Abstract
In the small intestine the nature of the environment leads to a highly heterogeneous mucus layer primarily composed of the MUC2 mucin. We set out to investigate whether the soluble dietary fibre sodium alginate could alter the permeability of the mucus layer. The alginate was shown to freely diffuse into the mucus and to have minimal effect on the bulk rheology when added at concentrations below 0.1%. Despite this lack of interaction between the mucin and alginate, the addition of alginate had a marked effect on the diffusion of 500 nm probe particles, which decreased as a function of increasing alginate concentration. Finally, we passed a protein stabilised emulsion through a simulation of oral, gastric and small intestinal digestion. We subsequently showed that the addition of 0.1% alginate to porcine intestinal mucus decreased the diffusion of fluorescently labelled lipid present in the emulsion digesta. This reduction may be sufficient to reduce problems associated with high rates of lipid absorption such as hyperlipidaemia.
Keywords: Mucus, Digestion, Alginate, Dietary fibre, Permeability
Graphical abstract
Highlights
-
•
There are no specific interactions between alginate and intestinal mucus.
-
•
Mucus rheology was hardly altered by small additions of alginate.
-
•
Alginate freely diffused into mucus and reduced diffusion of 500 nm beads.
-
•
Intestinal mucus permeability was decreased by a factor of two.
1. Introduction
The putative health benefits of dietary fibre (DF) have been investigated over a number of years and have been shown to be many and varied (Brownlee, 2011; European Food Safety, 2010; Gunness & Gidley, 2010). By definition DF is not digested in the upper GI tract therefore early investigations into health benefits focused on the colon and the use of DF as a nutrient source for intestinal microbiota (Gibson & Roberfroid, 1995). However, more recently attention has also focused on interactions in the upper GI tract. In particular, the ability of DF to increase both gastric and intestinal viscosity has been investigated by a number of groups but a key early work showed that dietary fibre could be used to increase the viscosity of digesta and reduce the rate of absorption of glucose (Jenkins et al., 1978). More recent studies have confirmed the health benefits of a wide range of dietary fibre (Anderson et al., 2009; Frassetto, Schloetter, Mietus-Synder, Morris, & Sebastian, 2009; Nilsson, Ostman, Holst, & Bjorck, 2008). These include decreasing risk factors for CVD, lowering energy intake and glycaemic index, which have an impact on risk factors for type 2 diabetes. However, the mechanisms by which such health benefits are gained is still poorly understood (Lattimer & Haub, 2010). The DF sodium alginate has also been shown to reduce energy intake. In a study where participants consumed a preload sodium alginate formulation, daily preprandial ingestion of the sodium alginate formulation produced a significant 134.8 kcal (7%) reduction in mean daily energy intake (Paxman, Richardson, Dettmar, & Corfe, 2008).
Alginates are linear polysaccharides produced by marine brown algae (Phaeophyceae). They are used extensively in the food and chemical industries as thickening and stabilising agents. Alginate (E401) is a linear polysaccharide polymer with homopolymeric blocks of (1-4)-linked β-d-mannuronate (M) and α-l-guluronate (G) residues covalently linked together in different blocks. The monomers can appear in homopolymeric G-blocks, consecutive M-blocks or alternating MG-blocks. The ratio of M to G has a marked effect on the properties of the polymer. In a study by Jensen et al. (Jensen, Knudsen, Viereck, Kristensen, & Astrup, 2012) alginate solutions with a low M:G ratio (0.8) exhibited higher gel strength than solutions with higher M:G ratios (1.3 and 2.5). The gelation of alginate (sol/gel transition) is almost independent of temperature but is induced by the presence of divalent cations such as Ca2+, which is associated with the G-blocks. Indeed there is a strong link between the strength of calcium alginate gels and the average length of the G-blocks (Smidsrod & Draget, 1997).
Digestion in the upper GI tract releases nutrients, which then diffuse through the unstirred mucus layer to the intestinal enterocytes where they are absorbed. However, many nutrients such as lipids and some vitamins are not water soluble and so spontaneously form self-assembled structures such as micelles and liposomes (Mu & Hoy, 2004) before diffusing away from the intestinal lumen. Both small particles and soluble hydrocolloids are free to diffuse from the lumen but have the potential to be trapped by the intestinal mucus layer. The ability of dietary fibre to increase luminal viscosity is known to be an important factor affecting both transit times and rates of hydrolysis. Thus fibre may trap the products of digestion such as mixed micelles and consequently slow absorption. It has also been suggested that soluble dietary fibre may be trapped on the surface of the mucus and that this in turn prevents the diffusion of other hydrocolloids or mixed micelles (Gunness & Gidley, 2010; Theuwissen & Mensink, 2008). Such a mechanism has been used as a possible explanation of the cholesterol lowering ability of DF (Othman, Moghadasian, & Jones, 2011) as it would also interrupt the recycling of bile salts leading to the conversion of cholesterol into bile in the liver. In the absence of an attractive interaction between the DF and mucus the trapping of the DF by the mucus is dependent on the relative sizes of the DF polymers and the pores in the mucus network. Recent work has shown that the pore size of the mucus varies quite widely from 25 to 200 nm (Round et al., 2012). This compares to the size of a typical DF such as β-glucan, molecular weight 100–1000 kDa (150–250 nm) (Shelat, Vilaplana, Nicholson, Gidley, & Gilbert, 2011) or alginate of ∼190 KDa and ∼100 nm in diameter (Strand, Boe, Dalberg, Sikkeland, & Smidsrod, 1982).
Examples of alginate/mucin interaction with biological relevance have been shown previously (Taylor, Pearson, Draget, Dettmar, & Smidsrod, 2005). Indeed, the authors of the article have a patent for using oligo-guluronates alone (polyguluronic acid) to disrupt airway mucus in cystic fibrosis patients (Taylor, Draget, & Smidsrod, 2009). In addition to the function of the oligo-guluronates, the mixed block polymers have been suggested to be mucin secretagogs (Shimotoyodome, Meguro, Hase, Tokimitsu, & Sakata, 2001). This appears to be a general affect for high viscosity fibre (Ito et al., 2009) and this is partly supported by the fact that the use of low molecular weight sodium alginate in rats showed no activity after feeding for 10 days (Hino et al., 2013). Thus it appears that while G-block alginate polymers disrupt mucus structure, other alginates enhance the mucus layer.
