Abstract
Cordycepin, an adenosine analog derived from Cordyceps militaris has been shown to exert anti-tumor activity in many ways. However, the mechanisms by which cordycepin contributes to the anti-tumor still obscure. Here our present work showed that cordycepin inhibits cell growth in NB-4 and U937 cells by inducing apoptosis. Further study showed that cordycepin increases the expression of p53 which promotes the release of cytochrome c from mitochondria to the cytosol. The released cytochrome c can then activate caspase-9 and trigger intrinsic apoptosis. Cordycepin also blocks MAPK pathway by inhibiting the phosphorylation of ERK1/2, and thus sensitizes the apoptosis. In addition, our results showed that cordycepin inhibits the expression of cyclin A2, cyclin E, and CDK2, which leads to the accumulation of cells in S-phase. Moreover, our study showed that cordycepin induces DNA damage and causes degradation of Cdc25A, suggesting that cordycepin-induced S-phase arrest involves activation of Chk2-Cdc25A pathway. In conclusion, cordycepin-induced DNA damage initiates cell cycle arrest and apoptosis which leads to the growth inhibition of NB-4 and U937 cells.
Keywords: apoptosis, cordycepin, cytochrome c, Cdc25A, DNA damage, p53, S-phase arrest
Abbreviations
- ATM
Ataxia telangiectasia mutated
- ATR
ATM and Rad3-related
- PI
Propidium iodide
- PFT-α
Pifithrin-α
- XIAP
X-linked inhibitor of apoptosis protein.
Introduction
Cordyceps is a caterpillar-shaped mushroom that has been used in lung and kidney tonics in traditional Chinese medicine. This mushroom possesses numerous biological activities, including anti-oxidant,1 anti-hyperglycemic,2 anti-fatigue,3 anti-inflammatory,4 anti-bacterial,5 anti-viral,6 and anti-tumor7 activities. It has also been shown to enhance sexual function.8 It has been used in the treatment of chronic bronchitis, asthma, and chronic renal failure. Cordycepin (3´-deoxyadenosine) is the main functional component of Cordyceps militaris.9 As a derivative of the nucleoside adenosine it can inhibit mRNA polyadenylation by incorporating into RNA.10 Numerous studies have shown that cordycepin has many anti-tumor activities, such as inhibition of cell proliferation,11,12 inhibition of cell migration,13,14 and induction of apoptosis.15–17 In 1996, researchers from the Boston Medical Center showed that cordycepin could induce apoptosis in adenosine deaminase (ADA)-inhibited terminal deoxynucleotidyl transferase-positive (TdT+) leukemia cells.15 A clinical trial was also carried out to study the effectiveness of cordycepin combined with pentostatin in the treatment of patients with refractory acute lymphocytic or chronic myelogenous leukemia together with National Cancer Institute in 1999. In 2008, Oncovista Inc. organized a phase I/II study of cordycepin combined with pentostatin treatment in patients with refractory TdT-positive leukemia. And it was reported that patient with acute lymphocytic leukemia demonstrated a clinical response to the combination therapy of cordycepin and pentostatin while no significant toxicity was observed.18 So the cordycepin may have synergistic action with pentostatin for the treatment of leukemia patients. However, the molecular mechanisms of its anti-cancer activities are not well understood, so more studies are still needed.
In this study, we tested the cytotoxicity of cordycepin on a series of leukemia cell lines and found that cordycepin can inhibit the growth of these leukemia cells. The study showed that cordycepin induces apoptosis in NB-4 and U937 cells through activation of caspase-8, - 9, and -3. Further study showed that cordycepin-induced apoptosis involves upregulation of p53 and the release of cytochrome c from mitochondria to the cytosol. Moreover, cordycepin blocks MAPK pathway which results in sensitization of drug-induced apoptosis. Cordycepin also induces DNA damage which causes the accumulation of phosphorylated Chk2 and degradation of Cdc25A, and then leads to the S-phase delay. Our findings support the mechanism that cordycepin inhibits the growth of NB-4 and U937 cells through cell cycle arrest and cell apoptosis.
Results
Cordycepin induces apoptosis in NB-4 and U937 cells
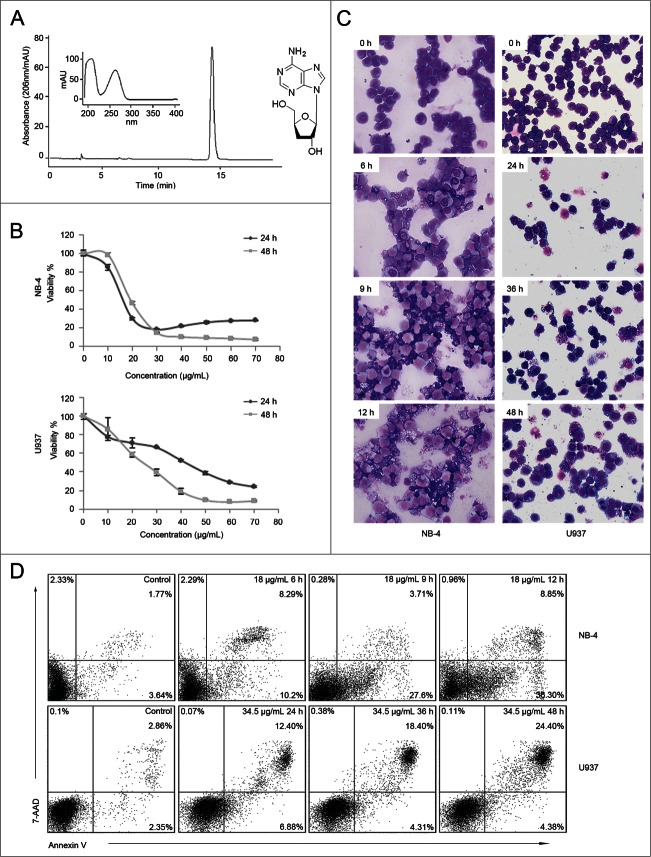
Cordycepin was extracted from cultured Cordyceps kyushuensis. The HPLC analysis and UV spectrum of purified cordycepin and its chemical structure is shown in Fig. 1A. To investigate the cytotoxicity of cordycepin on leukemia cell lines, cells were exposed to increasing concentrations of cordycepin. CCK-8 assay data showed that leukemia cells can be inhibits by cordycepin (Fig. S1). And cordycepin inhibits the growth of NB-4 and U937 cells in a dose-dependent manner (Fig. 1B), and the IC50 value were 18.4 μg/mL (73.2 μM) and 22.7 μg/mL (90.4 μM) respectively. To confirm the effects of cordycepin on cell survival, both cell morphology and Annexin V-PE/7-AAD double staining were analyzed. Results showed that NB-4 and U937 cells presented morphological changes characteristic of apoptosis, such as cell shrinkage, chromatin condensation, and cytoplasmic blebbing (Fig. 1C). After the treatment of cordycepin, the percentage of Annexin V+ cells were increased from 5.41% to 45.15% in NB-4 cells and were increased from 5.21% to 28.78% in U937 cells in a time-dependent manner (Fig. 1D). Taken together, these data suggested that cordycepin induces apoptosis in both cell lines.
