Abstract
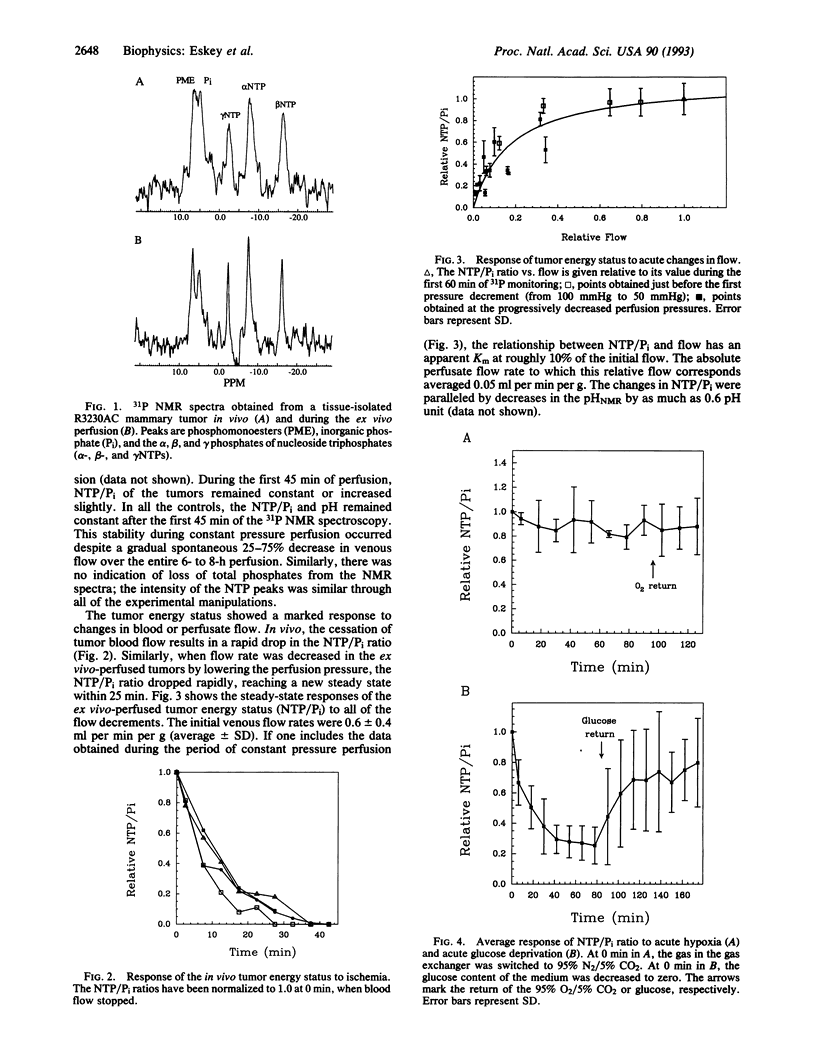
The role of glycolysis vs. respiration in tumor energy metabolism has been studied, to date, primarily in vitro by using single cells, multicellular spheroids, or tissue slices. With the advent of in vivo NMR spectroscopy, several investigators have shown that tumor energy status depends on its blood flow. Since manipulation of blood flow alters both oxygen and glucose delivery to a solid tumor, these studies have not been able to separate the relative contribution of oxygen vs. glucose in energy metabolism in vivo. In the present study, we have overcome this problem by combining two methods: the tissue-isolated R3230AC mammary adenocarcinoma perfused ex vivo and 31P NMR spectroscopy. The isolated tumor permits one to control the perfusion pressure as well as the metabolite concentrations in the perfusate. NMR spectroscopy permits one to measure the ratio of nucleoside triphosphate to inorganic phosphate (NTP/Pi) and pH. Our results show that (i) the NTP/Pi ratio ex vivo is similar to that observed in vivo prior to surgery, (ii) the NTP/Pi ratio is insensitive to flow changes at high flow rates but is proportional to flow rate at flows comparable to those found in vivo, (iii) the NTP/Pi ratio of these tumors is resistant to hypoxia and is not maintained when glucose is removed or replaced with glutamine, and (iv) although both O2 and glucose are consumed by these tumors, the effect of perfusate flow rate appears to be mediated largely through glucose delivery. The current approach not only provides information about the role of glycolysis vs. respiration in a rodent tumor but also is general and versatile enough to provide similar data in human tumors perfused ex vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhujwalla Z. M., Tozer G. M., Field S. B., Proctor E., Busza A., Williams S. R. The combined measurement of blood flow and metabolism in RIF-1 tumours in vivo. A study using H2 flow and 31P NMR spectroscopy. NMR Biomed. 1990 Aug;3(4):178–183. doi: 10.1002/nbm.1940030406. [DOI] [PubMed] [Google Scholar]
- Blasberg R. G., Kobayashi T., Patlak C. S., Shinohara M., Miyoaka M., Rice J. M., Shapiro W. R. Regional blood flow, capillary permeability, and glucose utilization in two brain tumor models: preliminary observations and pharmacokinetic implications. Cancer Treat Rep. 1981;65 (Suppl 2):3–12. [PubMed] [Google Scholar]
- Demetrakopoulos G. E., Linn B., Amos H. Rapid loss of ATP by tumor cells deprived of glucose: contrast to normal cells. Biochem Biophys Res Commun. 1978 Jun 14;82(3):787–794. doi: 10.1016/0006-291x(78)90851-3. [DOI] [PubMed] [Google Scholar]
- Evanochko W. T., Sakai T. T., Ng T. C., Krishna N. R., Kim H. D., Zeidler R. B., Ghanta V. K., Brockman R. W., Schiffer L. M., Braunschweiger P. G. NMR study of in vivo RIF-1 tumors. Analysis of perchloric acid extracts and identification of 1H, 31P and 13C resonances. Biochim Biophys Acta. 1984 Sep 14;805(1):104–116. doi: 10.1016/0167-4889(84)90042-9. [DOI] [PubMed] [Google Scholar]
