Abstract
Our previous studies revealed that offspring from rat dams fed fish oil (at 8% and 18% energy), developed impaired intestinal barriers sensitizing the colon to exacerbated injury later in life. To discern the mechanism, we hypothesized that in utero exposure to fish oil, rich in n-3 polyunsaturated fatty acid (PUFA), caused abnormal intestinal reparative responses to mucosal injury through differences in intestinal microbiota and the presence of naïve immune cells. To identify such mechanisms, gut microbes and naïve immune cells were compared between rat pups born to dams fed either n-6 PUFA, n-3 PUFA or breeder chow. Maternal exposure to either of the PUFA rich diets altered the development of the intestinal microbiota with an overall reduction in microbial density. Using qPCR, we found that each type of PUFA differentially altered the major gut phyla; fish oil increased Bacteroidetes and safflower oil increased Firmicutes. Both PUFA diets reduced microbes known to dominate the infant gut like Enterobacteriaceae and Bifidobacteria spp. when compared to the chow group. Uniquely, maternal fish oil diets resulted in offspring showing blooms of opportunistic pathogens like Bilophila wadsworthia, Enterococcus faecium and Bacteroides fragilis in their gut microbiota. As well, fish oil groups showed a reduction in colonic CD8+ T cells, CD4+ Foxp3+ T cells and arginase+ M2 macrophages. In conclusion, fish oil supplementation in pharmacological excess, at 18% by energy as shown in this study, provides an example where excess dosing in utero can prime offspring to harbor intestinal pathobionts and alter immune cell homeostasis.
Keywords: colonic inflammation, infant immunity, intestinal microbiota, maternal diets, n-6 and n-3 polyunsaturated fatty acids
Introduction
Humans have co-evolved with vast numbers of microorganisms that inhabit the skin, nasal-oral cavity, urogenital and gastrointestinal (GI) tracts. Among all sites, the GI tract is the most densely populated area; the colon alone harbors over 70% of all microbes and up to a 1000 species.1 Initial microbial colonization of the GI tract occurs during birth and develops rapidly thereafter with maternal and environmental microbes. During infancy, the GI tract is typically dominated by facultative anaerobic bacteria like Streptococcus spp, Veillonellaceae (Firmicutes), Enterobacteriaceae (Proteobacteria) and Bifidobacteria spp. (Actinobacteria).2 By childhood and into adulthood the majority of the microbes belong in only 2 phyla: the Firmicutes and Bacteroidetes (>90%).3 Correspondingly, the colonization and diversity of microbes present during immune development of an infant primes balanced responses important in tolerance and homeostasis while ensuring the infant is immunocompetent.4 For example, some microbes can suppress the production of the regulatory T cells,5 whereas B. fragilis bacterial polysaccharide can influence the ratio of Th1 and Th2 effector cells.6 Although a newborn's immune system is not completely developed at birth, it develops rapidly afterwards due to increasing exposure to bacterial antigens following birth. Recently, it has been shown that infants have unique effector CD4+ T cells and CD8+ T cells that produce IL-8 to allow for neutrophil chemotaxis.4
While many gut microbes are harmless or even beneficial, some microbes have the potential to be pathogenic (known as pathobionts). A dysbiotic intestinal microbial ecosystem, low in diversity and rich in harmful microbes and less beneficial microbes, has been associated with various diseases and is hypothesized to have clinical consequences (reviewed in Chan et al)7. In support of this hypothesis, microbial ecosystems taken from diseased rodents and transplanted into healthy rodents can induce diseases like obesity, metabolic disease and inflammatory bowel disease (IBD) in the previously healthy host (reviewed in Brown et al)8. Most recently, in utero exposure to a “Western” diet has been shown to alter the offspring's intestinal microbes leaving a “lard legacy,”9 presumably through the alteration of maternal microbes that are passed onto offspring during delivery and lactation. However, in light of the current practice of extensive prenatal and postnatal supplementation of fish oil and/or its derivatives,10 this study was undertaken to identify the specific in utero effects of pharmacological doses of fish oil, rich in long chain n-3 polyunsaturated fatty acids (n-3 PUFA), on the intestinal microbiota and immune cell homeostasis of the offspring.