In the work described here we have studied the ability of two different types of alginate with differing M:G ratios to diffuse into intestinal mucus and subsequently change its permeability to a range of particles. We have used 500 nm carboxylated latex beads as a probe particle in order to determine the micro-viscosity of the mucus alginate mixture and fluorescently labelled chyme resulting from simulated digestion of a fat containing meal in order to determine changes in mucus permeability to the self-assembled products of lipid digestion.
2. Materials and methods
2.1. Mucus
Porcine mucus was prepared as described previously (Macierzanka et al., 2011). Briefly, fresh porcine small intestine was obtained from a local abattoir and stored on crushed ice for transport to the laboratory. The gut was rinsed through with ice cold phosphate buffer (10 mM phosphate pH 6.5, 5 mM EDTA) followed by a further rinse with the same buffer containing a protease inhibitor (0.5 mM Pefabloc (AEBSF)). The gut was then opened out flat and mucus was collected by gently scraping the jejunal surface. Samples were frozen and stored at −20 °C for further use. MUC2 mucin was prepared for DPI measurements using the methods described elsewhere (Macierzanka et al., 2011).
2.2. Alginate
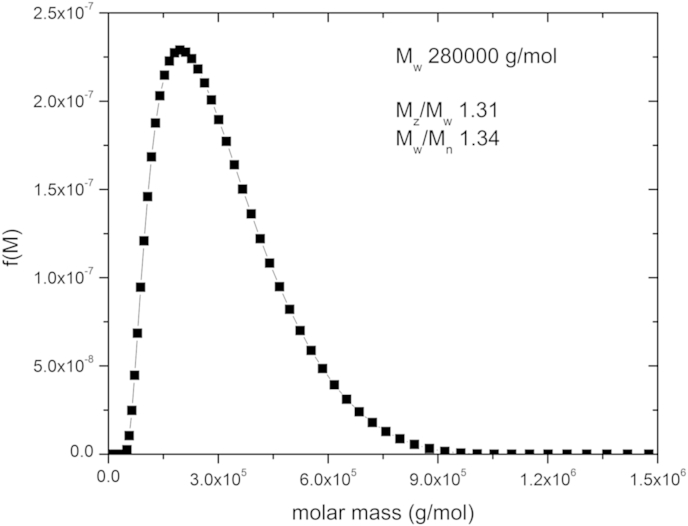
Alginates with a range of mannuronic/guluronic (M/G) ratios were kindly donated by Danisco. Their composition, molecular weight and the viscosity of a 1% (w/w) solution are as follows: Sample D1 had an M/G ratio of 60/40, a molecular weight of 350 kDa. The D4 sample had an M/G ratio of 35/65, a quoted molecular weight of 315 kDa. Subsequent measurement of the molecular weight yielded a weight average of 280 kDa and the size distribution given in Fig. 1. Samples of both forms of alginate were fluorescently labelled using DTAF (5-(4,6-dichlorotriazinyl) aminofluorescein) (Life Technologies Ltd, Paisley, UK). Polysaccharide was dissolved at 10 mg/mL in 50 mM sodium bicarbonate adjusted to pH 9.0 with 1.0 M NaOH. This was mixed overnight at 1:0.4 v/v with a solution of DTAF (10 mg/mL in DMSO) at room temperature. The reaction mixture was dialysed in 10 kDa cut-off dialysis tubing against PBS until no residual DTAF could be detected in the dialysate by UV absorbance at 490 nm. The labelling density of the resulting alginate was an average of 2.5 fluorophore molecules per alginate molecule. Although the labelling involved prolonged exposure of the alginate to pH 9, this is unlikely to have degraded the alginate by β-elimination and certainly no evidence of this was seen.
Fig. 1.
Molecular weight distribution for D4 alginate in 0.3 NaCl obtained from transformation of the sedimentation coefficient distribution at concentration (0.03 mg/mL), using the Extended Fujita method (Harding et al., 2011).
2.3. Alginate heterogeneity and molecular weight characterisation
Sedimentation velocity was performed on a solution of alginate. A very low concentration (∼0.03 mg/mL) and high ionic strength solvent (0.3 M NaCl) were used so as to render negligible the effects of non-ideality. A high ionic strength was chosen to minimise non-ideality effects an Optima XL-I (Beckman Instruments, Palo Alto, USA) equipped with Rayleigh interference optics was used. Sample solution (400 μl) and reference buffer (400 μl) were injected into the respective channels of double sector 12 mm optical path length cells. The Rayleigh interference optical system was used to record changes in the concentration versus radial displacement profiles with time. An initial low rotor speed of 3000 rpm was used to check for the presence of any large sedimenting species and then adjusted to a rotor speed of 40,000 rpm. Scans were taken at 2 min intervals for a run time of ∼24 h. The data was analysed using the least squares (ls-g(s)) procedure of SEDFIT (Dam & Schuck, 2004), which solves the Lamm equation describing the change of concentration distribution with radial position with time in terms of a distribution of sedimentation coefficients, g(s) vs s, where s is the sedimentation coefficient. The standard conditions of density and viscosity of water at 20.0 °C were used for adjustment of the sedimentation coefficients s to s20,w values. Knowledge of the weighted average molecular weight (molar mass), Mw from SEC MALS and the corresponding weighted average sedimentation coefficient from sedimentation velocity measured by analytical ultracentrifugation allows the sedimentation coefficient g(s) vs s to be transformed into an estimate for the distribution of molecular weight, f(M) vs M using the Extended Fujita method of Harding et al., and built into the SEDFIT routine. Assuming a value for the conformation parameter for alginate ∼0.33 (Harding et al., 2011), and taking Mw = 280,000 and the corresponding s value at 0.03 mg/mL = 4.3 S this yields a value for κs = 0.0685. The distribution obtained is shown in Fig. 1.
