Abstract
Endoplasmic reticulum (ER) stress is caused by the accumulation of misfolded or unfolded proteins in the lumen of the endoplasmic reticulum. CCAAT/enhancer binding proteins are one of the cellular proteins whose expression is upregulated during ER stress. Previously, we have identified C/EBPbeta isoforms, especially LIP, as a negative regulator of polyomavirus JC (JCV), the causative agent of the demyelinating disease progressive multifocal leukoencephalopathy (PML). Here, we show that the induction of ER stress by thapsigargin increase the expression of endogenous LIP and the degradation of JCV T-antigen in a JCV-transgenic mouse tumor cell line. Our results also revealed that overexpression of LIP significantly reduced the level of T-Ag and this effect is reversed upon siRNA-mediated silencing of LIP. Immunoprecipitation/Western blot experiments indicated that LIP interacts with T-antigen directly. Treatment of cells that overexpress LIP with MG115, a proteasome inhibitor, partially rescued LIP-mediated degradation of T-antigen. Our observations point to a role of LIP in ER stress regulation of T-antigen stability and may open a new avenue to study host-virus interaction during ER stress.
Keywords: CAAT/enhancer binding protein-beta, endoplasmic reticulum stress, large transforming antigen, liver-inhibitory isoform, polyomavirus JC
Introduction
The human neurotropic polyomavirus JC (JCV) is a DNA tumor virus and the etiological agent of the often fatal demyelinating disease progressive multifocal leukoencephalopathy (PML). The virus can exist in a persistent asymptomatic state or in a replicative pathogenic state where it infects glial cells to produce demyelinating lesions in the white matter typical of PML pathology.1 The activity of the virus is regulated by a transcriptional control region that determines the extent of the expression of the early genes, large-T antigen (T-Ag) and small t-antigen, and the late genes, agnoprotein and the capsid proteins VP1, VP2 and VP3.2 We have found that key transcription factors in this regulation are NF-κB p65,3 NFAT44 and C/EBPβ.3 T-Ag is the major regulatory protein of the JCV life cycle by driving cell cycle progression to S-phase for viral DNA replication and it is also tumorigenic in animals.5 Recently, it has been reported that T-Ag can also be regulated post-transcriptionally through mechanisms involving its degradation by the proteasome6 and by autophagy.7 In U-87 MG human glioblastoma cells, neurofibromatosis type 2 protein (NF2) suppresses T-Ag protein expression by binding to T-Ag and promoting degradation of ubiquitin bound T-Ag protein via a proteasome dependent pathway.6 On the other hand in BsB8 cells derived from primitive neuroectodermal tumors arising in T-Ag-transgenic mice, protein-protein interaction between T-Ag and the Bcl−2-associated athanoprotein BAG3 is important for the autophagic degradation of T-Ag.7
Infection of cells with viruses initiates a struggle between the virus, which tries to drive host cell proliferation and biosynthetic processes to further its lice cycle, and the host cell, which responds by mobilizing cellular processes that might limit infection such as apoptosis8,9 autophagy10,11 and the unfolded protein response.9,13 A key player in the interplay is the liver-inhibitory isoform (LIP) of CAAT/enhancer binding protein-β (C/EBPβ), which is translated from an alternate initiation codon from a common C/EBPβ mRNA that also encodes the full-length (FL) C/EBPβ isoform and the liver-activating (LAP) isoform of C/EBPβ.14 LIP has been found to be regulated by the stress response program induced by ER stress15 and LIP regulates stress-triggered cell death16 and autophagy.17 In the present study, we examine the effect of C/EBPβ LIP on JCV T-Ag.
Results
Treatment with thapsigargin induces LIP and downregulates JCV T-antigen
Thapsigargin is an inhibitor of the endoplasmic reticulum Ca2+ ATPase18 and raises cytosolic calcium concentration by blocking the ability of the cell to pump calcium into the endoplasmic reticulum (ER) which causes these stores to become depleted and ER stress.19 In order to induce ER stress in a cell line that expresses T-Ag, BsB8 cells were treated with 35 μM thapsigargin and analyzed for LIP and T-Ag expression as described in Materials and Methods. As shown in Figure 1A, LIP expression was increased up to 12 hours whereas T-Ag expression declined (Fig. 1B). Thapsigargin at concentrations up to 70 μM had little effect on cell viability as measured by MTT assay (Fig. 1C).
Figure 1.
