precise contribution to cell division and embryonic development is unclear. Genetic ablation of STIL, an essential component of the centriole replication machinery in mammalian cells, causes embryonic lethality in mice around mid gestation associated with defective Hedgehog signaling. Here, we describe, by focused ion beam scanning electron microscopy, that STIL−/− mouse embryos do not contain centrioles or primary cilia, suggesting that these organelles are not essential for mammalian development until mid gestation. We further show that the lack of primary cilia explains the absence of Hedgehog signaling in STIL−/− cells. Exogenous re-expression of STIL or STIL microcephaly mutants compatible with human survival, induced non-templated, de novo generation of centrioles in STIL−/− cells. Thus, while the abscence of centrioles is compatible with mammalian gastrulation, lack of centrioles and primary cilia impairs Hedgehog signaling and further embryonic development.
Keywords: centriole, centrosome, electron microscopy, embryo, microcephaly, STIL
Abbreviations
- CDK6
cyclin-dependent kinase 6
- CEP
centrosomal protein
- COILEDX
coiled-coil domain deletion
- E
embryonic day
- FIB/SEM
focused ion beam scanning electron microscopy
- MCPH
autosomal recessive primary microcephaly
- MEFs
mouse embryonic fibroblasts
- MTOC
microtubule organizing center
- nm
nanometer
- PLK4
polo kinase 4
- SHH
sonic hedgehog
- siRNA
small interfering RNA
- STAN
STIL/ANA2
- STANX
STAN domain deletion
- STIL
SCL/TAL1 interrupting locus
Introduction
Centrioles are key structural elements of centrosomes and primary cilia. Centriole duplication is a tightly ordered event that involves the growth of a procentriole orthogonal to a preexisting centriole.1,2 A core of only a few evolutionary conserved proteins including SPD2/CEP192, ZYG1/SAK/PLK4, SAS4/CPAP, SAS6, and SAS5/ANA2/STIL have thus far been identified to be required for centriole duplication in worms, flies, and humans.3–23 siRNA and immunoelectron microscopy approaches have revealed that templated centriole assembly in human cells is triggered by the activation of PLK4 and the recruitment of CEP152 to the surface of the parental centriole.23–26 STIL and SAS6 are then recruited to the base of the nascent procentriole and, together with CEP135 form the centriole cartwheel whereas CPAP subsequently promotes the assembly of the outer 9 microtubule triplets.19–22,27–31
Although centrosomes are the major microtubule organizing centers (MTOC) during animal cell division, they are not essential for mitotic spindle assembly. Some animal cells normally organize their spindles without canonical centrosomes, cultured cells can form bipolar spindles after centrosome removal by laser ablation or microsurgery and DSAS4-mutant flies, which start to lose centrioles during embryonic development nevertheless develop into morphologically normal adults.11,32,33 On the other hand, centrosomes seem to be essential for early Drosophila embryogenesis because both DSAS4- and SAK/PLK4-mutant syncytial embryos fail to develop into larvae.34,35
Primary microcephaly (MIM 251200) is an autosomal recessive neurodevelopmental defect of prenatal brain growth and results in a cerebral cortex which is reduced in size whereas the overall organization of the brain is mostly unaffected. Up to date, homozygous mutations in 10 genes including microcephalin, CDK5RAP2/CEP215, ASPM, CENPJ/CPAP, STIL, CEP135, CEP152, CEP63, WDR62, and CDK6 have been identified in indiviuals with primary microcephaly.36–45 Most of the proteins encoded by these genes localize to centrosomes or mitotic spindles. It has thus been proposed that primary microcephaly is caused by a reduced number of neurons owing to defective mitoses of fetal neuronal precursor cells.46
Mouse oocytes and early embryos lack centrioles until the blastocyst stage.47–49 Thereafter, centrioles seem to be generated de novo and are believed to be essential for further mammalian development.49,50 STIL−/− mouse embryos die at mid gestation with marked growth retardation, defects in the developing neural fold and randomization of left-right asymmetry associated with an impaired response to Sonic Hedgehog (SHH) signaling.51,52 As judged by immunofluorescence microscopy both mouse embryonic fibroblasts (MEFs) derived from STIL−/− embryos and U2OS or HeLa cells transfected with STIL-specific siRNA lack identifiable centriolar structures as well as primary cilia derived thereof and required for SHH signaling.19–21,53
As it is unclear whether mammalian development can proceed beyond the blastocyst stage in the absence of centrioles, we here employed focused ion beam scanning electron microscopy (FIB/SEM) and subsequent 3-dimensional reconstruction of serial images from STIL−/− mouse embryos to demonstrate that mouse embryonic development occurs until mid gestation without centrioles. Consequential to a lack of centrioles, we show that STIL−/− mouse embryos are also devoid of primary cilia and SHH signaling, findings that might explain the phenotype of STIL−/− mouse embryos. We further show that STIL microcephaly mutants compatible with successful completion of human embryogenesis are the only structural mutants of STIL that partially rescue the centriolar and primary cilia phenotype of STIL−/− MEFs. Our data reveal that STIL is one key component of non-templated, de novo centriole biogenesis in mammalian cells and suggest that centrioles are essential for successful completion of mammalian embryogenesis.
Results
STIL−/− mouse embryos lack centrioles and primary cilia
Recently, we have shown that MEFs derived from STIL−/− mouse embryos lack both centrioles and primary cilia, as determined by immunofluorescence microscopy.20,53 To unequivocally demonstrate that centrioles are not essential for early mammalian embryonic development we analyzed both wildtype and STIL−/− mouse embryos (Fig. S1) using focused ion beam scanning electron microscopy (FIB/SEM).54 This methodology automatically generates large sequential series of images that can be 3-dimensionally reconstructed, by combining a scanning electron microscope with a focused ion beam to sequentially mill away the sample surface, and a backscattered electron detector to image the milled surfaces. 621 and 1787 serial images from embryonic day (E) 8.5 and E9.5 STIL−/− embryos were examined. 1220 and 983 serial images from E8.5 and E9.5 wildtype embryos served as controls. Centrioles as well as primary cilia were readily identifiable in single sections, or through their visualization in 3-dimensional reconstructions of wildtype embryos (sections of 20 nm thickness, spanning a total of 24.4 and 19.7 μm from E8.5 and E9.5 wildtype embryos, respectively) (Fig. 1A, Movie S1 and S2). While each of the completely pictured cells and about half of the partially pictured cells from E8.5 and E9.5 wildtype embryos harbored both centrioles and primary cilia, none of the cells examined from E8.5 and E9.5 STIL−/− embryos contained these organelles (Movie S3, Table 1, P <0.001, χ2 test). Thus, STIL−/− mouse embryos develop until mid gestation in the absence of centrioles. Consistent with the presence of acentriolar MTOCs, immunofluorescence microscopy of histologic sections from E9.5 STIL−/− mouse embryos revealed cytoplasmic foci enriched in the pericentriolar material component pericentrin (Fig. 1B). However, in accordance with our FIB/SEM results, these acentriolar MTOCs were unable to support the outgrowth of primary cilia in E9.5 STIL−/− mouse embryos as demonstrated by immunostaining to acetylated tubulin (Fig. 1B).
Figure 1.