2.4. Rheology
The measurements of bulk viscosity were measure using a TA instrument G2 rheometer (TA Instruments, Crawley, West Sussex, UK) equipped with a cone and plate geometry (aluminium cone; 6°/40 mm cone angle/diameter, truncation gap 12 μm). The temperature was held at 37 °C and samples were allowed to equilibrate in the instrument for 30 min prior to measurement. A viscosity ramp test was performed over a shear rate range from 0.01 to 500 s−1 over 15 min.
2.5. Alginate diffusion
The ability of the alginate polymers (D1 and D4) to penetrate into porcine mucus was determined using time-lapse microscopy. Ex vivo porcine mucus and fluorescein-labelled alginate were layered in a 9 mm diameter by 0.9 mm depth perfusion well (CoverWell™, Sigma, Poole. UK). Laser scanning confocal microscopy was used to follow the diffusion of the alginate into the mucus for 90 min using a Leica SP1 with a 20x objective (Leica Microsystems, Mannheim, Germany). Linear fluorescent intensity profiles were generated from the time-lapse images using ImagePro-Plus 7.0 (Media Cybernetics Inc., Silver Springs, MD, USA). The diffusion coefficient was calculated from the fluorescence profiles using the following equation, , where the fluorescence F is described as a function of distance from the starting boundary x and time t. Here, a is an arbitrary scalar, erfc is the complimentary error function and D is the diffusion coefficient of the fluorescent alginate.
Diffusion of alginate in mucus was assessed using fluorescence recovery after photo-bleaching (FRAP). Briefly, fluorescein-labelled alginate D4 was mixed into mucus samples at concentrations between 0 and 0.1%. Samples were gently mixed and loaded onto glass slides using 9 mm × 120 μm SecureSeal spacers (Sigma, Poole, UK). The FRAP was conducted using a Leica SP5 (II) Laser scanning confocal microscope with an 8 kHz resonance scanner. A bleach spot of 50 μm diameter was used with an initial post bleach of 3.7 s at 37 ms/frame, followed by 25 s at 250 ms/frame. Diffusion coefficients were calculated from the fluorescence recovery data using nonlinear least-square fitting as described by (Ladha et al., 1996), using the following equation, , where F(t) is the observed fluorescence as a function of time, F(0) is the fluorescence immediately after bleaching, F(∞) is the fluorescence at infinite time after bleaching, β is the depth of bleach and τD is the diffusion time. The lateral diffusion coefficient, D is then given by D = ω2/4τD, where ω is the radius of the bleach region.
2.6. Dual polarisation interferometry (DPI)
Solutions of MUC2 mucin were prepared using 10 mM phosphate, 137 mM NaCl, 3.7 mM KCl, pH 6.5 buffer. Mucin concentrations used were 20, 100 and 500 μg/mL. Alginate solution at 0.6 mg/mL was prepared from a high guluronic acid alginate (D4). Calcium chloride solution to test for calcium-mediated alginate crosslinking was at concentration of 3 mM. Details of the dual polarisation interferometer are given in the accompanying paper (Westwood, Noel, & Parker, 2010). Standard silicon oxynitride chip was used, typically described as being essentially a silicon dioxide surface. After establishing a baseline with the standard buffer increasing concentrations of mucin were run over the chip surface at a flow rate of 10 μL/min for 10 min each followed by a 15 μL/min 10 min rinsing step. After the four mucin concentrations the alginate solution was applied using the same injection conditions. This was followed by a calcium chloride injection and a second alginate injection.
2.7. Particle tracking and stokes viscosity
The Stokes viscosity was calculated from ensemble data from multiple particle tracking of 500 nm latex beads. The mean square displacement (MSD) of 500 nm carboxylated latex beads was determined at 22 °C over 50 frames at 2 frames/s using multiple particle tracking as outlined in more detail previously (Macierzanka et al., 2011). The diffusion coefficient was calculated from the MSD as D = MSD/4t, where t is the timescale over which the displacement has occurred. From the measured diffusion coefficient of 500 nm latex beads, we calculated the local viscosity using the Stokes–Einstein relation.
2.8. In vitro digestion
A 3.0 mg/mL sodium caseinate solution in 150 mm NaCl pH 6.5 stabilised emulsion containing 18% triglyceride (sunflower oil) was prepared by passing a premix of oil and Na-Cas for a total of 6 times at 20,000 psi through a Microfluidiser (Microfluidics, Massachusetts, USA). The emulsion was digested using the standardised in vitro digestion protocol recommended by the Infogest COST Action (Minekus et al., 2014), involving 2 h gastric followed by 2 h of small intestinal simulation. The mean size (D3,2) of the original emulsion was 1.0 μm.
2.9. Digested emulsion diffusion
The ability of digested emulsion to diffuse through mucus was assessed at the beginning of the duodenal phase (5 min) and after 2 h (120 min) and with or without alginate at 0.1%. The lipid in the emulsion was visualised using Nile Red at a final concentration of 50 μM (Sigma, Poole, UK). The FRAP experiments were conducted as described for Alginate diffusion using bleach spot of 36 μm diameter with an initial post bleach of 7.36 s at 74 ms/frame, followed by 50 s at 500 ms/frame.
3. Results
In this manuscript we have shown that the dietary fibre sodium alginate can combine with intestinal mucus to decrease the diffusion of lipid digestion products. All measurements were made using D4 alginate. However, measurements of alginate diffusion used the combined data from fluorescently labelled D1 and D4 alginates as no differences were seen between the two. We first looked at the macroscopic viscosity. Even though it is not a direct indicator of the properties of a gel network seen by object diffusing at the molecular scale, it is nevertheless important as a measure of molecular interactions.