Figure 1 (See previous page).
Cordycepin induces cell apoptosis in NB-4 and U937 cells. (A) HPLC analysis and UV spectrum of cordycepin purified by HSCCC (left panel). Chemical structure of cordycepin (right panel). (B) Cell viability of NB-4 and U937 cells treated with cordycepin for 24h and 48h at the indicated concentrations. Each data point represents the mean ± SD of triplicate experiments. (C) Morphology characteristic of NB-4 and U937 cells treated with cordycepin. NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h, and 12 h, and U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h. (D) Annexin V analysis of NB-4 and U937 cells treated with cordycepin. NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h, and 12 h, and U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h.
Cordycepin triggers caspase-dependent apoptosis
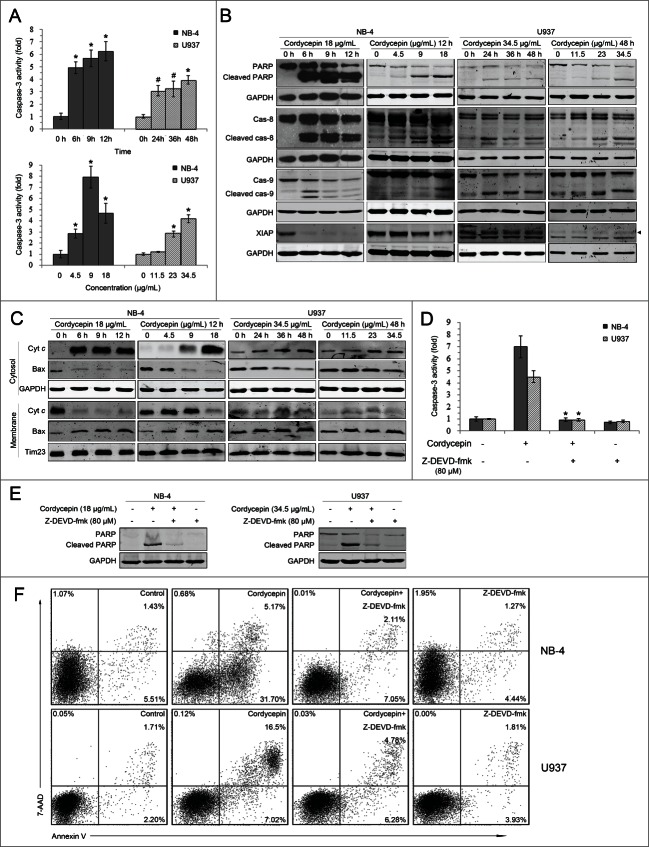
Because activation of caspases play a critical role in apoptosis, the activation of caspases in cells treated with cordycepin was investigated. Caspase-3 activity was found to increase significantly in a time-dependent manner in both NB-4 and U937 cells (Fig. 2A). Results also showed that cordycepin induced the cleavage of PARP, caspase-8, and caspase-9 (Fig. 2B). The levels of anti-apoptotic protein XIAP were decreased in cordycepin treated NB-4 cells, but were not changed in cordycepin treated U937 cells (Fig. 2B). Further study showed that exposure to cordycepin led to the release of cytochrome c into the cytosol (Fig. 2C). In contrast, the levels of Bax were decreased in the cytosolic fractions and increased in the mitochondrial fractions after the treatment of cordycepin (Fig. 2C). These results indicated that cordycepin activates initiator and executioner caspases involved in both the extrinsic and the intrinsic pathways.
Figure 2 (See previous page).
Cordycepin triggers caspase-dependent apoptosis. (A) NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h and 12 h (upper panel), or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h (bottom panel). U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h (upper panel), or treated with 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM), 34.5 μg/mL (137.3 μM) cordycepin for 48 h (bottom panel). The extracts from cells were assayed for caspase-3 activity by using colorimetric assay. #, P <0.05 versus 0 h group. *, P<0.01 vs. 0 h group. Each data point represents the mean ± SD of 3 independent experiments. (B) NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h and 12 h, or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h. U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h, or treated with 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM), 34.5 μg/mL (137.3 μM) cordycepin for 48 h. Whole cell lysates were analyzed by Western blot with the indicated antibodies. s, no specific bands. (C) NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h and 12 h, or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h. U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h, or treated with or treated with 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM), 34.5 μg/mL (137.3 μM) cordycepin for 48 h. Cytosolic and membrane fractions were generated as described in Materials and Methods. Bax and cytochrome c were detected by Western blot analysis. (D and E) NB-4 cells were preincubated with 80 μM Z-DEVD-fmk for 2 h before treatment with 18 μg/mL (71.6 μM) cordycepin for another 12 h. U937 cells were preincubated with 80 μM Z-DEVD-fmk for 1 h before treatment with 34.5 μg/mL (137.3 μM) cordycepin for another 36 h. Extracts from cells were assayed for caspase-3 activity using a colorimetric assay. *, P<0.01 versus cordycepin treated group. Each data point represents the mean ± SD of 3 independent experiments. Cleavage of PARP was evaluated by Western blot analysis. (F) Annexin V analysis of NB-4 and U937 cells treated with Z-DEVD-fmk and cordycepin. NB-4 cells were preincubated with 80 μM Z-DEVD-fmk for 2 h before treated with 18 μg/mL (71.6 μM) cordycepin for another 12 h. U937 cells were preincubated with 80 μM Z-DEVD-fmk for 1 h before treatment with 34.5 μg/mL (137.3 μM) cordycepin for another 36 h.
To further demonstrate that the apoptosis induced by cordycepin is caspase-dependent, the caspase-3 inhibitor Z-DEVD-fmk was used to block the activation of caspase-3. Results showed that 80 μM Z-DEVD-fmk could significantly inhibit cordycepin-induced caspase-3 activation (Fig. 2D) and PARP cleavage (Fig. 2E). Similarly, cordycepin-induced apoptosis was blocked in the presence of this caspase-3 inhibitor (Fig. 2F). Altogether, these findings demonstrated that cordycepin induces caspase-dependent apoptosis.
