Abstract
Box H/ACA ribonucleoproteins (RNPs), each consisting of one unique guide RNA and 4 common core proteins, constitute a family of complex enzymes that catalyze, in an RNA-guided manner, the isomerization of uridines to pseudouridines (Ψs) in RNAs, a reaction known as pseudouridylation. Over the years, box H/ACA RNPs have been extensively studied revealing many important aspects of these RNA modifying machines. In this review, we focus on the composition, structure, and biogenesis of H/ACA RNPs. We explain the mechanism of how this enzyme family recognizes and specifies its target uridine in a substrate RNA. We discuss the substrates of box H/ACA RNPs, focusing on rRNA (rRNA) and spliceosomal small nuclear RNA (snRNA). We describe the modification product Ψ and its contribution to RNA function. Finally, we consider possible mechanisms of the bone marrow failure syndrome dyskeratosis congenita and of prostate and other cancers linked to mutations in H/ACA RNPs.
Keywords: dyskeratosis congenita, H/ACA, prostate cancer, pseudouridine, ribonucleoproteins, RNA-guided, RNA modification, rRNA, spliceosomal small nuclear RNA, snoRNA
Abbreviations
- DC
dyskeratosis congenita
- HH
hoyeraal-hreidarsson syndrome
- PIKK
phosphatidylinositol 3-kinase-related kinase
- ψ
pseudouridine, 5-ribosyluracil
- PUA
pseudouridylase and archaeosine transglycosylase
- RNP
ribonucleoprotein
- rRNA
ribosomal RNA
- sca
small Cajal body
- SMN
survival of motor neuron protein
- sno
small nucleolar
- snoRNA
small nucleolar RNA
- snRNA
small nuclear RNA
- SSD
SHQ1 specific domain
- tRNA
transfer RNA
- U
uridine
- X-DC
X-linked dyskeratosis congenita
Introduction
Posttranscriptional modification of RNA is a common and diverse event with over 140 distinct RNA modifications.1 Of all these, pseudouridine (Ψ, 5-ribosyluracil) is the most common and at the same time perhaps the most unusual. Unlike other RNA modifications that simply add functional groups while leaving the nucleoside untouched, pseudouridylation involves the detachment of the base from the ribose, its rotation by 180° and reattachment to form a nearly unique carbon-carbon glycosidic bond, i.e. the isomerization of uridine to Ψ (Fig. 1A). Ψ is so abundant that it was originally recognized as a “fifth” nucleotide.2 This is not surprising given that it so far has been identified as significant component of only the most abundant stable RNAs in the cell, transfer RNA (tRNA), ribosomal RNA (rRNA) and spliceosomal small nuclear RNA (snRNA). Pseudouridylation is performed by phylogenetically related enzymes, known as pseudouridine synthases, with or without the help of additional proteins and guide RNAs. Guide RNAs identify target uridines by site-directed base pairing. Pseudouridylation guide RNAs are known as H/ACA RNAs, one of the 2 major classes of small nucleolar RNAs (snoRNAs), the other being C/D RNAs responsible for guiding 2′-O-methylation. The ribonucleoproteins (RNPs), formed by H/ACA RNAs, the pseudouridine synthase, and 3 other core proteins, are called H/ACA RNPs. In this review, we will concentrate on RNA-guided pseudouridylation, which limits us to the following modification targets, archaeal and eukaryotic rRNA, archaeal tRNA and yeast and mammalian snRNA. For the abundant pseudouridylation of tRNAs and its dedicated stand-alone enzymes, we refer to the following excellent sources.3-5
Figure 1.
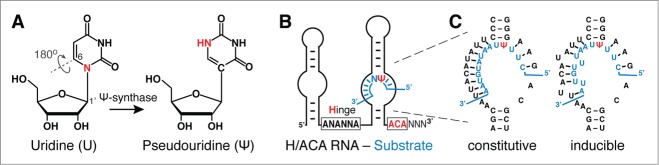
Pseudouridylation, guide RNA, and guide mechanism. (A) Chemical structure of uridine (U) and pseudouridine (Ψ). The 180° rotation of the base frees up the N-glycosidic nitrogen in U as an additional hydrogen donor in Ψ (red). The numbering of base and ribose positions for potential nucleophilic attacks by a conserved aspartate of the Ψ-synthase is indicated. Note the aspartate is highlighted in yellow in the dark green catalytic domain of the Ψ-synthase (Fig. 2, inset). (B) Schematic of an H/ACA guide RNA hybridized to a substrate RNA (blue) in its 3′ pseudouridylation pocket. The hinge region and ACA conserved sequence elements responsible for the H/ACA name are highlighted (red; N = any nucleotide). (C) Example of the pseudouridylation pocket of the yeast H/ACA RNA snR81 hybridized to its substrate RNA (blue). Perfect Watson-Crick base-pairs between the guide sequence and the substrate RNA result in constitutive pseudouridylation at the target site (left panel, constitutive pseudouridylation). In contrast, if the guide sequence forms imperfect base-pairs (there are 2 mismatches) with the substrate RNA, pseudouridylation occurs only under stress (nutrient-deprivation) conditions (right panel, inducible pseudouridylation).
Pseudouridine
Ψ, 5-ribosyluracil, is the most abundant modification of RNA (Fig. 1A). The isomerization of uridine to Ψ changes the chemical properties of the nucleoside in 2 ways, the additional imino group in the base provides an extra hydrogen bond donor and the carbon-carbon glycosidic bond is more stable than the nearly universal nitrogen-carbon bond.4 Compared to uridine, these differences improve local base stacking, rigidify the phosphate-sugar backbone (through bound water molecules) and enhance the stability of pseudouridylated duplexes.6-12 Although measurable, the differences between uridine and Ψ containing RNAs are subtle and Ψs are thought to fine-tune the function of their RNAs (see below).
Guide RNA Machinery
H/ACA RNP overview
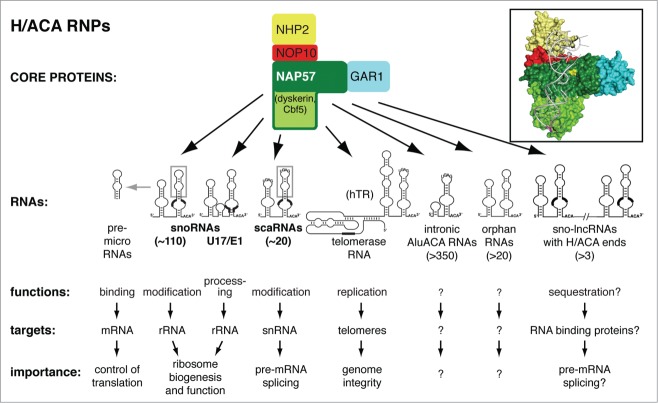
All H/ACA RNPs consist of one name and function specifying H/ACA RNA and the same 4 core proteins (Fig. 2). The core proteins are essential for the stability of all H/ACA RNAs and of each other, and for catalysis of pseudouridylation. Surprisingly, it appears that for the majority of H/ACA RNPs, at least in mammalian cells, the enzymatic activity of the pseudouridine synthase is irrelevant but its function in maintaining the RNAs is paramount. These structural classes of H/ACA RNPs include the telomerase RNP, intron-encoded Alu (AluACA) RNPs and small nucleolar-long noncoding (sno-lnc) RNPs (Fig. 2)13-15. Although it is not clear on which side the orphan H/ACA RNPs will come down, structural H/ACA RNPs outnumber catalytic ones in diversity by about 4 to 1, whereas the catalytic RNPs are far more abundant in terms of copy number. While the structural aspects of the RNPs discussed below apply to all H/ACA particles, we will focus here specifically on those guiding pseudouridylation, i.e., H/ACA small nucleolar (sno) and small Cajal body-specific (sca) RNPs. For information on all H/ACA RNPs, we refer to the following reviews.16-22
Figure 2.
H/ACA RNPs. Schematic of the different classes of H/ACA RNPs defined by the H/ACA RNAs. One set of the 4 core proteins assembles with each hairpin of every H/ACA RNA. Guide sequences of the RNAs are indicated (thickening of lines) and the snoRNAs and scaRNAs that are the focus of this review are highlighted (bold). The functions, targets, and importance, where known, are listed underneath. The approximate number of species within each class of H/ACA RNA (which in most cases is still growing) is specified in parentheses. Inset: model of the structure of a single hairpin of a human H/ACA RNP. The color code of the core proteins is the same as in the schematic. The RNA is in gray and the ACA triplet at the bottom of the molecule is in purple. The catalytic aspartate of the green Ψ-synthase is highlighted (yellow). Archaeal and bacterial structures served as basis for the model.140
Guide RNAs
In RNA-guided pseudouridylation, site-directed base pairing of small H/ACA RNAs with the target RNA positions the uridine at the active site of the modification machinery. Identified in 1996/7,23,24 H/ACA RNAs have a median length of 133 nucleotides (137.6 +/-18.8; average +/-standard deviation)25 forming an evolutionarily conserved hairpin-hinge-hairpin-tail secondary structure that carries a consensus ANANNA sequence in the hinge region (H box) and an ACA triplet exactly 3 positions from the 3′-end (ACA box) (Fig. 1B). Identification of target uridines is achieved by 2 3-10 nucleotide-long antisense elements in the bulge of one or both hairpins (pseudouridylation pocket) directly 5′ and 3′ of the upper stem (Fig. 1B). These 2 elements hybridize to sequences immediately 5′ and one nucleotide 3′ of the target uridine thereby framing it.26,27 This positions the target uridine 14-15 nucleotides from the H or ACA box.
Although this is the basic structure and guide mechanism for most H/ACA RNAs, there are exceptions. For example in archaea, H/ACA RNAs are simply termed small (s) RNAs and consist mostly of single hairpins but may have up to 3 hairpins.28,29 In mammalian cells, H/ACA RNAs that guide pseudouridylation are snoRNAs, which guide the modification of rRNA, and scaRNAs, which guide the modification of spliceosomal snRNAs. A fraction of some of the sno and scaRNAs is further processed to pre-microRNAs (Fig. 2, gray boxes). In yeast, all H/ACA RNAs are snoRNAs, irrespective of target RNA. A few of the H/ACA scaRNAs are hybrids that also contain C/D boxes and that guide both pseudouridylation and 2′-O-methylation.30,31 This is particularly of practical importance because isolation of box H/ACA RNPs will therefore also yield box C/D components and vice versa.32
H/ACA core proteins
The central core protein is the enzyme of H/ACA RNPs, the pseudouridine synthase. It was originally identified in yeast and mammalian cells before its function was known and subsequently revealed its true identity through sequence homology to the bacterial pseudouridine synthase specific for U55 in tRNA and through genetic analysis in yeast.33-37 The protein goes under a variety of names, NAP57 and dyskerin in mammalian cells, Cbf5 in yeast, protists and archaea, minifly and Nop60B in fly, and as the stand-alone enzyme TruB in bacteria.33-35,38-42 The 514-amino acid human NAP57 (Fig. 2, green) features highly charged, lysine-rich N- and C-termini, a central catalytic domain followed by an RNA-binding pseudouridylase and archaeosine transglycosylase (PUA) domain. The X-ray structure of the bacterial, archaeal and yeast enzymes show a high degree of structural conservation, particularly in the catalytic and PUA domains.43-48 The charged and low complexity terminal extremes are apparently unstructured and were omitted. The PUA domain and part of the N-terminus combine to form a domain that is separate from the catalytic module. The catalytic domain is conserved in all pseudouridine synthases including all stand-alone enzymes.22,49 The 2 domains form an L-shaped structure with one arm representing the catalytic (Fig. 2, dark green) and the other the N- and C-terminal domains (light green).