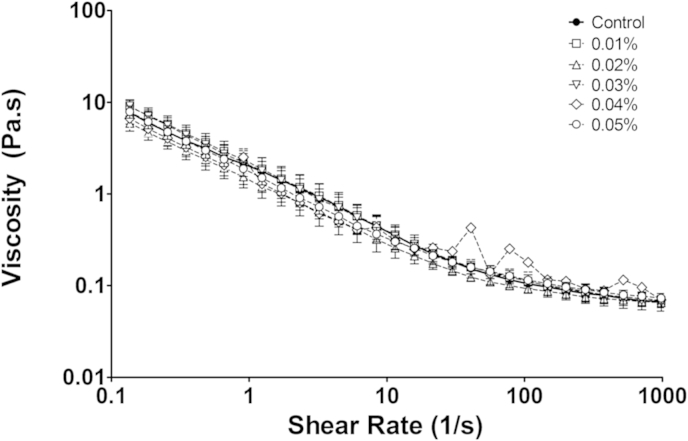
Previous experiments on porcine ex vivo mucus removed from the small intestine yielded a mean viscosity of ∼10 Pa at a shear rate of 0.2 s−1 and at 37 °C and the Stokes viscosity determined by particle tracking was 3.7 mPa at 37 °C for 500 nm beads (Macierzanka et al., 2011). Using the same conditions, the ex vivo mucus samples used in this study yielded similar values for the bulk viscosity as shown by the control sample in Fig. 2. Having established the shear thinning behaviour of the mucus, we studied the effect of adding D4 alginate in increasing concentrations up to 0.05%. No significant effect of alginate addition was seen even for the highest concentration.
Fig. 2.
Viscosity of ex vivo small intestinal mucus at 37 °C plotted as a function of increasing shear rate. The D4 alginate was gently mixed into the mucus and then allowed to equilibrate for 30 min before measurement. Mean ± sem, n = 3.
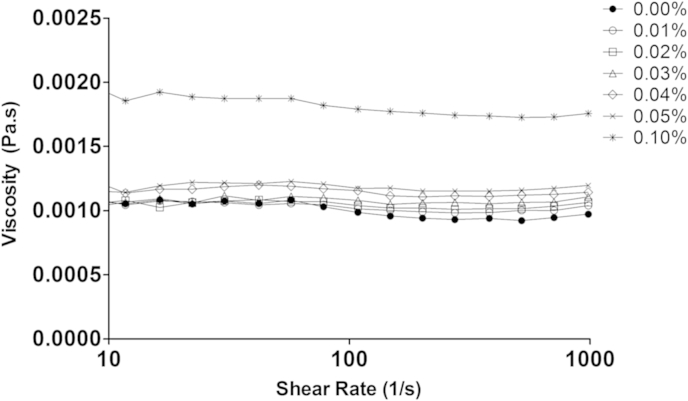
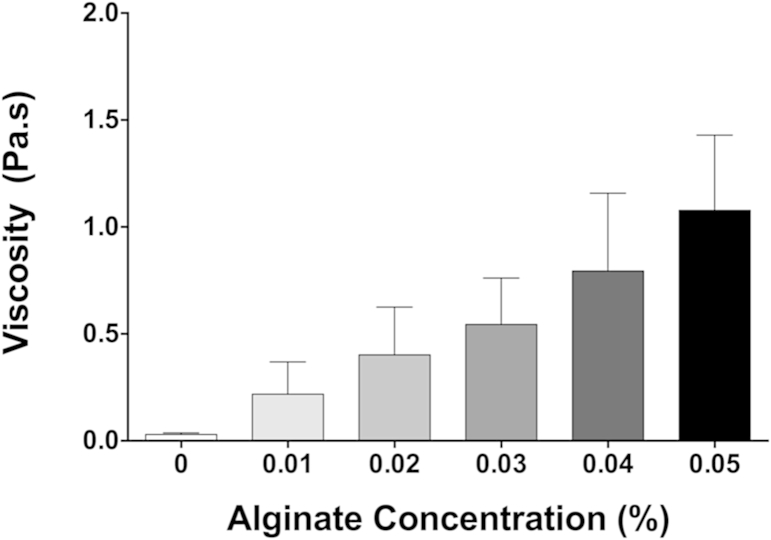
Despite having seen no change in the viscosity of mucus after the addition of alginate, it is still important to know whether the alginate alone exhibited significant viscosity under conditions simulating those in the small intestine. Fig. 3 shows the viscosity of D4 alginate as a function of shear rate over the same range of concentrations used in Fig. 2. The rheology was measured in the ionic environment recommended by the Infogest protocol (Minekus et al., 2014) as representing the small intestine. Thus, the pH was 7.0, the calcium concentration was 0.6 mM, the ionic strength was 184 mmol/L and the temperature was 37 °C.
Fig. 3.
Viscosity of solutions of increasing concentrations of D4 alginate in intestinal conditions.
It is clear from Fig. 3 that the low concentrations of alginate that we used in these experiments (0.015–0.05%) cause only a very minor increase in viscosity over that of water and thus are likely to have a minimal effect on the rate of digestion and the diffusion of digestion products in vivo in the intestinal lumen. Only by increasing the concentration of the alginate to 0.1% was a significant increase in viscosity achieved.