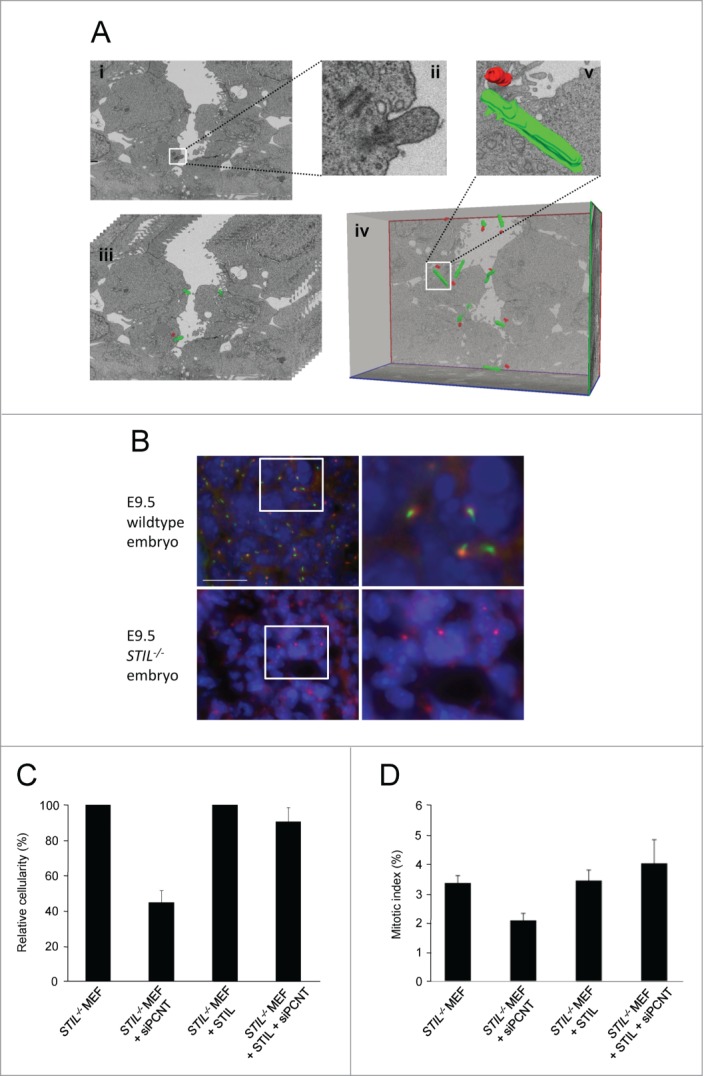
STIL−/− mouse embryos lack centrioles and primary cilia. (A) Focused ion beam scanning electron microscopy of an E8.5 wildtype mouse embryo. (i) Single image captured by FIB/SEM showing centrioles and a primary cilium (magnification 5700 x, scale bar 1 μm) which are depicted enlarged in (ii). (iii) Stack of serial sections with centrioles and cilia manually segmented and centrioles marked in red and cilia marked in green. Only 10 sections are shown out of the 361 serial images that compose the complete stack. (iv) Three-dimensional reconstruction of the image stack shown in (iii) after manual segmentation of centrioles and primary cilia in each section of the stack. As a representative example a 3-dimensionally reconstructed centriole/cilium pair is shown enlarged in (v). (B) Wildtype and STIL−/− E9.5 mouse embryos stained for acetylated tubulin (green), pericentrin (red) and DNA (DAPI, blue). Scale bar, 23 μm. Pictures on the right represent fold6- magnifications of the framed regions in the pictures on the left. (C) Proliferation of STIL−/− MEFs and STIL−/− MEFs reconstituted with Flag-STIL, 96 hrs after transfection with pericentrin-specific siRNA. Cellularity has been calculated as the ratio of living cells after transfection with pericentrin-specific siRNA versus a non-specific control siRNA. Error bars represent mean ± SE from 3 independent experiments. (D) Mitotic index of STIL−/− MEFs and STIL−/− MEFs reconstituted with Flag-STIL, 72 hrs after transfection with pericentrin-specific siRNA. The frequency of mitotic cells has been determined by counting at least 300 cells after transfection with pericentrin-specific siRNA vs. a non-specific control siRNA. Error bars represent mean ± SE from 3 independent experiments.
Table 1.
Centrioles and primary cilia found in mouse embryos
| Centrioles | Cilia | Total cells scored | |
|---|---|---|---|
| Wildtype | |||
| E8.0–8.5 (1220*) | |||
| Complete cells | 6 | 6 | 6 |
| Partial cells | 8 | 7 | 17 |
| E9.0–9.5 (983*) | |||
| Complete cells | 2 | 2 | 2 |
| Partial cells | 4 | 4 | 11 |
| Total (2203*) | 20 | 19 | 36† |
| STIL−/− | |||
| E8.0–8.5 (621*) | |||
| Complete cells | 0 | 0 | 1 |
| Partial cells | 0 | 0 | 9 |
| E9.0–9.5 (1787*) | |||
| Complete cells | 0 | 0 | 10 |
| Partial cells | 0 | 0 | 15 |
| Total (2408*) | 0 | 0 | 35† |
E, embryonic day; MEFs, mouse embryonic fibroblasts.
Number of images analyzed.
P < 0 .001 for both centrioles and primary cilia (2 test).
Recruitment of pericentriolar material to acentrosomal MTOCs is required for proliferation of STIL−/− MEFs
To examine if the recruitment of pericentriolar material to acentrosomal MTOCs is required for proliferation of STIL−/− MEFs, we next analyzed whether siRNA-mediated depletion of pericentrin reduces the proliferation capacity of STIL−/− MEFs with and without Flag-STIL reconstitution. siRNA-mediated reduction of pericentrin expression to about 50% of mRNA wildtype levels (Fig. S2A) led to reduced proliferation of STIL−/− MEFs (45 ± 12% relative to control siRNA, p=0.015) (Fig. 1C) but not of Flag-STIL reconstituted STIL−/− MEFs (90 ± 14% relative to control siRNA, p=0.809). Similarly, in an independent experiment, the mitotic index of STIL−/− MEFs (p=0.021) but not Flag-STIL reconstituted STIL−/− MEFs (p=0.530) was reduced after siRNA-mediated pericentrin protein depletion from about 70% of mitotic spindle poles (Fig. 1D, Figs. S2B and C). Thus recruitment of pericentrin to acentrosomal MTOCs is required to compensate for the proliferation defect caused by the absence of centrioles.
Primary cilia mediate STIL-dependence of SHH signaling
Primary cilia are required for vertebrate SHH signaling.55 We have reported earlier that in STIL−/− mouse embryos SHH signaling is compromised.51,52 To determine whether SHH signaling depends on STIL itself or on STIL-dependent primary cilia formation, both STIL−/− MEFs and MEFs exogenously expressing Flag-STIL were grown in control or SHH-conditioned medium for 3 d, and subsequently harvested for quantitative real-time PCR analysis of GLI1 mRNA levels (Fig. 2A).56 C166 mouse yolk sac derived endothelial cells, known to be responsive to SHH were used as a control. While mRNA expression of the SHH target GLI1 was almost absent in STIL−/− MEFs, exogenous expression of Flag-STIL led to restored GLI1 expression in these cells in response to SHH. Constitutively active GLI2ΔN, which lacks the amino-terminal repressor domain of GLI2 does not require cilia for its activity.57,58 Therefore, we next determined the impact of GLI2ΔN on GLI1 mRNA expression in both STIL−/− MEFs and STIL−/− MEFs with restored STIL expression by lentiviral transduction. In contrast to the virtually complete lack of GLI1 mRNA expression after exposure to SHH-conditioned medium (Fig. 2A), GLI1 expression in STIL−/− MEFs was restored after transfection with GLI2ΔN (Fig. 2B). These findings support the notion that the requirement of STIL for SHH signaling, which may explain the specific developmental defects in STIL−/− embryos,51,52 is mediated by STIL-dependent formation of primary cilia.
Figure 2.
