Highlights
-
•
We present a robust method for VOC analysis from rocket salad packaging headspace.
-
•
TD-GC–TOF-MS putatively identified 39 volatile compounds.
-
•
VOC profiles for Eruca sativa varied significantly between accessions.
-
•
Isothiocyanate compounds degrade significantly over shelf-life.
-
•
Changes in VOC profiles could provide a useful tool for assessment of leaf quality.
Keywords: Eruca sativa, Diplotaxis tenuifolia, Brassicaceae, Rocket salad, Rucola, Gas chromatography mass spectrometry, Volatile organic chemicals, Isothiocyanates
Abstract
An important step in breeding for nutritionally enhanced varieties is determining the effects of the post-harvest supply chain on phytochemicals and the changes in VOCs produced over time. TD-GC–TOF-MS was used and a technique for the extraction of VOCs from the headspace using portable tubes is described. Forty-two compounds were detected; 39 were identified by comparison to NIST libraries. Thirty-five compounds had not been previously reported in Eruca sativa. Seven accessions were assessed for changes in headspace VOCs over 7 days. Relative amounts of VOCs across 3 time points were significantly different – isothiocyanate-containing molecules being abundant on ‘Day 0’. Each accession showed differences in proportions/types of volatiles produced on each day. PCA revealed a separation of VOC profiles according to the day of sampling. Changes in VOC profiles over time could provide a tool for assessment of shelf life.
1. Introduction
Rocket (or arugula, rucola, roquette) species are of increasing commercial importance across the world. Leaves of the crop are usually sold in mixed salad bags or whole bags, and in some niche markets as gourmet microleaves. The nutritional and sensory quality of leaves throughout the supply chain is of major concern to producers and supermarkets, as they will ultimately be accepted or rejected, by the consumer, based on these attributes. Much of the current rocket supply chain is designed to preserve predominantly visual and morphological traits of leaves (such as stem browning/yellowing) before they reach the consumer. Very little research has been conducted to determine the phytochemical and volatile organic compound (VOC) losses incurred post-harvest (Verkerk, Dekker, & Jongen, 2001).
Rocket varieties are genetically very diverse, and morphological uniformity can be an issue for plant breeders and growers alike. One plant can have very different leaf shapes from another, even within the same variety (Egea-Gilabert et al., 2009). Rocket species are generally preferential outbreeders, making production of uniform breeding lines difficult. This variability has been shown to extend to concentrations of phytochemicals (Bell, Oruna-Concha, & Wagstaff, 2015), where significant differences in glucosinolate (GSL) and flavonols have been detected amongst accessions. An important step in breeding for nutritionally enhanced varieties is determining the effects of the post-harvest supply chain on phytochemicals and the changes in volatile degradation products produced over time. The concentrations and/or relative abundances of both of these, which include isothiocyanates, (ITCs) may also vary greatly, depending on the levels of physical damage during processing of the leaves. The VOC bouquet is the term used to describe the collection of volatiles within the headspace of a plant or other foodstuff, often giving rise to aromas. These aromas will affect the sensory attributes perceived by the consumer when the product is eaten, and they influence re-purchase (Ragaert, Verbeke, Devlieghere, & Debevere, 2004). This may have consequences on consumers’ nutritional intake, and hence long-term health.
VOCs found in rocket comprise ITCs, alkanes, aliphatic alcohols, carbonyl compounds, fatty acids, esters, phenols and C13-norisoprenoids (Blazevic & Mastelic, 2008). However, comparison of the relative abundance amongst cultivars has not been established, as earlier studies only utilized one commercially bought, bagged variety, and leaves from a wild population (Blazevic & Mastelic, 2008; Jirovetz, Smith, & Buchbauer, 2002). Given the very different sample sources, differences between the two studies may be representative of environmental stresses, as well as genetic variation (Varming et al., 2004). These include exposure to fungal diseases, wounding (pre- and post-harvest), and variations in temperature and humidity during growth and while in controlled environment conditions – all of which can lead to changes in phytochemical content and VOCs produced (Schouten et al., 2009).
In this study rocket salad was grown under controlled environment conditions, thus reducing environmental stress responses, enabling effects of post-harvest storage on VOC profiles to be assessed. Thermal desorption gas chromatography time-of-flight mass spectrometry (TD-GC–TOF-MS) was used to determine changes in VOCs during storage of seven different accessions, demonstrating that collection of headspace volatiles onto thermal desorption tubes is a rapid and robust method for assessing post-harvest changes and identifying differences in these changes amongst accessions.
2. Materials and methods
2.1. Plant material
Six Eruca sativa accessions were obtained from European gene banks, four from the Leibniz-Institut für Pflanzengenetik und Kulturpflanzenforschung (IPK Gatersleben, Germany), one from the Centre for Genetic Resources in the Netherlands (CGN, Wageningen, The Netherlands) and one from The University of Warwick Crop Centre Genetic Resources Unit (Wellesbourne, UK; formerly Warwick HRI). The Elsoms Seeds variety SR3 was used as a commercial comparator. Accessions have been coded to protect commercially sensitive information.
2.2. Growing conditions and simulated shelf life
Each accession was germinated under controlled environmental conditions (Fitotron, Weiss-Technik UK, Loughborough, UK). Long-day lighting was used (16 h light, 8 h dark) at an intensity of 200 μmol m−2 s−1. Day temperatures were set at 20 °C and night temperatures at 14 °C. Seedlings were grown for 10 days in seedling trays and then transplanted to larger trays. Subsequently, plants were grown for a further 20 days and then leaves were harvested at 30 days. Leaves were collected in batches of 70 g in three experimental replicates per accession. Amount of leaf material was chosen based on preliminary experiments, where abundant yields of VOCs were obtained.
2.3. Sample collection
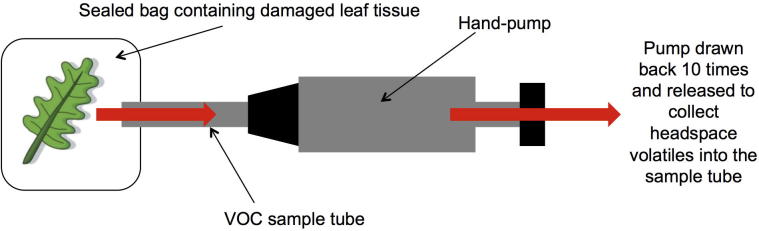
Whole leaves were placed into a multi-purpose roasting bag (25 cm × 38 cm, TJM Ltd.) and sealed, using an elastic band and an Eppendorf tube with the end cut off, which served as a sampling port for the TD tubes (see supplementary material for diagram). Leaves were then disrupted manually within the bags for 10 s by crushing the leaves between the hands and making a vigorous rubbing motion. Care was taken not to perforate the bags and inadvertently release VOCs. Three replicates were taken for each sample, including three ‘blank’ samples of atmosphere within empty bags to rule out any possible contaminating VOCs. These were prepared and sampled in an identical fashion, with the exception that no leaves were contained in the bag.
2.4. Post-harvest storage simulation
Harvested rocket leaves were stored in a dark, controlled temperature room at 4 °C, to simulate industrial storage conditions for 7 days. Bags were only removed from the environment while samples were taken (room temperature, ∼22 °C).
2.5. TD-GC–MS-TOF analysis
All tubes were desorbed by a TD100 thermal desorption system (Markes International Ltd., Llantrisant, Wales, UK), using the following settings for tube desorption: 5 min at 100 °C, followed by 5 min at 280 °C, trap flow of 40 ml/min and trap desorption and transfer: 20 °C/s to 300 °C, split flow of 20 ml/min into GC (7890A; Agilent Technologies, Inc., Stockport, UK). VOCs were separated over 60 min, 0.32 mm ID, 0.5 μm film thickness Rxi-5ms (Restek) at 2 ml continuous flow of helium, using the following temperature programme: initial temperature 40 °C for 2 min, 5 °C/min to 240 °C, final hold 5 min. The BenchTOF-dx mass spectrometer (Almsco International, Cincinnati, OH, USA) was operated in EI mode at an ion source temperature of 275 °C and a mass range of 35–350 m/z. A retention time standard (C8–C20, Sigma Aldrich, Gillingham, UK) was prepared by injection of 1 μl of the standard mixture directly onto a thermal desorption tube, and analysed under the same conditions as the samples.
Data from GC–MS measurements were processed with MSD ChemStation software (E.02.01.1177; Agilent Technologies, Inc., Stockport, UK) and deconvoluted and integrated with AMDIS (NIST 2011), using a custom retention-indexed mass spectral library. MS spectra from deconvolution were searched against the NIST 2011 library (Mallard, Sparkman, & Sparkman, 2008) and only compounds scoring >80% in forward and backward fit were included into the custom library. Putative identifications were based on match of mass spectra (>80%) and retention index (RI ± 15) (Beaulieu & Grimm, 2001).
Compounds abundant in controls or in only one of the three replicates were excluded from statistical analyses. Areas of remaining compounds were normalized to total area of chromatograms prior to averaging within samples.