Cordycepin-induced apoptosis involves p53
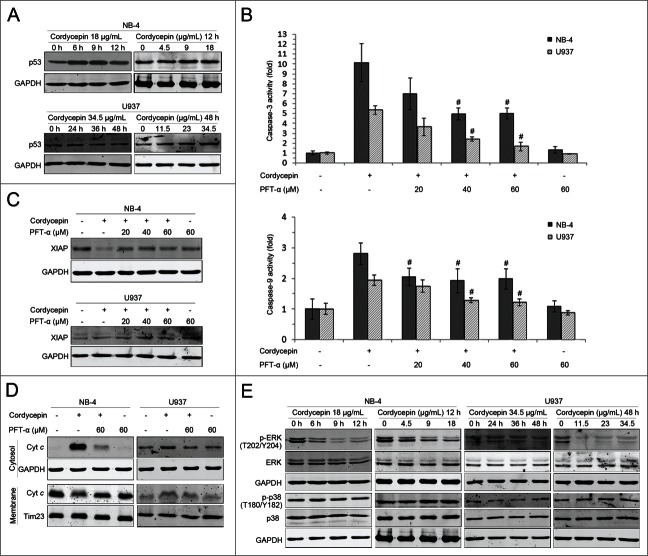
Cordycepin treatment also upregulated expression of p53 (Fig. 3A). To investigate the role of p53 in cordycepin-mediated apoptosis, caspase-3 and caspase-9 activity levels were assessed in the presence and absence of the p53-specific inhibitor PFT-α. Results showed that pretreatment of NB-4 and U937 cells with PFT-α led to a decrease in the activities of caspase-3 and -9 (Fig. 3B). PFT-α was also found to restore XIAP levels decreased by cordycepin in NB-4 cells (Fig. 3C), and reduce cordycepin-induced cytochrome c release in both cell lines (Fig. 3D). These results suggested that cordycepin-induced apoptosis is both p53-dependent and -independent.
Figure 3.
Effects of cordycepin on p53 and MAPK signaling pathways. (A) NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h and 12 h, or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h. U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h, or treated with 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM), 34.5 μg/mL (137.3 μM) cordycepin for 48 h. Whole cell lysates were evaluated by Western blot analysis with anti-p53 antibody. (B) NB-4 cells were preincubated with PFT-α for 2 h before treatment with 18 μg/mL (71.6 μM) cordycepin for another 12 h. U937 cells were preincubated with PFT-α for 1 h before treatment with 34.5 μg/mL (137.3 μM) cordycepin for another 48 h. Extracts from cells were assayed for caspase-3/9 activity by using colorimetric assay. #, P <0.05 vs. cordycepin treated group. Each data point represents the mean ± SD of 3 independent experiments. (C) NB-4 cells were preincubated with PFT-α for 2 h before treatment with 18 μg/mL (71.6 μM) cordycepin for another 9 h. U937 cells were preincubated with PFT-α for 1 h before treatment with 34.5 μg/mL (137.3 μM) cordycepin for another 48 h. Whole-cell lysates were analyzed by Western blot with anti-XIAP antibody. (D) NB-4 cells were preincubated with PFT-α for 2 h before treatment with 18 μg/mL (71.6 μM) cordycepin for another 12 h. U937 cells were preincubated with PFT-α for 1 h before treatment with 34.5 μg/mL (137.3 μM) cordycepin for another 48 h. Cytosolic and membrane fractions were generated as described in Materials and Methods. Cytochrome c was detected by Western blot analysis. (E) NB-4 cells were treated with 18 μg/mL (71.6 μM). cordycepin for 6 h, 9 h and 12 h, or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h. U937 cells were treated with 34.5 μg/mL (137.3 μM) cordycepin for 24 h, 36 h, and 48 h, or treated with 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM), 34.5 μg/mL (137.3 μM) cordycepin for 48 h. Whole cell lysates were evaluated using Western blot analysis with the indicated antibodies.
Effect of cordycepin on MAPK pathway
The mitogen-activated protein kinase (MAPK) pathway plays a critical role in regulation of cell survival, and interruption of this pathway results in sensitization to spontaneous and drug-induced apoptosis.19 In the present work, the contributions of ERK and p38 to cordycepin-induced cell growth inhibition were evaluated. Results showed that ERK1/2 phosphorylation was markedly reduced after cordycepin treatment, but p38 phosphorylation was not affected (Fig. 3E).
Cordycepin induces cell cycle arrest in NB-4 and U937 cells
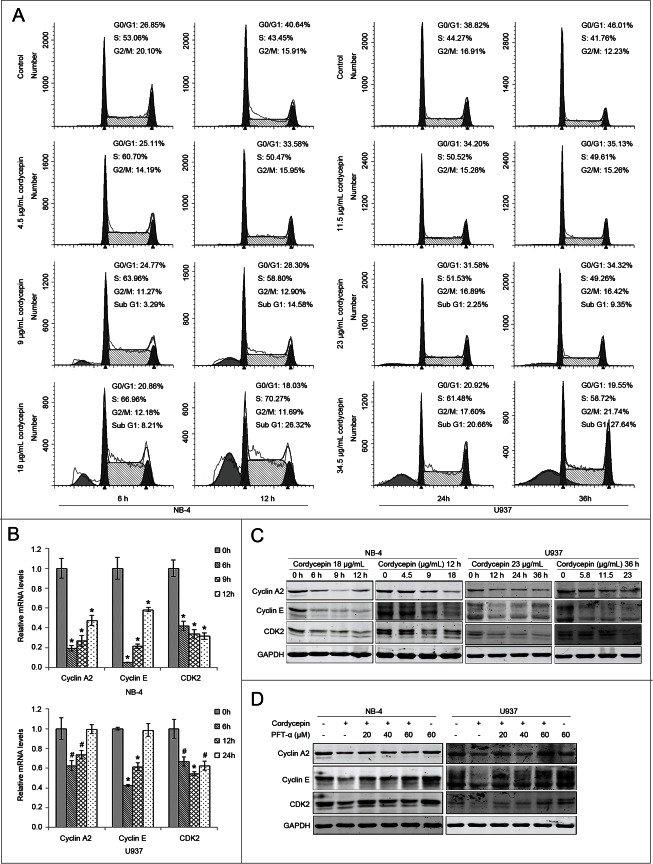
In addition to cell viability, effect on cell cycle progression is an important parameter of anti-tumor drug action. Thus, we analyzed the effects of cordycepin on cell cycle, and the results showed that treatment with cordycepin led to accumulation of NB-4 and U937 cells in S-phase (Fig. 4A). Consistent with previous studies, the percentage of cells in the sub G1-phase population was found to increase after treatment with cordycepin.
Figure 4 (See previous page).