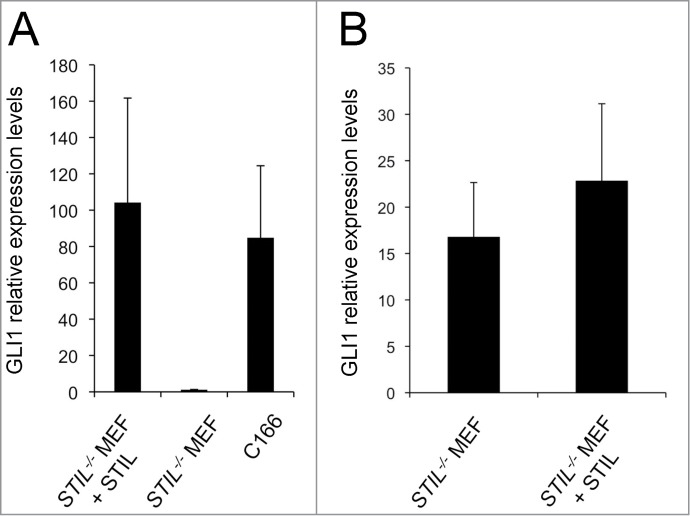
Primary cilia mediate STIL dependence of SHH signaling. (A) mRNA expression of GLI1 in STIL−/− MEFs and STIL−/− MEFs reconstituted with Flag-STIL 3 d after exposure to SHH-conditioned medium. C166 endothelial cells were used as a positive control. (B) mRNA expression of GLI1 in STIL−/− MEFs and STIL−/− MEFs reconstituted with Flag-STIL which have been transiently transfected with a vector expressing constitutively active GLI2 (GLI2ΔN); p=0.71. Error bars represent mean ± SE from 4 (A) and 3 (B) independent experiments. Differences in Y axis scaling in (A) and (B) are due to a lack of exposure of cells to SHH-conditioned medium in (B).
Exogenous STIL causes de novo formation of centrioles in STIL−/− MEFs
Overexpression of SAK/PLK4, DSAS6 and DSAS4 has been reported to induce non-templated generation of many hundreds of centriole-like structures in unfertilized Drosophila eggs.12,59 In contrast, only very little data exist regarding proteins causing de novo centriole formation in mammalian somatic cells. We have previously reported that re-introduction of STIL into STIL−/− MEFs results in the re-appearance of centrioles.53 FIB/SEM and 3-dimensional reconstruction of 1918 serial images revealed neither centrioles nor primary cilia in 14 cells from STIL−/− MEFs. 1024 serial images from STIL−/− MEFs with restored STIL expression by lentiviral transduction of Flag-STIL showed de novo appearance of multiple centrioles in one out of 6 partially pictured cells (Figs. 3A and B). To confirm the FIB/SEM result, we next co-immunostained Flag-STIL-reconstituted STIL−/− MEFs with antibodies to Flag, centrin, CP110 and □-tubulin, respectively. These stainings demonstrated that 31.4 ± 8.2% of Flag-STIL-reconstituted STIL−/− MEFs indeed contained multiple Flag-STIL-, centrin- and CP110-decorated centriolar structures capable of recruiting pericentriolar material as shown by accumulation of γ-tubulin (Fig. 3C). These centriolar structures assembled into regular centrosomes consisting of 2 centrioles each and tend to be clustered into bipolar spindle arrays during mitosis (Fig. 3D). We conclude that similar to SAK/PLK4, DSAS6 and DSAS4 in unfertilized Drosophila eggs, STIL is one key component of de novo centriole biogenesis in mammalian cells.
Figure 3.
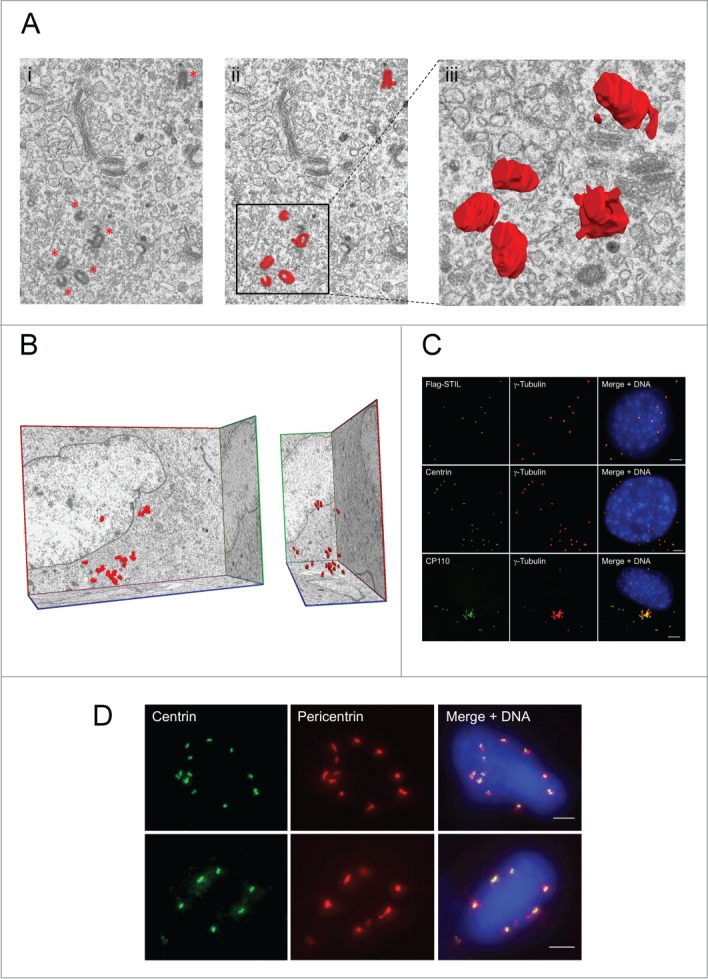
Exogenous STIL causes de novo formation of supernumerary centrioles in STIL−/− MEFs. (A) Single image captured by focused ion beam scanning electron microscopy (FIB/SEM) from STIL−/− MEFs reconstituted with Flag-STIL (i) before (centrioles marked with asterics) and (ii) after manual segmentation of centrioles (red, magnification 5700 x). A 3-dimensional reconstruction of an image stack consisting out of 16 single pictures showing a group of centrioles is depicted enlarged in (iii). (B) Front (left) and side view (right) of a 3-dimensional reconstruction of 301 serial sections from STIL−/− MEFs reconstituted with Flag-STIL after manual segmentation of centrioles in each section of the stack; Magnification 5700×. (C, D) STIL−/− MEFs reconstituted with Flag-STIL by lentiviral transduction were co-immunostained with antibodies to Flag (green) and γ-tubulin (red, upper panel), centrin (green) and γ-tubulin (red, middle panel), or CP110 (green) and γ-tubulin (red, lower panel) (C) as well as centrin (green) and pericentrin (red) (D) and analyzed by immunofluorescence microscopy. DNA was stained with DAPI (blue). Scale bars: 5 μm.