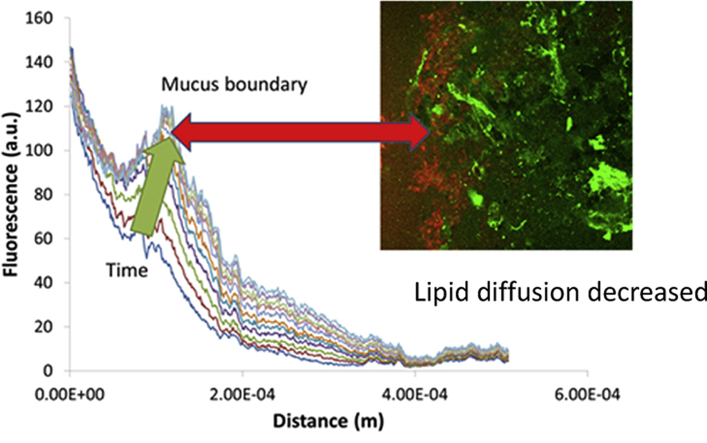
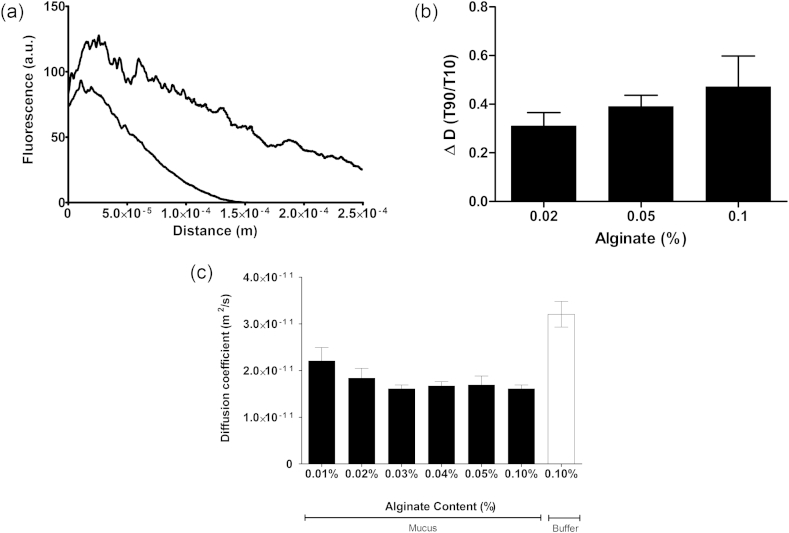
The ability of the alginate and mucus to mix is likely to be of importance if the permeability is to be affected. Thus, we were also interested to see whether the alginate could itself diffuse freely into the intestinal mucus. Both polymers carry significant negative charge and results from the rheology suggest that there was no significant interaction between the two. Experiments were conducted using fluorescently labelled alginate as described above. Fig. 4a shows the profile of fluorescence intensity and thus alginate concentration normal to the surface of the ex vivo mucus. The peak in intensity at the boundary of the mucus layer indicates that either entrapment or possibly binding of the alginate to the mucus increased the local concentration. The diffusion of the alginate into the mucus is shown by the increase in fluorescence some 250 μm from the surface. It was immediately clear from the change in fluorescence as a function of time that the alginate was able to diffuse into the mucus layer with a relatively high diffusion coefficient (10−12–10−11 m2/s). These values correspond to the alginate diffusing through little more than water. It was also clear that the apparent diffusion tended to decrease as a function of time. Fig. 4b shows the decrease in diffusion coefficient of the fluorescent alginate from 10 min to 90 min after the start of penetration into the mucus. The data is shown as the ratio of diffusion coefficients and indicates that there was consistently a drop to about 30% of the initial diffusion coefficient after 90 min and although there was a correlation between the size of the drop in diffusion and the alginate concentration, it was not statistically significant. There was also no difference observed between the different types of alginate and so the data was pooled. Despite the lack of significant concentration dependence, the trend suggests that at higher alginate concentrations the drop in diffusion was faster leading to a smaller decrease from 10 to 90 min after penetration.
Fig. 4.
(a) Fluorescence intensity profiles normal to the mucus surface taken at 5 (lower line) and 90 min (upper line) after the initial penetration of the mucus layer. (b)The ratio of diffusion coefficients of the fluorescent alginates at 10 and 90 min after initial penetration of the mucus layer plotted as a function of alginate concentration. The data for D1 and D4 alginates was pooled. (c) Diffusion coefficients of D4 alginate in ex vivo mucus or buffer as measured by FRAP. Mean ± sem, n = 3.
In addition to measuring the diffusion of alginate over 90 min as it slowly diffused into a layer of mucus, we also studied the local diffusion over a few seconds using FRAP. The data (Fig. 4c) show that in buffer alone the diffusion coefficient of the alginate was 3.2 × 10−11 m2/s, which yields a hydrodynamic radius of ∼7 nm if we assume that the conditions for the Stokes Einstein equation hold. This low figure highlights the fact that the smallest polymers diffuse the fastest, heavily biasing the outcome to the lowest molecular weight fraction in Fig. 1. Diffusion through the mucus was slightly affected by the concentration of the alginate but at the highest concentration measured (0.1%) was only reduced by a factor of two from diffusion in the buffer. All of these measurements suggest that there were no interactions between the polymer and the mucus and that the alginate was free to diffuse into the intestinal mucus.
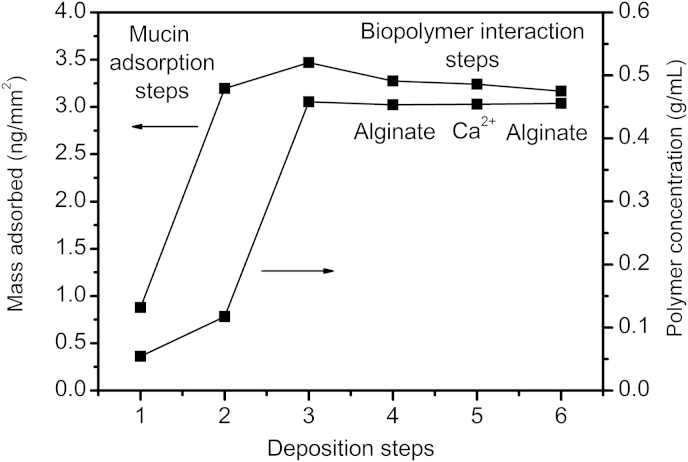
In addition to assessing the ability of the alginate to diffuse into intestinal mucus, we also used DPI to determine whether there was any specific interaction between the two polymers. The data in Fig. 5 shows the initial adsorption of mucin onto the silicon dioxide surface of the DPI chip at three increasing concentrations from 0.004 to 0.5 mg/mL. There was negligible adsorption at the lowest mucin concentration. At the second and third concentrations there was progressively more adsorption and a low density indicating an open structure with loops and tails stretching from the surface. After the final adsorption step the density of the residual adsorbed material jumped to a value of 0.45 g/mL and, the thickness dropped from 25–30 nm to 7–8 nm (data not shown) implying the mucin was relatively flat on the surface. Subsequently exposing this mucin surface to alginate, calcium and more alginate showed no changes in the adsorbed layer and no interaction, suggesting that for these polymers to interact they need to interpenetrate in bulk solution rather than at an interface.