Cordycepin induces cell cycle arrest in NB-4 and U937 cells. (A) Flow cytometric analysis of cell cycle distribution of NB-4 and U937 cells. Cells were treated with or without cordycepin for indicated time and stained with propidium iodide for DNA content. (B) Expression of cyclin A2, cyclin E, and CDK2 mRNAs in NB-4 and U937 cells treated with cordycepin. NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h, and 12 h. U937 cells were treated with 23 μg/mL (91.5 μM) cordycepin for 6 h, 12 h, and 24 h. #, P <0.05 vs. 0 h group. *, P<0.01 versus 0 h group. (C) NB-4 cells were treated with 18 μg/mL (71.6 μM) cordycepin for 6 h, 9 h and 12 h, or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h. U937 cells were treated with 23 μg/mL (91.5 μM) cordycepin for 12 h, 24 h, and 36 h, or treated with 5.8 μg/mL (23.1 μM), 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM) cordycepin for 36 h. Whole cell lysates were evaluated by Western blot analysis with the indicated antibodies. (D) NB-4 cells were preincubated with PFT-α for 2 h before treatment with 18 μg/mL (71.6 μM) cordycepin for another 9 h. U937 cells were preincubated with PFT-α for 1 h before treatment with 23 μg/mL (91.5 μM) cordycepin for another 36 h. Whole cell lysates were evaluated by Western blot analysis with the indicated antibodies.
To further explore the mechanism of cordycepin-induced cell cycle arrest, the expression of cell cycle proteins was evaluated. Real-time PCR analysis showed a strong downregulation of cyclin A2, cyclin E, and CDK2 in cordycepin treated groups in NB-4 and U937 cells, although the mRNA levels of cyclin A and cyclin E were restored after 24 h of cordycepin treatment in U937 cells (Fig. 4B). Western blots showed a dramatic reduction in the levels of CDK2, cyclin A2, and cyclinE proteins (Fig. 4C). These results suggested that cordycepin induces S-phase arrest through downregulation of the expression of cell cycle proteins.
As cordycepin treatment upregulated expression of p53, we supposed that the activation of p53 pathway is necessary for cordycepin-mediated cell cycle arrest. To verify our hypothesis, NB-4 and U937 cells were incubated with cordycepin in the presence or absence of the p53-specific inhibitor PFT-α. As shown in Fig. 4D, PFT-α reversed the cordycepin-induced reduction in the levels of cyclin A2, cyclin E, and CDK2 proteins. These results indicated that cordycepin-induced cell cycle arrest in NB-4 and U937 cells involves p53.
Cordycepin induces DNA damage
To determine whether cordycepin induces DNA damage, we measured the accumulation of γH2AX, a sensitive marker of DNA double strand breaks (DSBs).20 Results showed a dramatically increase of γH2AX in cordycepin treated cells (Fig. 5A). However, ATM and ATR which involved in the response of mammalian cells to DSBs were not activated (Fig. 5A). As Cdc25A-degradation pathway is involved in the intra-S-phase checkpoint,21 we analyzed the effects of cordycepin on Chk2 and Cdc25A. Our data showed that cordycepin induced the phosphorylation of Chk2 and Cdc25A, and decreased the levels of Cdc25A (Fig. 5B). We conclude that cordycepin-induced S-phase arrest involves the activation of Chk2-Cdc25A pathway.
Figure 5.
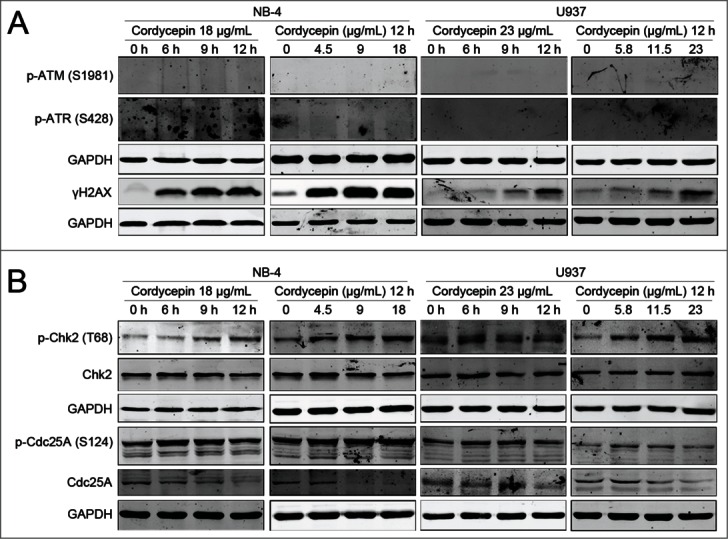
Cordycepin induces DNA damage in NB-4 and U937 cells. (A and B) NB-4 cells were treated with 18 μg/mL cordycepin for 6 h, 9 h and 12 h, or treated with 4.5 μg/mL (17.9 μM), 9 μg/mL (35.8 μM), 18 μg/mL (71.6 μM) cordycepin for 12 h. U937 cells were treated with 23 μg/mL (91.5 μM) cordycepin for 6 h, 9 h, and 12 h, or treated with 5.8 μg/mL (23.1 μM), 11.5 μg/mL (45.8 μM), 23 μg/mL (91.5 μM) cordycepin for 12 h. Whole cell lysates were evaluated by Western blot analysis with the indicated antibodies.
Discussion
In the present study, we showed that the growth of NB-4 and U937 cells can be inhibited by cordycepin. Further study showed that this inhibition was caused by apoptosis and cell cycle arrest. Cordycepin was found to activate both the extrinsic and intrinsic apoptotic pathways through caspase-8 and caspase-9 activation. Our results also showed a release of cytochrome c from the mitochondria to the cytosol in cordycepin treated NB-4 cells. Oddly, the levels of cytochrome c were found to be increased in both cytosolic and membrane fractions in U937 cells (Fig.2C), this maybe because the expression of cytochrome c was increased in cordycepin treated U937 cells. The released cytochrome c can then activate caspase-9 and trigger intrinsic apoptosis.22 XIAP is a member of the inhibitor of apoptosis family of proteins (IAP). These proteins can inhibit the activity of caspase-9 and caspase-3 by binding directly to them.23 Cordycepin treatment decreased the levels of XIAP in NB-4 cells, and thus sensitized cells to caspase-dependent apoptosis.
MAPK signaling is related to cell survival, and constitutive activation of this pathway was found in acute leukemia cells.24 The blockage of MAPK pathway impairs survival of acute myeloid leukemia (AML) cell lines by promoting cell apoptosis.19 The results of the present work showed that cordycepin inhibits the phosphorylation of ERK1/2, and blocks the activation of MAPK pathway. The blockage of MAPK signaling therefore sensitizes cordycepin-induced cell apoptosis.