The other 3 core proteins are NOP10, NHP2 and GAR1. Like NAP57, they are all essential proteins and depletion of their yeast orthologs, with the exception of GAR1,50,51 causes the loss of all H/ACA RNAs. NOP10 (Fig. 2, red) is a 64-amino acid polypeptide that stabilizes and lines the catalytic domain of NAP57 serving as a dock for NHP2 binding.43-45,47 NHP2 (Fig. 2, yellow) is a 153-amino acid protein with a phylogenetically conserved central RNA binding domain, which in the case of NHP2 binds RNA non-specifically.52,53 Unlike the other H/ACA core proteins, NHP2 remains stable when the other core proteins are depleted in yeast, but depletion of NHP2 leads to loss of the other 3 proteins.54,55 The 217-amino acid long GAR1 (Fig. 2, light blue) also binds to the catalytic domain of NAP57 but independently of NOP10 and NHP2. As suggested by its name, the hallmarks of GAR1 are its 2 terminal glycine–arginine-rich domains that together account for about half of the protein.50,51 GAR1 can contact the active site of the particle and is required for substrate turnover during the pseudouridylation reaction48,53 (see below).
Structure
The crystal structures of many pseudouridine synthases have been solved. In case of NAP57 homologs, this starts with the structure of the bacterial TruB in complex with part of a tRNA, then encompasses the structures of several archaeal partial and complete H/ACA protein complexes with guide RNA and substrate RNA and finally, the structure of a yeast H/ACA RNP lacking Nhp2.43-46,48,56-58 Although the structure of the human complex is still elusive, copious information can be gained from these structures because of the high degree of phylogenetic conservation of the enzymes and RNPs. All structures include only a single hairpin of an H/ACA RNA leaving the organization of the intact particle open to speculation. However, based on low resolution structures derived from negative stained EM images of purified yeast H/ACA RNPs, the holoparticle forms a v-shaped structure with each arm apparently representing one hairpin and 4 core proteins.41,59 Therefore, as documented for the telomerase RNP, one H/ACA RNA accommodates 2 sets of the 4 core proteins to yield an intact eukaryotic H/ACA RNP.60 The structure of a human H/ACA RNP modeled after that of the complete structure of an archaeal RNP shows the organization of the proteins and the single hairpin RNA (Fig. 2, inset). NAP57 forms the core of the particle, with NOP10 and GAR1 binding independently to its catalytic domain and NHP2 docking on NOP10. The RNA is draped over the core trimer of NAP57, NOP10, and NHP2 without seemingly engaging GAR1. The conserved ACA triplet is anchored in the PUA domain of NAP57, whereas the loop of the hairpin binds to NHP2 thereby placing the pseudouridylation pocket over the central catalytic domain (Fig. 2, inset). Although this structure is firmly established through crystallization of several homologs and based on biochemical evidence, it is somewhat misleading as significant parts of the individual proteins were truncated or are not visible in the structure. For example, the charged termini of eukaryotic NAP57 and the glycine–arginine-rich domains of GAR1 are missing from the archaeal orthologs and were removed from the yeast proteins for crystallization purposes. Nevertheless, these “partial” structures are functional readily pseudouridylating substrate RNAs.48,61,62 In general, H/ACA RNPs are very stable. Although all core proteins, except NAP57 can exchange, the H/ACA RNA cannot be replaced without disassembly or degradation of the particle, i.e. requiring de novo synthesis.53,63
Mechanism
Isomerization of uridine to Ψ requires significant molecular gymnastics, the breakage of the N-glycosidic bond and the 180° rotation of the base. Based on the universal conservation of an aspartic acid residue in the active site of all pseudouridine synthases identified to date, all proposed mechanisms involve a nucleophilic attack by the aspartate. However, the site of this attack has been questioned, be it at the base (Fig. 1A, C6) or at the ribose (C1′).64 This confusion has been mainly brought about by the use of 5-fluorouridine (5-FU) in substrate RNAs. Such substrates lead to covalent attachment of 5-FU to some pseudouridine synthases concomitant with their inhibition, whereas others efficiently convert 5-FU to 5-fluoro-6-hydroxy-Ψ (5FhΨ).46,65,66 The latter is true for the TruB class of enzymes to which NAP57 belongs. Indeed, in vitro pseudouridylation experiments with 5-FU substrates indicated that also mammalian H/ACA RNPs isomerize substrates with 5-FU at their target sites67 (Wang and Meier, unpublished results). The more recent identification of arabinose (instead of ribose) in a minor product of 5-FU isomerization by TruB, suggests a reaction mechanism wherein the nucleophilic attack by the aspartate occurs at C1′ of the ribose ring and proceeds via a glycal or acylal intermediate.68,69 However, analysis of structures of uridine substrate analogs bound to archaeal H/ACA RNPs reveals a closer proximity of the catalytic aspartate to the C6 of the base favoring the originally proposed mechanism.65,70 So, the discussion about the reaction mechanism continues. How the enzymes accomplish the separation and rotation of the base will have to await more sensitive technology that allows following detailed conformational changes, live and in structures.
Regardless, it has become abundantly clear that the target uridine within a substrate RNA is being flipped out for isomerization into the active pocket of the enzymes, be that in the stand-alone enzymes or in H/ACA RNPs.46,56,57 An important role in substrate RNA binding and turnover is played by a thumb-loop element (b7_10 loop) in the catalytic domain of both kinds of pseudouridine synthases, guide-RNA dependent and independent.46,56,57,71 Thus, upon substrate binding, the thump-loop swings over to lock the substrate RNA in place. In H/ACA RNPs this movement is supported by GAR1, which is required for multi-turnover reactions.57,72 The central conserved domain of GAR1 is sufficient for the reaction, with its 11 C-terminal residues playing a crucial role by directly interacting with the thumb loop.48 This interaction is essential for the multi-turnover reaction of the enzyme complex.48 Unlike the rest of the central GAR1 domain, these 11 amino acids are not conserved in the assembly factor NAF1, which, while binding to the same domain of NAP57, is unable to support pseudouridylation.48,73,74 The function of assembly factors is explored in the next section.
Biogenesis
Biogenesis of the only 5-component pseudouridylation guide machinery is surprisingly complex involving at least 6 assembly factors in the cytoplasm and nucleus (Fig. 3). NAF1 and SHQ1 are H/ACA-specific chaperones and were identified in yeast through their interaction with the yeast NAP57 ortholog, Cbf5.74-76 They are required for stable expression of all H/ACA RNAs without being part of the mature particles in nucleolus and Cajal bodies. SHQ1 functions first by tightly binding to NAP57, apparently right after translation.77 Doing so, SHQ1 achieves 2 purposes, protecting the degradation and aggregation prone NAP57 and physically preventing non-cognate RNAs from binding to NAP57 before its cotranscriptional association with its guide RNAs. Specifically, SHQ1 is an RNA mimic taking up the same position on NAP57 as an H/ACA RNA will in the mature RNP and forming many of the same molecular interactions (compare insets in Figs. 2 and 3).78,79 Thus, the C-terminal SHQ1-specific domain (SSD, 487 amino acids) of SHQ1 binds predominantly to the domain of NAP57 formed by its C-terminal PUA and N-terminal parts (Figs. 2 and 3, light green). Additionally, the HSP20-like N-terminal CS domain of SHQ1 (90 amino acids) binds to the same domain of NAP57 but on the opposite side thereby closing a tight clamp around NAP57.77,80-82 In fact, the NAP57-SHQ1 interaction is so tight that its resolution depends on additional chaperones, the R2TP complex.82
Figure 3.
Schematic of the surprisingly complex biogenesis of H/ACA RNPs. Details of the at least 4-step process are described in the text. The approximate size and sites of interactions of the proteins is drawn to scale. Inset: Surface rendering of the structures of yeast NAP57 alone and in complex with the SHQ1 specific domain (SSD; pdb: 3uai). Amino acid positions equivalent to those mutated in human NAP57 in patients with dyskeratosis congenita are highlighted (orange). Note many of these positions cluster in the C-terminal segment (CTS, knob on the left) that serves as handle for the SSD, although they are generally poorly visible in this surface rendering.