Fig. 5.
DPI data showing six separate experimental steps. The first three steps are the adsorption of mucin from solutions at 0.02, 0.1 and 0.5 mg/mL respectively. The last three show the result of exposure of the adsorbed mucin to 0.6 mg/mL alginate, 3 mM calcium and further 0.6 mg/mL alginate respectively.
Having established that sodium alginate was able to diffuse into the mucus with a diffusion coefficient similar to that in water or slightly lower, we investigated the ability of the increasing concentrations of D4 alginate to increase the micro-viscosity of the mucus. The Stokes viscosity was calculated from the diffusion of 500 nm latex beads through the mucus/alginate polymer mixture and is shown in Fig. 6 as a function of alginate concentration. Interestingly, even though the measurement of these samples showed no increase in bulk viscosity of the mucus after introducing the alginate, the micro-viscosity increased, even for the lowest concentration of alginate. The Stokes viscosity increased as a linear function of alginate concentration indicating that the effect of the two polymers was additive.
Fig. 6.
The Stokes viscosity of ex vivo mucus incorporating different concentrations of D4 sodium alginate from 0 to 0.05%. The viscosity was calculated from the diffusion coefficient of 500 nm latex beads using the Stokes–Einstein relation. Mean ± sem, n = 5.
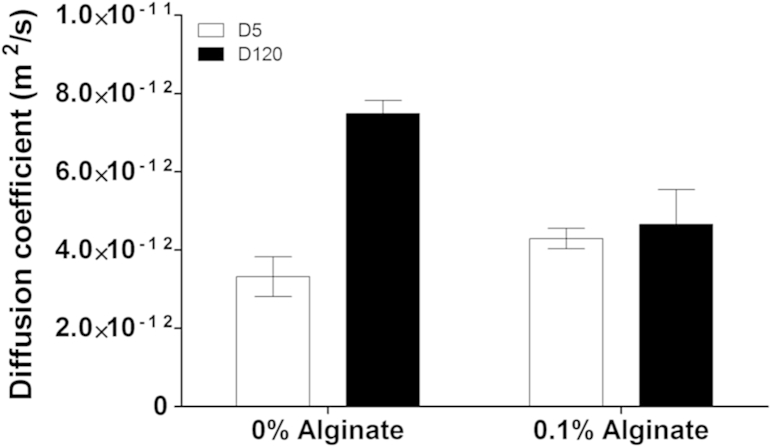
Although the diffusion of 500 nm latex beads was decreased by the addition of alginate to intestinal mucus at relatively low concentrations, this does not mean that the diffusion of digestion products from the intestinal lumen to the site of absorption would be decreased. It is likely that the majority of the self-assembled products of lipid digestion will be much smaller in size than 500 nm. Therefore, we fluorescently labelled the lipid from digesta generated as outlined in the methods section. The surface area mean size of the lipid droplets in the original emulsion was 1.0 μm. The mean size of the lipid structures undergoing Brownian motion in the digesta from the 5 and 120 min samples was much more difficult to determine because of the presence of a wide range of particulates. These were partly from the pancreatin used in the simulation of intestinal digestion but also from the self-assembly of the products of lipid hydrolysis. The size distributions of the three samples are given in the supplementary information (Figure S1). The ability of the digestion products to penetrate the mucus in the presence of various concentrations of alginate is shown in Fig. 7. In the mucus alone the extent of hydrolysis had a significant impact on the rate of diffusion. The 5 min sample was still largely in the form of 1 μm lipid droplets that were slow to diffuse whereas the 120 min digesta was more than twice as mobile. This is presumably due to a decrease the average size of the diffusing particles. The addition of 0.1% alginate had no effect on the 5 min sample but decreased the diffusion of the 120 min sample by almost a factor of two.
Fig. 7.
The mean diffusion coefficient of fluorescently labelled digesta in intestinal mucus containing 0% or 0.1% alginate. The open and closed bars are digesta samples after 5 min and 120 min of simulated intestinal digestion respectively. Mean ± sem, n = 3.
4. Discussion
In this article we have investigated the ability of the dietary fibre sodium alginate to decrease the diffusion of the products of lipid digestion through porcine intestinal mucus. The concentrations of alginate we have used are equivalent to those that might be encountered after consumption of a food containing alginate as a stabiliser. For example, typical concentrations in foods are around range from 0.5% to 1.5%. Thus, if this food is consumed we can assume a dilution of around a factor of 10 by the time it reaches the mid small intestine. This is based on the same assumptions as the in vitro digestion where each of the three phases of digestion (oral, gastric, small intestinal) represents a 50% dilution (Minekus et al., 2014) giving an overall dilution factor of 8. In addition we can assume some further dilution from other food/drink components. It is clear from the rheological measurements shown in Figs. 2 and 3 that such low concentrations are likely to have little effect on the bulk viscosity of the chyme in the intestinal lumen or the mucus layer of the intestinal wall.
Whilst bulk rheological and tribological properties may be important for lubrication of the GI tract, the barrier function involves much smaller length-scales. We first considered the scale of the polymer molecules themselves. The porcine intestinal mucus used in this study comprises primarily MUC2 mucin and was characterised with a mean molecular weight of 4.6 × 106 Da. This is considerably larger than the 2.8 × 105 Da of the alginate. In recent work we showed that the pore size of the MUC2 mucin varied from 25 to 200 nm (Round et al., 2012), which is rather smaller than the MUC2 mucin structure proposed by Ambort et al. (Ambort et al., 2012) in which the pore size is in excess of 1 μm. In the former case, the alginate would be able to diffuse through all the pores but not the 500 nm latex beads. In the case of the Ambort model, both could freely diffuse through the mucin. This model nominally describes the properties of the MUC2 mucus network in the large intestine. However, the mucus layer in the small intestine is much more heterogeneous with only the denser regions having these properties (Johansson, Larsson, & Hansson, 2011). Nonetheless, according to the Ambort model both the alginate and the 500 nm latex should be able to diffuse through all regions of the small intestine mucus. The fact that the apparent diffusion of the alginate decreased as a function of time suggests that the pores at the surface of the mucus became clogged with the alginate. This was confirmed by an increase in fluorescence in the boundary region of the mucus over that seen in the pool of alginate introduced into the luminal space as shown in Fig. 4a. The lack of any attractive interaction between the alginate and mucin in the DPI experiments illustrated in Fig. 5, strongly suggests that the mucus was indeed only able to trap the alginate in this way. However, despite this apparent entrapment, the bulk rheology of the mixed mucus, alginate systems is in agreement with previous measurements on alginate and porcine gastric mucin (PGM) (Taylor-Nordgard & Draget, 2011). The results suggest that potentially any high molecular weight non-interacting fibre might have the same general effect.