STIL microcephaly mutants can induce de novo centriole and primary cilia formation
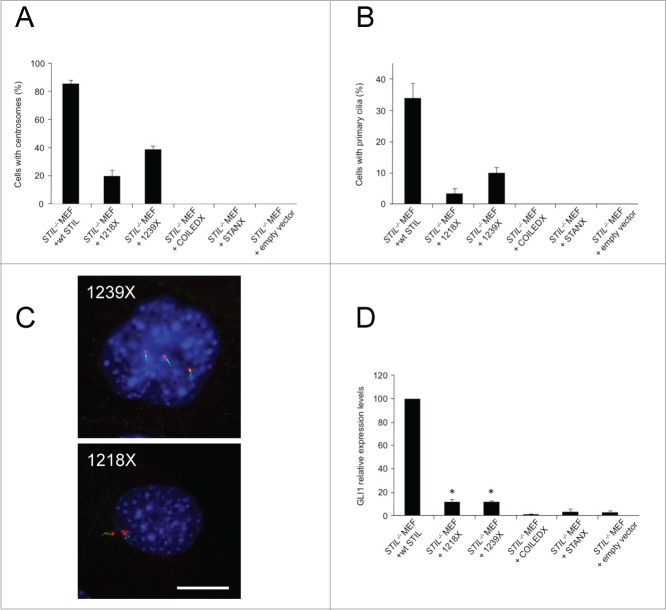
Unlike the embryonic lethality caused by complete elimination of STIL in vertebrate embryos, truncation of its carboxy terminus is compatible with life, as such mutations have been identified in humans with autosomal recessive primary microcephaly (MCPH7, MIM 612703).36 We therefore next asked whether STIL microcephaly mutants are capable of mediating centriole and primary cilia den novo formation as well. For that, 4 different STIL mutant versions were expressed in STIL−/− MEFs by lentiviral transduction (Fig. S3): STIL microcephaly mutants with premature stop codons at amino acid positions 1218 (1218×) and 1239 (1239×) of human STIL (1287 aa)36 and larger STIL deletion mutants lacking the conserved STAN (STANX) or coiled-coiled domains (COILEDX).15 In contrast to 1218× and 1239×, STANX and COILEDX mutant versions of STIL have never been described in individuals with primary microcephaly and might, therefore, not support embryonic development and survival. The expression of all STIL mutant versions in STIL−/− MEFs was verified by Western blot analysis of transduced cells (Fig. S3B).
Using STIL−/− MEFs expressing different STIL mutant versions we then examined the effect of STIL construct expression on centriole and primary cilia formation as well as on the response to SHH. Expression of the microcephaly mutants 1218× and 1239× but not the larger deletion mutants STANX and COILEDX partially rescued centriole and primary cilia formation. Expression of wildtype human STIL led to de novo formation of centrosomes in 86.3 ± 2.4% of the cells while expression of the microcephaly mutants 1218× and 1239× lead to centrosome formation in 20.2 ± 4.4% and 39.5 ± 2.2% of STIL−/− MEFs, respectively (Fig. 4A). Similarly, expression of STIL microcephaly mutants led to de novo formation of primary cilia, albeit with reduced frequency compared to wildtype STIL (Figs. 4B and C). Similar to the occurrence of multiple centrioles in wildtype Flag-STIL-reconstituted STIL−/− MEFs, expression of the microcephaly mutants 1218× and 1239× induced the formation of supernumerary primary cilia in 50% and 48.8% of STIL−/− MEFs with cilia de novo formation (Fig. 4C). Accordingly, the STIL microcephaly mutants but not the larger deletion mutants STANX and COILEDX partially rescued SHH signaling, although to a significantly lower degree than wildtype STIL, consistent with the reduced number of ciliated cells (Fig. 4D). Thus, STIL mutants that are compatible with human development retain the ability to support de novo formation of centrioles and primary cilia and consequently support SHH signaling, albeit with clear quantitative differences compared to wildtype STIL.
Figure 4.
Microcephaly mutant versions of STIL partially rescue de novo centrosome formation, primary cilia formation and SHH signaling in STIL−/− MEFs. STIL mutant versions were exogenously expressed in STIL−/− MEFs and subsequently analyzed for centrosome and primary cilium formation as well as SHH signaling as a readout for cilia function. STIL−/− MEFs reconstituted with wildtype Flag-STIL (wt STIL), Flag-STIL-1218× (1218×), Flag-STIL-1239× (1239×), Flag-STIL-COILEDX (COILEDX) and Flag-STIL-STANX (STANX) by lentiviral transduction were immunostained with antibodies to γ-tubulin and poly-glutamylated tubulin. Subsequently, cells containing centrosomes (A) and primary cilia (B) were counted. Error bars represent mean ± SE from 3 independent experiments. (C) Representative examples of STIL−/− MEFs reconstituted with Flag-STIL-1239× (1239×; upper panel) or Flag-STIL-1218× (1218×; lower panel) by lentiviral transduction, co-immunostained with antibodies to γ-tubulin (red) and poly-glutamylated tubulin (green). DNA was stained with DAPI (blue). Scale bar: 14 μm. (D) mRNA expression levels of GLI1 in STIL−/− MEFs reconstituted with Flag-STIL-1218× (1218×), Flag-STIL-1239× (1239×), Flag-STIL-COILEDX (COILEDX) and Flag-STIL-STANX (STANX) by lentiviral transduction 3 d after exposure to SHH-conditioned medium, normalized to Gli1 levels in STIL−/− MEFs reconstituted with wildtype Flag-STIL (wt STIL). *, Gli1 levels in Flag-STIL-1218× (1218×) and Flag-STIL-1239× (1239×) reconstituted STIL−/− MEFs are statistically significantly different from levels of Gli1 in STIL−/− MEFs reconstituted with empty vector. Error bars represent mean ± SE from 3 independent experiments.
Discussion
Using STIL−/− mouse embryos as a model system, we demonstrate here that neither centrioles nor primary cilia are required for early mouse embryo development until mid gestation. Subsequently, STIL−/− mouse embryos die with multiple abnormalities including growth retardation, midline as well as left-right axial defects, and impaired SHH signaling.51
With regard to growth retardation, we have shown earlier that MEFs derived from STIL−/− mouse embryos are characterized by a low proliferation rate and a low mitotic index.53 Although acentrosomal vertebrate cells are capable of forming functional amphiastral mitotic spindles, the fidelity of bipolar spindle formation is compromised.11,32,33,60 Failure of a fraction of acentrosomal cells to form a bipolar spindle has been speculated to be caused by insufficient recruitment of pericentriolar material components including pericentrin to MTOCs.60 These results are corroborated by our finding that recruitment of pericentrin to acentrosomal MTOCs is required to allow for proliferation of STIL−/− MEFs lacking centrioles.
Secreted Hedgehog proteins bind to and inactivate the transmembrane protein Patched1, thereby allowing activation of signaling which plays an important role in embryonic development.55 STIL−/− Patched−/− mouse embryos do not activate SHH signaling and display a phenotype that is indistinguishable from STIL−/− mouse embryos.51 These observations suggest that STIL is required for the response to SHH signaling downstream to its receptor. Our results now suggest that the requirement of STIL for SHH signaling strictly depends on the formation of primary cilia, as SHH-conditioned medium is unable to induce GLI1 mRNA expression in STIL−/− MEFs whereas expression of constitutively active GLI2ΔN downstream of primary cilia rescues this defect.
OFD1, the gene underlying oro-facial-digital type I syndrome, is a component of the distal centriole that controls centriole length and has been implicated in SHH signaling as well.61,62 Absence of OFD1 leads to abnormally long centrioles but prevents the formation of primary cilia in OFD1−/− mouse embryos, which die at mid gestation with failure of left-right axis specification and impaired patterning of the neural tube, reminiscent of STIL−/− mouse embryos.51,52 From these data and our results presented here it might be speculated that centrioles are dispensable for early mouse development but primary cilia and SHH signaling may be required for morphogenesis during gastrulation.