The R2TP complex consists of the 2 closely related AAA+ ATPases (ATPases associated with diverse cellular activities) pontin and reptin (Rvb1/2, TIP48/49, RuvBL1/2, etc.), PIH1D1 (Nop17, Pih1) and RPAP3 (hSpagh, Tah1), arranged in (hetero)hexameric rings, and is named after the yeast homologs Rvb1, Rvb2, Tah1 and Pih1 (Fig. 3).83 In addition to snoRNP biogenesis of both, box H/ACA and C/D RNPs, the R2TP complex has been implicated in the assembly of RNA polymerase II and of phosphatidylinositol 3-kinase-related kinases (PIKKs, i.e., mTOR and SMG1).82,84-90 Pontin and reptin alone or together are associated with many other macromolecular assemblies involved in a wide variety of cellular activities, e.g. chromatin remodeling, histone acetyl transfer, transcription, and mitotic spindle formation.83,91,92 Studies on the separation of the NAP57•SHQ1 complex resulted in mechanistic insight into the function of the ATPases (Fig. 3). They bind to both proteins and require the charged C- (but not N-) terminus of NAP57 for SHQ1 release.82 Presumably, freeing of NAP57 from SHQ1 occurs immediately before its association with a guide RNA at the site of transcription to prevent aggregation and binding to non-cognate RNAs.77-79
At the site of H/ACA RNA transcription, the core proteins NOP10 and NHP2 as well as the assembly factor NAF1 are present in and likely join the nascent RNP complex (Fig. 3).93-95 As stated above, NAF1 lacks the amino acids for interaction with the thumb loop domain of NAP57 from its central GAR1-homology domain.73 In this manner, NAF1 keeps the pseudouridylation machinery inactive until replacement of NAF1 with GAR1 thus preventing modification of random RNA targets until formation of the mature particles.95 The latter, unlike the assembly factors, concentrate in nucleoli and Cajal bodies, their major sites of action. Exactly where in the cell these exchanges and maturation steps occur remains to be elucidated; however, they are essential for viability, as cells depleted of the individual assembly factors lose all H/ACA RNAs and consequently fail to properly process and modify rRNA.74-77,82,96
Substrates
Overview
Pseudouridylation guide RNAs are known to target mainly 2 kinds of RNAs, rRNA and spliceosomal snRNA.97,98 In vertebrate cells, these 2 types of RNAs are pseudouridylated mostly (if not exclusively) by an RNA-guided mechanism. Additionally, vertebrate snoRNAs U3 and U8 can also be pseudouridylated by this pathway.99,100 In contrast, yeast snRNA is pseudouridylated by both RNA-guided and stand-alone enzymes, the latter recognize and modify the RNA without the help of guide RNAs.101 This mixed mechanism of pseudouridylation of the same RNA may mark an evolutionary transition from single-protein to RNA-guided pseudouridine synthases.101 This transition is further evident with the switch to RNA-guided pseudouridylation concomitant with increasing number of modification sites in target RNAs, e.g., from 11 sites in bacterial rRNA (stand-alone enzymes) to ∼50 in yeast and ∼100 in mammalian rRNA (both RNA-guided).102,103 Despite the extensive pseudouridylation of all tRNAs in all species, archaea offer the only known example of RNA-guided modification of tRNA.104,105 In summary, only stable noncoding RNAs are targets of RNA-guided pseudouridylation, which shall be the focus of this review. Nevertheless, given the difficulty of detecting partial pseudouridylation in low-abundant RNAs (e.g. mRNAs) and the existence of many H/ACA RNAs without identified target (orphan H/ACA RNAs) or with imprecise complementarity, it is reasonable to predict pseudouridylation extending beyond these types of noncoding RNAs.
Eukaryotic rRNAs
Eukaryotic rRNAs contain a large number of pseudouridines. For instance, there are 46 pseudouridines in S. cerevisiae rRNAs.106 In mammalian rRNAs, the amount of pseudouridine is even higher; currently, 97 pseudouridines have been identified.103 Since the discovery of the box H/ACA RNA-guided pseudouridylation mechanism approximately 17 years ago,26,27 a great effort has been made to search for new box H/ACA RNAs. Using a combination of computational and experimental approaches, researchers have identified a large number of new box H/ACA RNAs in a variety of organisms. Subsequent computational analysis and visual inspection (comparing the guide sequences of box H/ACA RNAs with the target sequences of rRNAs) have generated nearly complete sets of box H/ACA RNAs that are potentially capable of directing rRNA pseudouridylation, not only in S. cerevisiae, but also in humans.103,106,107 For some, the predicted guide RNA-substrate relationship has been experimentally verified. For instance, it has been shown that deleting several box H/ACA RNAs individually or in combination results in loss of pseudouridine(s) at expected sites in yeast rRNAs. Importantly, introduction of a plasmid(s) containing the box H/ACA RNA gene(s) into the deletion strain leads to restoration of rRNA pseudouridylation.26,27,108-113 Using various reconstitution systems, several labs have recapitulated box H/ACA RNA-guided rRNA pseudouridylation in vitro.31,48,61,62,67,113 Although quite successful, the experiments performed to date have tested only a small fraction of predicted box H/ACA RNA-guided rRNA pseudouridylation; for most sites, box H/ACA RNA guided rRNA pseudouridylation has yet to be experimentally verified. However, given the clear base-pairing interactions between the guide sequences of box H/ACA RNAs and their predicted substrate sequences in rRNAs, it is widely believed that eukaryotic rRNA pseudouridylation is catalyzed mostly (if not exclusively) by the box H/ACA RNA-guided mechanism.
Eukaryotic spliceosomal snRNAs
Pseudouridine is the most abundant modified nucleotide in eukaryotic spliceosomal snRNAs.100 There are a total of 24 pseudouridines in the 5 vertebrate snRNAs (U1, U2, U4, U5 and U6), and 13 of them are concentrated in U2 snRNA accounting for approximately 7% of all nucleotides.100,114 A total of 6 pseudouridines have been identified in S. cerevisiae snRNAs (3 in U2, 2 in U1 and one in U5).115 Over the years, an intense search for enzymes responsible for snRNA pseudouridylation has been carried out, resulting in the identification of a large number of snRNA-specific box H/ACA RNAs in various eukaryotic organisms, including, among others, S. cerevisiae, X. laevis, mouse, human, and fruit fly.101,107,116,117 Some of the identified putative box H/ACA RNAs have been experimentally proven to guide spliceosomal snRNA pseudouridylation. For instance, it has been shown that depletion of pugU2-34/44, a box H/ACA RNA that was predicted to guide U2 pseudouridylation at positions 34 and 44, results in the loss of Ψ34 and Ψ44 in U2 snRNA in Xenopus oocytes; injection of in vitro-transcribed pugU2-34/44 into the depleted oocytes restores the formation of Ψ34 and Ψ44 in U2 snRNA.117 Likewise, it has also been experimentally demonstrated that mammalian U85 is capable of directing U5 pseudouridylation at position 46 both in vitro and in vivo.31 Recently, the Gall group has identified several box H/ACA RNAs in Drosophila, and shown experimentally that these RNAs are capable of guiding spliceosomal snRNA pseudouridylation in fruit fly.116 Although a large number of snRNA-specific H/ACA RNAs have been identified, there are still many known pseudouridylation sites in spliceosomal snRNAs that are unaccounted for. Thus, it remains a challenge to identify the missing H/ACA RNAs for these sites. However, it is still possible that Ψ formation at some of these sites is not guided by H/ACA RNAs. In this regard, of the 3 characterized pseudouridylation enzymes responsible for S. cerevisiae U2 pseudouridylation, only one is a box H/ACA RNP (snR81), catalyzing the formation of Ψ42101; the other 2 are stand-alone protein enzymes (responsible for the formation of Ψ35 and Ψ44, respectively).118 Similarly, pseudouridylation of S. cerevisiae U6 snRNA (Ψ28) is catalyzed by the stand-alone Ψ synthase Pus1.119 Further research is necessary to address this issue.
Potential substrates
Both computational and experimental approaches have been used in the search for novel box H/ACA RNAs. Because computational searches often involve sequence alignments with known pseudouridine sites/sequences in rRNAs and spliceosomal snRNAs, the thus identified H/ACA RNAs are specific for those already known. In contrast, the experimental approach uses biochemical techniques to detect and identify any H/ACA RNAs present in the cell, and is thus unbiased. As a result, although many biochemically identified H/ACA RNAs match known pseudouridine sites, many others have no known target sites and are thus called orphan H/ACA RNAs107 (Fig. 2). It is quite possible that these orphan H/ACA RNAs are able to target other types of RNA (other than rRNA and snRNA) for pseudouridylation. In this sense, it is expected that a simple BLAST search against RNA databases (e.g. mRNA database) will generate a number of novel target sites. However, whether these predicted sites are in fact pseudouridylated will have to be experimentally tested. Finally, an entire new set of pseudouridylation sites are potentially out there due to imprecise base pairing of known H/ACA guide RNAs, exemplified by the case of regulated pseudouridylation below (Fig. 1C).
Artificial substrates
In an effort to study possible mRNA/pre-mRNA pseudouridylation, Karijolich and Yu designed an artificial H/ACA RNA, derived from snR81 (a verified endogenous H/ACA RNA), to target mRNA in S. cerevisiae.120 All sequence elements remain unchanged from the original gene, with the exception of the single-stranded guides, which are targeted to specific mRNA sequences. Experiments using this system have demonstrated that the artificial H/ACA RNA is capable of directing mRNA pseudouridylation at the target site. Although the modification efficiency is not as robust (∼7–10%) as in rRNAs and snRNAs, the fact that the artificial H/ACA RNA can target mRNA pseudouridylation indicates that different types of RNA can indeed be modified, and that these additional RNAs may even be naturally modified, perhaps at low enough levels that have eluded detection by current techniques. Likewise, using a similar strategy, Chen et al. show that such artificial H/ACA RNAs can also site-specifically pseudouridylate pre-mRNA in Xenopus oocytes.121 BLAST searches using the guide sequences of some known S. cerevisiae H/ACA RNAs have revealed a handful of complementary sequences in naturally occurring S. cerevisiae mRNAs, suggesting that these sites might indeed be pseudouridylated.120 Unfortunately, the pseudouridylation assays currently available are generally not sensitive enough to check the modification status of low-abundance mRNA species, and thus, development of more sensitive pseudouridylation assays is necessary to clarify this issue.
Function of Pseudouridines
Overview
Given the fact that pseudouridylation endows the modified uridine with chemical properties that are distinct from those of all other known nucleotides, introducing Ψ(s) into an RNA will likely alter its function. Close inspection of the sequences and structures of both rRNAs and spliceosomal snRNAs has revealed that Ψs are not only abundantly present in these RNAs, but are also clustered in regions/structures of functional importance,100,122 thus further underlining the notion that pseudouridylation may affect RNA function. Indeed, over the years, it has been reported that pseudouridines contribute significantly to the functions of rRNAs and spliceosomal snRNAs in protein translation and pre-mRNA splicing, respectively.