Diet has been speculated to be an important factor in modulating risk of developing IBD as well as controlling symptoms in IBD patients inclusive of Crohn's disease and ulcerative colitis. Several prospective cohort studies identified PUFA as a potential risk factor in the etiology of ulcerative colitis.11,12 In particular, diets high in n-6 PUFA and low in n-3 PUFA appear to increase ulcerative colitis risk.13,14 Dietary PUFAs are known to be crucial in regulating inflammation where n-6 PUFA metabolism leads to production of pro-inflammatory metabolites, while n-3 PUFA leads to production of anti-inflammatory metabolites. While it had been speculated that fish oil or n-3 PUFA would reduce inflammation in IBD, clinical trials have not consistently supported this. In fact, a meta-analysis including placebo-controlled studies concluded no benefit of n-3 PUFA in ameliorating IBD symptoms or remission maintenance.15 Moreover, in a murine model of colitis, a high intake of n-3 PUFA in diets (8% w/w fish oil) have been shown to exacerbate colitic responses through decreased expression of adiponectin.16 In contrast, other studies in experimental mouse models have shown lower doses of fish oil to protect against colitis.17,18
We have recently shown that post-natal fish oil supplementation (2% energy) results in dysregulated immune responses resulting in sepsis and mortality during enteric infection,10 and increased oxidative stress in aged mice,19 through the intestinal-microbe nexus. Additionally, our group had shown that excessive dietary n-3 PUFA (8 and 18% energy fish oil) fed to rat dams during gestation and lactation primed offspring to display impaired intestinal barrier development evident by reduced intestinal crypt length associated with altered permeability and heightened susceptibility to DNBS-colitis at 3 months of age.20 The objective of this study was to determine if altered intestinal microbial ecosystem is a pre-determining factor that leads to increased susceptibility to colitis in offspring of rat dams fed a diet rich in n-3 PUFA, like fish oil, compared to a diet rich in n-6 PUFA, like safflower oil. We examined the intestinal luminal microbes as well as mucosal immune cell populations of their newborn offspring. We found that the colonic microbial ecology in offspring from dams fed a diet rich in fish oil had an ecosystem with more pathogenic features including the presence of several pathobionts and reduced CD8+ T cells, CD4+ Foxp3+ T cells and M2 macrophages. These findings reveal that excessive doses of fish oil during pregnancy and lactation prime offspring to harbor intestinal pathobionts that could contribute to altered susceptibility to colitis later in life.
Results
Maternal diets rich in either n-3 or n-6 PUFA affect the development of the intestinal microbiota in their offspring
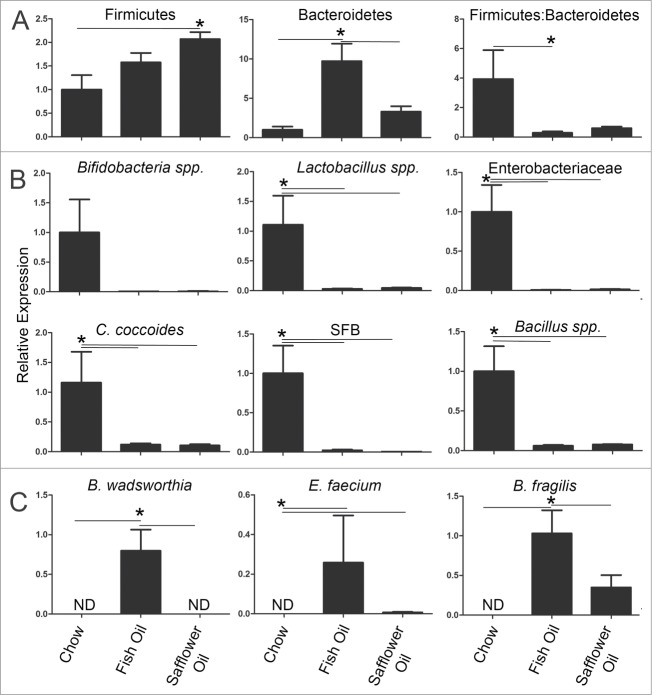
Intestinal bacterial density reaches that of an adult within the first 1-2 weeks of life.21 Since bacterial density could be an important factor in an infant's developing immune system, total bacteria was quantified from the caeca of rat pups using SYBR green nucleic acid stain as described previously.22 Both the fish oil and safflower oil groups showed a decrease in overall bacterial loads compared to the chow group at day 15 of age (Fig. 1). This suggests that maternal diets dominant in either n-3 or n-6 PUFA impaired or delayed the overall development of their offspring's gut microbiota. To determine what specific microbes had changed in the ecosystem in response to maternal exposure to dietary PUFAs, we used qPCR and primers specific to the 16S rRNA sequences from microbes known to dominate infant microbiota,23 or found to be altered during intestinal inflammation.22 The major phyla in the gut, Bacteroidetes and Firmicutes, were altered in the pups exposed to PUFA (Fig. 2A). The fish oil maternal diet resulted in microbial ecosystems with enriched populations of bacteria from the phyla Bacteroidetes while the safflower oil maternal diet had blooms in the bacteria from the phyla Firmicutes (Fig. 2A). As a result, pups from both fish and safflower oil fed dams had an overall decreased ratio of Firmicutes to Bacteroidetes compared to the chow group. Several microbes known to dominate the GI tract during infancy were similarly decreased with both maternal PUFA diets. This included Enterobacteriaceae, Bifidobacteria and Lactobacillus spp (Fig. 2B). We also examined microbes associated with immune system maturation and found that Clostridia coccoides, Bacillus spp. and segmented filamentous bacteria were similarly decreased in the PUFA diets (Fig. 2B). Overall, both n-3 and n-6 PUFA rich diets fed during gestation and lactation resulted in similarly altered microbes in offspring colons.