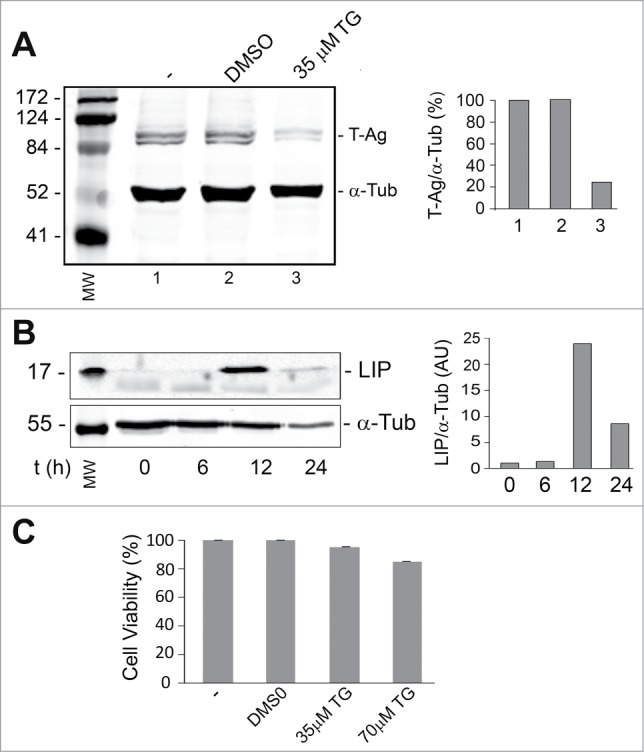
Effect of thapsigargin on the expression of LIP and T-Ag. BsB8 cells were treated with thapsigargin (TG) and then analyzed by Western blot for expression of T-Ag (A) and LIP (B) as described in Materials and Methods. The loading control was α-tubulin. The left-hand lane contains the molecular weight markers (MW). The effect of TG on BsB8 cell viability was measured by MTT assay (C).
Since LIP has been found to be regulated by the ER stress response program15 and can regulate stress-triggered processes,16,17 we examined the effect of ectopically expressed LIP on T-Ag expression levels. As shown in Figure 2A, transfecting increasing amounts LIP expression plasmid into BsB8 cells caused a progressive decline in T-Ag expression. On the other hand, using an adenovirus that we constructed to deliver siRNA to LIP resulted in a reduction of the level of LIP and an induction in T-Ag expression (Fig. 2B). These data indicate the involvement of LIP in suppression of T-Ag expression.
Figure 2.
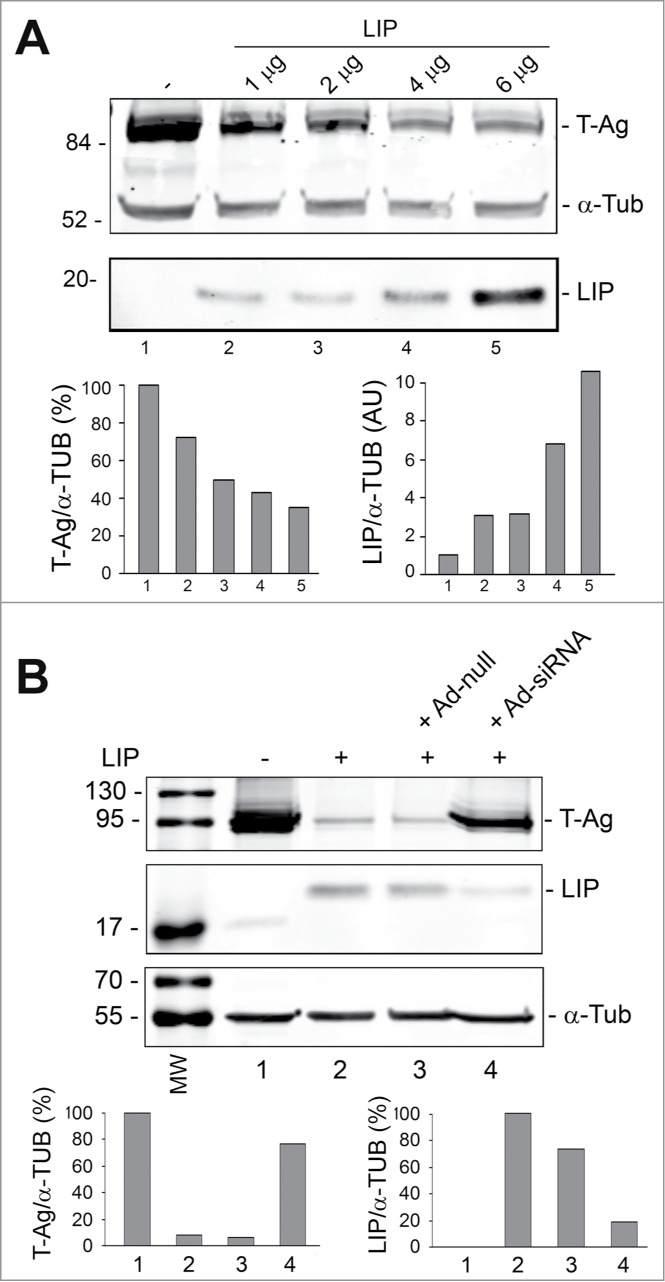
Effect of overexpression of LIP and LIP siRNA on T-Ag expression. (A) BsB8 cells were transfected with different amounts of expression plasmid for LIP as indicated and T-Ag expression analyzed by Western blot. The loading control was α-tubulin. The left-hand lane contains the molecular weight markers (MW). (B) BsB8 cells were transfected with expression plasmid for LIP and transduced with adenovirus vectors as indicated and T-Ag expression analyzed by Western blot. The loading control was α-tubulin. The left-hand lane contains the molecular weight markers (MW).