In rRNA
The consequences of Ψ deletion from rRNA have been studied in yeast ribosomes, where Ψs can be individually and globally ablated. For instance, the Fournier group has shown that the deletion of 5 H/ACA RNAs responsible for the formation of pseudouridines in the ribosome peptidyl transferase center results in defects in protein translation and cell growth.109 Likewise, eliminating pseudouridylation in either the ribosome decoding center of 18S rRNA and the A-site finger region of 25S rRNA leads to functional defects.111,123 Surprisingly, ablation of Ψs from helix 69 of 25S rRNA demonstrated variable effects, such that deletion of 5 Ψs showed less effect on cell growth/translation than that of only 3, even if they were contained within the 5 Ψs, highlighting the subtleties and intricacies of Ψ function.110,112 Furthermore, the Dinman group has shown that hypo-pseudouridylated rRNAs—resulting from catalytically defunct H/ACA RNPs—form impaired ribosomes that exhibit low affinity for tRNA and poor fidelity in protein translation.124
In spliceosomal snRNA
The functional importance of snRNA-specific H/ACA RNPs has also been extensively studied over the past 17 years. For instance, depletion of pugU2-34/44 from Xenopus oocytes results in the loss of 2 pseudouridines (Ψ34 and Ψ44) in the U2 branch site recognition region, which, in turn, impacts pre-mRNA splicing.117,125 Deletion of snR81 from yeast cells eliminates the formation of Ψ42 in the U2 branch site recognition region, creating a mutant strain, which, when combined with a U2 point mutation, exhibits a synthetic growth (and splicing) defect phenotype.126 In an exciting recent observation, a single Ψ residue in U6 snRNA was demonstrated to initiate the yeast filamentous growth program highlighting potential effects of Ψs in development.119
Artificially introduced
When artificially introduced into stop codons, Ψs cause nonsense suppression.120 X-ray crystallography revealed that pseudouridine is capable of promoting unusual base-pairing interactions between the stop codon and the tRNA anticodon in the ribosome decoding center, allowing effective amino acid incorporation at the pseudouridylated stop codon.127 Experimental results, although restricted to artificial H/ACA RNAs targeting reporter mRNAs, have demonstrated that mRNA pseudouridylation can play an important role in protein translation.120 For the reasons discussed above, pseudouridylation probably occurs naturally to mRNA, and thus, pseudouridine-mediated translation recoding is likely to be biologically relevant. One consequence of pseudouridine-mediated codon-specificity change is proteome diversity: by pseudouridylating specific codons, multiple proteins could be produced from a single mRNA (gene), representing gene regulation at the RNA modification level.
Regulation
For decades, RNA pseudouridylation, including RNA-guided RNA pseudouridylation, had been considered a constitutive process. However, this notion was challenged 3 years ago, with the discovery of inducible pseudouridylation.128,129 Specifically, S. cerevisiae cells, when stressed, can catalyze RNA pseudouridylation at novel sites. For instance, when cells are deprived of nutrients or grown to stationary phase, novel pseudouridylation activities are activated, resulting in spliceosomal snRNA pseudouridylation at several new sites that are otherwise unmodified when cells are grown in nutrient-rich media (or under log-phase conditions). One of these induced pseudouridylation activities involves snR81 H/ACA RNP, which catalyzes U2 pseudouridylation at position 93 under stress conditions. Interestingly, in this guide-substrate base-pairing scheme, there are 2 mismatches (Fig. 1C). It appears that the 2 mismatches are required for induced pseudouridylation, as changes from 2 mismatches to 3 (or more) mismatches, or to complete complementarity, result in no pseudouridylation or constitutive pseudouridylation at position 93, respectively. Importantly, induced spliceosomal snRNA pseudouridylation seems to have a negative impact on pre-mRNA splicing.
Thus, besides the known constitutive pseudouridines, at least 2 new inducible pseudouridines have been identified in spliceosomal snRNAs.128 The fact that Ψ93 of U2 snRNA affects pre-mRNA splicing suggests that, like the regulatory modifications of DNA and protein, induced pseudouridylation of RNA may play an important regulatory role in gene expression. However, the question of whether induced pseudouridylation is reversible remains to be addressed. Given that induction requires imperfect or less stringent base-pairing (2 mismatches) between the guide sequence and the substrate sequence, it is possible that induced pseudouridylation is widespread (perhaps also occurring to mRNA) and that current computer algorithms and visual inspection are inadequate to identify these sites. Further investigation is necessary.
Disease
The pseudouridine synthase NAP57 is the target of the predominant and severe X-linked form of dyskeratosis congenita (X-DC). Hence, NAP57 is also known as dyskerin and its gene is termed DKC1.40 DC is an inherited bone marrow failure syndrome caused mainly by the depletion of haematopoietic but also other stem cells. The classic phenotypical triad of abnormal skin pigmentation, mucosal leukoplakia, and nail dystrophy manifests itself in the first 2 decades of life, but is often followed by severe anemia in the third decade with dire consequences.130-132 In the autosomal forms of DC, mutations have been identified in 5 other H/ACA RNP components, the core proteins NOP10 and NHP2, telomerase RNA (which ends in an H/ACA domain), telomerase reverse transcriptase, and WDR79 (WRAP53, TCAB1; responsible for binding to the CAB box motif of scaRNAs and for their Cajal body localization). Additionally, the telomere proteins TIN2, CTC1 and RTEL1 are affected.133,134 Given the abundance of telomeric targets in patients with DC and Hoyeraal-Hreidarsson syndrome (HH, a severe variant of DC), it is not surprising that telomeres are affected in these individuals.135,136 However, it is still debated to what extent translation and pre-mRNA splicing are impaired through insufficiently modified rRNA and snRNA, respectively.137,138 In this context, it was recently demonstrated that even minor fluctuations in protein synthesis rate can impair the function of haematopoietic stem cells.139
Regardless, mutations in the NAP57 gene DKC1 account for some 40% of all known DC mutations and include cases of HH. Most of the 55 missense mutations identified so far cluster in the N- and PUA containing C-terminal domain, which is enveloped by SHQ1 in the NAP57•SHQ1 complex (Fig. 3, inset).134 In fact, about a quarter of all X-DC amino acid substitutions are present in the short C-terminal segment (CTS, 35 amino acids) that is right adjacent to the PUA domain and serves as a handle for binding of the SSD of SHQ1 (Fig. 3 inset, left knob).78,79 This clearly implicates the interface between NAP57 and SHQ1 as a target of X-DC. Indeed, also binding of the N-terminal CS domain of SHQ1 is affected by X-DC mutations.82,140 A consequence of impaired or enhanced binding of NAP57 to SHQ1 is reduced cellular availability of NAP57 through degradation or sequestration. This predicts insufficient NAP57 for the stabilization of all cellular H/ACA RNAs, such that the least abundant RNAs, i.e. telomerase RNA, may lose out. Such a mechanism is analogous to that proposed for spinal muscular atrophy where reduced levels of the survival of motor neuron protein (SMN) affects levels of snRNAs varyingly and in a cell type-specific manner.141 Moreover, when focusing on the molecular consequences of single mutations, it is important to keep in mind that bone marrow failure is additionally strongly influenced by genetic background. This is perhaps best illustrated by the alanine 353 to valine mutation in NAP57 that accounts for about half of X-DC cases. Thus, the penetrance of this amino acid substitution ranges from causing growth retardation and immunodeficiency in utero (in HH) to going unnoticed until over 30 years of age (in DC).142 Additionally, the equivalent amino acid position in the yeast ortholog Cbf5p is already a valine. Obviously, there is an enormous complexity underlying the pathogenesis, which we have yet to understand. Perhaps, whole genome sequencing will make a significant contribution. Another confounding issue is the fact that with the many NAP57 mutations adorning the interface of the 2 proteins, no mutations in SHQ1 have been observed in DC.143 Does SHQ1 have other functions that elude this simple concept?
In the case of somatic mutations observed in several tumors, the NAP57-SHQ1 interaction is affected by mutations in both proteins. Thus, in an integrative genomic profiling study of prostate cancer, the chromosomal 3p region that contains SHQ1 was identified as a tumor suppressor in conjunction with the androgen-driven serine protease-transcription factor fusion TMPRSS2-ERG, which is observed in some 50% of prostate cancers.144 Importantly, SHQ1 was the only gene in the 3p region that also harbored tumor associated mutations.144-146 Indeed, the growth-suppressive properties of SHQ1 and its loss in primary prostate tumors has been observed.147,148 A more general role of the 3p region in tumorigenesis is supported by its tumor suppressing activity in the pathogenesis of lung and other cancers, as well as by the acquisition of invasiveness in cervical cancer after its loss.149,150 Importantly, tumor associated mutations have now also been identified in NAP57 (and more in SHQ1) in prostate, colon, lung, uterine and glioblastoma tumors.145,151-153 Many of these amino acid substitutions lie in the NAP57-SHQ1 interaction domains. However, even some mutations outside affect the interaction of the recombinant proteins likely through allosteric effects (Machado-Pinilla and Meier, in preparation). Finally, it is interesting to note that DC also predisposes to malignant tumor formation, possibly providing an intersection between the somatic and inherited mutations observed in NAP57. Regardless, nature teaches us that fine-tuning of the NAP57-SHQ1 interaction and with it the biogenesis of H/ACA RNPs is crucial for the wellbeing of the cell and organism.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
The work in the authors’ laboratories is supported by grants from the National Institutes of Health (GM104077 and AG039559 to Y.T.Y. and GM097752 to U.T.M.)