- Evelhoch J. L., Sapareto S. A., Nussbaum G. H., Ackerman J. J. Correlations between 31P NMR spectroscopy and 15O perfusion measurements in the RIF-1 murine tumor in vivo. Radiat Res. 1986 Apr;106(1):122–131. [PubMed] [Google Scholar]
- Fernandez E. J., Mancuso A., Murphy M. K., Blanch H. W., Clark D. S. Nuclear magnetic resonance methods for observing the intracellular environment of mammalian cells. Ann N Y Acad Sci. 1990;589:458–475. doi: 10.1111/j.1749-6632.1990.tb24264.x. [DOI] [PubMed] [Google Scholar]
- Gerweck L. E., Koutcher J. A., Zaidi S. T., Seneviratne T. Energy status in the murine FSaII and MCaIV tumors under aerobic and hypoxic conditions: an in-vivo and in-vitro analysis. Int J Radiat Oncol Biol Phys. 1992;23(3):557–561. doi: 10.1016/0360-3016(92)90011-6. [DOI] [PubMed] [Google Scholar]
- Graham R. A., Brown T. R., Meyer R. A. An ex vivo model for the study of tumor metabolism by nuclear magnetic resonance: characterization of the phosphorus-31 spectrum of the isolated perfused Morris hepatoma 7777. Cancer Res. 1991 Feb 1;51(3):841–849. [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Courtney A. H. Glucose consumption by transplanted tumors in vivo. Cancer Res. 1967 Jun;27(6):1031–1040. [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Courtney A. H., Losonczy I. Relationship between oxygen and glucose consumption by transplanted tumors in vivo. Cancer Res. 1967 Jun;27(6):1041–1052. [PubMed] [Google Scholar]
- Gullino P. M., Grantham F. H., Courtney A. H. Utilization of oxygen by transplanted tumors in vivo. Cancer Res. 1967 Jun;27(6):1020–1030. [PubMed] [Google Scholar]
- Hilf R., Murant R. S., Narayanan U., Gibson S. L. Relationship of mitochondrial function and cellular adenosine triphosphate levels to hematoporphyrin derivative-induced photosensitization in R3230AC mammary tumors. Cancer Res. 1986 Jan;46(1):211–217. [PubMed] [Google Scholar]
- Jain R. K. Delivery of novel therapeutic agents in tumors: physiological barriers and strategies. J Natl Cancer Inst. 1989 Apr 19;81(8):570–576. doi: 10.1093/jnci/81.8.570. [DOI] [PubMed] [Google Scholar]
- Jain R. K. Determinants of tumor blood flow: a review. Cancer Res. 1988 May 15;48(10):2641–2658. [PubMed] [Google Scholar]
- Kallinowski F., Schlenger K. H., Runkel S., Kloes M., Stohrer M., Okunieff P., Vaupel P. Blood flow, metabolism, cellular microenvironment, and growth rate of human tumor xenografts. Cancer Res. 1989 Jul 15;49(14):3759–3764. [PubMed] [Google Scholar]
- Karczmar G. S., Arbeit J. M., Toy B. J., Speder A., Weiner M. W. Selective depletion of tumor ATP by 2-deoxyglucose and insulin, detected by 31P magnetic resonance spectroscopy. Cancer Res. 1992 Jan 1;52(1):71–76. [PubMed] [Google Scholar]
- Koutcher J. A., Fellenz M. P., Vaupel P. W., Gerweck L. E. FSaII mouse tumor metabolic changes with different doses of glucose measured by 31P nuclear magnetic resonance. Cancer Res. 1988 Nov 1;48(21):5917–5921. [PubMed] [Google Scholar]