P53 tumor suppressor plays a critical role in regulation of cell cycle and apoptosis, when cells were under cellular stress, p53 could be triggered, and thus induces cell cycle arrest or apoptosis.25,26 In the present work, it was observed that treatment of NB-4 and U937 cells with cordycepin leads to upregulation of p53 expression. As the p53-specific inhibitor PFT-α can partially inhibit cordycepin-induced caspase-3 and caspase-9 activation, we proposed that cordycepin-induced apoptosis in NB-4 and U937 cells is both p53-dependent and -independent. The increased levels of p53 can also act as a repressor to reduce the transcription of genes relevant to the cell cycle.27 Results showed that cordycepin-induced decreases in cyclin A2, cyclin E, and CDK2 levels could be reversed by the p53-specific inhibitor PFT-α. This indicated that cordycepin-induced S-phase arrest is p53-dependent.
Cyclin-dependent kinases (CDKs) are a family of proteins that reactivated at specific points during the cell cycle to control the progression of cells through the cycle. We demonstrated that cordycepin downregulates the expression of CDK2, an essential kinase for the G1/S transition28 and S phase progression.29 Cordycepin also induced a remarkable decrease in cyclin E and cyclin A2 protein levels, and thus reduced the rate of formation of cyclin E-CDK2 and cyclin A2-CDK2, facilitating cell cycle arrest.
DNA damage was indicated by the accumulation of γH2AX (Fig. 5A). Previous study showed that γH2AX is modulated through a complex signaling pathway which involves activation of ATM and ATR. However, neither ATM nor ATR was activated by cordycepin (Fig. 5A), suggesting that cordycepin-induced DNA damage is ATM/ATR independent. Although ATM was not affected, Chk2 was phosphorylated after the treatment of cordycepin (Fig. 5B). The activated Chk2 in turn phosphorylates Cdc25A and leads to the degradation of Cdc25A (Fig.5B). Lack of Cdc25A prevents dephosphorylation of CDK2 and locks CDK2 in its inactive form.21 As a result, DNA synthesis is inhibited and S-phase delay occurs.
In conclusion, the present study showed that cordycepin induced apoptosis in NB-4 and U937 cells involves upregulation of p53, release of cytochrome c from the mitochondria to the cytosol and blockage of MAPK pathway. Results also showed that cordycepin caused S-phase arrest by activation of Chk2-Cdc25A pathway, and by inhibiting expression of cyclinA2, cyclinE, and CDK2. These results suggested a specific model for the mechanism of cordycepin in leukemia cells (Fig. 6). Taken together, cordycepin inhibits the growth of leukemia cells by inducing apoptosis and cell cycle arrest, and may be a potential chemotherapeutic agent for the treatment of leukemia patients.
Figure 6.
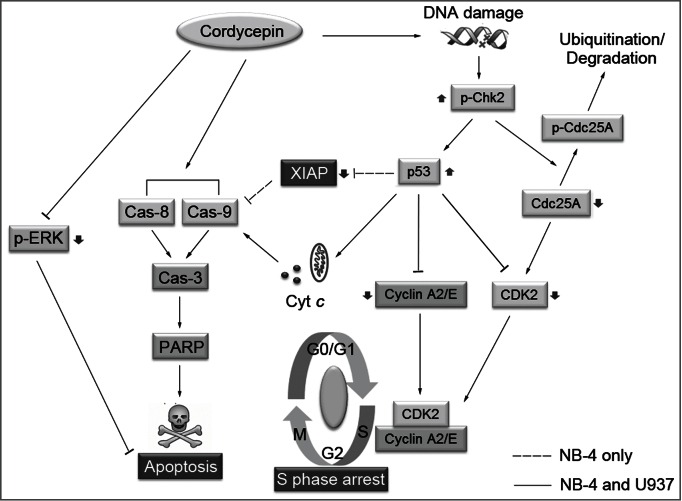
Schematic representation of proposed mechanism of cordycepin-induced apoptosis and cell cycle arrest in NB-4 and U937 cells. In response to cordycepin induced DNA damage, Chk2 is phosphorylated independent of ATM/ATR. The activated Chk2 can then induce ubiquitin-mediated degradation of Cdc25A by phosphorylating Cdc25A on S124. The degradation of Cdc25A leads to the accumulation of CDK2 in its inactive form, and results in the inhibition of DNA synthesis. Cordycepin increases the levels of p53 which repress the expression of cyclin A2, cyclin E, and CDK2, which then cause S-phase arrest. Cordycepin leads to activation of caspase-8 and caspase-9, which then cleave executioner caspase-3. Once activated, caspase-3 cleaves its substrates, such as PARP, and triggers cell apoptosis. The increased levels of p53 can also induce the release of cytochrome c from mitochondria to cytosol promoting the activation of caspase-9. In addition, the expression of anti-apoptotic protein XIAP was inhibited by the upregulation of p53 in NB-4 cells, while the levels of XIAP were not affected in U937 cells. Cordycepin also inhibits the phosphorylation of ERK1/2, and blocks the activation of MAPK pathway. The blockage of MAPK signaling therefore sensitizes cordycepin-induced cell apoptosis.