2.6. Statistical analysis
Results from the three biological replicates for each time point were averaged and analysed using XL Stat software (Addinsoft, New York City, New York, USA). ANOVA + Tukey’s HSD test (p ⩽ 0.05) and principal component analyses (PCA; Pearson n − 1) were performed on the data to determine significant differences and correlations of compounds detected over time and on each respective sampling day. Tukey’s test was chosen because of its high stringency (compared to Fisher’s test, for example) and a reduced possibility of Type I statistical errors.
3. Results and discussion
3.1. Composition and functional analysis of E. sativa VOC bouquet
We focussed on E. sativa accessions to assess the range of VOCs produced by different genotypes from the same species, and then to determine whether, within this narrower genetic range, there were sufficient VOC differences to discriminate amongst accessions. A total of 39 compounds were putatively identified by comparison to NIST libraries, and a further three unknown volatile compounds were also detected (see Table 1 for compounds, their identification codes, retention indices and CAS numbers). Compounds comprised several classes of aliphatic organic compounds, including alcohols, aldehydes, ketones, isothiocyanates and furanones; some of which have been reported in other studies (Blazevic & Mastelic, 2008; Jirovetz et al., 2002). However, to our knowledge, only four of these compounds have been previously reported in E. sativa (C8, C14, C20 and C38). Fifteen of the compounds have been detected in other members of the Brassicaceae family. These include broccoli (Brassica oleracea var. italica) (C10, C15, C9) (Hansen, Laustsen, Olsen, Poll, & Sorensen, 1997), radish (Raphanus sativus) (C8, C20, C33, C25) (Blazevic & Mastelic, 2009), kale (B. oleracea var. acephala) (C3, C10, C8, C31, C20, C29) (Fernandes, de Pinho, Valentao, Pereira, & Andrade, 2009), oilseed rape (Brassica napus) (C20, C6, C7), Thale cress (Arabidopsis thaliana) (C20, C29, C3, C9, C14) (Rohloff & Bones, 2005) and mustard (Brassica juncea) (C15, C9, C14, C23) (Zhao, Tang, & Ding, 2007).
Table 1.
VOC compounds putatively identified in rocket accessions by TD-GC–MS-TOF with retention indices, CAS registry number, chemical group, and references for identifications in previous studies.
| Compound number | Compound name | RI | CAS No. | Chemical group | References |
|---|---|---|---|---|---|
| C1 | (E)-4-oxohex-2-enal⁎ | 989 | EPA-374042 | Aldehyde | Sanda, Zacek, Streinz, Dracinsky, and Koutek (2012) |
| C2 | 4-Isothiocyanato-1-butene⁎ | 1021 | 3386-97-8 | Isothiocyanate | Guo, Yang, Wang, Guo, and Gu (2014) |
| C3 | 1-Penten-3-ol⁎ | 677 | 616-25-1 | Alcohol | Fernandes et al. (2009) |
| C4 | 1-Penten-3-one⁎ | 683 | 1629-58-9 | Ketone | Servili, Selvaggini, Taticchi, Esposto, and Montedoro (2003) |
| C5 | 2-(1,1-Dimethylethyl)-1H-indole⁎ | 1582 | 1805-65-8 | Aromatic compound | – |
| C6 | 2-Methyl-2-butenal⁎ | 702 | 1115-11-3 | Aldehyde | Starkenmann (2003) |
| C7 | 2-Hexenal⁎ | 865 | 505-57-7 | Aldehyde | Servili et al. (2003) |
| C8 | (E)-2-hexenal | 858 | 6728-26-3 | Aldehyde | Blazevic and Mastelic (2008) |
| C9 | (Z)-2-penten-1-ol⁎ | 782 | 1576-95-0 | Alcohol | Rohloff and Bones (2005), Servili et al. (2003) |
| C10 | (E)-2-pentenal⁎ | 760 | 1576-87-0 | Aldehyde | Fernandes et al. (2009) |
| C11 | (E,E)-2,4-hexadienal⁎ | 933 | 142-83-6 | Aldehyde | Farag, Ryu, Sumner, and Paré (2006) |
| C12 | 5-Ethyl-2(5H)-furanone⁎ | 1003 | 2407-43-4 | Furanone | Buttery and Takeoka (2004) |
| C13 | 3-Ethyl-1,5-octadiene⁎ | 961 | EPA-114877 | Alkene | Rohloff and Bones (2005) |
| C14 | 3-Hexen-1-ol | 872 | 3681-71-8 | Alcohol | Ruther and Kleier (2005), Blazevic and Mastelic (2008, 2009) |
| C15 | 3-Hexenal⁎ | 810 | 4440-65-7 | Aldehyde | Tandon, Baldwin, and Shewfelt (2000) |
| C16 | (Z)-3-hexenal⁎ | 898 | 6789-80-6 | Aldehyde | Buttery and Takeoka (2004) |
| C17 | 3-Octyne⁎ | 921 | 15232-76-5 | Alkyne | – |
| C18 | 3-Pentanone⁎ | 691 | 96-22-0 | Ketone | Servili et al. (2003) |
| C19 | 5-Methyl-4-hexen-3-one⁎ | 1073 | 13905-10-7 | Ketone | – |
| C20 | 4-Methylpentyl isothiocyanate | 1184 | 17608-07-0 | Isothiocyanate | Beevi, Mangamoori, Subathra, and Edula (2010) |
| C21 | 5-Nonanone oxime⁎ | 791 | 14475-42-4 | Imine | – |
| C22 | O-methyloxime-butanal⁎ | 672 | 31376-98-4 | Imine | – |
| C23 | 1-Isothiocyanato-3-methyl-butane⁎ | 1082 | 628-03-5 | Isothiocyanate | Zhao et al. (2007) |
| C24 | Ethylidene-cyclopropane⁎ | 564 | 18631-83-9 | Cycloalkane | Bajpai, Rahman, and Kang (2008) |
| C25 | Dimethyl-sulphide⁎ | 570 | 75-18-3 | Sulphur compound | Manchali, Chidambara Murthy, and Patil (2012) |
| C26 | 2-Ethyl-furan⁎ | 696 | 3208-16-0 | Aromatic compound | Farag et al. (2006) |
| C27 | 3-Methyl-furan⁎ | 605 | 930-27-8 | Aromatic compound | – |
| C28 | 3-Methyl-hexadecane⁎ | 1658 | 6418-43-5 | Alkane | Wang, Xing, Chin, Ho, and Martin (2001) |
| C29 | n-Hexyl-isothiocyanate⁎ | 1223 | 4404-45-9 | Isothiocyanate | Fernandes et al. (2009) |
| C30 | 2-Oxo-hexanoic acid methyl ester⁎ | 1085 | 6395-83-1 | Ester | – |
| C31 | n-Pentyl isothiocyanate⁎ | 1120 | 629-12-9 | Isothiocyanate | Grob and Matile (1980) |
| C32 | Oxalic acid diallyl ester⁎ | 808 | EPA-309229 | Ester | – |
| C33 | Iberverin⁎ | 1355 | 505-79-3 | Isothiocyanate | Munday and Munday (2004) |
| C34 | Propanoic acid anhydride⁎ | 1086 | 123-62-6 | Acid anhydride | – |
| C35 | 4-Methyl-2-(2-methyl-propenyl)-pyridine⁎ | 1424 | 104188-16-1 | Pyridine derivative | – |
| C36 | Pyrrolidine-1-dithiocarboxylic acid 2-oxocyclopentyl ester⁎ | 1391 | 147723-50-0 | Sulphur aromatic compound | – |
| C37 | 3-Ethyl-thiophene⁎ | 885 | 1795-01-3 | Sulphur aromatic compound | – |
| C38 | Tetrahydrothiophene | 826 | 110-01-0 | Sulphur aromatic compound | Blazevic and Mastelic (2008) |
| C39 | [Unknown 2] | 693 | - | – | – |
| C40 | [Unknown 8] | 1129 | - | – | – |
| C41 | [Unknown 9] | 991 | - | – | – |
| C42 | Vinylfuran⁎ | 726 | 1487-18-9 | Aromatic compound | – |
Previously unreported in Eruca sativa.
3.2. Relative VOC abundance and differences amongst rocket gene bank accessions over simulated shelf life
3.2.1. Across-day variation
The abundance of the 42 VOCs was determined in each of the seven E. sativa accessions (Table 2) over three different storage time-points: ‘Day 0’ (at harvest) and following +3 and +7 days of storage at a commercially relevant temperature of 4 °C. Seven days is the typical time taken from harvest to retail (Favell, 1998), assuming a typical supply chain where rocket is imported by lorry from northern or southern Italy (depending on the season) to the UK. Thirty-one of the 42 compounds detected were significantly different in percentage-abundance amongst rocket accessions, across the three sampling days (ANOVA with post hoc Tukey’s HSD test; see Table 2). Fig. 1 (PCA loadings plot) shows how each of the genotypes is arranged spatially, according to the volatiles produced on each sampling day. On ‘Day 0’ there is a large degree of separation along the F1 axis, indicating that genotypic variability is high in terms of the types of volatiles produced and their relative abundance. Samples SR2 and SR12 are the most dissimilar in this respect with varying degrees of similarity with the other five genotypes. This indicates that there may be a high degree of genetic variability and control involved in VOC production in the early stages of shelf life, as environmental variation was minimal during plant growth and sampling. On ‘Day 3’ and ‘Day 7’, the distinction between accessions is somewhat reduced, and volatile profiles less variable for each respective accession. The profiles of SR2 on ‘Day 3’ and ‘Day 7’ are virtually indistinguishable in Fig. 1a, and similarly with SR5.