Figure 1.
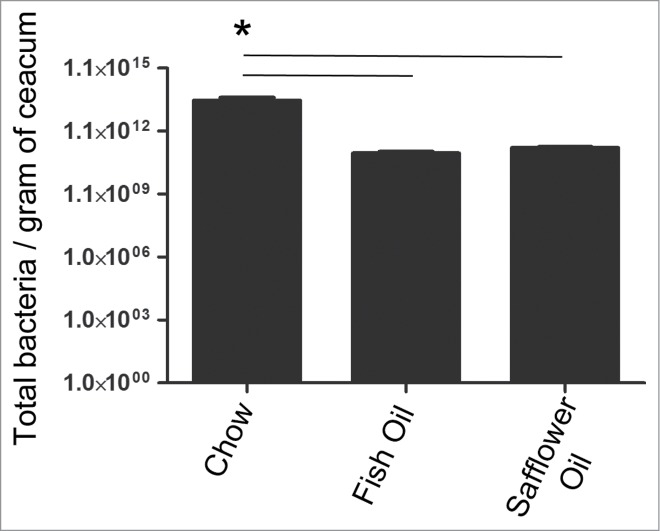
Rat pups born to dams fed diets rich in either n-3 PUFA (fish oil) or n-6 PUFA (safflower oil) have decreased total bacterial load compared to the pups born to dams fed chow which contains a ratio of 8:1 n-6 PUFA to n-3 PUFA. Total number of bacteria per gram of cecal tissue was quantified using SYBR green nucleic acid staining. Rat pups whose mothers consumed diets rich in either n-3 or n-6 PUFA had significantly less bacteria compared to the chow group (*, P < 0.05).
Figure 2.
Rat pups born to dams fed diets rich in either n-3 PUFA (fish oil) or n-6 PUFA (safflower oil) have similarly decreased microbes known to dominate the infant microbiota but the n-3 PUFA group have enriched bacteria that are opportunistic pathogens. Rat dams were fed either 20% fat diets rich in either n-3 or n-6 PUFA and the offspring colons were examined for the presence of specific intestinal microbes using qPCR. A) Both n-6 and n-3 rich diets reduced the overall Firmicutes:Bacteroidetes ratio and B) similarly reduced microbes known to dominate during infancy like Bifidobacteria and Lactobacillus spp and Enterobacteriace as well as C. cocoides, SFB and Bacillus spp. C) In contrast, only the omega-3 rich diet enriched pathobionts like Bilophila wadsworthia, Enterococcus faecium and Bacteroides fragilis. Expression is relative to the average of the chow group (*, P < 0.05).
Maternal n-3 PUFA rich diet results in increased abundance of pathobionts in the offspring gut microbiota
Since we previously found that offspring of dams fed fish oil rich diets are more susceptible to colitis,20 we examined the abundance of pathobionts in the colons of 15-day old rat pups to determine if fish oil creates an environment more favorable to the growth of potentially pathogenic microbes. We found that pups from dams fed fish oil diets, but not safflower oil diets enriched the presence of Bilophila wadsworthia, Enterococcus faecium and Bacteroides fragilis (Fig. 2C). Overall, maternal diets rich in n-3 PUFA consumed during gestation and lactation resulted in offspring harboring intestinal pathobionts.