References
- 1. Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Res 2013; 41:D262-7; PMID:23118484; http://dx.doi.org/ 10.1093/nar/gks1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Davis FF, Allen FW. Ribonucleic acids from yeast which contain a fifth nucleotide. J Biol Chem 1957; 227:907-15; PMID:13463012 [PubMed] [Google Scholar]
- 3. Ferre d'Amare A. RNA-modifying enzymes. Curr Opin Struct Biol 2003; 13:49-55; PMID:12581659; http://dx.doi.org/ 10.1016/S0959-440X(02)00002-7 [DOI] [PubMed] [Google Scholar]
- 4. Charette M, Gray MW. Pseudouridine in RNA: what, where, how, and why. IUBMB Life 2000; 49:341-51; PMID:10902565; http://dx.doi.org/ 10.1080/152165400410182 [DOI] [PubMed] [Google Scholar]
- 5. Boschi-Muller S, Motorin Y. Chemistry enters nucleic acids biology: enzymatic mechanisms of RNA modification. Biochem Moscow 2014; 78:1392-404; http://dx.doi.org/ 10.1134/S0006297913130026 [DOI] [PubMed] [Google Scholar]
- 6. Hudson GA, Bloomingdale RJ, Znosko BM. Thermodynamic contribution and nearest-neighbor parameters of pseudouridine-adenosine base pairs in oligoribonucleotides. RNA 2013; 19:1474-82; PMID:24062573; http://dx.doi.org/ 10.1261/rna.039610.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kierzek E, Malgowska M, Lisowiec J, Turner DH, Gdaniec Z, Kierzek R. The contribution of pseudouridine to stabilities and structure of RNAs. Nucleic Acids Res 2014; 42:3492-501; PMID:24369424; http://dx.doi.org/ 10.1093/nar/gkt1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Davis DR. Stabilization of RNA stacking by pseudouridine. Nucleic Acids Res 1995; 23:5020-6; PMID:8559660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meroueh M, Grohar PJ, Qiu J, SantaLucia J, Jr, Scaringe SA, Chow CS. Unique structural and stabilizing roles for the individual pseudouridine residues in the 1920 region of Escherichia coli 23S rRNA. Nucleic Acids Res 2000; 28:2075-83; PMID:10773075; http://dx.doi.org/ 10.1093/nar/28.10.2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Newby MI, Greenbaum NL. Sculpting of the spliceosomal branch site recognition motif by a conserved pseudouridine. Nat Struct Biol 2002; 9:958-65; PMID:12426583; http://dx.doi.org/ 10.1038/nsb873 [DOI] [PubMed] [Google Scholar]
- 11. Hall KB, McLaughlin LW. Properties of a U1/mRNA 5' splice site duplex containing pseudouridine as measured by thermodynamic and NMR methods. Biochemistry 1991; 30:1795-801; PMID:1993194; http://dx.doi.org/ 10.1021/bi00221a010 [DOI] [PubMed] [Google Scholar]
- 12. Arnez JG, Steitz TA. Crystal structure of unmodified tRNA(Gln) complexed with glutaminyl-tRNA synthetase and ATP suggests a possible role for pseudo-uridines in stabilization of RNA structure. Biochemistry 1994; 33:7560-7; PMID:8011621; http://dx.doi.org/ 10.1021/bi00190a008 [DOI] [PubMed] [Google Scholar]
- 13. Mitchell JR, Cheng J, Collins K. A box H/ACA small nucleolar RNA-like domain at the human telomerase RNA 3′ end. Mol Cell Biol 1999; 19:567-76; PMID:9858580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jády BE, Ketele A, Kiss T. Human intron-encoded Alu RNAs are processed and packaged into Wdr79-associated nucleoplasmic box H/ACA RNPs. Genes Dev 2012; 26:1897-910; http://dx.doi.org/ 10.1101/gad.197467.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yin Q-F, Yang L, Zhang Y, Xiang J-F, Wu Y-W, Carmichael GG, Chen L-L. Long Noncoding RNAs with snoRNA Ends. Mol Cell 2012; 48:219-30; PMID:22959273; http://dx.doi.org/ 10.1016/j.molcel.2012.07.033 [DOI] [PubMed] [Google Scholar]
- 16. Reichow SL, Hamma T, Ferre-D'amare AR, Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res 2007; 35:1452-64; PMID:17284456; http://dx.doi.org/ 10.1093/nar/gkl1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grozdanov P, Meier UT. Multicomponent machines in RNA modification: H/ACA ribonucleoproteins. In: DNA and RNA Modification Enzymes: Structure, Mechanism, Function and Evolution. Grosjean H. editor. Landes Bioscience, Austin, TX: 2009; 450-60. [Google Scholar]
- 18. Meier UT. The many facets of H/ACA ribonucleoproteins. Chromosoma 2005; 114:1-14; PMID:15770508; http://dx.doi.org/ 10.1007/s00412-005-0333-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watkins NJ, Bohnsack MT. The box C/D and H/ACA snoRNPs: key players in the modification, processing and the dynamic folding of ribosomal RNA. Wiley Interdiscip Rev RNA 2012; 3:397-414; PMID:22065625; http://dx.doi.org/ 10.1002/wrna.117 [DOI] [PubMed] [Google Scholar]
- 20. Kiss T, Fayet-Lebaron E, Jády BE. Box H/ACA small ribonucleoproteins. Mol Cell 2010; 37:597-606; PMID:20227365; http://dx.doi.org/ 10.1016/j.molcel.2010.01.032 [DOI] [PubMed] [Google Scholar]
- 21. Ye K. H/ACA guide RNAs, proteins and complexes. Curr Op Struct Biol 2007; 17:287-92; http://dx.doi.org/ 10.1016/j.sbi.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 22. Liang B, Li H. Structures of ribonucleoprotein particle modification enzymes. Q Rev Biophys 2011; 44:95-122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ganot P, Caizergues-Ferrer M, Kiss T. The family of box ACA small nucleolar RNAs is defined by an evolutionarily conserved secondary structure and ubiquitous sequence elements essential for RNA accumulation. Genes Dev 1997; 11:941-56; PMID:9106664; http://dx.doi.org/ 10.1101/gad.11.7.941 [DOI] [PubMed] [Google Scholar]
- 24. Balakin AG, Smith L, Fournier MJ. The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell 1996; 86:823-34; PMID:8797828; http://dx.doi.org/ 10.1016/S0092-8674(00)80156-7 [DOI] [PubMed] [Google Scholar]
- 25. Lestrade L, Weber MJ. snoRNA-LBME-db, a comprehensive database of human H/ACA and C/D box snoRNAs. Nucleic Acids Res 2006; 34:D158-62; PMID:16381836; http://dx.doi.org/ 10.1093/nar/gkj002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ni J, Tien AL, Fournier MJ. Small nucleolar RNAs direct site-specific synthesis of pseudouridine in ribosomal RNA. Cell 1997; 89:565-73; PMID:9160748; http://dx.doi.org/ 10.1016/S0092-8674(00)80238-X [DOI] [PubMed] [Google Scholar]
- 27. Ganot P, Bortolin ML, Kiss T. Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell 1997; 89:799-809; PMID:9182768; http://dx.doi.org/ 10.1016/S0092-8674(00)80263-9 [DOI] [PubMed] [Google Scholar]
- 28. Tang TH, Rozhdestvensky TS, d'Orval BC, Bortolin M-L, Huber H, Charpentier B, Branlant C, Bachellerie J-P, Brosius J, Hüttenhofer A. RNomics in Archaea reveals a further link between splicing of archaeal introns and rRNA processing. Nucleic Acids Res 2002; 30:921-30; PMID:11842103; http://dx.doi.org/ 10.1093/nar/30.4.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rozhdestvensky TS, Tang TH, Tchirkova IV, Brosius J, Bachellerie JP, Hüttenhofer A. Binding of L7Ae protein to the K-turn of archaeal snoRNAs: a shared RNA binding motif for C/D and H/ACA box snoRNAs in Archaea. Nucleic Acids Res 2003; 31:869-77; PMID:12560482; http://dx.doi.org/ 10.1093/nar/gkg175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darzacq X, Jády BE, Verheggen C, Kiss AM, Bertrand E, Kiss T. Cajal body-specific small nuclear RNAs: a novel class of 2'-O-methylation and pseudouridylation guide RNAs. EMBO J 2002; 21:2746-56; PMID:12032087; http://dx.doi.org/ 10.1093/emboj/21.11.2746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jády BE, Kiss T. A small nucleolar guide RNA functions both in 2'-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA. EMBO J 2001; 20:541-51; http://dx.doi.org/ 10.1093/emboj/20.3.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meier UT. Studying and working with ribonucleoproteins that catalyze H/ACA guided RNA modification. In: RNA and DNA Editing. Smith HC. editor. John Wiley & Sons, Inc; Hoboken, NJ: 2008; 162-74. [Google Scholar]
- 33. Meier UT, Blobel G. NAP57, a mammalian nucleolar protein with a putative homolog in yeast and bacteria. J Cell Biol 1994; 127:1505-14; PMID:7798307; http://dx.doi.org/ 10.1083/jcb.127.6.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang W, Middleton K, Yoon HJ, Fouquet C, Carbon J. An essential yeast protein, CBF5p, binds in vitro to centromeres and microtubules. Mol Cell Biol 1993; 13:4884-93; PMID:8336724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nurse K, Wrzesinski J, Bakin A, Lane BG, Ofengand J. Purification, cloning, and properties of the tRNA psi 55 synthase from Escherichia coli. RNA 1995; 1:102-12; PMID:7489483 [PMC free article] [PubMed] [Google Scholar]
- 36. Lafontaine DL, Bousquet-Antonelli C, Henry Y, Caizergues-Ferrer M, Tollervey D. The box H + ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes Dev 1998; 12:527-37; PMID:9472021; http://dx.doi.org/ 10.1101/gad.12.4.527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zebarjadian YY, King TT, Fournier MJM, Clarke LL, Carbon JJ. Point mutations in yeast CBF5 can abolish in vivo pseudouridylation of rRNA. Mol Cell Biol 1999; 19:7461-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giordano E, Peluso I, Senger S, Furia M. minifly, a Drosophila gene required for ribosome biogenesis. J Cell Biol 1999; 144:1123-33; PMID:10087258; http://dx.doi.org/ 10.1083/jcb.144.6.1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phillips B, Billin AN, Cadwell C, Buchholz R, Erickson C, Merriam JR, Carbon J, Poole SJ. The Nop60B gene of Drosophila encodes an essential nucleolar protein that functions in yeast. Mol Gen Genet 1998; 260:20-9; PMID:9829824; http://dx.doi.org/ 10.1007/s004380050866 [DOI] [PubMed] [Google Scholar]
- 40. Heiss NS, Knight SW, Vulliamy TJ, Klauck SM, Wiemann S, Mason PJ, Poustka A, Dokal I. X-linked dyskeratosis congenita is caused by mutations in a highly conserved gene with putative nucleolar functions. Nat Genet 1998; 19:32-8; PMID:9590285; http://dx.doi.org/ 10.1038/ng0598-32 [DOI] [PubMed] [Google Scholar]