Table 2.
Means of percentage relative abundance with standard error and significant differences (p ⩽ 0.05) of volatile compounds detected in the headspace of rocket accessions. Values expressed as percentage of the total volatiles detected per sample.
| VOC | Cultivar and day of sampling (% of total volatiles detected) |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SR2 |
SR3 |
SR5 |
SR6 |
SR12 |
SR14 |
SR19 |
|||||||||||||||
| 0 | 3 | 7 | 0 | 3 | 7 | 0 | 3 | 7 | 0 | 3 | 7 | 0 | 3 | 7 | 0 | 3 | 7 | 0 | 3 | 7 | |
| C1 | 22.4 ± 0.4abc | 30.5 ± 2.7bc | 25.6 ± 6.2abc | 14.8 ± 2.9ab | 49.9 ± 0.9c | 22.5 ± 4.9abc | 10.5 ± 8.6ab | nda | 1.9 ± 1.0ab | 4.7 ± 3.8ab | 20.6 ± 2.9ab | 17.9 ± 7.3ab | 2.5 ± 2.0ab | 19.7 ± 4.1ab | 3.2 ± 1.5ab | 13.4 ± 8.6ab | 19.8 ± 4.9ab | 9.3 ± 4.1ab | 12.3 ± 5.1ab | 8.4 ± 1.4ab | 3.3 ± 1.9ab |
| C2 | nda | nda | nda | nda | nda | nda | <0.1 ± <0.1b | nda | nda | 0.1 ± <0.1c | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda |
| C3 | 1.2 ± 0.1ab | 1.5 ± 0.2b | 1.3 ± 0.2ab | 1.5 ± 0.3b | 0.7 ± 0.3ab | 1.4 ± 0.7ab | 0.5 ± 0.1ab | nda | nda | 1.2 ± 0.2ab | 0.7 ± 0.1ab | 0.5 ± 0.2ab | 1.5 ± 0.2b | nda | nda | 1.3 ± 0.1ab | nda | nda | 1.2 ± 0.2ab | nda | nda |
| C4 | 1.9 ± 0.1a | 4.7 ± 1.0a | 4.2 ± 1.0a | 2.0 ± 0.2a | 2.6 ± 0.9a | 4.3 ± 2.2a | 1.3 ± 0.5a | 1.0 ± 0.6a | 0.9 ± 0.6a | 1.4 ± 0.3a | 1.8 ± 0.3a | 1.8 ± 0.3a | 1.8 ± 0.2a | 1.2 ± 0.2a | 0.6 ± 0.2a | 1.9 ± 0.2a | 3.0 ± 0.4a | 2.7 ± 1.5a | 2.2 ± 0.3a | 1.8 ± 0.7a | 0.5 ± 0.1a |
| C5 | nda | nda | nda | nda | nda | nda | nda | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | nda | nda | nda | <0.1 ± <0.1a | nda | nda |
| C6 | nda | nda | nda | <0.1 ± <0.1a | nda | nda | nda | nda | nda | <0.1 ± <0.1a | nda | nda | 0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda |
| C7 | 4.3 ± 0.5abc | 6.5 ± 1.6abc | 4.8 ± 0.5abc | 7.7 ± 2.1abc | 5.8 ± 0.5abc | 4.9 ± 2.2abc | 4.7 ± 1.6abc | 1.4 ± 1.1ab | 1.2 ± 0.9ab | 7.3 ± 1.8abc | 2.5 ± 0.6abc | 2.1 ± 0.8abc | 9.9 ± 1.7c | 2.4 ± 0.7abc | 0.4 ± 0.2a | 7.7 ± 2.4abc | 2.2 ± 0.8abc | nda | 8.9 ± 0.9bc | 1.0 ± 0.5ab | 0.3 ± 0.2a |
| C8 | 0.7 ± 0.2ab | nda | 0.1 ± 0.1a | 3.1 ± 1.6ab | nda | 0.6 ± 0.2ab | 0.4 ± 0.1a | nda | nda | 3.5 ± 1.5ab | 0.2 ± 0.1a | 0.1 ± <0.1a | 5.5 ± 1.7b | 0.4 ± 0.2a | nda | 3.4 ± 1.9ab | nda | nda | 1.9 ± 1.0ab | nda | nda |
| C9 | 0.1 ± <0.1ab | nda | nda | 0.2 ± 0.1bc | nda | nda | nda | nda | nda | 0.2 ± 0.1bc | nda | nda | 0.3 ± 0.1c | nda | nda | 0.2 ± 0.1bc | nda | nda | 0.2 ± <0.1bc | nda | nda |
| C10 | 1.0 ± <0.1ab | 3.0 ± 0.6bc | 2.8 ± 0.7bc | 1.2 ± 0.2abc | 4.0 ± 0.8c | 2.1 ± 0.8abc | 1.0 ± 0.3ab | 0.8 ± 0.6ab | 0.6 ± 0.5ab | 1.0 ± 0.1ab | 1.1 ± 0.1ab | 1.1 ± 0.3ab | 1.3 ± 0.2abc | 0.9 ± 0.1ab | 0.4 ± 0.2ab | 1.3 ± 0.1abc | 1.5 ± 0.7abc | nda | 1.5 ± 0.1abc | 1.2 ± 0.4abc | 0.4 ± 0.1ab |
| C11 | 0.4 ± 0.1ab | nda | nda | 1.0 ± 0.4ab | nda | 0.2 ± 0.2ab | 0.2 ± 0.1ab | nda | nda | 1.4 ± 0.6ab | nda | nda | 1.6 ± 0.5b | 0.3 ± 0.1ab | nda | 1.1 ± 0.5ab | nda | nda | 0.8 ± 0.2ab | nda | nda |
| C12 | 0.2 ± <0.1ab | nda | nda | 0.2 ± 0.1ab | nda | 0.1 ± <0.1a | <0.1 ± <0.1a | nda | nda | 0.4 ± <0.1bc | nda | nda | 0.4 ± <0.1bc | nda | nda | 0.5 ± 0.1c | nda | nda | 0.4 ± 0.1bc | nda | nda |
| C13 | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | 0.4 ± <0.1ab | 0.2 ± 0.1ab | <0.1 ± <0.1a | 0.9 ± 0.4b | nda | nda | 0.4 ± 0.2ab | 0.2 ± 0.2ab | nda | 0.1 ± 0.1a | nda |
| C14 | 39.3 ± 5.4a | 1.0 ± 2.9a | 25.0 ± 2.5a | 36.9 ± 14.1a | 12.5 ± 4.0a | 12.5 ± 3.8a | 14.1 ± 1.9a | 9.9 ± 5.0a | 20.7 ± 14.0a | 17.0 ± 1.4a | 23.9 ± 7.8a | 17.0 ± 6.6a | 14.3 ± 1.1a | 10.4 ± 3.6a | 4.7 ± 2.1a | 15.7 ± 1.9a | 38.0 ± 5.5a | 8.6 ± 4.3a | 24.2 ± 5.2a | 17.5 ± 3.4a | 4.9 ± 3.2a |
| C15 | 12.2 ± 2.6abc | 4.8 ± 2.8abc | 6.8 ± 2.8abc | 18.7 ± 5.6c | 3.4 ± 1.6abc | 12.9 ± 5.4abc | 6.9 ± 1.6abc | 2.1 ± 1.4ab | 1.7 ± 1.2ab | 15.3 ± 1.8abc | 6.1 ± 1.2abc | 5.2 ± 2.0abc | 16.5 ± 1.2bc | 7.3 ± 2.5abc | 2.1 ± 0.5ab | 11.7 ± 3.3abc | nda | nda | 17.4 ± 3.2bc | 2.0 ± 1.4ab | nda |
| C16 | nda | nda | nda | nda | nda | nda | nda | nda | nda | 0.1 ± <0.1a | nda | nda | 1.4 ± 1.1a | nda | nda | nda | nda | nda | nda | nda | nda |
| C17 | 0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | <0.1 ± <0.1a | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | 0.1 ± 0.1a | nda | nda | nda | nda | <0.1 ± <0.1a | nda | nda |
| C18 | 0.2 ± <0.1a | nda | 0.1 ± <0.1a | 0.1 ± 0.1a | nda | 0.1 ± 0.1a | 0.1 ± <0.1a | 0.2 ± 0.1a | 0.1 ± <0.1a | 0.1 ± <0.1a | 0.2 ± 0.1a | nda | 0.1 ± <0.1a | 0.1 ± <0.1a | nda | 0.2 ± 0.1a | 0.2 ± 0.1a | nda | 0.3 ± 0.1a | nda | nda |
| C19 | nda | nda | nda | nda | nda | nda | 0.9 ± 0.4a | 1.2 ± 0.9a | nda | 2.3 ± 1.4a | nda | nda | 3.3 ± 1.4a | nda | <0.1 ± <0.1a | 1.8 ± 1.4a | nda | nda | 2.2 ± 1.8a | nda | nda |