Given that genetic ablation of STIL results in mid-gestational embryonic lethality of both mice51 and fish,63 it was unexpected to discover humans with germline mutations in STIL.36 Most recently, however, it has been shown that truncating microcephaly mutations render STIL resistant to proteasomal degradation and cause centriole amplification instead of the expected loss-of-function phenotype.64 In line with these findings, we show here that microcephaly mutant versions of STIL retain the capacity to support centriole biogenesis and, consequently, primary cilia formation which can transmit Hedgehog signals in STIL−/− MEFs as well. Moreover, similar to wildtype STIL they even support de novo formation of multiple centrosomes and primary cilia in these cells. While it is unclear how these mutations cause microcephaly, it is likely that the presence of centrioles and primary cilia ensures completion of embryogenesis and survival. This view is also supported by the fact that while ablation of STIL results in embryonic lethality,51 knockout of microcephalin or CDK6, 2 other genes mutated in primary microcephaly but not required for centriole replication, leads to viable offspring in mice.65,66 Also, ablation of pericentrin, a major pericentriolar material constituent, of which homozygous loss of function mutations cause microcephalic osteodysplastic primordial dwarfism type II, a disorder related to primary microcephaly, seems to be compatible with life.67,68
Besides assembling through the canonical templated pathway, in some circumstances centrioles can also form de novo.12,13 De novo centriole formation occurs during ciliogenesis and also as a normal part of the development of some organisms including the early mouse embryo. In contrast to templated centriole replication, the number of centrioles assembled is not precisely regulated through the de novo pathway. Whereas no data exist regarding proteins required for de novo centriole formation in mammalian cells, overexpression of SAK/PLK4, DSAS6 and DSAS4 has been reported to induce non-templated generation of many hundreds of centriole-like structures in unfertilized Drosophila eggs, which normally do not contain any centrioles.12,13 Our results presented here demonstrate that STIL is one key component of de novo centriole biogenesis in mammalian cells. As microcephaly mutants of STIL but not larger C-terminal STIL deletion mutants allow for centriole and primary cilia de novo formation as well, primary human microcephaly seems not to be due to a complete lack of centrioles or primary cilia.
While this paper was under review, very similar data showing that SAS4−/− mouse embryos lack centrioles and primary cilia, cannot respond to SHH signals but survive until midgestation were published online.72
Materials and Methods
Mouse strain
STIL−/− mice have been described.51
Immunofluorescence microscopy of mouse embryo sections and MEFs
E8.5–9.5 mouse embryos were dissected and fixed in 4% paraformaldehyde in PBS, mounted in 2% agarose in PBS to facilitate positioning, embedded in TissueTec optimum cutting temperature (OCT) compound 4583 (Sakura), frozen, and sectioned. Frozen 20 μm sections on slides were dried for 30 min at room temperature, rehydrated in PBS for 5 min, incubated with blocking solution (5% normal goat serum and 0.5% Triton X-100 in PBS) for 1 h, and then incubated with primary antibodies for 4 h at room temperature (anti-pericentrin; Abcam and anti-acetylated-tubulin; Sigma). Slides were washed 3 times with PBS for 10 min and incubated with the secondary antibodies (Alexa Flour 488 and 568; Invitrogen) for 2 h at room temperature. The coverslips were mounted using ProLong Gold antifade reagent with DAPI (Invitrogen). Images were acquired using an Olympus IX81 fluorescence microscope. STIL−/− embryos were distinguished from wildtype embryos by their profound morphological differences as described in detail earlier.51 For verification, yolk sacs were collected during embryo dissection for genotyping. For immunostaining of STIL−/− MEFs a rabbit polyclonal antibody against g-tubulin (Sigma) and STIL (Bethyl Laboratories Inc.) and mouse monoclonal antibodies to centrin-3 (Abnova) and Flag (Sigma) and poly-glutamylated tubulin (Enzo) were used. A mouse monoclonal anti-CP110 antibody was generously provided by Erich Nigg (Biozentrum, University of Basel, Switzerland).
Focused ion beam scanning electron microscopy (FIB/SEM)
Cells and embryos were fixed in Karnovsky fixative for 90 min (cells) or several days (embryos) at room temperature. After incubation with osmium tetroxide (1.25%) for 90 min and uranylacetate in 70% ethanol for 30 min, cells and tissues were embedded into araldite (Huntsman). After cutting, semithin sections (0.5 μm) were stained with methylene blue to judge suitability of the samples. Subsequently, surfaces of the araldite blocks were coated with a 20 nm layer of gold. Blocks were fixed on a sample stub. Focused ion beam milling and acquisition of serial scanning electron microscopy images started in and by a crossbeam workstation (Auriga, Zeiss, Oberkochen, Germany). A coarse cross-section was milled (ion beam current 10 nA) as a viewing channel for SEM. Layers were serially milled by scanning the ion beam (ion beam current 2 nA) parallel to the surface of the cutting plane. Layer thickness was 20 nm. Images were acquired by an ESB (energy selective backscattered) detector at 1.5 kV. Magnification of the images was 5700 x covering an area of 20.1 × 15 μm.
Image segmentation and 3-dimensional reconstruction of image stacks
Three-dimensional reconstruction of centrioles and cilia from microscopy data is based on a segmentation which was performed manually using the Medical Imaging Interaction Toolkit (MITK).69 The process of segmentation divides an image into multiple segments, in this case in centrioles and cilia. In a second step, a 3-dimensional isosurface is created from the segmentations using the marching cubes approach.70 For a representative visualization, the 3-dimensional surfaces were imaged as an overlay of the original microscope data.
Plasmids, infection, and transfection
Flag-tagged human STIL constructs were cloned into a lentiviral 2 promoter plasmid. MEFs were infected as previously described.53 Construction of Flag-tagged STIL-1218×, STIL-1239×, STIL-STANX and STIL-COILEDX mutants was done as previously described.20 Forty 8 hours after infection, cells were selected for puromycin resistance and a stably infected polyclonal population was generated. pCS2-MT-GLI2□N, missing the 328 N-terminal amino acids of GLI257,58 and plasmid 17649 (Addgene) were transfected into MEFs using jetPEI (Polyplus).
Cell culture
MEFs were grown in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal calf serum, penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C and 5% CO2.
RNA interference
Target sequences against mouse pericentrin A and B (5′-AAUGAGGUUG-UCCACAGGAGA-3′) were as previously described.71 Mission siRNA Universal Negative Control #1 (Sigma) was used as a control. siRNAs were introduced into cells using SiImporter siRNA transfection reagent (Millipore), in accordance with the manufacturer's instructions. 96 h after transfection, cells were harvested for cellularity determination by counting living cells under the microscope and for RT-PCR analysis of pericentrin levels.
Sonic hedgehog activation assay
SHH conditioned medium was produced as reported previously.56 Briefly, 293T cells were transiently transfected with a SHH-N encoding plasmid, kindly provided by Philip A. Beachy, or with a control pcDNA3.1 plasmid (Invitrogen). Conditioned medium was collected 3 d after transfection, centrifuged at 3000 × g for 10 min, transferred through a 0.45 □m filter to remove cell debris and then diluted in starvation medium to a final fetal calf serum concentration of 2%. MEFs were grown in control or SHH-conditioned medium for 3 d, and then harvested for quantitative real-time PCR analysis of GLI1 levels. Medium potency was checked using C166 endothelial cells, known to be responsive to SHH, as a control.
Primers used for quantitative real-time polymerase chain reaction
GLI1 forward primer: 5′-CAAGTGCACGTTTGAAGGCT-3′, GLI1 reverse primer: 5′-CAACCTTCTTGCTCACACATGTAAG-3′, pericentrin forward primer: 5′-CTTCCCGAGAGA CAGTCAGG-3′, pericentrin reverse primer: 5′-GCAAAAGCTCCTCACTCCAC-3′.
Western-Blot
For detection of human STIL expression, STIL was precipitated with Flag-M2-agarose conjugated beads (Sigma), followed by SDS-PAGE and immunoblotting with mouse monoclonal anti Flag-HRP antibody (Sigma).
Funding Statement
This research was supported by a joint DKFZ-MOST grant to S.I. and A.K., the Israel Science Foundation (S.I.), the Israeli Ministry of Science Eshkol program (A.D.), the Israel Cancer Research Foundation (SI), and the NCT-IFP program (A.K.).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Maximilian Muenke for providing a GLI2□N expression plasmid, Philip Beachy for a Shh-N encoding plasmid and Erich Nigg for an antibody to CP110. This research was done as partial fulfillment of the requirement for Ph.D. degrees of A.D. at the Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel and F.L. at the German Cancer Research Center (DKFZ), Heidelberg, Germany.