The limited levels of entrapment and the small increases in bulk rheology discussed above, suggest that the addition of alginate to intestinal mucus would have only a limited effect on the local microrheology. However, it is clear from Fig. 6 that the viscosity encountered by 500 nm particles was increased after alginate addition even at the lowest concentrations used and that the effect was purely additive. The data here contrasts with previous measurements on PGM showing limited diffusion of 200 nm latex beads (Taylor-Nordgard, Nonstad, Olderoy, Espevik, & Draget, 2014). The data suggest that even though the beads were able to diffuse through the mucus, the pores did not need much alginate to limit the diffusion significantly. Finally the addition of the range of particles present in digesta to mucus in order to assess their ability to diffuse through it in the presence or absence of alginate gave slightly conflicting results. The mean particle size of the original emulsion was 1.0 μm but the addition of the emulsion to gastric and then small intestinal simulation altered this significantly. After 5 min of simulated duodenal digestion of a protein stabilised emulsion, there were a large number of particles present ranging in size from less than 100 nm to more than 100 μm. Consequently, the degree of penetration was low as was the mean diffusion coefficient and the presence of 0.1% alginate made little difference to the diffusion coefficients obtained. It is not clear however, what the mean size of the diffusing particles was under those circumstances. After 120 min simulated digestion, the particle size distribution was still very broad, although one might expect the droplet size to have decreased as a result of hydrolysis of the oil. This was confirmed as the diffusion coefficient was significantly higher. In this case the addition of 0.1% alginate decreased the diffusion to about half of the original value. The data here confirm our original hypothesis and may be sufficient to limit the type of fast lipid absorption that leads to hyperlipidaemia, which has been seen as a potential health issue. However, a 50% decrease in the rate of lipid diffusion may not be enough to have an impact on lipid absorption in vivo or bile salt recycling.
In conclusion, we have investigated the ability of sodium alginate to decrease the permeability of small intestinal mucus. We have shown there are no interactions between sodium alginate and the small intestinal mucus at the molecular level. As a result the bulk rheological properties of intestinal mucus were little altered by the addition of low concentrations of alginate. The alginate was able to freely diffuse into the mucus where it had a significant effect on the diffusion of 500 nm latex beads, indicating an additive effect on the microviscosity and suggesting that the mucus pore size was close to 500 nm. However, while the permeability to 500 nm latex beads was significantly affected, the permeability to lipid digestion products was only decreased to a limited extent. While the low concentrations of alginate may have limited effect on lipid absorption in vivo, further research investigating the role of alginates and other dietary fibres on reducing rates of lipid absorption in the small intestine could lead to strategies to combat health issues such as hyperlipidaemia, obesity and type-2 diabetes.
Acknowledgements
The authors from IFR would like to acknowledge the BBSRC for funding through an institute strategic programme grant (research grant BB/J004545/1). We would also like to thank Mike Ridout for useful discussion of the bulk rheology and Richard Gillis and Gary Adams for discussion on the ultracentrifugation. The authors are participants of the EU funded COST action INFOGEST (COST FA 1005).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.foodhyd.2015.08.004.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Ambort D., Johansson M.E.V., Gustafsson J.K., Nilsson H.E., Ermund A., Johansson B.R. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(15):5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.W., Baird P., Davis R.H., Jr., Ferreri S., Knudtson M., Koraym A. Health benefits of dietary fiber. Nutrition Reviews. 2009;67(4):188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- Brownlee I.A. The physiological roles of dietary fibre. Food Hydrocolloids. 2011;25(2):238–250. [Google Scholar]
- Dam J., Schuck P. Calculating sedimentation coefficient distributions by direct modeling of sedimentation velocity concentration profiles. Numerical Computer Methods, Pt E. 2004;384:185–212. doi: 10.1016/S0076-6879(04)84012-6. [DOI] [PubMed] [Google Scholar]
- European Food Safety, A Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA Journal. 2010;8(3):1462. [Google Scholar]
- Frassetto L.A., Schloetter M., Mietus-Synder M., Morris R.C., Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. European Journal of Clinical Nutrition. 2009;63(8):947–955. doi: 10.1038/ejcn.2009.4. [DOI] [PubMed] [Google Scholar]
- Gibson G.R., Roberfroid M.B. Dietary modulation of the human colonic microbiota - introducing the concept of prebiotics. Journal of Nutrition. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- Gunness P., Gidley M.J. Mechanisms underlying the cholesterol-lowering properties of soluble dietary fibre polysaccharides. Food & Function. 2010;1(2):149–155. doi: 10.1039/c0fo00080a. [DOI] [PubMed] [Google Scholar]
- Harding S.E., Schuck P., Abdelhameed A.S., Adams G., Kok M.S., Morris G.A. Extended Fujita approach to the molecular weight distribution of polysaccharides and other polymeric systems. Methods. 2011;54(1):136–144. doi: 10.1016/j.ymeth.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hino S., Sonoyama K., Bito H., Kawagishi H., Aoe S., Morita T. Low-methoxyl pectin stimulates small intestinal mucin secretion irrespective of goblet cell proliferation and is characterized by jejunum Muc2 upregulation in rats. Journal of Nutrition. 2013;143(1):34–40. doi: 10.3945/jn.112.167064. [DOI] [PubMed] [Google Scholar]