- 41. Watkins NJ, Gottschalk A, Neubauer G, Kastner B, Fabrizio P, Mann M, Luhrmann R. Cbf5p, a potential pseudouridine synthase, and Nhp2p, a putative RNA-binding protein, are present together with Gar1p in all H BOX/ACA-motif snoRNPs and constitute a common bipartite structure. RNA 1998; 4:1549-68; PMID:9848653; http://dx.doi.org/ 10.1017/S1355838298980761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Watanabe Y-I, Gray MW. Evolutionary appearance of genes encoding proteins associated with box H/ACA snoRNAs: Cbf5p in Euglena gracilis, an early diverging eukaryote, and candidate Gar1p and Nop10p homologs in archaebacteria. Nucleic Acids Res 2000; 28:2342-52; PMID:10871366; http://dx.doi.org/ 10.1093/nar/28.12.2342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rashid R, Liang B, Baker D, Youssef O, He Y, Phipps K, Terns R, Terns M, Li H. Crystal structure of a Cbf5-Nop10-Gar1 complex and implications in RNA-Guided pseudouridylation and dyskeratosis congenita. Mol Cell 2006; 21:249-60; PMID:16427014; http://dx.doi.org/ 10.1016/j.molcel.2005.11.017 [DOI] [PubMed] [Google Scholar]
- 44. Hamma T, Reichow SL, Varani G, Ferré-D'amaré AR. The Cbf5–Nop10 complex is a molecular bracket that organizes box H/ACA RNPs. Nat Struct Mol Biol 2005; 12:1101-7; PMID:16286935; http://dx.doi.org/ 10.1038/nsmb1036 [DOI] [PubMed] [Google Scholar]
- 45. Manival X. Crystal structure determination and site-directed mutagenesis of the Pyrococcus abyssi aCBF5-aNOP10 complex reveal crucial roles of the C-terminal domains of both proteins in H/ACA sRNP activity. Nucleic Acids Res 2006; 34:826-39; PMID:16456033; http://dx.doi.org/ 10.1093/nar/gkj482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hoang C, Ferré-D'Amaré AR. Cocrystal structure of a tRNA Psi55 pseudouridine synthase: nucleotide flipping by an RNA-modifying enzyme. Cell 2001; 107:929-39; PMID:11779468; http://dx.doi.org/ 10.1016/S0092-8674(01)00618-3 [DOI] [PubMed] [Google Scholar]
- 47. Li L, Ye K. Crystal structure of an H/ACA box ribonucleoprotein particle. Nature 2006; 443:302-7; PMID:16943774; http://dx.doi.org/ 10.1038/nature05151 [DOI] [PubMed] [Google Scholar]
- 48. Li S, Duan J, Li D, Yang B, Dong M, Ye K. Reconstitution and structural analysis of the yeast box H/ACA RNA-guided pseudouridine synthase. Genes Dev 2011; 25:2409-21; PMID:22085967; http://dx.doi.org/ 10.1101/gad.175299.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCleverty CJ, Hornsby M, Spraggon G, Kreusch A. Crystal structure of human Pus10, a novel pseudouridine synthase. J Mol Biol 2007; 373:1243-54; PMID:17900615; http://dx.doi.org/ 10.1016/j.jmb.2007.08.053 [DOI] [PubMed] [Google Scholar]
- 50. Girard JP, Lehtonen H, Caizergues-Ferrer M, Amalric F, Tollervey D, Lapeyre B. GAR1 is an essential small nucleolar RNP protein required for pre-rRNA processing in yeast. EMBO J 1992; 11:673-82; PMID:1531632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bousquet-Antonelli C, Henry Y, G'elugne JP, Caizergues-Ferrer M, Kiss T. A small nucleolar RNP protein is required for pseudouridylation of eukaryotic ribosomal RNAs. EMBO J 1997; 16:4770-6; PMID:9303321; http://dx.doi.org/ 10.1093/emboj/16.15.4770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Henras A, Dez C, Noaillac-Depeyre J, Henry Y, Caizergues-Ferrer M. Accumulation of H/ACA snoRNPs depends on the integrity of the conserved central domain of the RNA-binding protein Nhp2p. Nucleic Acids Res 2001; 29:2733-46; PMID:11433018; http://dx.doi.org/ 10.1093/nar/29.13.2733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang C, Meier UT. Architecture and assembly of mammalian H/ACA small nucleolar and telomerase ribonucleoproteins. EMBO J 2004; 23:1857-67; PMID:15044956; http://dx.doi.org/ 10.1038/sj.emboj.7600181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Henras AK, Capeyrou R, Henry Y, Caizergues-Ferrer M. Cbf5p, the putative pseudouridine synthase of H/ACA-type snoRNPs, can form a complex with Gar1p and Nop10p in absence of Nhp2p and box H/ACA snoRNAs. RNA 2004; 10:1704-12; PMID:15388873; http://dx.doi.org/ 10.1261/rna.7770604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Henras A, Henry Y, Bousquet-Antonelli C, Noaillac-Depeyre J, Gélugne JP, Caizergues-Ferrer M. Nhp2p and Nop10p are essential for the function of H/ACA snoRNPs. EMBO J 1998; 17:7078-90; PMID:9843512; http://dx.doi.org/ 10.1093/emboj/17.23.7078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liang B, Zhou J, Kahen E, Terns RM, Terns MP, Li H. Structure of a functional ribonucleoprotein pseudouridine synthase bound to a substrate RNA. Nat Struct Mol Biol 2009; 16:740-6; PMID:19478803; http://dx.doi.org/ 10.1038/nsmb.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Duan J, Li L, Lu J, Wang W, Ye K. Structural mechanism of substrate RNA recruitment in H/ACA RNA-guided pseudouridine synthase. Mol Cell 2009; 34:427-39; PMID:19481523; http://dx.doi.org/ 10.1016/j.molcel.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 58. Liang B, Xue S, Terns R, Terns M, Li H. Substrate RNA positioning in the archaeal H/ACA ribonucleoprotein complex. Nat Struct Mol Biol 2007; 14:1189-95; PMID:8059286 [DOI] [PubMed] [Google Scholar]
- 59. Lübben B, Fabrizio P, Kastner B, Luhrmann R. Isolation and characterization of the small nucleolar ribonucleoprotein particle snR30 from Saccharomyces cerevisiae. J Biol Chem 1995; 270:11549-54; PMID:7744794; http://dx.doi.org/ 10.1074/jbc.270.19.11549 [DOI] [PubMed] [Google Scholar]
- 60. Egan ED, Collins K. Specificity and stoichiometry of subunit interactions in the human telomerase holoenzyme assembled in vivo. Mol Cell Biol 2010; 30:2775-86; PMID:20351177; http://dx.doi.org/ 10.1128/MCB.00151-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Baker DL, Youssef OA, Chastkofsky MIR, Dy DA, Terns RM, Terns MP. RNA-guided RNA modification: functional organization of the archaeal H/ACA RNP. Genes Dev 2005; 19:1238-48; PMID:15870259; http://dx.doi.org/ 10.1101/gad.1309605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Charpentier B, Muller S, Branlant C. Reconstitution of archaeal H/ACA small ribonucleoprotein complexes active in pseudouridylation. Nucleic Acids Res 2005; 33:3133-44; PMID:15933208; http://dx.doi.org/ 10.1093/nar/gki630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kittur N, Darzacq X, Roy S, Singer RH, Meier UT. Dynamic association and localization of human H/ACA RNP proteins. RNA 2006; 12:2057-62; PMID:17135485; http://dx.doi.org/ 10.1261/rna.249306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Spedaliere CJ, Ginter JM, Johnston MV, Mueller EG. The pseudouridine synthases: revisiting a mechanism that seemed settled. J Am Chem Soc 2004; 126:12758-9; PMID:15469254; http://dx.doi.org/ 10.1021/ja046375s [DOI] [PubMed] [Google Scholar]
- 65. Gu X, Liu Y, Santi DV. The mechanism of pseudouridine synthase I as deduced from its interaction with 5-fluorouracil-tRNA. Proc Natl Acad Sci USA 1999; 96:14270-5; PMID:10588695; http://dx.doi.org/ 10.1073/pnas.96.25.14270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spedaliere CJ, Mueller EG. Not all pseudouridine synthases are potently inhibited by RNA containing 5-fluorouridine. RNA 2004; 10:192-9; PMID:14730018; http://dx.doi.org/ 10.1261/rna.5100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang C, Query CC, Meier UT. Immunopurified small nucleolar ribonucleoprotein particles pseudouridylate rRNA independently of their association with phosphorylated Nopp140. Mol Cell Biol 2002; 22:8457-66; PMID:12446766; http://dx.doi.org/ 10.1128/MCB.22.24.8457-8466.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Miracco EJ, Mueller EG. The products of 5-fluorouridine by the action of the pseudouridine synthase TruB disfavor one mechanism and suggest another. J Am Chem Soc 2011; 133:11826-9; PMID:21744792; http://dx.doi.org/ 10.1021/ja201179f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McDonald MK, Miracco EJ, Chen J, Xie Y, Mueller EG. The handling of the mechanistic probe 5-fluorouridine by the pseudouridine synthase TruA and its consistency with the handling of the same probe by the pseudouridine synthases TruB and RluA. Biochemistry 2011; 50:426-36; PMID:21142053; http://dx.doi.org/ 10.1021/bi101737z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhou J, Liang B, Li H. Functional and structural impact of target uridine substitutions on the H/ACA ribonucleoprotein particle pseudouridine synthase. Biochemistry 2010; 49:6276-81; PMID:20575532; http://dx.doi.org/ 10.1021/bi1006699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hoang C, Hamilton CS, Mueller EG, Ferré-D'Amaré AR. Precursor complex structure of pseudouridine synthase TruB suggests coupling of active site perturbations to an RNA-sequestering peripheral protein domain. Protein Sci 2005; 14:2201-6; PMID:15987897; http://dx.doi.org/ 10.1110/ps.051493605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liang B, Kahen EJ, Calvin K, Zhou J, Blanco M, Li H. Long-distance placement of substrate RNA by H/ACA proteins. RNA 2008; 14:2086-94; PMID:18755842; http://dx.doi.org/ 10.1261/rna.1109808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Leulliot N, Godin KS, Hoareau-Aveilla C, Quevillon-Cheruel S, Varani G, Henry Y, van Tilbeurgh H. The box H/ACA RNP assembly factor Naf1p contains a domain homologous to Gar1p mediating its interaction with Cbf5p. J Mol Biol 2007; 371:1338-53; PMID:17612558; http://dx.doi.org/ 10.1016/j.jmb.2007.06.031 [DOI] [PubMed] [Google Scholar]
- 74. Fatica A, Dlakić M, Tollervey D. Naf1 p is a box H/ACA snoRNP assembly factor. RNA 2002; 8:1502-14; PMID:12515383 [PMC free article] [PubMed] [Google Scholar]
- 75. Dez C, Noaillac-Depeyre J, Caizergues-Ferrer M, Henry Y. Naf1p, an essential nucleoplasmic factor specifically required for accumulation of box H/ACA small nucleolar RNPs. Mol Cell Biol 2002; 22:7053-65; PMID:12242285; http://dx.doi.org/ 10.1128/MCB.22.20.7053-7065.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Yang PK, Rotondo G, Porras T, Legrain P, Chanfreau G. The Shq1p.Naf1p complex is required for box H/ACA small nucleolar ribonucleoprotein particle biogenesis. J Biol Chem 2002; 277:45235-42. [DOI] [PubMed] [Google Scholar]