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website. http://dx.doi.org/10.4161/15384101.2014.946830
References
- 1. Azimzadeh J, Marshall WF. Building the centriole. Curr Biol 2010; 20:R816-25; PMID:20869612; http://dx.doi.org/ 10.1016/j.cub.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nigg EA, Stearns T. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 2011; 13:1154-60; PMID:21968988; http://dx.doi.org/ 10.1038/ncb2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dammermann A, Müller-Reichert T, Pelletier L, Habermann B, Desai A, Oegema K. Centriole assembly requires both centriolar and pericentriolar material proteins. Dev Cell 2004; 7:815-29; PMID:15572125; http://dx.doi.org/ 10.1016/j.devcel.2004.10.015 [DOI] [PubMed] [Google Scholar]
- 4. Delattre M, Leidel S, Wani K, Baumer K, Bamat J, Schnabel H, Feichtinger R, Schnabel R, Gönczy P. Centriolar SAS-5 is required for centrosome duplication in C. elegans. Nat Cell Biol 2004; 6:656-64; PMID:15232593; http://dx.doi.org/ 10.1038/ncb1146 [DOI] [PubMed] [Google Scholar]
- 5. Leidel S, Gönczy P. SAS-4 is essential for centrosome duplication in C elegans and is recruited to daughter centrioles once per cell cycle. Dev Cell 2003; 4:431-9; PMID:12636923; http://dx.doi.org/ 10.1016/S1534-5807(03)00062-5 [DOI] [PubMed] [Google Scholar]
- 6. Leidel S, Delattre M, Cerutti L, Baumer K, Gönczy P. SAS-6 defines a protein family required for centrosome duplication in C. elegans and in human cells. Nat Cell Biol 2005; 7:115-25; PMID:15665853; http://dx.doi.org/ 10.1038/ncb1220 [DOI] [PubMed] [Google Scholar]
- 7. Kemp CA, Kopish KR, Zipperlen P, Ahringer J, O’Connell KF. Centrosome maturation and duplication in C. elegans require the coiled-coil protein SPD-2. Dev Cell 2004; 6:511-23; PMID:15068791; http://dx.doi.org/ 10.1016/S1534-5807(04)00066-8 [DOI] [PubMed] [Google Scholar]
- 8. Kirkham M, Müller-Reichert T, Oegema K, Grill S, Hyman AA. SAS-4 is a C. elegans centriolar protein that controls centrosome size. Cell 2003; 112:575-87; PMID:12600319; http://dx.doi.org/ 10.1016/S0092-8674(03)00117-X [DOI] [PubMed] [Google Scholar]
- 9. O’Connell KF, Caron C, Kopish KR, Hurd DD, Kemphues KJ, Li Y, White JG. The C. elegans zyg-1 gene encodes a regulator of centrosome duplication with distinct maternal and paternal roles in the embryo. Cell 2001; 105:547-58; PMID:11371350; http://dx.doi.org/ 10.1016/S0092-8674(01)00338-5 [DOI] [PubMed] [Google Scholar]
- 10. Pelletier L, O’Toole E, Schwager A, Hyman AA, Müller-Reichert T. Centriole assembly in caenorhabditis elegans. Nature 2006; 444:619-23; PMID:17136092; http://dx.doi.org/ 10.1038/nature05318 [DOI] [PubMed] [Google Scholar]
- 11. Basto R, Lau J, Vinogradova T, Gardiol A, Woods CG, Khodjakov A, Raff JW. Flies without centrioles. Cell 2006; 125:1375-86; PMID:16814722; http://dx.doi.org/ 10.1016/j.cell.2006.05.025 [DOI] [PubMed] [Google Scholar]
- 12. Peel N, Stevens NR, Basto R, Raff JW. Overexpressing centriole-replication proteins in vivo induces centriole overduplication and de novo formation. Curr Biol 2007; 17:834-43; PMID:17475495; http://dx.doi.org/ 10.1016/j.cub.2007.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rodrigues-Martins A, Bettencourt-Dias M, Riparbelli M, Ferreira C, Ferreira I, Callaini G, Glover DM. DSAS-6 organizes a tube-like centriole precursor, and its absence suggests modularity in centriole assembly. Curr Biol 2007; 17:1465-72; PMID:17689959; http://dx.doi.org/ 10.1016/j.cub.2007.07.034 [DOI] [PubMed] [Google Scholar]
- 14. Bettencourt-Dias M, Rodrigues-Martins A, Carpenter L, Riparbelli M, Lehmann L, Gatt MK, Carmo N, Balloux F, Callaini G, Glover DM. SAK/PLK4 is required for centriole duplication and flagella development. Curr Biol 2005; 15:2199-207; PMID:16326102; http://dx.doi.org/ 10.1016/j.cub.2005.11.042 [DOI] [PubMed] [Google Scholar]
- 15. Stevens NR, Dobbelaere J, Brunk K, Franz A, Raff JW. Drosophila Ana2 is a conserved centriole duplication factor. J Cell Biol 2010; 188:313-23; PMID:20123993; http://dx.doi.org/ 10.1083/jcb.200910016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature 2003; 426:570-4; PMID:14654843; http://dx.doi.org/ 10.1038/nature02166 [DOI] [PubMed] [Google Scholar]
- 17. Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. The Polo kinase Plk4 functions in centriole duplication. Nat Cell Biol 2005; 7:1140-6; PMID:16244668; http://dx.doi.org/ 10.1038/ncb1320 [DOI] [PubMed] [Google Scholar]
- 18. Hung LY, Tang CJ, Tang TK. Protein 4.1 R-135 interacts with a novel centrosomal protein (CPAP) which is associated with the gamma-tubulin complex. Mol Cell Biol 2000; 20:7813-25; PMID:11003675; http://dx.doi.org/ 10.1128/MCB.20.20.7813-7825.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tang CJ, Lin SY, Hsu WB, Lin YN, Wu CT, Lin YC, Chang CW, Wu KS, Tang TK. The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO J 2011; 30:4790-804; PMID:22020124; http://dx.doi.org/ 10.1038/emboj.2011.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vulprecht J, David A, Tibelius A, Castiel A, Konotop G, Liu F, Bestvater F, Raab MS, Zentgraf H, Izraeli S, et al. STIL is required for centriole duplication in human cells. J Cell Sci 2012; 125:1353-62; PMID:22349705; http://dx.doi.org/ 10.1242/jcs.104109 [DOI] [PubMed] [Google Scholar]
- 21. Arquint C, Sonnen KF, Stierhof YD, Nigg EA. Cell-cycle-regulated expression of STIL controls centriole number in human cells. J Cell Sci 2012; 125:1342-52; PMID:22349698; http://dx.doi.org/ 10.1242/jcs.099887 [DOI] [PubMed] [Google Scholar]
- 22. Kitagawa D, Kohlmaier G, Keller D, Strnad P, Balestra FR, Flückiger I, Gönczy P. Spindle positioning in human cells relies on proper centriole formation and on the microcephaly proteins CPAP and STIL. J Cell Sci 2011; 124:3884-93; PMID:22100914; http://dx.doi.org/ 10.1242/jcs.089888 [DOI] [PubMed] [Google Scholar]
- 23. Kleylein-Sohn J, Westendorf J, Le Clech M, Habedanck R, Stierhof YD, Nigg EA. Plk4-induced centriole biogenesis in human cells. Dev Cell 2007; 13:190-202; PMID:17681131; http://dx.doi.org/ 10.1016/j.devcel.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 24. Cizmecioglu O, Arnold M, Bahtz R, Settele F, Ehret L, Haselmann-Weiss U, Antony C, Hoffmann I. Cep152 acts as a scaffold for recruitment of of Plk4 and CPAP to the centrosome. J. Cell Biol 2010; 191:731-39; PMID:21059844; http://dx.doi.org/ 10.1083/jcb.201007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dzhindzhev NS, Yu QD, Weiskopf K, Tzolovsky G, Cunha-Ferreira I, Riparbelli M, Rodrigues-Martins A, Bettencourt-Dias M, Callaini G, Glover DM. Asterless is a scaffold for the onset of centriole assembly. Nature 2010; 467:714-8; PMID:20852615; http://dx.doi.org/ 10.1038/nature09445 [DOI] [PubMed] [Google Scholar]