Maternal intake of a diet high in n-3 or n-6 PUFA alters the balance of immune cells present in the intestine of the developing offspring rat pup
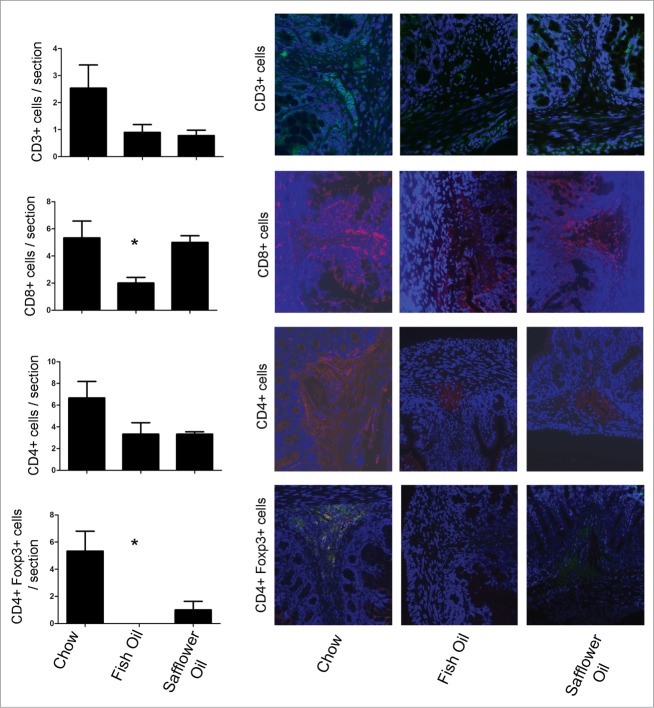
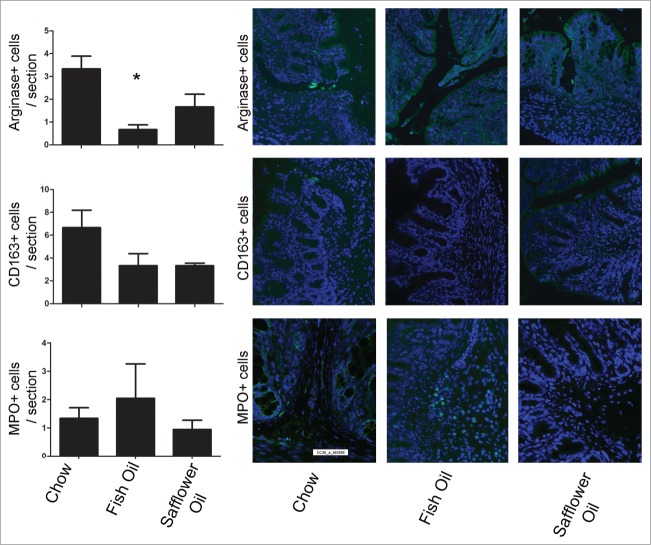
To address how maternal diet affected the development of intestinal mucosal immune system, we examined the presence of populations of T cells, monocytes/macrophages and neutrophils by immunofluorescence on colonic tissue sections from the pups (Figs. 3 and 4). Compared to the chow group, offspring of dams fed fish oil diets had an altered development of subsets of T cells in colonic tissues demonstrated by the reduction of CD8+ T cells and CD4+ Foxp3+ T cells. In addition to reduced T cell populations, maternal exposure to fish oil also resulted in reduced M2 macrophages evident by the reduction of arginase+ cells (Fig. 4). Both PUFA diets had similar levels of CD163+ monocytes/macrophages, and myeloperoxidase (MPO)+ polymorphonuclear leukocytes (Fig. 4). We examined the isotype control antibodies (mouse and rabbit IgG) which confirmed the specificity of primary antibody binding (data not shown). Overall, this data suggests that a maternal diet rich in fish oil alters the balance of immune cells present of the developing rat pup including a reduction of host defensive cytotoxic lymphocytes, T regulatory cells involved in promoting tolerance and M2 macrophages involved in cell proliferation and tissue repair.
Figure 3.
Rat pups born to dams fed diets rich in n-3 PUFA (fish oil) have altered colonic T cell balance. Rat dams were fed either 20% fat diets rich in either n-3 or n-6 PUFA and the offspring colons were examined for the presence of T cell markers via immunofluorescence and then quantified on colonic tissues sections. The n-3 rich diets depleted the presence of CD8+ T cells and CD4+ Foxp3+ T cells (*, P < 0.05).
Figure 4.
Rat pups born to dams fed diets rich in n-3 PUFA (fish oil) had decreased colonic M2 macrophages. Rat dams were fed either 20% fat diets rich in either n-3 or n-6 PUFA and the offspring colons were examined for the presence of M2 arginase+ macrophages, CD163+ macrophage/monocytes and MPO+ polymorphonuclear leukocytes via immunofluorescence and then quantified on colonic tissues sections. The n-3 diet group had decreased M2 arginase+ macrophages (*, P < 0.05).