Next, we investigated the role of the proteasomal pathway in T-Ag down regulation by LIP. MG115 inhibits the peptidase activities of the 20S and 26S proteasomes and reduces the cellular degradation of proteins.20 BsBb cells were transfected with increasing amounts of LIP expression plasmid in the absence (DMSO) or presence of MG115. As shown in Figure 3, MG115 partially rescued T-Ag from LIP-induced reduction in expression (compare lane 5 to lane 2 and lane 6 to lane 3). Thus, LIP-induced reduction is, in part, dependent on the proteasome.
Figure 3.
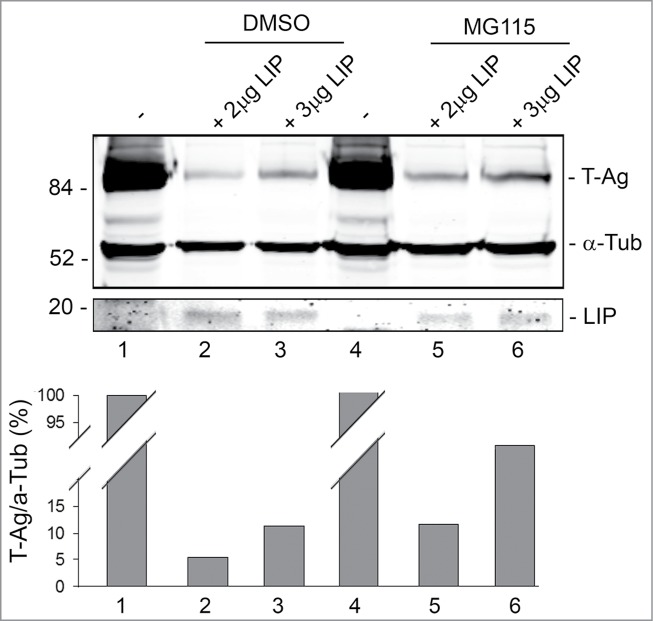
Effect of MG118 on LIP downregulation of T-Ag expression. BsB8 cells were transfected with different amounts of expression plasmid for LIP in the absence and presence of MG115 as indicated and T-Ag expression analyzed by Western blot. The loading control was α-tubulin.
Finally, we asked if the downregulation of T-Ag by LIP might involve a direct interaction between the 2 proteins using an immunoprecipitation/Western blot approach. As shown in Figure 4A, IP with antibody to T-Ag, but not nonimmune mouse serum, coprecipitated LIP in BsB8 cells transfected with expression plasmid for LIP. Reciprocally, antibody to LIP coprecipitated JCV T-Ag (Fig. 4B). Thus the 2 proteins directly interact in cells suggesting that this interaction may be involved in the turnover of T-Ag.
Figure 4.