| C20 | 1.2 ± 0.2a | 1.5 ± 0.6a | 1.5 ± 0.6a | 0.7 ± 0.3a | nda | 0.7 ± 0.2a | 4.3 ± 1.0a | 3.4 ± 1.6a | 0.3 ± 0.1a | 1.1 ± 0.3a | 1.6 ± 0.5a | 0.6 ± 0.2a | 1.3 ± 0.2a | 1.3 ± 0.4a | 0.1 ± <0.1a | 0.8 ± 0.1a | nda | nda | 2.4 ± 0.5a | 4.7 ± 2.4a | 0.3 ± 0.2a |
| C21 | 0.1 ± <0.1a | 14.3 ± 4.7abcd | 11.7 ± 3.5a | 0.1 ± <0.1a | 8.7 ± 2.8abc | 12.9 ± 9.9abcd | 0.2 ± <0.1a | 36.9 ± 7.5cd | 27.2 ± 5.7abcd | 0.1 ± <0.1a | 11.1 ± 2.8abcd | 24.7 ± 10.0abcd | <0.1 ± <0.1a | 15.8 ± 2.5abcd | 36.8 ± 2.6cd | 0.1 ± <0.1a | 13.3 ± 1.2abcd | 23.5 ± 7.1abcd | 0.3 ± <0.1ab | 30.4 ± 4.8bcd | 40.9 ± 3.5d |
| C22 | nda | 7.6 ± 3.4abc | 8.2 ± 4.1abc | nda | 4.2 ± 1.8ab | 17.7 ± 9.9abc | 0.2 ± 0.1a | 27.1 ± 7.6abc | 43.3 ± 12.8abc | nda | 9.9 ± 3.3abc | 21.3 ± 10.8abc | nda | 25.9 ± 6.4abc | 48.2 ± 3.0c | nda | 6.3 ± 3.4abc | 46.0 ± 19.4bc | nda | 30.9 ± 0.6abc | 48.9 ± 2.6c |
| C23 | nda | nda | nda | nda | nda | nda | <0.1 ± <0.1ab | nda | nda | <0.1 ± <0.1ab | nda | nda | <0.1 ± <0.1ab | nda | nda | nda | nda | nda | <0.1 ± <0.1b | nda | nda |
| C24 | <0.1 ± <0.1ab | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | 0.1 ± <0.1ab | nda | nda | 0.1 ± <0.1ab | nda | nda | 0.1 ± <0.1b | nda | nda | <0.1 ± <0.1ab | nda | nda |
| C25 | nda | 1.1 ± 0.7ab | 1.5 ± 0.8ab | nda | nda | nda | nda | 1.8 ± 0.6ab | 0.3 ± 0.2a | nda | 0.4 ± 0.2a | 1.5 ± 0.8ab | nda | 2.6 ± 0.8ab | 2.1 ± 1.3ab | nda | 10.0 ± 5.2b | 7.8 ± 2.7ab | nda | nda | nda |
| C26 | 1.9 ± 0.3abc | 5.0 ± 0.6c | 3.3 ± 0.7abc | 1.3 ± 0.3abc | 4.6 ± 1.4bc | 2.1 ± 0.9abc | 2.8 ± 0.4abc | 1.8 ± 0.8abc | 0.7 ± 0.4ab | 3.2 ± 0.3abc | 2.0 ± 0.3abc | 1.5 ± 0.4abc | 1.9 ± 0.3abc | 1.4 ± 0.3abc | 0.6 ± 0.3ab | 2.7 ± 0.4abc | 2.7 ± 0.7abc | 1.9 ± 1.0abc | 2.1 ± 0.1abc | 1.4 ± 0.5abc | 0.4 ± <0.1a |
| C27 | <0.1 ± <0.1c | nda | nda | <0.1 ± <0.1b | nda | nda | nda | nda | nda | <0.1 ± <0.1b | nda | nda | <0.1 ± <0.1b | nda | nda | <0.1 ± <0.1b | nda | nda | nda | nda | nda |
| C28 | nda | nda | 1.3 ± 0.6b | nda | nda | nda | nda | nda | 0.1 ± <0.1a | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda |
| C29 | 0.4 ± 0.1a | nda | nda | 0.2 ± 0.1a | nda | 0.1 ± 0.1a | 1.8 ± 0.7b | nda | nda | 0.4 ± <0.1a | 0.3 ± 0.1a | nda | 0.3 ± <0.1a | 0.4 ± 0.2a | nda | 0.2 ± 0.1a | nda | nda | 0.2 ± 0.1a | nda | nda |
| C30 | 10.4 ± 1.4cd | nda | nda | 7.0 ± 2.9abc | nda | 4.7 ± 2.1abc | 6.2 ± 1.1abc | nda | nda | 10.8 ± 1.3cd | 3.8 ± 0.4abc | 1.0 ± 0.4ab | 12.1 ± 1.6cd | 3.7 ± 1.6abc | 0.6 ± 0.1ab | 16.7 ± 3.5d | nda | nda | 8.9 ± 1.8bcd | nda | nda |
| C31 | nda | nda | nda | 0.1 ± <0.1a | nda | nda | 0.4 ± 0.2b | nda | nda | 0.1 ± <0.1a | nda | nda | 0.1 ± <0.1ab | nda | nda | 0.1 ± <0.1ab | nda | nda | 0.1 ± <0.1a | nda | nda |
| C32 | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | nda | 3.3 ± 1.4b | nda | nda | nda | nda | nda | nda | nda | nda |
| C33 | nda | nda | nda | <0.1 ± <0.1a | nda | nda | 0.1 ± 0.1b | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda |
| C34 | nda | nda | nda | nda | nda | nda | nda | nda | nda | 2.2 ± 0.9ab | nda | nda | 3.9 ± 0.5b | nda | nda | 3.1 ± 1.5b | nda | nda | nda | nda | nda |
| C35 | nda | nda | nda | 0.1 ± <0.1a | nda | nda | nda | nda | nda | 0.2 ± <0.1ab | nda | nda | 0.5 ± 0.1b | nda | nda | 0.2 ± 0.1ab | nda | nda | 0.2 ± 0.1ab | nda | nda |
| C36 | 1.5 ± 0.9a | 7.6 ± 3.3a | nda | 2.4 ± 1.1a | nda | nda | 41.7 ± 16.8b | 11.2 ± 2.9a | 0.4 ± 0.2a | 15.8 ± 7.0ab | 12.1 ± 6.2a | 2.9 ± 1.3a | 7.4 ± 3.2a | 4.8 ± 3.3a | nda | 8.2 ± 2.5a | nda | nda | 11.2 ± 1.6a | nda | nda |
| C37 | nda | nda | nda | nda | nda | nda | nda | nda | nda | <0.1 ± <0.1c | nda | nda | <0.1 ± <0.1b | nda | nda | nda | nda | nda | nda | nda | nda |
| C38 | 0.3 ± <0.1a | 1.6 ± 0.4ab | 1.4 ± 0.2ab | 0.2 ± 0.1a | 3.0 ± 1.2b | 0.1 ± 0.1a | 1.2 ± 0.1ab | 1.2 ± 0.3ab | 0.5 ± 0.2ab | 0.4 ± 0.1ab | 0.9 ± 0.4ab | 0.5 ± 0.2ab | 0.2 ± 0.1a | 0.4 ± 0.3ab | nda | 0.3 ± <0.1a | 2.3 ± 1.1ab | nda | 0.5 ± <0.1ab | 0.5 ± 0.3ab | 0.1 ± 0.1a |
| C39 | nda | nda | nda | 0.1 ± <0.1a | nda | nda | nda | nda | nda | 0.1 ± <0.1a | nda | nda | 0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | nda | nda |
| C40 | 0.1 ± <0.1a | nda | nda | 0.3 ± 0.1a | nda | nda | 0.3 ± 0.1a | 0.1 ± <0.1a | nda | 0.3 ± <0.1a | 0.5 ± 0.2a | nda | 0.3 ± 0.1a | nda | nda | 0.2 ± 0.1a | nda | nda | 0.3 ± 0.1a | nda | nda |
| C41 | nda | nda | nda | nda | nda | nda | nda | nda | nda | 9.0 ± 0.2b | nda | nda | 7.7 ± 0.4b | nda | nda | 6.9 ± 3.0b | nda | nda | nda | nda | nda |
| C42 | <0.1 ± <0.1a | 0.3 ± 0.1ab | 0.3 ± 0.1ab | <0.1 ± <0.1a | 0.7 ± 0.3b | 0.1 ± 0.1a | <0.1 ± <0.1a | nda | nda | <0.1 ± <0.1a | <0.1 ± <0.1a | <0.1 ± <0.1a | <0.1 ± <0.1a | <0.1 ± <0.1a | <0.1 ± <0.1a | 0.1 ± <0.1a | 0.2 ± 0.1ab | nda | 0.1 ± <0.1a | <0.1 ± <0.1a | <0.1 ± <0.1a |
nd = not detected.