Discussion
In this study, we examined the perinatal effects of diets rich in n-3 and n-6 PUFA on intestinal health. We found that maternal dietary intake high in either n-6 PUFA or n-3 PUFA during gestation and lactation altered the normal trajectory of intestinal microbes and the developing immune cell balance in the intestine of offspring rat pups. In this regard, maternal diets rich in either n-3 PUFA or n-6 PUFA lowered bacterial density and caused a decreased ratio of Firmicutes to Bacteroidetes and a decrease in several dominant microbes in the offspring's gut. In addition, excess n-3 PUFA was associated with blooms of potentially pathogenic microbes. In following with this, development of the intestinal immune system was also disrupted in the pups exposed to high levels of n-3 PUFA, exhibiting lower levels of CD8+ T cells important for host defense and also recently found to be present at high levels in a neonate.4 Furthermore, maternal fish oil diets resulted in offspring having less colonic T regulatory cells important for tolerance and a reduced number of M2 macrophages important for resolution of acute inflammation. Overall, this data suggests that high fish oil intake in utero resulted in an altered immune system which was associated with blooms of the pathobionts. Such early changes are associated with the predisposition of these rats to be more susceptibility to exacerbated injury later in life, as we had previously shown.20 The results from this study support that the influence of maternal diet is of considerable importance to the offspring's developing intestinal microbial ecosystem and their nascent mucosal immune system.
The bacterial ecosystem that develops during the first few weeks of life is, in part, specific to the maternal microbial signatures it has been exposed to.21 Within the first 2-4 years of life, the microbiota resembles that of an adult24 and remains relatively stable over time.25-27 This stability could impart resilience to disturbance and ensure continued normal gut physiological function. In contrast, in a disease context alteration in the microbiota with dominance of pathobionts and/or the reduction of beneficial microbes can contribute to disease pathogenesis. Here we found the opportunistic pathogens each associated with clinical sepsis Bilophila wadsworthia,28,29 Enterococcus faecium,30,31 and Bacteroides fragilis,32,33 were enriched in the intestinal ecosystem from rat pups born to mothers fed a diet rich in fish oil during gestation and lactation. Since these rat pups also had diminished levels of CD4+ Foxp3+ T cells and CD8+ T cells, this altered immune cell balance could have led to an imbalance in host defensive cells and contributed to the development of the blooms of opportunist pathogens. However, it is also possible that diet-induced alterations in the intestinal microbial ecosystem contributed to the alterations in mucosal immune cell populations.
We had previously shown that perinatal lipid nutrition alters early intestinal development ultimately affecting the susceptibility of the offspring to experimental colitis later in life.20 Here we provide a potential mechanism for the increased susceptibility through the modulatory effects of in utero exposure to various lipid diets during gestation and lactation. In support of this, we previously demonstrated that the intestinal microbiota is the major determinant, overriding natural susceptibility to experimental, lethal colitis.34 In the current study, we found that day 15 rat pups born to mothers fed either a diet high in either n-3 or n-6 PUFA, inherited a microbiota that was different than the chow offspring group where pregnant dams consumed a more balanced n-6: n-3 PUFA ratio at 8:1. It's possible that these microbial changes are not a function of fatty acids alone since the chow diet has a lower fat percentage and therefore other macro and micronutrients will differ compared to the safflower and fish oil diets. However, we did previously find that diet with a lower fish oil percentage had similar intestinal barrier defects as the high-fat fish oil diet suggesting perhaps that the other macronutrients are not responsible for the intestinal alterations seen here.20
Remarkably the total amount of bacteria reaches that of an adult within the first 1-2 weeks of life.21 Similarly, we found that rat pups from mothers fed the standard chow diet had a total microbial load of an adult rat, whereas rat pups from mothers fed either PUFA diet had approximately 1.5 fold reduction in microbial loads. Furthermore, both PUFA diets resulted in a decreased Firmicutes: Bacteroidetes ratio which has been previously correlated negatively with short chain fatty acids.35 Similarly, a previous study showed that a “Western” diet fed to pregnant mice resulted in their offspring inheriting a microbiota with an overall increase in bacteria from the phyla Firmicutes.9 This could be disadvantageous to the offspring considering several short chain fatty acids have important beneficial contributions to gut health. For example, butyrate produced by colonic microbes, is not only the main source of energy for colonocytes, but also inhibits intestinal cell proliferation and reduces colitis symptoms.36