- Lilly M. B., Katholi C. R., Ng T. C. Direct relationship between high-energy phosphate content and blood flow in thermally treated murine tumors. J Natl Cancer Inst. 1985 Nov;75(5):885–889. doi: 10.1093/jnci/75.5.885. [DOI] [PubMed] [Google Scholar]
- Malloy C. R., Cunningham C. C., Radda G. K. The metabolic state of the rat liver in vivo measured by 31P-NMR spectroscopy. Biochim Biophys Acta. 1986 Jan 23;885(1):1–11. doi: 10.1016/0167-4889(86)90032-7. [DOI] [PubMed] [Google Scholar]
- Ohkouchi K., Imoto H., Takakura Y., Hashida M., Sezaki H. Disposition of anticancer drugs after bolus arterial administration in a tissue-isolated tumor perfusion system. Cancer Res. 1990 Mar 1;50(5):1640–1644. [PubMed] [Google Scholar]
- Okunieff P., McFarland E., Rummeny E., Willett C., Hitzig B., Neuringer L., Suit H. Effects of oxygen on the metabolism of murine tumors using in vivo phosphorus-31 NMR. Am J Clin Oncol. 1987 Dec;10(6):475–482. doi: 10.1097/00000421-198712000-00003. [DOI] [PubMed] [Google Scholar]
- Okunieff P., Rummeny E., Vaupel P., Skates S., Willett C., Neuringer L. J., Suit H. D. Effects of pentobarbital anesthesia on the energy metabolism of murine tumors studied by in vivo 31P nuclear magnetic resonance spectroscopy. Radiat Res. 1988 Aug;115(2):361–372. [PubMed] [Google Scholar]
- Senior A. E., McGowan S. E., Hilf R. A comparative study of inner membrane enzymes and transport systems in mitochondria from R3230AC mammary tumor and normal rat mammary gland. Cancer Res. 1975 Aug;35(8):2061–2067. [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Blood flow and venous pH of tissue-isolated Walker 256 carcinoma during hyperglycemia. Cancer Res. 1988 Mar 1;48(5):1201–1207. [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Geometric resistance to blood flow in solid tumors perfused ex vivo: effects of tumor size and perfusion pressure. Cancer Res. 1989 Jul 1;49(13):3506–3512. [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Measurement of capillary filtration coefficient in a solid tumor. Cancer Res. 1991 Feb 15;51(4):1352–1355. [PubMed] [Google Scholar]
- Sevick E. M., Jain R. K. Viscous resistance to blood flow in solid tumors: effect of hematocrit on intratumor blood viscosity. Cancer Res. 1989 Jul 1;49(13):3513–3519. [PubMed] [Google Scholar]
- Steen R. G., Graham M. M. 31P magnetic resonance spectroscopy is sensitive to tumor hypoxia: perfusion and oxygenation of rat 9L gliosarcoma after treatment with BCNU. NMR Biomed. 1991 Jun;4(3):117–124. doi: 10.1002/nbm.1940040302. [DOI] [PubMed] [Google Scholar]
- Sutherland R. M. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science. 1988 Apr 8;240(4849):177–184. doi: 10.1126/science.2451290. [DOI] [PubMed] [Google Scholar]
- WARBURG O. On the origin of cancer cells. Science. 1956 Feb 24;123(3191):309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]