Materials and Methods
Reagents
Cordycepin was extracted from cultured Cordyceps kyushuensis by supercritical fluid extraction (SFE), and purified using high-speed counter-current chromatography (HSCCC). HSCCC extracts were analyzed using the Agilent 1100 HPLC system (Agilent) with a Kromasil 100-C18 column (250 mm×4.6 mm I.D., 5 μm) at 260 nm and at a column temperature of 30°C. Anti-caspase-8 (http://www.cellsignal.com/products/primary-antibodies/9746?hit=productId &Ntt=9746 , caspase-9 (http://www.cellsignal.com/products/primary-antibodies/9502?hit=productId &Ntt=9502), CDK2 (http://www.cellsignal.com/products/primary-antibodies/2546?hit=productId &Ntt=2546), cyclin A2 (http://www.cellsignal.com/products/primary-antibodies/4656?hit=productId &Ntt=4656), cyclin E (http://www.cellsignal.com/products/primary-antibodies/4129?hit=productId &Ntt=4129), ERK1/2 (http://www.cellsignal.com/products/primary-antibodies/9102?hit=productId &Ntt=9102), phospho-ERK1/2 (T202/Y204) (http://www.cellsignal.com/products/primary-antibodies/9101?hit=productId &Ntt=9101), γ-H2A.X. (http://www.cellsignal.com/products/primary-antibodies/9718?hit=productId &Ntt=9718), GAPDH (http://www.cellsignal.com/products/primary-antibodies/2118?hit=productId &Ntt=2118), p53 (http://www.cellsignal.com/products/primary-antibodies/9282?hit=productId &Ntt=9282), phospho-ATR (S428) (http://www.cellsignal.com/products/primary-antibodies/2853?hit=productId &Ntt=2853), poly (ADP-ribose) polymerase (PARP, http://www.cellsignal.com/products/primary-antibodies/9542?hit=productId &Ntt=9542) and XIAP (http://www.cellsignal.com/products/primary-antibodies/2045?hit=productId &Ntt=2045) antibodies were purchased from Cell Signaling Technology. Anti-Bax (http://www.abcam.com/Bax-antibody-E63-ab32503.html), cytochrome c (http://www.abcam.com/Cytochrome-C-antibody-EP1326Y-ab76107.html), phospho-ATM (S1981) (http://www.abcam.com/ATM-phospho-S1981-antibody-EP1890Y-ab81292.html), phospho-Cdc25A (S124) (http://www.abcam.com/Cdc25A-phospho-S124-antibody-EPR8888-ab156574.html), phosphor-Chk2 (T68) (http://www.abcam.com/Chk2-phospho-T68-antibody-Y171-ab32148.html), p38 (http://www.abcam.com/p38-antibody-Y122-ab32142.html) and phospho-p38 (T180/Y182) (#1229–1) antibodies were purchased from Epitomics. Anti-Tim23 (http://www.bdbiosciences.com/ptProduct.jsp?prodId=36427 andkey=611222 andparam=search andmterms=true andfrom=dTable) antibody was purchased from BD Biosciences. Anti-Cdc25A (http://www.abclonal.com/index.php?a=show andm=Product andid=3958) and Chk2 (http://www.abclonal.com/index.php?a=show andm=Product andid=1379) antibodies were purchased from Abclonal. Alexa Fluor 680 goat anti-mouse (http://www.lifetechnologies.com/order/catalog/product/A21057?ICID=search - a21057) and -rabbit (https://www.lifetechnologies.com/order/catalog/product/A21076?ICID=search -a21076) secondary antibodies were obtained from Invitrogen. Z-DEVD-fmk (http://www.bdbiosciences.com/ptProduct.jsp?prodId=61127 andkey=550378 andparam=search andmterms=true andfrom=dTable ) was purchased from BD Bioscience. Propidium iodide (PtdIns, http://www.sigmaaldrich.com/catalog/product/sigma/p4170?lang=zh andregion=CN) and Pifithrin-α (http://www.sigmaaldrich.com/catalog/product/sigma/p4359?lang=zh andregion=CN) were purchased from Sigma-Aldrich.
Cell culture
Cells were cultured in RPMI-1640 medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen). The cells were grown in a humidified incubator at 37°C under an atmosphere of 5% CO2 and used for assays during the exponential phase of growth.
Measurement of cell death
Cytotoxicity was measured using a Cell Counting Assay Kit-8 (Dojindo Molecular Technologies, http://www.dojindo.cn/products/C/cck-8.htm) according to the manufacturer's instructions. Cells were plated on 96-well plates and treated with various concentrations of cordycepin for 24 and 48 hours. Cell Counting Assay Kit-8 solution was added to each well, the cells were incubated for another 2–3 hours, and the absorbance at 450 nm was measured using a microplate reader (Synergy 2 Multi-Mode Microplate Reader, BioTek).
Cell morphology
Cells treated with cordycepin were washed twice with PBS and spun onto slides by using cytospin centrifugation (700 rpm, 5 min; Thermo Shandon). The slides were dipped in Wright's stain for 5 min, and phosphate buffer (0.03% KH2PO4, 0.02% Na2HPO4, pH 6.4) was added so that a metallic sheen would appear on the surface. After 5 min, the slides were washed with distilled water and tilted to drain. Then the slides were dipped in Giemsa stain (Beijing Leagene Biotech.Co,Ltd, http://www.leagene.com/product/shiji/dbzl/2013–06–24/18628.html) for 5 min, washed with distilled water, and air-dried in a vertical position. Once the slides were dry, morphology was evaluated by conventional light field microscopy under magnification 400×.
Subcellular fractionation
The subcellular fractionation was performed as described previously.30 Briefly, cells were collected, washed with ice-cold PBS and incubated on ice in buffer (250 mM sucrose, 20 mM HEPES pH 7.4, 5 mM MgCl2, 10 mM KCl, 1 mM EDTA, 1 mM EGTA, 0.05% digitonin, and protease inhibitor cocktail) for 20 min. Cytosolic (supernatant) and membrane (pellet) fractions were separated by centrifugation at 13,000×g for 10 min.
Western blot analysis
Proteins were separated by SDS-PAGE and transferred to nitrocellulose membranes (Whatman). Membranes were probed with the indicated primary antibodies overnight at 4°C. After washing 3 times with TBST, Alexa Fluor 680 goat anti-mouse or -rabbit was used to visualize the Western blotting using an infrared imaging system (Odyssey, LI-COR Biosciences). GAPDH served as an internal control.
Flow cytometric analysis of cell cycle
To measure cell cycle distribution, cells were harvested and analyzed by flow cytometry. Briefly, cells treated with cordycepin were harvested, washed with PBS, fixed with ice-cold 70% ethanol and finally stained with 20 μg/mL Propidium iodide (PI) and RNase A (0.2 mg/mL, Takara, http://www.takara.com.cn/?action=Page &Plat=pdetail andnewsid=1099 andsubclass=1) diluted in PBS (with 0.1% Triton X-100) for 15 min at 37°C. At least 20,000 cells were acquired per sample. Data were collected using CellQuestTM software and analyzed using ModFitLT.
Flow cytometric analysis of apoptosis
Cells treated with cordycepin were stained using a PE Annexin V Apoptosis Detection Kit I (BD Biosciences, http://www.bdbiosciences.com/ptProduct.jsp?prodId=13189 andcatyId=745077 andpage=product) according to the manufacturer's instructions. Briefly, cells were washed twice with cold phosphate-buffered saline (PBS) and stained in 100 μL of binding buffer containing Annexin V-PE and 7-AAD for 15 min at room temperature in the dark. Then 400 μL of binding buffer was added. The samples were analyzed with FACSCalibur (BD Biosciences). Then 10,000 cells were counted per sample. Data were collected using CellQuestTM software and analyzed using FlowJo 7.6.
Caspase-3/9 activity assay
Caspase-3/9 activity was assessed using caspase-3/9 colorimetric assay kits (BioVision, http://www.biovision.com/caspase-3-colorimetric-assay-kit-3478.html, http://www.biovision.com/caspase-9-colorimetric-assay-kit-3523.html) according to the manufacturer's instructions. Briefly, cells (5×106) were harvested and lysed in 50 μL of chilled lysis buffer and then incubated for 10 min on ice. After centrifugation at 12,000 g for 1 min, the supernatants were collected and approximately 100 μg of protein from the cell lysate was incubated with reaction buffer (containing 10 mM DTT) and conjugated substrates (DEVD-pNA for caspase-3 and LEHD-pNA for caspase-9) at 37°C for 2–4 hours. Caspase activity was measured using a fluorometer at an absorbance of 400 nm.