Fish oil contains eicosapentaenoic acid (EPA) and docasahexaenoic acid (DHA) both long chain n-3 PUFAs. DHA is accrued by the brain during the last intrauterine trimester and in the first months after birth. DHA concentration in the phospholipid membranes of an infant's central nervous system varies as a result of postnatal nutrition.37 The n-3 PUFAs are precursors of eicosanoids, which regulate inflammation. EPA and DHA lead to the series 3 prostaglandins, series 5 leukotrienes and resolvins, which reduce production and translocation of inflammatory mediators to the site of injury.38-40 Overall, evidence has shown that dietary n-3 PUFA intake decreases pro-inflammatory responses by down-regulating lymphocyte proliferation, antigen presentation and pro-inflammatory cytokine expression,41,42 and increasing anti-inflammatory cytokine expression.43 In contrast, diets containing an excessive amount of n-3 PUFA can also exacerbate colitis,16,20 and results in adverse outcomes in murine models of pathogen exposure (reviewed in Fenton et al).10 It is possible that there are upper limits of dietary n-3 PUFA with excessive intake associated with pathological consequences. An optimal dose of n-3 PUFA should be determined to maintain a controlled and balanced immune response that can defend against pathogens while preventing chronic inflammatory conditions. In this study, we found that rat pups whose mothers were fed high levels of fish oil had decreased M2 macrophages in the gut. This may be associated with the exacerbated colitis and increased intestinal TNF-α production previously found in this group of animals.20
The gut mucosa participates in a complex relationship with the intestinal microbiota influencing local and systemic immunity, tolerance and homeostasis. Both clinical and experimental studies have established that altered microbial communities rich in pathobionts and deficient in beneficial microbes are correlated with many chronic diseases including obesity, IBD, diabetes and metabolic syndrome. Thus, initial intestinal colonization by the microbiota may represent a critical control point for disease susceptibilities through the development of appropriate immune responses to enteric bacteria. In conclusion, maternal diets containing fish oil at 18% energy result in offspring harboring a microbial community with pathogenic features including the presence of several pathobionts and altered mucosal immune cell balance. These findings highlight the importance of dietary choices including the potential dangers of pregnant and lactating mothers over supplementing with fish oil pills, which could modify their offspring's potential disease profiles later in life through the passage and modification of maternal microbes. In conclusion, fish oil supplementation in pharmacologically excess doses in utero can prime offspring to harbor intestinal pathobionts and alter immune cell homeostasis.
Materials and Methods
Rats and diet
Female Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were housed in a temperature-controlled (23°C) animal facility with a 12-h light-dark cycle and fed food and tap water under specific pathogen-free conditions. Two weeks prior to mating, animals were assigned to diets that were identical in energy, protein, vitamins, and minerals per kilogram diet but varied in fatty acid content as previously described.20 Female rats were fed diets with 20% energy from safflower oil or 18% fish oil plus 2% safflower oil throughout gestation and lactation. The breast milk of lactating rats was shown to reflect dietary fatty acid content.20 The safflower oil diet was dominant in n-6 PUFA and the fish oil diet dominant in n-3 PUFA (Table 1). The chow group was fed Picolab rodent diet 5053 (LabDiets) formulated for rat breeding colonies which contained 13.2% energy from fat where the ratio of n-6:n-3 PUFA was 8:1 (Table 1). The fish oil and safflower diets were isocaloric, isonitrogenous with each other. The control group was fed Picolab rodent diet 5053 (LabDiets) formulated for rat breeding colonies. It contained 13.2% energy from fat where the ratio of n-6:n-3 PUFA was 8:1 (Table 1) represented a low fat chow diet only. There were differences between the macronutrient compositions of the experimental diets and the control chow (Table 2). Diets were fed to dams ad libitum throughout gestation and post-partum and fresh diet was provided daily. The pups were sacrificed for analysis at day 15. All procedures involving the care and handling of the rats were approved by the University of British Columbia Committee on Animal Care Ethics and under the guidelines of the Canadian Council on the Use of Laboratory Animals.
Table 1.
Major fatty acid compositions of the maternal diets. Lipids are listed as gram per 100 grams of diet
| Fatty acids | Chow | Safflower oil | Fish oil |
|---|---|---|---|
| Saturated | 19.1 | 9.38 | 30.8 |
| Linoleic Acid (18:2n-6) | 49.3 | 72.0 | 9.82 |
| Arachidonic Acid (20:4n-6) | 0.17 | <0.01 | 1.55 |
| Oleic Acid (18:1n-9) | 20.8 | 18.0 | 8.25 |
| α-Linolenic Acid (18:3n-3) | 5.39 | 0.22 | 0.84 |
| DHA/EPA (22:6n3/20:5n-3) | 0.85 | NA | 49 |
| n-6:n-3 PUFA ratio | 8:1 | 327:1 | 0.2:1 |
Rat Chow: LabDiets. Picolab rodent diet 5053
Fish oil: Menhaden oil (Sigma F8020)
Table 2.