Superscript letters in the same row indicate differing levels of significance for each respective cultivar across all sampling days (ANOVA Tukey’s HSD test; (p = ⩽0.05)).
Fig. 1.
Loadings plot (a) and scores plot (b) from principal components analysis of seven accessions of E. sativa and the volatile organic compounds identified. Data points were averaged for each accession time point (consisting of three replicates). PC1 vs. PC2 (F1 and F2) accounted for 56.72% of the total variation within data. For compound identities refer to Table 1. SR codes refer to each of the 7 accessions used in the experiment (SR2, SR3, SR5, SR6, SR12 SR14 and SR19) with the following colour coding, 0 (green), 3 (orange) and 7 (red) corresponding to the day of sampling.
3.2.2. Within-day variation
3.2.2.1. General
A separate ANOVA Tukey’s HSD test (p ⩽ 0.05) was performed on data from each of the three sampling days. Several significant differences were observed between cultivars on each day. Twelve compounds were significantly different between accessions on ‘Day 0’, nine on ‘Day 3’ and six on ‘Day 7’. The dwindling abundances of VOCs in the latter shelf-life samples would seem to indicate the depletion of GSLs and other defense related compounds. The most interesting of these are discussed in this section.
3.2.2.2. Isothiocyanates
On ‘Day 0’ SR6 and SR5 were the only accessions where 4-isothiocyanato-1-butene (C2, both 0.1 ± <0.1%) was detected. 4-methylpentyl ITC (C20) was detected in all accessions with SR5 being significantly different (4.3 ± 1.0%) from all others with the exception of SR19 (2.4 ± 0.5%). SR5 contained over six times greater abundance of this ITC than SR3.
Hexyl ITC (C29) was similarly detected in all accessions (‘Day 0’), with SR5 having the highest abundance (1.8 ± 0.7%) – significantly different in this case from SR19, which had the lowest recorded abundance (0.2 ± 0.1%; nine times less abundant in relative terms). Bell et al. (2015) found that SR5 had high total GSL concentrations (11.5 mg g−1 DW) compared with other Eruca and Diplotaxis accessions, potentially making it a valuable source of genetic material for breeding programmes interested in enhancing GSL/ITC accumulation traits.
3.2.2.3. Alcohols
(Z)-2-penten-1-ol (C9) was detected in all accessions except SR5, with the highest abundance detected in SR12 (0.3 ± 0.1%; ‘Day 0’) which was significantly different from zero (i.e. significantly different from samples with 0% abundance). Other alcohols, such as 1-penten-3-ol (C3) displayed no significant differences on this sampling day. Some of these differences may indicate a genetic component to VOC production, i.e. the types and abundances produced may be under direct genetic regulation. Alcohols are typically used by plants as a defensive mechanism (Ruther & Kleier, 2005), and often provide the ‘cut grass’ aroma found in leafy vegetables. Plant defence mechanisms are known to rely on genetic regulation via enzymatic regulation in other species (D’Auria, Pichersky, Schaub, Hansel, & Gershenzon, 2007), and it is reasonable to assume that the same may be true for rocket. D’Auria et al. (2007), showed this to be the case in Arabidopsis for production of (Z)-3-hexen-1-yl acetate via a BAHD acetyltransferase enzyme. It is also widely known that nitriles, epithionitriles and thiocyanates are produced enzymatically in Brassicaceae through specifier proteins in the GSL-myrosinase reaction (Kuchernig, Backenköhler, Lübbecke, Burow, & Wittstock, 2011).
3.2.2.4. Sulphur aromatic compounds
3-Ethyl-thiophene (C37) was detected in only two accessions, SR6 and SR12 (both <0.1 ± <0.1%, ‘Day 0’) and were significantly different from each other, despite the exceedingly small abundances observed. Tetrahydrothiophene (C38) was detected in every accession, but SR5 had the highest abundance (1.2 ± 0.1%, ‘Day 0’) which was significantly different from the other samples. It is likely that these types of VOCs also contribute to the characteristic pungent/mustard/pepper sensory attributes of rocket, as well as ITCs.
By ‘Day 7’, pyrrolidine-1-dithiocarboxylic acid 2-oxocyclopentyl ester (C36) was detected in only two accessions: SR5 (0.4 ± 0.2%) and SR6 (2.9 ± 1.3%). Tetrahydrothiophene (C38) was present in all accessions with the exception of SR12 and SR14.
3.2.2.5. Imines
On ‘Day 3’ 5-nonanone-oxime (C21) was most abundant in SR5 (36.9 ± 7.5%), significantly higher than in either SR3 or SR6 (8.7 ± 2.8%; 11.1 ± 2.8%). Abundances were generally much higher across all accessions than on ‘Day 0’, though with fewer significant differences between accessions, possibly because of more variation between replicates. SR3 also contained the lowest abundance of O-methyloxime-butanal (C22) (4.2 ± 1.8%). In contrast, SR19 had the highest abundance (30.9 ± 0.6%) of this compound.
3.2.2.6. Aldehydes
A very large range of abundances was observed for (E)-4-oxohex-2-enal (C1) amongst accessions. This compound was not observed at all in SR5 on ‘Day 3’, yet accounted for 49.9 ± 0.9% of VOCs produced by SR3 on this sampling day. SR3 also contained significantly higher abundance of (E)-2-pentenal (C10) (4.0 ± 0.8%) than did SR5 (0.8 ± 0.6%) on ‘Day 3’. In contrast, a narrow range of abundance was observed for 2-hexenal (C7); SR19 had the lowest (1.0 ± 0.5%) and SR2 the highest (6.5 ± 1.6%), with both being the only accessions significantly different from each other.
The more exotic aldehyde (E,E)-2,4-hexadienal (C11) was only observed in SR12 (0.3 ± 0.1%) on ‘Day 3’. These observations are interesting, in contrast with ‘Day 0’, where no significant differences were observed in any of the aldehyde compounds that were present. Despite the apparent differences in the percentage abundances in compound C1 (ranging from 2.5% to 22.4%), the fact that no significant differences were observed can most likely be explained by the very large standard errors of each accession, and the highly variable nature of VOC production. It might suggest, however, that some varieties are more genetically predisposed to differing rates of lipid oxidation, that are characteristic of plants kept in storage (Varming et al., 2004).
Figs. 1–4, which display the PCA plots, also show how individual replicates from each accession vary in VOC abundance and profile within each sampling day. In some cases this can be quite marked, illustrating how profiles can be affected by multiple and very small factors, despite efforts to maintain constant experimental conditions. Further independent experiments will allow better elucidation and confirmation of VOC profiles and relative abundances in rocket in terms of within-day and within-cultivar production.
Fig. 2.
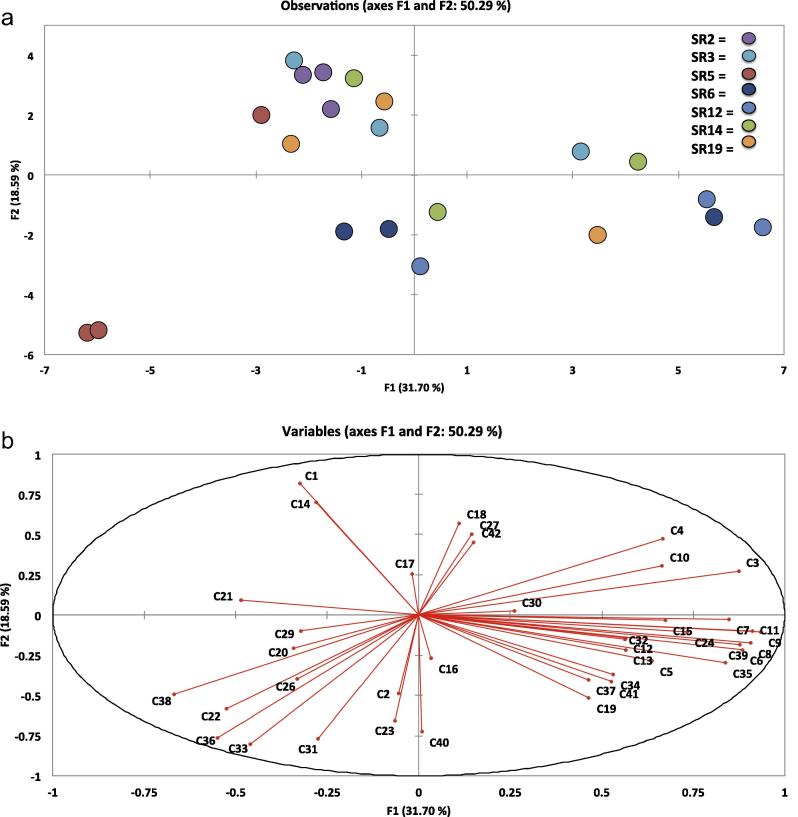
Loadings plot (a) and scores plot (b) from principal components analysis of seven accessions of E. sativa, and the volatile organic compounds identified on ‘Day 0’. Individual replicates were plotted to visualize variation within and between accessions. PC1 vs. PC2 (F1 and F2) accounted for 50.29% of the total variation within data. For compound identities refer to Table 1. SR codes refer to each of the seven accessions used in the experiment (SR2, SR3, SR5, SR6, SR12, SR14 and SR19). See inset for accession colour coding.