- 77. Grozdanov PN, Roy S, Kittur N, Meier UT. SHQ1 is required prior to NAF1 for assembly of H/ACA small nucleolar and telomerase RNPs. RNA 2009; 15:1188-97; PMID:19383767; http://dx.doi.org/ 10.1261/rna.1532109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Walbott H, Machado-Pinilla R, Liger D, Blaud M, Réty S, Grozdanov PN, Godin K, van Tilbeurgh H, Varani G, Meier UT, et al. The H/ACA RNP assembly factor SHQ1 functions as an RNA mimic. Genes Dev 2011; 25:2398-408; PMID:22085966; http://dx.doi.org/ 10.1101/gad.176834.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li S, Duan J, Li D, Ma S, Ye K. Structure of the Shq1-Cbf5-Nop10-Gar1 complex and implications for H/ACA RNP biogenesis and dyskeratosis congenita. EMBO J 2011; 30:5010-20; PMID:22117216; http://dx.doi.org/ 10.1038/emboj.2011.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Godin KS, Walbott H, Leulliot N, van Tilbeurgh H, Varani G. The box H/ACA snoRNP assembly factor Shq1p is a chaperone protein homologous to Hsp90 cochaperones that binds to the Cbf5p enzyme. J Mol Biol 2009; 390:231-44; PMID:19426738; http://dx.doi.org/ 10.1016/j.jmb.2009.04.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Singh M, Gonzales FA, Cascio D, Heckmann N, Chanfreau G, Feigon J. Structure and functional studies of the CS domain of the essential H/ACA ribonucleoparticle assembly protein SHQ1. J Biol Chem 2009; 284:1906-16; PMID:19019820; http://dx.doi.org/ 10.1074/jbc.M807337200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Machado-Pinilla R, Liger D, Leulliot N, Meier UT. Mechanism of the AAA+ ATPases pontin and reptin in the biogenesis of H/ACA RNPs. RNA 2012; 18:1833-45; PMID:22923768; http://dx.doi.org/ 10.1261/rna.034942.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kakihara Y, Houry WA. The R2TP complex: discovery and functions. Biochim Biophys Acta 2012; 1823:101-7; PMID:21925213; http://dx.doi.org/ 10.1016/j.bbamcr.2011.08.016 [DOI] [PubMed] [Google Scholar]
- 84. King TH, Decatur WA, Bertrand E, Maxwell ES, Fournier MJ. A well-connected and conserved nucleoplasmic helicase is required for production of box C/D and H/ACA snoRNAs and localization of snoRNP proteins. Mol Cell Biol 2001; 21:7731-46; PMID:11604509; http://dx.doi.org/ 10.1128/MCB.21.22.7731-7746.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Boulon S, Pradet-Balade B, Verheggen C, Molle D, Boireau S, Georgieva M, Azzag K, Robert M-C, Ahmad Y, Neel H, et al. HSP90 and its R2TP/Prefoldin-like cochaperone are involved in the cytoplasmic assembly of RNA polymerase II. Mol Cell 2010; 39:912-24; PMID:20864038; http://dx.doi.org/ 10.1016/j.molcel.2010.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boulon S, Marmier-Gourrier N, Pradet-Balade B, Wurth L, Verheggen C, Jády BE, Rothé B, Pescia C, Robert M-C, Kiss T, et al. The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. J Cell Biol 2008; 180:579-95; PMID:18268104; http://dx.doi.org/ 10.1083/jcb.200708110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhao R, Kakihara Y, Gribun A, Huen J, Yang G, Khanna M, Costanzo M, Brost RL, Boone C, Hughes TR, et al. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J Cell Biol 2008; 180:563-78; PMID:18268103; http://dx.doi.org/ 10.1083/jcb.200709061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Horejsí Z, Takai H, Adelman CA, Collis SJ, Flynn H, Maslen S, Skehel JM, de Lange T, Boulton SJ. CK2 phospho-dependent binding of R2TP complex to TEL2 is essential for mTOR and SMG1 stability. Mol Cell 2010; 39:839-50; http://dx.doi.org/ 10.1016/j.molcel.2010.08.037 [DOI] [PubMed] [Google Scholar]
- 89. McKeegan KS, Debieux CM, Boulon S, Bertrand E, Watkins NJ. A dynamic scaffold of pre-snoRNP factors facilitates human box C/D snoRNP assembly. Mol Cell Biol 2007; 27:6782-93; PMID:17636026; http://dx.doi.org/ 10.1128/MCB.01097-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McKeegan KS, Debieux CM, Watkins NJ. Evidence that the AAA+ proteins TIP48 and TIP49 bridge interactions between 15.5K and the related NOP56 and NOP58 proteins during box C/D snoRNP biogenesis. Mol Cell Biol 2009; 29:4971-81; PMID:19620283; http://dx.doi.org/ 10.1128/MCB.00752-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Jha S, Dutta A. RVB1/RVB2: running rings around molecular biology. Mol Cell 2009; 34:521-33; PMID:19524533; http://dx.doi.org/ 10.1016/j.molcel.2009.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Huen J, Kakihara Y, Ugwu F, Cheung KLY, Ortega J, Houry WA. Rvb1-Rvb2: essential ATP-dependent helicases for critical complexes. Biochem Cell Biol 2010; 88:29-40; PMID:20130677; http://dx.doi.org/ 10.1139/O09-122 [DOI] [PubMed] [Google Scholar]
- 93. Yang PK, Hoareau C, Froment C, Monsarrat B, Henry Y, Chanfreau G. Cotranscriptional recruitment of the pseudouridylsynthetase Cbf5p and of the RNA binding protein Naf1p during H/ACA snoRNP assembly. Mol Cell Biol 2005; 25:3295-304; PMID:15798213; http://dx.doi.org/ 10.1128/MCB.25.8.3295-3304.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ballarino M, Morlando M, Pagano F, Fatica A, Bozzoni I. The cotranscriptional assembly of snoRNPs controls the biosynthesis of H/ACA snoRNAs in Saccharomyces cerevisiae. Mol Cell Biol 2005; 25:5396-403; PMID:15964797; http://dx.doi.org/ 10.1128/MCB.25.13.5396-5403.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Darzacq X, Kittur N, Roy S, Shav-Tal Y, Singer RH, Meier UT. Stepwise RNP assembly at the site of H/ACA RNA transcription in human cells. J Cell Biol 2006; 173:207-18; PMID:16618814; http://dx.doi.org/ 10.1083/jcb.200601105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoareau-Aveilla C. hNaf1 is required for accumulation of human box H/ACA snoRNPs, scaRNPs, and telomerase. RNA 2006; 12:832-40; PMID:16601202; http://dx.doi.org/ 10.1261/rna.2344106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kiss T. Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs. 2001; 20:3617-22; PMID:11447102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Yu Y-T, Terns RM, Terns MP. Mechanisms and functions of RNA-guided RNA modification. In: Fine-Tuning of RNA Functions by Modification and Editing. Springer: Berlin Heidelberg; 2005; 223-62. [Google Scholar]
- 99. Harada F, Kato N. Nucleotide sequences of 4.5S RNAs associated with poly (A) -containing RNAs of mouse and hamster cells. Nucleic Acids Res 1980; 8:1273-86; PMID:6159592; http://dx.doi.org/ 10.1093/nar/8.6.1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Reddy R, Busch H. Small nuclear RNAs: RNA sequences, structure, and modifications. In: Structure and Function of Major and Minor Small Nuclear Ribonucleoprotein Particles. Birnstiel ML, editor. Springer: Berlin Heidelberg; 1988; 1-37. [Google Scholar]
- 101. Ma X, Yang C, Alexandrov A, Grayhack EJ, Behm-Ansmant I, Yu Y-T. Pseudouridylation of yeast U2 snRNA is catalyzed by either an RNA-guided or RNA-independent mechanism. EMBO J 2005; 24:2403-13; PMID:15962000; http://dx.doi.org/ 10.1038/sj.emboj.7600718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ofengand J, Fournier MJ. The pseudouridine residues of rRNA: number, location, biosynthesis, and function. In: Modification and Editing of RNA. American Society of Microbiology; Grosjean H and Benne R, Washington, DC; 1998; 229-53 . [Google Scholar]
- 103. Schattner P. A computational screen for mammalian pseudouridylation guide H/ACA RNAs. RNA 2006; 12:15-25; PMID:16373490; http://dx.doi.org/ 10.1261/rna.2210406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Muller S, Urban A, Hecker A, Leclerc F, Branlant C, Motorin Y. Deficiency of the tRNATyr:Psi 35-synthase aPus7 in Archaea of the Sulfolobales order might be rescued by the H/ACA sRNA-guided machinery. Nucleic Acids Res 2009; 37:1308-22; PMID:19139072; http://dx.doi.org/ 10.1093/nar/gkn1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bernick DL, Dennis PP, Höchsmann M, Lowe TM. Discovery of pyrobaculum small RNA families with atypical pseudouridine guide RNA features. RNA 2012; 18:402-11; PMID:22282340; http://dx.doi.org/ 10.1261/rna.031385.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schattner P, Decatur WA, Davis CA, Ares M, Fournier MJ, Lowe TM. Genome-wide searching for pseudouridylation guide snoRNAs: analysis of the Saccharomyces cerevisiae genome. Nucleic Acids Res 2004; 32:4281-96; PMID:15306656; http://dx.doi.org/ 10.1093/nar/gkh768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Hüttenhofer A, Kiefmann M, Meier-Ewert S, O'Brien J, Lehrach H, Bachellerie JP, Brosius J. RNomics: an experimental approach that identifies 201 candidates for novel, small, non-messenger RNAs in mouse. EMBO J 2001; 20:2943-53; PMID:11387227; http://dx.doi.org/ 10.1093/emboj/20.11.2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Baudin-Baillieu A, Fabret C, Liang X-H, Piekna-Przybylska D, Fournier MJ, Rousset J-P. Nucleotide modifications in three functionally important regions of the Saccharomyces cerevisiae ribosome affect translation accuracy. Nucleic Acids Res 2009; 37:7665-77; PMID:19820108; http://dx.doi.org/ 10.1093/nar/gkp816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. King TH, Liu B, McCully RR, Fournier MJ. Ribosome structure and activity are altered in cells lacking snoRNPs that form pseudouridines in the peptidyl transferase center. Mol Cell 2003; 11:425-35; PMID:12620230; http://dx.doi.org/ 10.1016/S1097-2765(03)00040-6 [DOI] [PubMed] [Google Scholar]
- 110. Liang X-H, Liu Q, Fournier MJ. rRNA modifications in an intersubunit bridge of the ribosome strongly affect both ribosome biogenesis and activity. Mol Cell 2007; 28:965-77; PMID:18158895; http://dx.doi.org/ 10.1016/j.molcel.2007.10.012 [DOI] [PubMed] [Google Scholar]
- 111. Liang X-H, Liu Q, Fournier MJ. Loss of rRNA modifications in the decoding center of the ribosome impairs translation and strongly delays pre-rRNA processing. RNA 2009; 15:1716-28; PMID:19628622; http://dx.doi.org/ 10.1261/rna.1724409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Piekna-Przybylska D, Przybylski P, Baudin-Baillieu A, Rousset J-P, Fournier MJ. Ribosome performance is enhanced by a rich cluster of pseudouridines in the A-site finger region of the large subunit. J Biol Chem 2008; 283:26026-36; PMID:18611858; http://dx.doi.org/ 10.1074/jbc.M803049200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Xiao M, Yang C, Schattner P, Yu Y-T. Functionality and substrate specificity of human box H/ACA guide RNAs. RNA 2009; 15:176-86; PMID:19033376; http://dx.doi.org/ 10.1261/rna.1361509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yu YT, Scharl EC, Smith CM, Steitz JA. The growing world of small nuclear ribonucleoproteins. In: The RNA World. Gesteland RF, Cech TR, Atkins JF, editors. Cold Spring: Harbor, NY: 1999; 1-38. [Google Scholar]