- Ito H., Satsukawa M., Arai E., Sugiyama K., Sonoyama K., Kiriyama S. Soluble fiber viscosity affects both goblet cell number and small intestine mucin secretion in rats. Journal of Nutrition. 2009;139(9):1640–1647. doi: 10.3945/jn.109.110171. [DOI] [PubMed] [Google Scholar]
- Jenkins D.J.A., Wolever T.M.S., Leeds A.R., Gassull M.A., Haisman P., Dilawari J. Dietary fibers, fiber analogs, and glucose-tolerance - importance of viscosity. British Medical Journal. 1978;1(6124):1392–1394. doi: 10.1136/bmj.1.6124.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M.G., Knudsen J.C., Viereck N., Kristensen M., Astrup A. Functionality of alginate based supplements for application in human appetite regulation. Food Chemistry. 2012;132(2):823–829. [Google Scholar]
- Johansson M.E.V., Larsson J.M.H., Hansson G.C. The two mucus layers of colon are organized by the MUC2 mucin, whereas the outer layer is a legislator of host-microbial interactions. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:4659–4665. doi: 10.1073/pnas.1006451107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladha S., Mackie A.R., Harvey L.J., Clark D.C., Lea E.J.A., Brullemans M. Lateral diffusion in planar lipid bilayers: a fluorescence recovery after photobleaching investigation of its modulation by lipid composition, cholesterol, or alamethicin content and divalent cations. Biophysical Journal. 1996;71(3):1364–1373. doi: 10.1016/S0006-3495(96)79339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattimer J.M., Haub M.D. Effects of dietary fiber and its components on metabolic health. Nutrients. 2010;2(12):1266–1289. doi: 10.3390/nu2121266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macierzanka A., Rigby N.M., Corfield A.P., Wellner N., Böttger F., Mills E.N.C. Adsorption of bile salts to particles allows penetration of intestinal mucus. Soft Matter. 2011;7:8077–8084. [Google Scholar]
- Minekus M., Alminger M., Alvito P., Ballance S., Bohn T., Bourlieu C. A standardised static in-vitro digestion method suitable for food – an international consensus. Food and Function. 2014;5:1113–1124. doi: 10.1039/c3fo60702j. [DOI] [PubMed] [Google Scholar]
- Mu H.L., Hoy C.E. The digestion of dietary triacylglycerols. Progress in Lipid Research. 2004;43(2):105–133. doi: 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- Nilsson A.C., Ostman E.M., Holst J.J., Bjorck I.M. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. Journal of Nutrition. 2008;138(4):732–739. doi: 10.1093/jn/138.4.732. [DOI] [PubMed] [Google Scholar]
- Othman R.A., Moghadasian M.H., Jones P.J.H. Cholesterol-lowering effects of oat beta-glucan. Nutrition Reviews. 2011;69(6):299–309. doi: 10.1111/j.1753-4887.2011.00401.x. [DOI] [PubMed] [Google Scholar]
- Paxman J.R., Richardson J.C., Dettmar P.W., Corfe B.M. Daily ingestion of alginate reduces energy intake in free-living subjects. Appetite. 2008;51(3):713–719. doi: 10.1016/j.appet.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Round A.N., Rigby N.M., Garcia de la Torre A., Macierzanka A., Mills E.N.C., Mackie A.R. Lamellar structures of MUC2-rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules. 2012;13(10):3253–3261. doi: 10.1021/bm301024x. [DOI] [PubMed] [Google Scholar]
- Shelat K.J., Vilaplana F., Nicholson T.M., Gidley M.J., Gilbert R.G. Diffusion and rheology characteristics of barley mixed linkage beta-glucan and possible implications for digestion. Carbohydrate Polymers. 2011;86(4):1732–1738. [Google Scholar]
- Shimotoyodome A., Meguro S., Hase T., Tokimitsu I., Sakata T. Sulfated polysaccharides, but not cellulose, increase colonic mucus in rats with loperamide-induced constipation. Digestive Diseases and Sciences. 2001;46(7):1482–1489. doi: 10.1023/a:1010644021888. [DOI] [PubMed] [Google Scholar]
- Smidsrod O., Draget K.I. Alginate gelation technologies. In: Dickinson E., Bergenstahl B., editors. Vol. 1. RSC; Ystad, Sweden: 1997. pp. 279–293. (Food colloids – Proteins, lipids and polysaccharides). [Google Scholar]
- Strand K.A., Boe A., Dalberg P.S., Sikkeland T., Smidsrod O. Dynamic and static light-scattering on aqueous-solutions of sodium alginate. Macromolecules. 1982;15(2):570–579. [Google Scholar]
- Taylor-Nordgard C., Draget K.I. Oligosaccharides as modulators of rheology in complex mucous systems. Biomacromolecules. 2011;12(8):3084–3090. doi: 10.1021/bm200727c. [DOI] [PubMed] [Google Scholar]
- Taylor-Nordgard C., Nonstad U., Olderoy M.O., Espevik T., Draget K.I. Alterations in mucus barrier function and matrix structure induced by guluronate oligomers. Biomacromolecules. 2014;15(6):2294–2300. doi: 10.1021/bm500464b. [DOI] [PubMed] [Google Scholar]
- Taylor C., Pearson J.P., Draget K.I., Dettmar P.W., Smidsrod O. Rheological characterisation of mixed gels of mucin and alginate. Carbohydrate Polymers. 2005;59(2):189–195. [Google Scholar]
- Taylor, C., Draget, K. I., & Smidsrod, O. A. (2009), Mucosal Treatment. In.
- Theuwissen E., Mensink R.P. Water-soluble dietary fibers and cardiovascular disease. Physiology & Behavior. 2008;94(2):285–292. doi: 10.1016/j.physbeh.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Westwood M., Noel T.R., Parker R. The characterisation of polygalacturonic acid-based layer-by-layer deposited films using a quartz crystal microbalance with dissipation monitoring, a dual polarization interferometer and a Fourier-transform infrared spectrometer in attenuated total reflectance mode. Soft Matter. 2010;6(21):5502–5513. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.