Fig. 3.
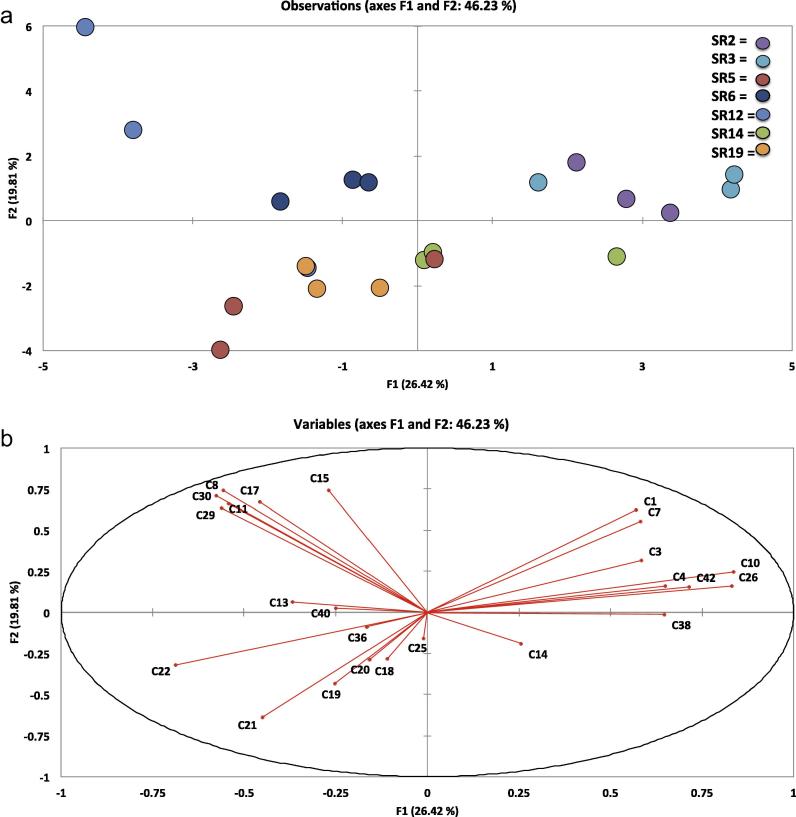
Loadings plot (a) and scores plot (b) from principal components analysis of seven accessions of E. sativa, and the volatile organic compounds identified on ‘Day 3’. Individual replicates were plotted to visualize variation within and between accessions. PC1 vs. PC2 (F1 and F2) accounted for 46.23% of the total variation within data. For compound identities refer to Table 1. SR codes refer to each of the seven accessions used in the experiment (SR2, SR3, SR5, SR6, SR12, SR14 and SR19). See inset for accession colour coding.
Fig. 4.
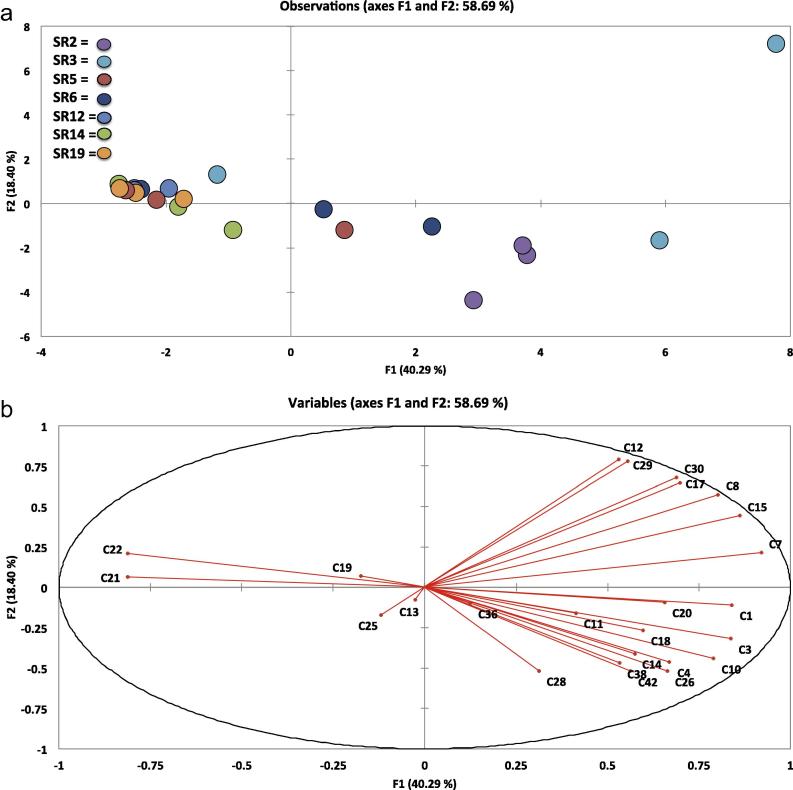
Loadings plot (a) and scores plot (b) from principal components analysis of seven accessions of E. sativa, and the volatile organic compounds identified on ‘Day 7’. Individual replicates were plotted to visualize variation within and between accessions. PC1 vs. PC2 (F1 and F2) accounted for 58.69% of the total variation within data. For compound identities refer to Table 1. SR codes refer to each of the seven accessions used in the experiment (SR2, SR3, SR5, SR6, SR12, SR14 and SR19). See inset for accession colour coding.
3.3. Correlation of VOC abundance with shelf-life time points: PCA
3.3.1. General
PCA of VOCs across and within the three time-points revealed significant correlations between accessions and the prevalence of different types of compounds during storage, indicating sizeable variation amongst the seven accessions. All data are represented as sample scores and loadings plots (Figs. 1–4).
3.3.2. Across-day PCA
PC1 vs. PC2 (F1 and F2; Fig. 1) accounted for 56.72% of the total variation within data and r-values became significant at ±0.434 (p ⩽ 0.05). ‘Day 0’ samples formed a distinct, linear cluster (green; Fig. 1a) spanning the first principal component. In contrast, however, ‘Day 3’ (yellow) and ‘Day 7’ (red) samples were not separable and both formed a linear cluster along the second principal component (F2).
Table 2 illustrates where significant differences between compounds for each accession were observed. When compared with the scores plot, it can be seen that a distinct cluster of volatiles is highly correlated with ‘Day 0’ along the first principal component, and two other separate localizations of VOCs can be seen correlating in the top and bottom left of Fig. 1b. This suggests that there may be some genotypic differentiation, in terms of the volatiles produced on both ‘Day 3’ and ‘Day 7’. Compounds with strong correlations along the first principal component include (Z)-2-penten-1-ol (C9 r = 0.950), (E)-2-hexenal (C8 r = 0.946), (E,E)-2,4-hexadienal (C11 r = 0.964), 2-methyl-2-butanal (C6 r = 0.916), 5-ethyl-2(5H)-furanone (C12 r = 0.915), 4-methyl-2-(2-methyl-1-propenyl)-pyridine (C35 r = 0.907), ethylidene-cyclopropane (C24 r = 0.912) and 5-methyl-4-hexen-3-one (C19 r = 0.886).
Compounds correlating with accessions along the second principal component, and with SR3 and SR2, on ‘Day 3’ and ‘Day 7’ consist of vinylfuran (C42 r = 0.874), (E)-4-oxohex-2-enal (C1 r = 0.889) and tetrahydrothiophene (C38 r = 0.716).
The presence of aldehydes within the ‘Day 3’ and ‘Day 7’ clusters is consistent with extensive and prolonged lipid degradation over the shelf life period. The exact role of many of these VOCs is unknown, and the exact significance they may have in affecting human sensory attributes when rocket is consumed is similarly not well understood.
3.3.3. Within-day PCA
3.3.3.1. ‘Day 0’
The first two principal components (F1 and F2; Fig. 2) explained 50.29% of the total variation present within the sample set and correlations between accessions became significant at r = ±0.433 (p ⩽ 0.05). Four clusters are apparent in the loadings plot (Fig. 2a); however, only one of these clusters contains all three respective sample replicates for the accessions (SR2). This spread of individual replicates across clusters indicates that VOC production for different germplasm accessions is inherently variable, even within genotypes.
The cluster located in the top left of the plot contains all three replicates of SR2, indicating a high degree of uniformity in terms of VOC production on the initial sampling day. This cluster also contains two replicates of accessions SR3 and SR19. It is interesting to note that the commercial accession (SR3) displays more variation than do many of the ‘wild’ germplasm accessions. It would be expected that commercial cultivars would be more uniform than cultivars of open pollinated accessions, as they (in theory) would have had at least some rudimentary plant breeding before commercial sale. Artificial selections would have been made in a breeding programme to select plants that had desirable characteristics, such as a pungent odour. Throughout successive generations, one would expect the variation associated with such a trait to decrease, but that does not appear to be the case and is perhaps indicative of little concerted breeding to improve varietal uniformity. When compared with Fig. 2b, it can be seen that two compounds in particular are correlated with this cluster: (E)-4-oxohex-2-enal (C1 r = 0.816) and 3-hexen-1-ol (C14 r = 0.699). The former of these was highlighted in the previous section as being an abundant compound in accessions, but on sampling ‘Day 3’ and ‘Day 7’.