RNA extraction, reverse transcription, and quantitative real-time PCR
RNA was isolated using Trizol reagent (Invitrogen, http://www.lifetechnologies.com/order/catalog/zh/CN/adirect/lt?cmd=catDisplayStyle andcatKey=101 andfilterDispName=TRIzol %26reg%3B+ReagentandfilterType=1 &OP=filter andfilter=ft _1201%2Ff_2056459*and_bcs_=H4sIAAAAAAAAAH-2MwQqDMBBEv2ZP0qARxKuXngU%2FIU6bQOKWzcb8-frc%2FUBjegxmYx0TjugufL-Wgd%0AyC%2FDAblTQP3TR-9UPzRv5p6X37nJ6QRHixZnfCdUFLja1asBliFxgYjkhLmrJ9kJ-%2B%2FmVcVRpMXxkG%0AQ8CLAAAA) according to the manufacturer's instructions. First-strand cDNA was synthesized from approximately 1 μg total RNA using a transcriptor-first strand cDNA synthesis kit (Roche, http://lifescience.roche.com/shop/en/cn/products/transcriptor-first-strand-cdna-synthesis-kit). RNA was denatured for 10 min at 65°C with anchored-oligo (dT)18 primer, then reaction mix was added (8 mM MgCl2, 20 U protector RNase inhibitor, 1 mM dNTPs, 10 U transcriptor Reverse Transcriptase). The reaction was held at 55°C for 30 min, followed by a 5 min step at 85°C. cDNA products were used for real-time PCR reactions using SYBR Green Master (Roche, http://lifescience.roche.com/shop/en/cn/products/faststart-universal-sybr-master-rox-). Reactions were run on an ABI 7500 real-time PCR system (Applied Biosystems). The GAPDH gene was used as an internal amplification control. The forward primer for GAPDH was 5′-GCA CCG TCA AGG CTG AGA AC-3′, and the reverse primer was 5′-CCA CTT GAT TTT GGA GGG ATC T-3′;31 the forward primer for CDK2 was 5′-CCA GGA GTT ACT TCT ATG CCT GA-3′, and the reverse primer was 5′-AAT CCG CTT GTT AGG GTC GTA-3′;32 the forward primer for cyclin A2 was 5′-CCT GCA AAC TGC AAA GTT GA-3′, and the reverse primer was 5′-AAA GGC AGC TCC AGC AAT AA-3′;33 the forward primer for cyclin E was 5′-AGA AAT GGC CAA AAT CGA CA-3′, and the reverse primer was 5′-CCC GGT CAT CT CTT CTT TG-3′.
Statistical analysis
Data are expressed as mean ± SD. Student t test was performed to determine the significance between the control group and the experimental ones. P values of <0 .05 were considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work is supported in part by the State Key Development Program for Basic Research of China (No. 2010CB529205), National Key Basic Research Project of China (No. 2012CB966900) and Shanghai Jiao Tong University “Chen-xin” Young Scholars project.
Supplemental Material
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Hwang IK, Lim SS, Yoo KY, Lee YS, Kim HG, Kang IJ, Kwon HJ, Park J, Choi SY, Won MH. A Phytochemically characterized extract of cordyceps militaris and cordycepin protect hippocampal neurons from ischemic injury in gerbils. Planta Med 2008; 74:114-9; PMID:18214814; http://dx.doi.org/ 10.1055/s-2008-1034277 [DOI] [PubMed] [Google Scholar]
- 2. Choi SB, Park CH, Choi MK, Jun DW, Park S. Improvement of insulin resistance and insulin secretion by water extracts of cordyceps militaris, phellinus linteus, and paecilomyces tenuipes in 90% pancreatectomized rats. Biosci Biotechnol Biochem 2004; 68:2257-64; PMID:15564662; http://dx.doi.org/ 10.1271/bbb.68.2257 [DOI] [PubMed] [Google Scholar]
- 3. Jung K, Kim IH, Han D. Effect of medicinal plant extracts on forced swimming capacity in mice. J Ethnopharmacol 2004; 93:75-81; PMID:15182908; http://dx.doi.org/ 10.1016/j.jep.2004.03.022 [DOI] [PubMed] [Google Scholar]
- 4. Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol 2005; 96:555-61; PMID:15619578; http://dx.doi.org/ 10.1016/j.jep.2004.10.009 [DOI] [PubMed] [Google Scholar]
- 5. Ahn YJ, Park SJ, Lee SG, Shin SC, Choi DH. Cordycepin: selective growth inhibitor derived from liquid culture of cordyceps militaris against clostridium spp. J Agri Food Chem 2000; 48:2744-8; PMID:10898616; http://dx.doi.org/ 10.1021/jf990862n [DOI] [PubMed] [Google Scholar]
- 6. Muller WE, Weiler BE, Charubala R, Pfleiderer W, Leserman L, Sobol RW, Suhadolnik RJ, Schroder HC. Cordycepin analogues of 2',5'-oligoadenylate inhibit human immunodeficiency virus infection via inhibition of reverse transcriptase. Biochemistry 1991; 30:2027-33; PMID:1705437; http://dx.doi.org/ 10.1021/bi00222a004 [DOI] [PubMed] [Google Scholar]
- 7. Wu JY, Zhang QX, Leung PH. Inhibitory effects of ethyl acetate extract of cordyceps sinensis mycelium on various cancer cells in culture and B16 melanoma in C57BL/6 mice. Phytomedicine 2007; 14:43-9; PMID:16423520; http://dx.doi.org/ 10.1016/j.phymed.2005.11.005 [DOI] [PubMed] [Google Scholar]
- 8. Chang Y, Jeng KC, Huang KF, Lee YC, Hou CW, Chen KH, Cheng FY, Liao JW, Chen YS. Effect of cordyceps militaris supplementation on sperm production, sperm motility and hormones in sprague-dawley rats. Am J Chin Med 2008; 36:849-59; PMID:19051352; http://dx.doi.org/ 10.1142/S0192415X08006296 [DOI] [PubMed] [Google Scholar]
- 9. Cunningham KG, Manson W, Spring FS, Hutchinson SA. Cordycepin, a metabolic product isolated from cultures of cordyceps militaris (Linn.) Link. Nature 1950; 166:949; PMID:14796634. [DOI] [PubMed] [Google Scholar]
- 10. Muller WE, Seibert G, Beyer R, Breter HJ, Maidhof A, Zahn RK. Effect of cordycepin on nucleic acid metabolism in L5178Y cells and on nucleic acid-synthesizing enzyme systems. Cancer Res 1977; 37:3824-33; PMID:332340. [PubMed] [Google Scholar]