- 26. Hatch EM, Kulukian A, Holland AJ, Cleveland DW, Stearns T. Cep152 interacts with Plk4 and is required for centriole duplication. J Cell Biol 2010; 191:721-9; PMID:21059850; http://dx.doi.org/ 10.1083/jcb.201006049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strnad P, Leidel S, Vinogradova T, Euteneuer U, Khodjakov A, Gönczy P. Regulated HsSAS-6 levels ensure formation of a single procentriole per centriole during the centrosome duplication cycle. Dev Cell 2007; 13:203-13; PMID:17681132; http://dx.doi.org/ 10.1016/j.devcel.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin YC, Chang CW, Hsu WB, Tang CJ, Lin YN, Chou EJ, Wu CT, Tang TK. Human microcephaly protein CEP135 bind to hSAS-6 and CPAP, and is required for centriole assembly. EMBO J 2013; 32:1141-54; PMID:23511974; http://dx.doi.org/ 10.1038/emboj.2013.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohlmaier G, Loncarek J, Meng X, McEwen BF, Mogensen MM, Spektor A, Dynlacht BD, Khodjakov A, Gönczy P. Overly long centrioles and defective cell division upon excess of the SAS-4-related protein CPAP. Curr Biol 2009; 19:1012-8; PMID:19481460; http://dx.doi.org/ 10.1016/j.cub.2009.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmidt TI, Kleylein-Sohn J, Westendorf J, Le Clech M, Lavoie SB, Stierhof YD, Nigg EA. Control of centriole length by CPAP and CP110. Curr Biol 2009; 19:1005-11; PMID:19481458; http://dx.doi.org/ 10.1016/j.cub.2009.05.016 [DOI] [PubMed] [Google Scholar]
- 31. Tang CJ, Fu RH, Wu KS, Hsu WB, Tang TK. CPAP is a cell-cycle regulated protein that controls centriole length. Nat Cell Biol 2009; 11:825-31; PMID:19503075; http://dx.doi.org/ 10.1038/ncb1889 [DOI] [PubMed] [Google Scholar]
- 32. Khodjakov A, Cole RW, Oakley BR, Rieder CL. Centrosome-independent mitotic spindle formation in vertebrates. Curr Biol 2000; 10:59-67; PMID:10662665; http://dx.doi.org/ 10.1016/S0960-9822(99)00276-6 [DOI] [PubMed] [Google Scholar]
- 33. Hinchcliffe EH, Miller FJ, Cham M, Khodjakov A, Sluder G. Requirement of a centrosomal activity for cell cycle progression through G1 into S phase. Science 2001; 291:1547-50; PMID:11222860; http://dx.doi.org/ 10.1126/science.1056866 [DOI] [PubMed] [Google Scholar]
- 34. Stevens NR, Raposo AA, Basto R, Johnston D, Raff JW. From stem cell to embryo without centrioles. Curr Biol 2007; 17:1498-503; PMID:17716897; http://dx.doi.org/ 10.1016/j.cub.2007.07.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. From centriole biogenesis to cellular function. Cell Cycle 2008; 7:11-6; PMID:18196975; http://dx.doi.org/ 10.4161/cc.7.1.5226 [DOI] [PubMed] [Google Scholar]
- 36. Kumar A, Girimaji SC, Duvvari MR, Blanton SH. Mutations in STIL, encoding a pericentriolar and centrosomal protein, cause primary microcephaly. Am J Hum Genet 2009; 84:286-90; PMID:19215732; http://dx.doi.org/ 10.1016/j.ajhg.2009.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bond J, Roberts E, Mochida GH, Hampshire DJ, Scott S, Askham JM, Springell K, Mahadevan M, Crow YJ, Markham AF, et al. ASPM is a major determinant of cerebral cortical size. Nat Genet 2002; 32:316-20; PMID:12355089; http://dx.doi.org/ 10.1038/ng995 [DOI] [PubMed] [Google Scholar]
- 38. Bond J, Roberts E, Springell K, Lizarraga SB, Scott S, Higgins J, Hampshire DJ, Morrison EE, Leal GF, Silva EO, et al. A centrosomal mechanism involving CDK5RAP2 and CENPJ controls brain size. Nat Genet 2002; 37:353-5; PMID:15793586; http://dx.doi.org/ 10.1038/ng1539 [DOI] [PubMed] [Google Scholar]
- 39. Guernsey DL, Jiang H, Hussin J, Arnold M, Bouyakdan K, Perry S, Babineau-Sturk T, Beis J, Dumas N, Evans SC, et al. Mutations in centrosomal protein CEP152 in primary microcephaly families linked to MCPH4. Am J Hum Genet 2010; 87:40-51; PMID:20598275; http://dx.doi.org/ 10.1016/j.ajhg.2010.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jackson AP, Eastwood H, Bell SM, Adu J, Toomes C, Carr IM, Roberts E, Hampshire DJ, Crow YJ, Mighell AJ, et al. Identification of microcephalin, a protein implicated in determining the size of the human brain. Am J Hum Genet 2002; 71:136-42; PMID:12046007; http://dx.doi.org/ 10.1086/341283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nicholas AK, Khurshid M, Desir J, Carvalho OP, Cox JJ, Thornton G, Kausar R, Ansar M, Ahmad W, Verloes A, et al. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet 2010; 42:1010-14; PMID:20890279; http://dx.doi.org/ 10.1038/ng.682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu TW, Mochida GH, Tischfield DJ, Sgaier SK, Flores-Sarnat L, Sergi CM, Topcu M, McDonald MT, Barry BJ, Felie JM, et al. Mutations in WDR62, encoding a centrosome-associated protein, cause microcephaly with simplified gyri and abnormal cortical architecture. Nat Genet 2010; 42:1015-20; PMID:20890278; http://dx.doi.org/ 10.1038/ng.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hussain MS, Baig SM, Neumann S, Peche VS, Szczepanski S, Nürnberg G, Tariq M, Jameel M, Khan TN, Fatima A, et al. CDK6 associates with the centrosome during mitosis and is mutated in a large Pakistani family with primary microcephaly. Hum Mol Genet 2013; 22:5199-214; PMID:23918663; http://dx.doi.org/ 10.1093/hmg/ddt374 [DOI] [PubMed] [Google Scholar]
- 44. Sir JH, Barr AR, Nicholas AK, Carvalho OP, Khurshid M, Sossick A, Reichelt S, D'Santos C, Woods CG, Gergely F. A primary microcephaly protein complex forms a ring around parental centrioles. Nat Genet 2011; 43:1147-53; PMID:21983783; http://dx.doi.org/ 10.1038/ng.971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hussain MS, Baig SM, Neumann S, Nürnberg G, Farooq M, Ahmad I, Alef T, Hennies HC, Technau M, Altmüller J, et al. A truncating mutation of CEP135 causes primary microcephaly and disturbed centrosomal function. Am J Hum Genet 2012; 90:871-8; PMID:22521416; http://dx.doi.org/ 10.1016/j.ajhg.2012.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thornton GK, Woods CG. Primary microcephaly: do all roads lead to Rome? Trends Genet 2009; 25:501-10; PMID:19850369; http://dx.doi.org/ 10.1016/j.tig.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Szollosi D, Calarco P, Donahue RP. Absence of centrioles in the first and second meiotic spindles of mouse oocytes. J Cell Sci 1972; 11:521-41; PMID:5076360 [DOI] [PubMed] [Google Scholar]