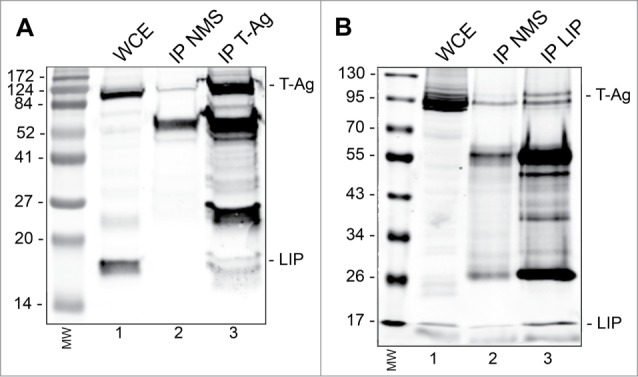
Immunoprecipitation(IP)/Western blot of LIP and T-Ag. BsB8 cells were transfected with expression plasmid for LIP, whole cell extract (WCE) prepared and: (A) IP performed with antibody to T-Ag or nonimmune mouse serum (NMS) followed by Western blot for LIP and T-Ag. The left-hand lane contains the molecular weight markers (MW). (B) IP performed with antibody to LIP or nonimmune mouse serum (NMS) followed by Western blot for LIP and T-Ag. The left-hand lane contains the molecular weight markers (MW).
Discussion
The endoplasmic reticulum (ER) has major functions in protein synthesis and transportation, the regulation of calcium homeostasis and carbohydrate metabolism. A variety of viruses can hijack the cellular translation machinery to produce large amount of viral proteins that lead to the disturbance ER homeostasis and causes ER stress. Liver-inhibitory isoform (LIP) of CCAAT/enhancer binding protein-β (C/EBPβ) has been implicated as a cellular protein that is upregulated upon ER stress and regulates stress-triggered cell death and autophagy.8-13 We have previously identified LIP is form as negative regulator of JCV transcription from both the early and late promoters in presence or absence of T-Ag by binding a specific DNA element within the viral promoter.3 The present study uncovers a new role of LIP during ER stress on the stability of T-Ag.
The data presented here indicate that thapsigargin, a well-known ER stressor, induces the expression of the LIP isoform of C/EBPβ and significantly decreases the expression of JCV T-Ag in BsB8 cells. Western blot analyses of BsB8 cells has shown that overexpression of LIP downregulates T-Ag expression in a concentration dependent manner, indicating that LIP is involved in regulating T-Ag expression level. This observation was further supported by experiment involving the silencing of LIP by an adeno-shRNA construct showing that T-Ag expression was rescued from LIP mediated downregulation. Although the exact mechanism of LIP-mediated regulation of T-Ag is not known, our results from the treatment of the cells with MG115, a potent proteasome inhibitor, show that it partially rescues T-Ag expression.
Previous studies have shown an increase in the level of C/EBPβ LIP in thapsigargin induced ER stress.21 It has been argued that LIP expression is regulated by preferential translation initiation site usage and that the ER stress response may regulate translation and the initiation codon for LIP may be efficient under ER stress.15 The study by Li et al.15 has shown an increase in the level of LIP during the late ER stress phase (>9 h), which is in line with our observation reported here where a high level of LIP was detected at 12 hours post-treatment with thapsigargin (Fig. 1). Two cellular proteins were identified in 2 previous studies involved in JCV T-Ag degradation. A study by Sariyer et al.7 showed that BAG3 induced autophagy was associated with decreased levels of T-Ag. On the other hand, Beltrami et al.6 reported proteasome-mediated degradation of T-Ag in human glioblastoma by Neurofibromatosis type 2 protein. The data presented here indicate the treatment of cells with MG115 partially reversed LIP-mediated T-Ag loss indicating a role for the proteasome. Although at this time the exact physiological role of ER stress-induced LIP is not clear, we speculate that LIP-mediated degradation of T-Ag may be seen as a host-virus interaction that might serve to contain viral infection.
In summary, our experimental results provide evidence that LIP overexpression is associated with T-Ag degradation. Further studies of the components of the ER stress pathway will provide new insight into the role of ER stress in the JCV life cycle.
Materials and Methods
Cell culture and plasmids
BsB8 is a mouse cell line from a tumor of cerebellar neuroectodermal origin arising in a mouse transgenic for the JCV early region, which expresses JCV T-Ag,22 were maintained in Dulbecco's Modified Eagle's Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) line as we have previously described.23 The expression plasmid pCMV-LIP and the adenoviruses for C/EBPβ siRNA (Ad-siRNA) and control (Ad-null) have been described.3
Antibodies
Mouse monoclonal anti-C/EBPβ (H7, sc-7962) was ordered from Santa Cruz Biotechnology, Inc., Santa Cruz, CA and recognizes all 3 C/EBPβ isoforms3 Mouse monoclonal anti-T-antigen Ab-2 PAb416 DP02 was obtained from Calbiochem (La Jolla, CA). Mouse anti-α-tubulin antibody clone B512 was from Sigma-Aldrich (St. Louis, MO).