- 11. Chang W, Lim S, Song H, Song BW, Kim HJ, Cha MJ, Sung JM, Kim TW, Hwang KC. Cordycepin inhibits vascular smooth muscle cell proliferation. Europ J Pharmacol 2008; 597:64-9; PMID:18782572; http://dx.doi.org/ 10.1016/j.ejphar.2008.08.030 [DOI] [PubMed] [Google Scholar]
- 12. Lee SJ, Kim SK, Choi WS, Kim WJ, Moon SK. Cordycepin causes p21WAF1-mediated G2/M cell-cycle arrest by regulating c-jun N-terminal kinase activation in human bladder cancer cells. Arch Biochem Biophys 2009; 490:103-9; PMID:19733546; http://dx.doi.org/ 10.1016/j.abb.2009.09.001 [DOI] [PubMed] [Google Scholar]
- 13. Lee EJ, Kim WJ, Moon SK. Cordycepin suppresses TNF-α-induced invasion, migration and matrix metalloproteinase-9 expression in human bladder cancer cells. Phytother Res 2010; 24:1755-61; PMID:20564512; http://dx.doi.org/ 10.1002/ptr.3132 [DOI] [PubMed] [Google Scholar]
- 14. Jeong JW, Jin CY, Park C, Han MH, Kim GY, Moon SK, Kim CG, Jeong YK, Kim WJ, Lee JD, et al. Inhibition of migration and invasion of LNCaP human prostate carcinoma. Int J Oncol 2012; PMID:22246470; 10.3892/ijo.2012.1332 [DOI] [PubMed] [Google Scholar]
- 15. Koc Y, Urbano AG, Sweeney EB, McCaffrey R. Induction of apoptosis by cordycepin in ADA-inhibited TdT-positive leukemia cells. Leukemia 1996; 10:1019-24; PMID:8667637 [PubMed] [Google Scholar]
- 16. Chen Y, Chen YC, Lin YT, Huang SH, Wang SM. Cordycepin induces apoptosis of CGTH W-2 thyroid carcinoma cells through the calcium-calpain-caspase 7-PARP pathway. J Agri Food Chem 2010; 58:11645-52; PMID:20961042; http://dx.doi.org/ 10.1021/jf1028976 [DOI] [PubMed] [Google Scholar]
- 17. Pan BS, Lin CY, Huang BM. The effect of cordycepin on steroidogenesis and apoptosis in MA-10 mouse leydig tumor cells. EvidBased Complement Alternat Med 2011; 2011:750468; PMID:21716681; 10.1155/2011/750468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Foss FM. Combination therapy with purine nucleoside analogs. Oncology (Williston Park) 2000; 14:31-5; PMID:10887642 [PubMed] [Google Scholar]
- 19. Milella M, Kornblau SM, Estrov Z, Carter BZ, Lapillonne H, Harris D, Konopleva M, Zhao S, Estey E, Andreeff M. Therapeutic targeting of the MEK/MAPK signal transduction module in acute myeloid leukemia. J Clin Invest 2001; 108:851-9; PMID:11560954; http://dx.doi.org/ 10.1172/JCI12807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273:5858-68; PMID:9488723; http://dx.doi.org/ 10.1074/jbc.273.10.5858 [DOI] [PubMed] [Google Scholar]
- 21. Falck J, Mailand N, Syljuasen RG, Bartek J, Lukas J. The ATM-Chk2-Cdc25A checkpoint pathway guards against radioresistant DNA synthesis. Nature 2001; 410:842-7; PMID:11298456; http://dx.doi.org/ 10.1038/35071124 [DOI] [PubMed] [Google Scholar]
- 22. Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol 1998; 60:619-42; PMID:9558479; http://dx.doi.org/ 10.1146/annurev.physiol.60.1.619 [DOI] [PubMed] [Google Scholar]
- 23. Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem 1998; 273:7787-90; PMID:9525868; http://dx.doi.org/ 10.1074/jbc.273.14.7787 [DOI] [PubMed] [Google Scholar]
- 24. Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia 1997; 11(4):479-84; PMID:9096686; http://dx.doi.org/ 10.1038/sj.leu.2400617 [DOI] [PubMed] [Google Scholar]
- 25. Wong VC, Cash HL, Morse JL, Lu S, Zhitkovich A. S-phase sensing of DNA-protein crosslinks triggers topBP1-independent ATR activation and p53-mediated cell death by formaldehyde. Cell Cycle 2012; 11:2526-37; PMID:22722496; http://dx.doi.org/ 10.4161/cc.20905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hopker K, Hagmann H, Khurshid S, Chen S, Schermer B, Benzing T, Reinhardt HC. Putting the brakes on p53-driven apoptosis. Cell Cycle 2012; 11:4122-8; PMID:22983126; http://dx.doi.org/ 10.4161/cc.21997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang B, Xiao Z, Ko HL, Ren EC. The p53 response element and transcriptional repression. Cell Cycle 2010; 9:870-9; PMID:20160511; http://dx.doi.org/ 10.4161/cc.9.5.10825 [DOI] [PubMed] [Google Scholar]
- 28. van den Heuvel S, Harlow E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 1993; 262:2050-4; PMID:8266103; http://dx.doi.org/ 10.1126/science.8266103 [DOI] [PubMed] [Google Scholar]
- 29. Hu B, Mitra J, van den Heuvel S, Enders GH. S and G2 phase roles for cdk2 revealed by inducible expression of a dominant-negative mutant in human cells. Mol Cell Biol 2001; 21:2755-66; PMID:11283255; http://dx.doi.org/ 10.1128/MCB.21.8.2755-2766.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene 2003; 22:2643-54; PMID:12730678; http://dx.doi.org/ 10.1038/sj.onc.1206326 [DOI] [PubMed] [Google Scholar]
- 31. Lanaro C, Franco-Penteado CF, Albuqueque DM, Saad ST, Conran N, Costa FF. Altered levels of cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydroxyurea therapy. J Leukoc Biol 2009; 85:235-42; PMID:19004988; http://dx.doi.org/ 10.1189/jlb.0708445 [DOI] [PubMed] [Google Scholar]
- 32. Chen K, Perez-Stable C, D'Ippolito G, Schiller PC, Roos BA, Howard GA. Human bone marrow-derived stem cell proliferation is inhibited by hepatocyte growth factor via increasing the cell cycle inhibitors p53, p21 and p27. Bone 2011; 49:1194-204; PMID:21907315; http://dx.doi.org/ 10.1016/j.bone.2011.08.023 [DOI] [PubMed] [Google Scholar]
- 33. Olivero OA, Tejera AM, Fernandez JJ, Taylor BJ, Das S, Divi RL, Poirier MC. Zidovudine induces S-phase arrest and cell cycle gene expression changes in human cells. Mutagenesis 2005; 20:139-46; PMID:15784690; http://dx.doi.org/ 10.1093/mutage/gei019 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.