- 48. Calarco-Gillam PD, Siebert MC, Hubble R, Mitchison T, Kirschner M. Centrosome d evelopment in early mouse embryos as defined by an autoantibody against pericentriolar material. Cell 1983; 35:621-29; PMID:6652679; http://dx.doi.org/ 10.1016/0092-8674(83)90094-6 [DOI] [PubMed] [Google Scholar]
- 49. Gueth-Hallonet C, Antony C, Aghion J, Santa-Maria A, Lajoie-Mazenc I, Wright M, Maro B. Gamma-tubulin is present in acentriolar MTOCs during early mouse development. J Cell Sci 1993; 105:157-66; PMID:8360270 [DOI] [PubMed] [Google Scholar]
- 50. Courtois A, Schuh M, Ellenberg J, Hiiragi T. The transition from meiotic to mitotic spindle assembly is gradual during early mammalian development. J Cell Biol 2012; 198:357-70; PMID:22851319; http://dx.doi.org/ 10.1083/jcb.201202135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Izraeli S, Lowe LA, Bertness VL, Good DJ, Dorward DW, Kirsch IR, Kuehn MR. The SIL gene is required for mouse embryonic axial development and left-right specification. Nature 1999; 399:691-4; PMID:10385121; http://dx.doi.org/ 10.1038/21429 [DOI] [PubMed] [Google Scholar]
- 52. Izraeli S, Lowe LA, Bertness VL, Campaner S, Hahn H, Kirsch IR, Kuehn MR. Genetic evidence that Sil is required for the sonic hedgehog response pathway. Genesis 2001; 31:72-7; PMID:11668681; http://dx.doi.org/ 10.1002/gene.10004 [DOI] [PubMed] [Google Scholar]
- 53. Castiel A, Danieli MM, David A, Moshkovitz S, Aplan PD, Kirsch IR, Brandeis M, Krämer A, Izraeli S. The stil protein regulates centrosome integrity and mitosis through suppression of Chfr. J Cell Sci 2011; 124:532-9; PMID:21245198; http://dx.doi.org/ 10.1242/jcs.079731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bushby AJ, P'ng KM, Young RD, Pinali C, Knupp C, Quantock AJ. Imaging three-dimensional tissue architectures by focused ion beam scanning electron microscopy. Nat Protoc 2011; 6:845-58; PMID:21637203; http://dx.doi.org/ 10.1038/nprot.2011.332 [DOI] [PubMed] [Google Scholar]
- 55. Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 2003; 426:83-7; PMID:14603322; http://dx.doi.org/ 10.1038/nature02061 [DOI] [PubMed] [Google Scholar]
- 56. Hochman E, Castiel A, Jacob-Hirsch J, Amariglio N, Izraeli S. Molecular pathways regulating pro-migratory effects of Hedgehog signaling. J Biol Chem 2006; 281:33860-70; PMID:16943197; http://dx.doi.org/ 10.1074/jbc.M605905200 [DOI] [PubMed] [Google Scholar]
- 57. Roessler E, Ermilov AN, Grange DK, Wang A, Grachtchouk M, Dlugosz AA, Muenke M. A previously unidentified amino-terminal domain regulates transcriptional activity of wild-type and disease-associated human GLI2. Hum Mol Genet 2005; 14:2181-8; PMID:15994174; http://dx.doi.org/ 10.1093/hmg/ddi222 [DOI] [PubMed] [Google Scholar]
- 58. Wong SY, Seol AD, So PL, Ermilov AN, Bichakjian CK, Epstein EH, Dlugosz AA, Reiter JF. Primary cilia can both mediate and suppress Hedgehog pathway-dependent tumorigenesis. Nat Med 2009; 15:1055-61; PMID:19701205; http://dx.doi.org/ 10.1038/nm.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rodrigues-Martins A, Riparbelli M, Callaini G, Glover DM, Bettencourt-Dias M. Revisiting the role of the mother centriole in centriole biogenesis. Science 2007; 316:1046-50; PMID:17463247; http://dx.doi.org/ 10.1126/science.1142950 [DOI] [PubMed] [Google Scholar]
- 60. Hornick JE, Mader CC, Tribble EK, Bagne CC, Vaughan KT, Shaw SL, Hinchcliffe EH. Amphiastral mitotic spindle assembly in vertebrate cells lacking centrosomes. Curr Biol 2011; 21:598-605; PMID:21439826; http://dx.doi.org/ 10.1016/j.cub.2011.02.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nat Genet 2006; 38:112-7; PMID:16311594; http://dx.doi.org/ 10.1038/ng1684 [DOI] [PubMed] [Google Scholar]
- 62. Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell 2010; 18:410-24; PMID:20230748; http://dx.doi.org/ 10.1016/j.devcel.2009.12.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pfaff KL, Straub CT, Chiang K, Bear DM, Zhou Y, Zon LI. The zebra fish cassiopeia mutant reveals that SIL is required for mitotic spindle organization. Mol Cell Biol 2007; 27:5887-97; PMID:17576815; http://dx.doi.org/ 10.1128/MCB.00175-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Arquint C, Nigg EA. STIL microcephaly mutations interfere with APC/C-mediated degradation and cause centriole amplification. Curr Biol 2014; 24:351-60; PMID:24485834; http://dx.doi.org/ 10.1016/j.cub.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 65. Gruber R, Zhou Z, Sukchev M, Joerss T, Frappart PO, Wang ZQ. MCPH1 regulates the neuroprogenitor division mode by coupling the centrosomal cycle with mitotic entry through the Chk1-Cdc25 pathway. Nat Cell Biol 2011; 13:1325-34; PMID:21947081; http://dx.doi.org/ 10.1038/ncb2342 [DOI] [PubMed] [Google Scholar]
- 66. Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004; 118:493-504; PMID:15315761; http://dx.doi.org/ 10.1016/j.cell.2004.08.002 [DOI] [PubMed] [Google Scholar]
- 67. Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, et al. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 2008; 319:816-9; PMID:18174396; http://dx.doi.org/ 10.1126/science.1151174 [DOI] [PubMed] [Google Scholar]
- 68. Miyoshi K, Kasahara K, Miyazaki I, Shimizu S, Taniguchi M, Matsuzaki S, Tohyama M, Asanuma M. Pericentrin, a centrosomal protein related to microcephalic primordial d warfism, is required for olfactory cilia assembly in mice. FASEB J 2009; 23:3289-97; PMID:19470799; http://dx.doi.org/ 10.1096/fj.08-124420 [DOI] [PubMed] [Google Scholar]
- 69. Nolden M, Zelzer S, Seitel A, Wald D, Müller M, Franz AM, Maleike D, Fangerau M, Baumhauer M, Maier-Hein L, et al. The medical imaging interaction toolkit: challenges and advances: 10 years of open-source development. Int J Comput Assist Radiol Surg 2013; 8:607-20; PMID:23588509; http://dx.doi.org/ 10.1007/s11548-013-0840-8 [DOI] [PubMed] [Google Scholar]
- 70. Lorensen WE, Cline HE. Marching cubes: a high resolution 3D surface construction algorithm. ACM Comput Graph 1987; 21:163-9; http://dx.doi.org/ 10.1145/37402.37422 [DOI] [Google Scholar]
- 71. Zimmerman WC, Sillibourne J, Rosa J, Doxsey SJ. Mitosis-specific anchoring of gamma tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol Biol Cell 2004; 15:3642-57; PMID:15146056; http://dx.doi.org/ 10.1091/mbc.E03-11-0796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bazzi H, Anderson KV. Acentriolar mitosis activates a p53-dependent apoptosis pathway in the mouse embryo. Proc Natl Acad Sci U S A 2014; published online March 31:E1491-500; PMID:24706806; http://dx.doi.org/ 10.1073/pnas.1400568111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.