Thapsigargin treatment
Cells were plated for 24 h and then treated with or without 35 μM thapsigargin in DMSO vehicle for 6, 12 and 24 h. Total cell extracts were prepared and analyzed by Western blot.
Western blot
Westerns were performed as previously described23 except that antibody was detected with the LI-COR system. Blots were incubated with IRDye® 680RD Goat Anti-Mouse Li-COR dyes and visualized with an Odyssey® CLx Imaging System (LI-COR, Inc., Lincoln, NE) using LI-COR Odyssey software. Band intensities were quantified using the Quantity One software (Bio-Rad, Hercules CA) and intensities normalized to α-tubulin.
Transient transfection and siRNA treatment
Transfection of LIP expression plasmid was performed using Lipofectamine 2000 (Life Technologies Inc., Carlsbad CA) and transduction with adenoviral vectors was we have previously described.3,23 The total amount of transfected DNA was normalized with empty vector DNA. MG115 (30 μM. Sigma) treatment was overnight.
Immunoprecipitation/Western blot
BsB8 cells were transfected with LIP expression plasmid, whole cell extracts prepared and IP/Western performed as we have described.23 T-Ag was immunoprecipitated followed by Western blot for LIP or vice versa for the reciprocal IP/Western.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgements
We thank Drs. Ilker Sariyer, Valeria Pietropaolo, Anna T. Palamara and Kamel Khalili for their helpful input into this project. We thank past and present members of the Center for Neurovirology for their insightful discussion and sharing of ideas and reagents.
funding
These studies were supported in part by grant AI077460 awarded by NIH to MKW and utilized services offered by the Temple University Comprehensive NeuroAIDS Center supported by NIH grant P30MH092177. Anna Bellizzi was partially supported by post-doctoral fellowship “Teresa Ariaudo 2013” from Institute Pasteur Cenci-Bolognetti Foundation.
References
- 1.Wollebo HS, White MK, Berger JR, Gordon J, Khalili K. Persistency and pathogenesis of the neurotropic polyomavirus JC. Ann Neurol 2015; (In Press); 77(4):560-70; PMID:25623836; http://dx.doi.org/ 10.1002/ana.24371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White MK, Safak M, Khalili K. Regulation of gene expression in primate polyomaviruses. J Virol 2009; 83:10846-56; PMID:19640999; http://dx.doi.org/ 10.1128/JVI.00542-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romagnoli L, Wollebo HS, Deshmane SL, Mukerjee R, Del Valle L, Safak M, Khalili K, White MK. Modulation of JC virus transcription by C/EBPbeta. Virus Res 2009; 146:97-106; PMID:19747512; http://dx.doi.org/ 10.1016/j.virusres.2009.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wollebo HS, Melis S, Khalili K, Safak M, White MK. Cooperative roles of NF-κB and NFAT4 in polyomavirus JC regulation at the KB control element. Virology 2012; 432:146-54; PMID:22749879; http://dx.doi.org/ 10.1016/j.virol.2012.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White MK, Khalili K. Polyomaviruses and human cancer: molecular mechanisms underlying patterns of tumorigenesis. Virology 2004; 324:1-16; PMID:15183048; http://dx.doi.org/ 10.1016/j.virol.2004.03.025 [DOI] [PubMed] [Google Scholar]
- 6.Beltrami S, Branchetti E, Sariyer IK, Otte J, Weaver M, Gordon J. Neurofibromatosis type 2 tumor suppressor protein, NF2, induces proteasome-mediated degradation of JC virus T-antigen in human glioblastoma. PLoS One 2013; 8:e53447; PMID:23308224; http://dx.doi.org/ 10.1371/journal.pone.0053447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sariyer IK, Merabova N, Patel PK, Knezevic T, Rosati A, Turco MC, Khalili K. Bag3-induced autophagy is associated with degradation of JCV oncoprotein, T-Ag. PLoS One 2012; 7:e45000; PMID:22984599; http://dx.doi.org/ 10.1371/journal.pone.0045000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev 2003; 17:2481-95; PMID:14561771; http://dx.doi.org/ 10.1101/gad.1126903 [DOI] [PubMed] [Google Scholar]