The second cluster to the extreme right of Fig. 2a is much less compact, containing two replicates of SR12 and individual replicates of SR3, SR14, SR6 and SR19. Compounds strongly correlated in this position along the principal component in Fig. 2b are much more numerous and diverse, including (Z)-2-penten-1-ol (C9 r = 0.934), (E,E)-2,4-hexadienal (C11 r = 0.911), (E)-2-hexenal (C8 r = 0.907), 2-methyl-2-butanal (C6 r = 0.884), 4-methyl-2-(2-methyl-1-propenyl)-pyridine (C35 r = 0.837), 2-hexenal (C7 r = 0.847) and ethylidene-cyclopropane (C24 r = 0.733). These compounds are indicative of ‘Day 0’ VOCs and seem to be produced in most abundance at the initial point of tissue damage, with levels declining or completely disappearing on subsequent sampling days.
The third cluster (located centrally and almost equidistant from clusters one and two) contains two replicates of SR6 and single replicates of SR14 and SR12. Compounds correlated in this vicinity of the scores plot are much more loosely distributed and have no strong correlation with either principal component. The presence of ITC compounds towards the lower section of the analysis plot indicates that production of these compounds is more dominant in certain accessions, or perhaps even individual plants within each accession.
The final cluster consists solely of two replicates of SR5, and is the most extreme within the sample set. As mentioned previously, SR5 is a promising cultivar that appears to be very different, in many respects, from other accessions of E. sativa, such as its higher GSL and ITC contents.
3.3.3.2. ‘Day 3’
Fig. 3 illustrates the PCA for ‘Day 3’ samples. 46.23% of variation within the data is explained by the analysis, with correlations becoming significant at r = ±0.434 (p ⩽ 0.05). Variation between replicates on this sampling day is much reduced, with replicates for each respective accession being much closer together spatially than on ‘Day 0’. Clusters are much less well defined, but can be broadly characterized into three groups.
The first cluster (Fig. 3a) consists of just two replicates of SR12 which seem to be relative outliers overall (much like SR5 on ‘Day 0’). These replicates are generally characterized by the prevalence of a set of VOCs correlated in this same direction: 3-hexenal (C15 r = 0.741), (E)-2-hexenal (C8 r = 0.742), 3-octyne (C17 r = 0.671), and hexyl ITC (C29 r = 0.634). Some of these compounds are more indicative of ‘Day 0’ VOCs, and may indicate a predisposition in this accession for these types of compounds to be produced for a prolonged period of time after the initial production stimuli. This may have implications for the food and agricultural industries, as it implies that beneficial health compounds can be sustained during shelf life by appropriate selection of varieties. The impact of the industrial supply chain on phytochemical content has yet to be properly determined.
The second cluster is central to the plot in Fig. 3a, stretching towards the lower left. This loose cluster contains all three replicates of SR6, SR5 and SR19, with two replicates of SR14, and a single replicate of SR12. Genotypically, this cluster represents the most diverse range of accessions.
The final cluster to the right side of Fig. 3a includes all three replicates of SR2 and SR3, and one replicate of SR14. Compounds correlating in this direction include (E)-4-oxohex-2-enal (C1 r = 0.571), 2-hexenal (C7 r = 0.582), 1-penten-3-ol (C3 r = 0.584), (E)-2-pentenal (C10 r = 0.835), 1-penten-3-one (C4 r = 0.650), vinylfuran (C42 r = 0.714), 2-ethyl-furan (C26 r = 0.830) and tetrahydrothiophene (C38 r = 0.647). These compounds are indicative of the ‘Day 3’ profile highlighted in the previous section.
3.3.3.3. ‘Day 7’
Fig. 4 displays the PCA plots for ‘Day 7’, where 58.69% of variation was explained by the data, and correlations became significant at r = ±0.434 (p ⩽ 0.05). In Fig. 4a, samples can be broadly divided into two clusters, left and right of the y-axis. Samples on the left are tightly clustered and those to the right are thinly spread along the x-axis. SR3, SR6 and SR5 have replicates contained in both clusters, which is unusual, considering that on ‘Day 3’ they were relatively close together. SR3 is the most extreme example, with replicates spread very far apart, indicating increased variability under controlled environmental conditions.
The dense left cluster contains all replicates of SR14, SR12 and SR19, two replicates of SR5 and one replicate of SR3, but it contained no significant correlations with any compounds.
The cluster on the right of the plot contains all replicates of SR2, two replicates of SR3 and SR6, and one replicate of SR5. Many of the compounds seem to be skewed on the scores plot in the direction of the SR3 and SR2 replicates. This suggests that these accessions have maintained a degree of VOC diversity later in the shelf-life period than the other accessions, which may be attributable to differing genetics. Compounds correlated with these samples along the first principal component include: 2-hexenal (C7 r = 0.920), 3-hexenal (C15 r = 0.862), (E)-4-oxohex-2-enal (C1 r = 0.840), 1-penten-3-ol (C3 r = 0.837), (E)-2-pentenal (C10 r = 0.791), (E)-2-hexenal (C8 r = 0.803), 3-octyne (C17 r = 0.697), 2-ethyl-furan (C26 r = 0.663). The presence of aldehyde compounds in this group is again indicative of extensive lipid breakdown.
3.4. Implications of detected VOCs for rocket quality and human nutrition
Between 1.0 ± 0.6% (SR3) and 6.8 ± 2.2% (SR5) of volatile compounds produced on ‘Day 0’ are isothiocyanates that may have potential benefits for human health (Verkerk et al., 2009). Although present at relatively low levels compared to other VOCs detected, isothiocyanates have been shown to be efficacious in eliciting health benefits at very low concentrations in previous in vitro studies with other crops (10 μM; Hanlon, Webber, & Barnes, 2007). Abundance of these compounds declined with storage to less than one percent on ‘Day 7’, indicating that, by the time the rocket leaves reach the consumer, it is possible that there has been a substantial drop in nutritional value. Processes that precede packaging, such as harvest, transport and washing, can be especially harsh on leaves, although some forms of stress can induce secondary metabolite production (Mewis et al., 2012). Thus, combined effects of storage and handling during the supply chain should be examined in relation to VOC loss.
4. Conclusions
Our results represent rocket plants grown under controlled environment conditions, and may differ from plants that are grown under variable field conditions. Many pre-harvest factors may affect the abundance and ratios of VOCs, such as disease status, soil type, light intensity and water status (Varming et al., 2004). Our method was established in order to minimize these stress effects and produce results based on genotypic and post-harvest storage factors, rather than pre-harvest environmental variation. Changes in phytochemical content between different growing environments are poorly documented in rocket species, and non-existent for VOCs. Future work will examine the effects that these factors may have on the crop and the end consumer, by conducting experiments in a commercial, field trial setting.
Our study has shown that there are significant differences amongst cultivars in the relative abundance of volatiles that they produce post-harvest and their retention during storage. Therefore, there is scope for plant breeders to consider basing selections, at least in part, on post-harvest VOC profiles in order to select for improved flavour and nutritionally valuable traits. By assessing such data in the supply chain, alongside phytochemical screening at harvest, rocket lines can feasibly be bred to limit losses of important VOCs, such as ITCs. More research is needed to fully understand where exactly these losses occur within the supply chain, and what (if anything) can be done to mitigate such potential losses.
The sampling methodology established here might also have potential applications within the food processing industry as part of quality assurance methods for rocket leaves. Sampling itself is straightforward and requires little specialist knowledge. Samples could be taken at critical points during processing to assess effects on VOC profiles with the aim of keeping ITC losses to a minimum. Most, if not all, producers routinely take and store samples of different batches of rocket salad to assess visual traits. In the near future, it is likely that quality assurance will expand beyond this to both phytochemical and volatile traits of many crops, not just rocket salad.
Funding
Luke Bell is supported by a BBSRC Case Award (Reference BB/J012629/1) in partnership with Elsoms Seeds Ltd. (Spalding, United Kingdom) and Bakkavor Group Ltd. (Spalding, United Kingdom).
Work conducted at the Cardiff University is supported by the European Union Seventh Framework Program for research, technological development and demonstration, as part of the QUAFETY (Quality/Safety) collaborative project (KBBE 2011 2.4-01).
Conflict of interest
The authors declare no conflict of interest.
Acknowledgement
The authors wish to thank Matthew Richardson (University of Reading) for assistance with controlled environment rooms.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.foodchem.2015.08.043.
Appendix A. Supplementary data
Supplementary Fig. S1.