- 115. Massenet S, Mougin A, Branlant C. Posttranscriptional modifications in the U small nuclear RNAs. In: Modification and Editing of RNA. American Society of Microbiology. Grosjean H and Benne R, Washington, DC; 1998; 201-27. [Google Scholar]
- 116.Deryusheva S, Gall J.G. Novel small Cajal-body-specific RNAs identified in Drosophila: probing guide RNA function. RNA 2013; 19:1802–1814. PMID: 24149844; http://dx.doi:12515384 10.1261/rna.042028.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhao X, Li ZH, Terns RM, Terns MP, Yu Y-T. An H/ACA guide RNA directs U2 pseudouridylation at two different sites in the branchpoint recognition region in Xenopus oocytes. RNA 2002; 8:1515-25; PMID:12515384 [PMC free article] [PubMed] [Google Scholar]
- 118. Ma X, Zhao X, Yu Y-T. Pseudouridylation (Psi) of U2 snRNA in S. cerevisiae is catalyzed by an RNA-independent mechanism. EMBO J 2003; 22:1889-97; PMID:12682021; http://dx.doi.org/ 10.1093/emboj/cdg191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Basak A., Query C.C. A pseudouridine residue in the core of the spliceosome is part of the filamentous growth program in yeast. Cell Rep. 2014; 8:966-73 in press, doi: 10.1016/j.celrep.2014.07.004; PMID:25127136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Karijolich J, Yu Y-T. Converting nonsense codons into sense codons by targeted pseudouridylation. Nature 2011; 474:395-8; PMID:21677757; http://dx.doi.org/ 10.1038/nature10165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Chen C, Zhao X, Kierzek R, Yu Y-T. A flexible RNA backbone within the polypyrimidine tract is required for U2AF65 binding and pre-mRNA splicing in vivo. Mol Cell Biol 2010; 30:4108-19; PMID:20606010; http://dx.doi.org/ 10.1128/MCB.00531-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ge J, Yu Y-T. RNA pseudouridylation: new insights into an old modification. Trends Biochem Sci 2013; 38:210-8; PMID:23391857; http://dx.doi.org/ 10.1016/j.tibs.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Badis G, Fromont-Racine M, Jacquier A. A snoRNA that guides the two most conserved pseudouridine modifications within rRNA confers a growth advantage in yeast. RNA 2003; 9:771-9; PMID:12810910; http://dx.doi.org/ 10.1261/rna.5240503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Jack K, Bellodi C, Landry DM, Niederer RO, Meskauskas A, Musalgaonkar S, Kopmar N, Krasnykh O, Dean AM, Thompson SR, et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell 2011; 44:660-6; PMID:22099312; http://dx.doi.org/ 10.1016/j.molcel.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Zhao X, Yu Y-T. Detection and quantitation of RNA base modifications. RNA 2004; 10:996-1002; PMID:15146083; http://dx.doi.org/ 10.1261/rna.7110804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yang C, McPheeters DS, Yu Y-T. Psi35 in the branch site recognition region of U2 small nuclear RNA is important for pre-mRNA splicing in Saccharomyces cerevisiae. J Biol Chem 2005; 280:6655-62; PMID:15611063; http://dx.doi.org/ 10.1074/jbc.M413288200 [DOI] [PubMed] [Google Scholar]
- 127. Fernández IS, Ng CL, Kelley AC, Wu G, Yu Y-T, Ramakrishnan V. Unusual base pairing during the decoding of a stop codon by the ribosome. Nature 2013; 500:107-10; http://dx.doi.org/ 10.1038/nature12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Wu G, Xiao M, Yang C, Yu Y-T. U2 snRNA is inducibly pseudouridylated at novel sites by Pus7p and snR81 RNP. EMBO J 2011; 30:79-89; PMID:21131909; http://dx.doi.org/ 10.1038/emboj.2010.316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Meier UT. Pseudouridylation goes regulatory. EMBO J 2011; 30:3-4; PMID:21206510; http://dx.doi.org/ 10.1038/emboj.2010.323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Marsh JC, Will AJ, Hows JM, Sartori P, Darbyshire PJ, Williamson PJ, Oscier DG, Dexter TM, Testa NG. “Stem cell” origin of the hematopoietic defect in dyskeratosis congenita. Blood 1992; 79:3138-44; PMID:1596563 [PubMed] [Google Scholar]
- 131. Walne AJ, Dokal I. Dyskeratosis Congenita: a historical perspective. Mech Ageing Dev 2008; 129:48-59; PMID:18054794; http://dx.doi.org/ 10.1016/j.mad.2007.10.006 [DOI] [PubMed] [Google Scholar]
- 132. Kirwan M, Dokal I. Dyskeratosis congenita: a genetic disorder of many faces. Clin Genet 2008; 73:103-12; PMID:18005359; http://dx.doi.org/ 10.1111/j.1399-0004.2007.00923.x [DOI] [PubMed] [Google Scholar]
- 133. Mason PJ, Bessler M. The genetics of dyskeratosis congenita. Cancer Genet 2011; 204:635-45; PMID:22285015; http://dx.doi.org/ 10.1016/j.cancergen.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Savage SA. Dyskeratosis congenita. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong C-T, Smith RJ, Stephens AN, editors. GeneReviews(R) [Internet]. Seattle: (WA: ): University of Washington, Seattle; 2013. [Google Scholar]
- 135. Bessler M, Wilson DB, Mason PJ. Dyskeratosis congenita. FEBS Lett 2010; 584:3831-8; PMID:20493861; http://dx.doi.org/ 10.1016/j.febslet.2010.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Dokal I. Dyskeratosis congenita. Hematol Am Soc Hematol Educ Program 2011; 2011:480-6; PMID:22160078; http://dx.doi.org/ 10.1182/asheducation-2011.1.480 [DOI] [PubMed] [Google Scholar]
- 137. Bellodi C, McMahon M, Contreras A, Juliano D, Kopmar N, Nakamura T, Maltby D, Burlingame A, Savage SA, Shimamura A, et al. H/ACA small RNA dysfunctions in disease reveal key roles for noncoding RNA modifications in hematopoietic stem cell differentiation. Cell Rep 2013; 3:1493-502; PMID:23707062; http://dx.doi.org/ 10.1016/j.celrep.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McMahon M, Bellodi C, Ruggero D. The “Fifth” RNA nucleotide: a role for ribosomal RNA pseudouridylation in control of gene expression at the translational level. Book Chapter 2013; 13:253-288. [Google Scholar]
- 139. Signer RAJ, Magee JA, Salic A, Morrison SJ. Haematopoietic stem cells require a highly regulated protein synthesis rate. Nature 2014; 509:49-54; PMID:24670665; http://dx.doi.org/ 10.1038/nature13035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Grozdanov PN, Fernández-Fuentes N, Fiser A, Meier UT. Pathogenic NAP57 mutations decrease ribonucleoprotein assembly in dyskeratosis congenita. Hum Mol Genet 2009; 18:4546-51; PMID:19734544; http://dx.doi.org/ 10.1093/hmg/ddp416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Zhang Z, Lotti F, Dittmar K, Younis I, Wan L, Kasim M, Dreyfuss G. SMN deficiency causes tissue-specific perturbations in the repertoire of snRNAs and widespread defects in splicing. Cell 2008; 133:585-600; PMID:18485868; http://dx.doi.org/ 10.1016/j.cell.2008.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vulliamy TJ, Marrone A, Knight SW, Walne A, Mason PJ, Dokal I. Mutations in dyskeratosis congenita: their impact on telomere length and the diversity of clinical presentation. Blood 2006; 107:2680-5; PMID:16332973; http://dx.doi.org/ 10.1182/blood-2005-07-2622 [DOI] [PubMed] [Google Scholar]
- 143. Dokal I, Vulliamy T, Mason P, Bessler M. Clinical utility gene card for: dyskeratosis congenita. Eur J Hum Genet 2014; 19:e1-e5; PMID: 25182133; http://dx.doi.org/20579941 10.1038/ejhg.2014.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18:11-22; PMID:20579941; http://dx.doi.org/ 10.1016/j.ccr.2010.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Cancer Genome Atlas Research Network . Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455:1061-8; PMID:18772890; http://dx.doi.org/ 10.1038/nature07385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Berger MF, Lawrence MS, Demichelis F, Drier Y, Cibulskis K, Sivachenko AY, Sboner A, Esgueva R, Pflueger D, Sougnez C, et al. The genomic complexity of primary human prostate cancer. Nature 2011; 470:214-20; PMID:21307934; http://dx.doi.org/ 10.1038/nature09744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hofmann ER, Nallar SC, Lin L, D'Cunha J, Lindner DJ, Weihua X, Kalvakolanu DV. Identification and characterization of GRIM-1, a cell-death-associated gene product. J Cell Sci 2010; 123:2781-91; PMID:20663920; http://dx.doi.org/ 10.1242/jcs.070250 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 148. Nallar SC, Lin L, Srivastava V, Gade P, Hofmann ER, Ahmed H, Reddy SP, Kalvakolanu DV. GRIM-1, a novel growth suppressor, inhibits rRNA maturation by suppressing small mucleolar RNAs. PLoS One 2011; 6:e24082; PMID:21931644; http://dx.doi.org/ 10.1371/journal.pone.0024082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Zabarovsky ER, Lerman MI, Minna JD. Tumor suppressor genes on chromosome 3p involved in the pathogenesis of lung and other cancers. Oncogene 2002; 21:6915-35; PMID:12362274; http://dx.doi.org/ 10.1038/sj.onc.1205835 [DOI] [PubMed] [Google Scholar]
- 150. Lando M, Wilting SM, Snipstad K, Clancy T, Bierkens M, Aarnes E-K, Holden M, Stokke T, Sundfør K, Holm R, et al. Identification of eight candidate target genes of the recurrent 3p12-p14 loss in cervical cancer by integrative genomic profiling. J Pathol 2013; 230:59-69; PMID:23335387; http://dx.doi.org/ 10.1002/path.4168 [DOI] [PubMed] [Google Scholar]
- 151. Reva BB, Antipin YY, Sander CC. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res 2011; 39:1-14; PMID:20805246; http://dx.doi.org/ 10.1093/nar/gkr407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012; 2:401-4; PMID:22588877; http://dx.doi.org/ 10.1158/2159-8290.CD-12-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wood LD, Parsons DW, Jones S, Lin J, Sjöblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, et al. The genomic landscapes of human breast and colorectal cancers. Science 2007; 318:1108-13; PMID:17932254; http://dx.doi.org/ 10.1126/science.1145720 [DOI] [PubMed] [Google Scholar]