Composition of experimental diets
| Ingredient | Safflower oil (g) | Fish oil (g) |
|---|---|---|
| Casein (vitamin free) | 395 | 395 |
| Sucrose (Teklad #160482) | 454 | 454 |
| Corn starch (Teklad #160170) | 908 | 908 |
| Safflower oil | 202 | 26.26 |
| Fish oil | 0 | 175.7 |
| Vitamin mix (Teklad #40060)*1 | 20 | 20 |
| Mineral mix (AIN 93)*2 | 100 | 100 |
| Choline chloride (Sigma C1879) | 2 | 2 |
| Methionine (Sigma M9500) | 6 | 6 |
| Cellulose (Teklad #160390) | 100 | 100 |
| SeO2 (Sigma S9379) | 0.168mg (200ml) | 0.168mg (200ml) |
| MnCl2 (Fisher M87) | 0.599 | 0.599 |
| Total | 2187 | 2187 |
| Analysis (% by energy) | Experimental Diets | Chow*3 |
| Protein | 17.8 | 24.6 |
| Carbohydrate | 61.6 | 62.2 |
| Fat | 20.6 | 13.2 |
1Vitamin mix: 20,000 IU retinyl palmitate, 2000 IU cholecalciferol, 100 IU α-tocopherol acetate, 5 mg menadione, 5 mg thiamine-HCL, 8 mg riboflavin, 40 mg pyridoxine-HCL, 40 mg niacin, 40 mg panthothenic acid, 2000 mg choline, 100 mg myoinositol, 100 mg para-aminobenzoic acid, 0.4 mg biotin, 2 mg folic acid, and 30 mg cyanocobalamine
2Mineral mix:1.1 g calcium carbonate, 36.8 g calcium phosphate, 0.1 g citric acid, 23 mg cupric citrate 0.5 H2O, 1.3 g magnesium oxide, 418 mg manganese citrate, 0.5 mg potassium iodide, 3.4 g potassium sulfate, 1.5 g sodium chloride, 1.1 g sodium phosphate, 66.5 mg zinc citrate 2 H2O with an additional 78 mg Mn2+, 60 μg Se2+
The chow diet (PicoLab Rodent Diet 5053; LabDiets, St. Louis, MO) used as a ‘control’ was semi-synthetic in origin with natural ingredients. On average, the diet composed of 20% w/w protein, 5.6% w/w fats [2.1linoleic, 0.29 α linolenic, 0.93 saturated and 0.99 monounsaturated fats] and 53% carbohydrates [starch 34, glucose0.19, sucrose3.18, fructose 0.23, lactose1.34] and 4.7% fiber. Minerals were provided by Ash (6.1%w/w). Minerals and vitamins were added according to the AIN76A guidelines. Detailed compositions of vitamins and minerals are found in http://www.labdiet.com/cs/groups/lolweb/@labdiet/documents/web_content/mdrf/mdi4/∼edisp/ducm04_028436.pdf
Tissue Preparation
Rat pups were euthanized on post-natal day 15 by decapitation. The caecum and colons were dissected, cut longitudinally, washed in a PBS solution, and then either a piece was immersed in 10% neutral buffered formalin (Fisher) for immunofluorescence or a piece was flash frozen in LN2 and stored at −80°C prior for bacterial analysis.
Microbiota analysis
To examine the mucosal associated colonic microbiota, the colonic washed sections were homogenized and bacterial genomic DNA was extracted using a DNA stool minikit (Qiagen) according to the manufacturer's instructions and quantified as above using 50 ng/μl of bacterial DNA. Primers are listed in Table 3. Relative values for bacterial groups were normalized to total bacteria present amplified using a universal Eubacterial probe. For total bacteria load, cecal samples were weighed, homogenized in 1 mL of PBS and 1:10 dilution of each homogenate was fixed in 3.7% formalin and then diluted samples were filtered onto Anodisc filters (Whatman International Ltd) with a pore size of 0.2 μm and 2.5 cm diameter. After complete drying, each sample was stained with SYBR green I nucleic acid gel stain (Invitrogen). Filters were dried and mounted on glass slides using ProLong Gold® Antifade (Invitrogen) and viewed with a Zeiss AxioImager 2 microscope operating through Axioview software. 6-10 fields per disc were randomly chosen and the number of SYBR+ cells counted and averaged per gram of tissue.
Table 3.