References
- Bajpai V.K., Rahman A., Kang S.C. Chemical composition and inhibitory parameters of essential oil and extracts of Nandina domestica Thunb. to control food-borne pathogenic and spoilage bacteria. International Journal of Food Microbiology. 2008;125(2):117–122. doi: 10.1016/j.ijfoodmicro.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Beaulieu J.C., Grimm C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. Journal of Agricultural and Food Chemistry. 2001;49(3):1345–1352. doi: 10.1021/jf0005768. [DOI] [PubMed] [Google Scholar]
- Beevi S.S., Mangamoori L.N., Subathra M., Edula J.R. Hexane extract of Raphanus sativus L. roots inhibits cell proliferation and induces apoptosis in human cancer cells by modulating genes related to apoptotic pathway. Plant Foods for Human Nutrition. 2010;65(3):200–209. doi: 10.1007/s11130-010-0178-0. [DOI] [PubMed] [Google Scholar]
- Bell L., Oruna-Concha M.J., Wagstaff C. Identification and quantification of glucosinolate and flavonol compounds in rocket salad (Eruca sativa, Eruca vesicaria and Diplotaxis tenuifolia) by LC–MS: Highlighting the potential for improving nutritional value of rocket crops. Food Chemistry. 2015;172:852–861. doi: 10.1016/j.foodchem.2014.09.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazevic I., Mastelic J. Free and bound volatiles of rocket (Eruca sativa Mill.) Flavour and Fragrance Journal. 2008;23(4):278–285. [Google Scholar]
- Blazevic I., Mastelic J. Glucosinolate degradation products and other bound and free volatiles in the leaves and roots of radish (Raphanus sativus L.) Food Chemistry. 2009;113(1):96–102. [Google Scholar]
- Buttery R.G., Takeoka G.R. Some unusual minor volatile components of tomato. Journal of Agricultural and Food Chemistry. 2004;52:6264–6266. doi: 10.1021/jf040176a. [DOI] [PubMed] [Google Scholar]
- D’Auria J.C., Pichersky E., Schaub A., Hansel A., Gershenzon J. Characterization of a BAHD acyltransferase responsible for producing the green leaf volatile (Z)-3-hexen-1-yl acetate in Arabidopsis thaliana. Plant Journal. 2007;49(2):194–207. doi: 10.1111/j.1365-313X.2006.02946.x. [DOI] [PubMed] [Google Scholar]
- Egea-Gilabert C., Fernandez J.A., Migliaro D., Martinez-Sanchez J.J., Vicente M.J., Fernández J.A., Martínez-Sánchez J.J. Genetic variability in wild vs. cultivated Eruca vesicaria populations as assessed by morphological, agronomical and molecular analyses. Scientia Horticulturae. 2009;121(3):260–266. [Google Scholar]
- Farag M.A., Ryu C.-M., Sumner L.W., Paré P.W. GC–MS SPME profiling of rhizobacterial volatiles reveals prospective inducers of growth promotion and induced systemic resistance in plants. Phytochemistry. 2006;67(20):2262–2268. doi: 10.1016/j.phytochem.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Favell D.J. A comparison of the vitamin C content of fresh and frozen vegetables. Food Chemistry. 1998;62(1):59–64. [Google Scholar]
- Fernandes F., de Pinho P.G., Valentao P., Pereira J.A., Andrade P.B. Volatile Constituents throughout Brassica oleracea L. Var. acephala Germination. Journal of Agricultural and Food Chemistry. 2009;57(15):6795–6802. doi: 10.1021/jf901532m. [DOI] [PubMed] [Google Scholar]
- Grob K., Matile P. Capillary GC of glucosinolate-derived horseradish constituents. Phytochemistry. 1980;19(8):1789–1793. [Google Scholar]
- Guo L., Yang R., Wang Z., Guo Q., Gu Z. Glucoraphanin, sulforaphane and myrosinase activity in germinating broccoli sprouts as affected by growth temperature and plant organs. Journal of Functional Foods. 2014;9:70–77. [Google Scholar]
- Hanlon P.R., Webber D.M., Barnes D.M. Aqueous extract from Spanish black radish (Raphanus sativus L. Var. niger) induces detoxification enzymes in the HepG2 human hepatoma cell line. Journal of Agricultural and Food Chemistry. 2007;55(16):6439–6446. doi: 10.1021/jf070530f. [DOI] [PubMed] [Google Scholar]
- Hansen M., Laustsen A.M., Olsen C.E., Poll L., Sorensen H. Chemical and sensory quality of broccoli (Brassica oleracea L. var italica) Journal of Food Quality. 1997;20(5):441–459. [Google Scholar]
- Jirovetz L., Smith D., Buchbauer G. Aroma compound analysis of Eruca sativa (Brassicaceae) SPME headspace leaf samples using GC, GC–MS, and olfactometry. Journal of Agricultural and Food Chemistry. 2002;50(16):4643–4646. doi: 10.1021/jf020129n. [DOI] [PubMed] [Google Scholar]
- Kuchernig J.-C., Backenköhler A., Lübbecke M., Burow M., Wittstock U. A thiocyanate-forming protein generates multiple products upon allylglucosinolate breakdown in Thalspi arvense. Phytochemistry. 2011;72:1699–1709. doi: 10.1016/j.phytochem.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Mallard W.G., Sparkman O.D., Sparkman J.A. The National Institute of Standards and Technology: NIST; 2008. NIST standard reference database 1A. 1–49. [Google Scholar]
- Manchali S., Chidambara Murthy K.N., Patil B.S. Crucial facts about health benefits of popular cruciferous vegetables. Journal of Functional Foods. 2012;4(1):94–106. [Google Scholar]
- Mewis I., Schreiner M., Nguyen C.N., Krumbein A., Ulrichs C., Lohse M., Zrenner R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant & Cell Physiology. 2012;53(9):1546–1560. doi: 10.1093/pcp/pcs096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday R., Munday C.M. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: Comparison of allyl isothiocyanate with sulforaphane and related compounds. Journal of Agricultural and Food Chemistry. 2004;52(7):1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- Ragaert P., Verbeke W., Devlieghere F., Debevere J. Consumer perception and choice of minimally processed vegetables and packaged fruits. Food Quality and Preference. 2004;15:259–270. [Google Scholar]
- Rohloff J., Bones A.M. Volatile profiling of Arabidopsis thaliana – Putative olfactory compounds in plant communication. Phytochemistry. 2005;66(16):1941–1955. doi: 10.1016/j.phytochem.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Ruther J., Kleier S. Plant–plant signaling: Ethylene synergizes volatile emission in Zea mays induced by exposure to (Z)-3-Hexen-1-ol. Journal of Chemical Ecology. 2005;31(9):2217–2222. doi: 10.1007/s10886-005-6413-8. [DOI] [PubMed] [Google Scholar]
- Sanda M., Zacek P., Streinz L., Dracinsky M., Koutek B. Profiling and characterization of volatile secretions from the European stink bug Graphosoma lineatum (Heteroptera: Pentatomidae) by two-dimensional gas chromatography/time-of-flight mass spectrometry. Journal of Chromatography B. 2012;881–882:69–75. doi: 10.1016/j.jchromb.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Schouten R.E., Zhang X.B., Verkerk R., Verschoor J.A., Otma E.C., Tijskens L.M.M., van Kooten O. Modelling the level of the major glucosinolates in broccoli as affected by controlled atmosphere and temperature. Postharvest Biology and Technology. 2009;53(1–2):1–10. [Google Scholar]
- Servili M., Selvaggini R., Taticchi A., Esposto S., Montedoro G.F. Volatile compounds and phenolic composition of virgin olive oil: Optimization of temperature and time of exposure of olive pastes to air contact during the mechanical extraction process. Journal of Agricultural and Food Chemistry. 2003;51(27):7980–7988. doi: 10.1021/jf034804k. [DOI] [PubMed] [Google Scholar]
- Starkenmann C. Analysis of a model reaction system containing cysteine and (E)-2-methyl-2-butenal, (E)-2-hexenal, or mesityl oxide. Journal of Agricultural and Food Chemistry. 2003;51(24):7146–7155. doi: 10.1021/jf0304268. [DOI] [PubMed] [Google Scholar]
- Tandon K.S., Baldwin E.A., Shewfelt R.L. Aroma perception of individual volatile compounds in fresh tomatoes (Lycopersicon esculentum, Mill.) as affected by the medium of evaluation. Postharvest Biology and Technology. 2000;20(3):261–268. [Google Scholar]
- Varming C., Jensen K., Møller S., Brockhoff P.B., Christiansen T., Edelenbos M.…Poll L. Eating quality of raw carrots – Correlations between flavour compounds, sensory profiling analysis and consumer liking test. Food Quality and Preference. 2004;15:531–540. [Google Scholar]
- Verkerk R., Dekker M., Jongen W.M.F. Post-harvest increase of indolyl glucosinolates in response to chopping and storage of Brassica vegetables. Journal of the Science of Food and Agriculture. 2001;81(9):953–958. [Google Scholar]
- Verkerk R., Schreiner M., Krumbein A., Ciska E., Holst B., Rowland I.…Dekker M. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Molecular Nutrition & Food Research. 2009;53:S219–S265. doi: 10.1002/mnfr.200800065. [DOI] [PubMed] [Google Scholar]
- Wang C., Xing J., Chin C.-K., Ho C.-T., Martin C.E. Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry. 2001;58(2):227–232. doi: 10.1016/s0031-9422(01)00233-3. [DOI] [PubMed] [Google Scholar]
- Zhao D.Y., Tang J., Ding X.L. Analysis of volatile components during potherb mustard (Brassica juncea, Coss.) pickle fermentation using SPME-GC–MS. LWT – Food Science and Technology. 2007;40(3):439–447. [Google Scholar]