- 9.Li S, Kong L, Yu X. The expanding roles of endoplasmic reticulum stress in virus replication and pathogenesis. Crit Rev Microbiol 2013; 9:1-15; PMID:25168431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silva LM, Jung JU. Modulation of the autophagy pathway by human tumor viruses. Semin Cancer Biol 2013; 23:323-8; PMID:23727156; http://dx.doi.org/ 10.1016/j.semcancer.2013.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovilovic G, Ristic B, Milenkovic M, Stanojevic M, Trajkovic V. The role and therapeutic potential of autophagy modulation in controlling virus-induced cell death. Med Res Rev. 2014; 34:744-67; PMID:24123125; http://dx.doi.org/ 10.1002/med.21303 [DOI] [PubMed] [Google Scholar]
- 12.Chan SW. The unfolded protein response in virus infections. Front Microbiol 2014; 5:518; PMID:25324837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inesi G, Sagara Y. Thapsigargin, a high affinity and global inhibitor of intracellular Ca2+ transport ATPases. Arch Biochem Biophys 1992; 298:313-7; PMID:1416963; http://dx.doi.org/ 10.1016/0003-9861(92)90416-T [DOI] [PubMed] [Google Scholar]
- 14.Ossipow V, Descombes P, Schibler U. CCAAT/enhancer-binding protein mRNA is translated into multiple proteins with different transcription activation potentials. Proc Natl Acad Sci USA 1993; 90:8219-23; PMID:8367486; http://dx.doi.org/ 10.1073/pnas.90.17.8219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Bevilacqua E, Chiribau CB, Majumder M, Wang C, Croniger CM, Snider MD, Johnson PF, Hatzoglou M. Differential control of the CCAAT/enhancer-binding protein beta (C/EBPbeta) products liver-enriched transcriptional activating protein (LAP) and liver-enriched transcriptional inhibitory protein (LIP) and the regulation of gene expression during the response to endoplasmic reticulum stress. J Biol Chem 2008; 283:22443-56; PMID:18550528; http://dx.doi.org/ 10.1074/jbc.M801046200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meir O, Dvash E, Werman A, Rubinstein M. C/EBP-beta regulates endoplasmic reticulum stress-triggered cell death in mouse and human models. PLoS One 2010; 5:e9516; PMID:20209087; http://dx.doi.org/ 10.1371/journal.pone.0009516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abreu MM, Sealy L. The C/EBPbeta isoform, liver-inhibitory protein (LIP), induces autophagy in breast cancer cell lines. Exp Cell Res 2010; 316:3227-38; PMID:20699097; http://dx.doi.org/ 10.1016/j.yexcr.2010.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhari N, Talwar P, Parimisetty A, Lefebvre d'Hellencourt C, Ravanan P. A molecular web: endoplasmic reticulum stress, inflammation, and oxidative stress. Front Cell Neurosci 2014; 8:213; PMID:25120434; http://dx.doi.org/ 10.3389/fncel.2014.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 1994; 78:761-71; PMID:8087844; http://dx.doi.org/ 10.1016/S0092-8674(94)90462-6 [DOI] [PubMed] [Google Scholar]
- 20.Vachirayonsti T, Ho KW, Yang D, Yan B. Suppression of the pregnane × receptor during endoplasmic reticulum stress is achieved by down-regulating hepatocyte nuclear factor-4α and up-regulating liver-enriched inhibitory protein. Toxicol Sci 2015. (In Press); 144(2):382-92; PMID:25616597; http://dx.doi.org/ 10.1093/toxsci/kfv008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krynska B, Del Valle L, Gordon J, Otte J, Croul S, Khalili K. Identification of a novel p53 mutation in JCV-induced mouse medulloblastoma. Virology 2000; 274:65-74; PMID:10936089; http://dx.doi.org/ 10.1006/viro.2000.0450 [DOI] [PubMed] [Google Scholar]
- 22.Wollebo HS, Safak M, Del Valle L, Khalili K, White MK. Role for tumor necrosis factor-α in JC virus reactivation and progressive multifocal leukoencephalopathy. J Neuroimmunol 2011; 233:46-53; PMID:21185609; http://dx.doi.org/ 10.1016/j.jneuroim.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White MK, Skowronska A, Gordon J, Del Valle L, Deshmane SL, Giordano A, Khalili K. Analysis of a mutant p53 protein arising in a medulloblastoma from a mouse transgenic for the JC virus early region. Anticancer Res 2006; 26:4079-92; PMID:17201119 [PubMed] [Google Scholar]