Bacterial primers used in this study
| Bacteria | Forward Primer | Reverse Primer | Reference |
|---|---|---|---|
| Bacillus spp | GCGGCGTGCCTAATACATGC | CTTCATCACTCACGCGGCGT | 22 |
| Bacteriodetes | GGARCATGTGGTTTAATTCGATGAT | AGCTGACGACAACCATGCAG | 44 |
| Bacteriodes fragilis | AYAGCCTTTCGAAAGRAAGA | CCAGTATCAACTGCAATTTT | 22 |
| Bifidobacteria spp. | CGCGTCYGGTGTGAAAG | CCCCACATCCAGCATCCA | 45 |
| Bilophila wadsworthia | CCAACATGCACGGYTCCA | CGTCGAACTTGAACTTGAACTTGTAGG | 46 |
| Clostridium coccoides | AAATGACGGTACCTGACTAA | CTTTGAGTTTCATTCTTGCGAA | 22 |
| Enterobacteriaceae | GTGCCAGCMGCCGCGGTAA | GCCTCAAGGGCACAACCTCCAAG | 22 |
| Enterococcus faecium | CCACCGGAGCTTGCTCCACCGGAAA | CCGTCAAGGGATGAACAGTTACTCTCA | 47 |
| Eubacteria | ACTCCTACGGGAGGCAGCAGT | GTATTACCGCGGCTGCTGGCAC | 48 |
| Firmicutes | GGAGYATGTGGTTTAATTCGAAGCA | AGCTGACGACAACCATGCAC | 44 |
| Lactobacillus spp | AGCAGTAGGGAATCTTCCA | CACCGCTACACATGGAG | 22 |
| Segmented filamentous bacteria | CGGAGCATGTGGTTTAATTC | GCTGTCTCGCTAAATGCTC | 22 |
Immunofluorescence
Paraffin-embedded colon sections cut in cross-sections (3 μm) were deparaffinized and antigen retrieval of rehydrated tissues was performed using a 1 mg/mL trypsin (Sigma) followed by incubation with the following primary antibodies: rabbit polyclonal IgG antibody raised against MPO (Thermo Scientific) to examine polymorphonuclear leukocytes; rabbit polyclonal IgG antibody raised against CD163 (Biorbyt) to examine monocytes/macrophages; fluorescein conjugated sheep IgG polyclonal antibody raised against arginase (R&D Systems) to examine M2 macrophages; rabbit monoclonal IgG antibody raised against CD8 (Cedarlane), rabbit polyclonal IgG antibody raised against CD4 (Santa Cruz Biotechnology), rabbit polyclonal IgG antibody raised against CD3 (GeneTex) and rabbit polyclonal IgG antibody raised against Foxp3 (Santa Cruz Biotechnology) were co-incubated with mouse monoclonal IgG2a antibody raised against CD4 (Abcam) to examine populations of T cells. For non-conjugated antibodies, the following secondary antibodies were used: goat anti-rabbit IgG AlexaFluor-conjugated 594-red antibody (Invitrogen) or goat anti-rabbit IgG AlexaFluor-conjugated 488-green antibody (Invitrogen). Tissue sections were mounted using fluoroshield with DAPI (Sigma) and imaged on an Olympus IX81 fluorescent microscope at 200X magnification and positively stained cells were quantified by counting florescent cells in the sub-mucosal region of the colon section for each mouse by a blinded observer and verified by another observer. The total number of positive cells for each mouse was then averaged to represent the mean number of positive cells found in each section.
Statistical analysis
The results are expressed as the mean ± standard error of the mean (SEM) from 3 rat offspring from 3 litters (n = 9) per diet experiment. For comparisons, a one-way analysis of variance with Tukey post hoc test was performed. All analyses were performed using GraphPad Prism 5 where P < 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank C Dai, E Rajendiran, M Lee, R Dryer, and S Wu for their contributions to this study.
Funding
SKG was supported through a summer-student award from the Canadian Association of Gastroenterology and NT was supported through an undergraduate research award from the I.K. Barber School. This work was supported by grants funded through the Intestinal Diseases Education Awareness Society and Crohn's and Colitis Canada to DLG, the Bill and Melinda Gates Foundation, a Dairy Farmers of Canada grant to DLG and SG, and the National Science and Engineering Research Council to SI and KJ. SG is a Michael Smith Foundations for Heath Research and Canadian Diabetes Association Scholar, SM Innis is a Senior Scholar at the Child and Family Research Institute, and K Jacobson is a Senior Clinician Scientist supported by the CHILD Foundation and the Child and Family Research Institute Clinician Scientists Award Program.
References
- 1. Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837-48; PMID:16497592; http://dx.doi.org/ 10.1016/j.cell.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 2. Fan W, Huo G, Li X, Yang L, Duan C, Wang T, Chen J. Diversity of the intestinal microbiota in different patterns of feeding infants by Illumina high-throughput sequencing. World J Microbiol Biotechnol 2013; 29:2365-72; PMID:23793940; http://dx.doi.org/ 10.1007/s11274-013-1404-3 [DOI] [PubMed] [Google Scholar]
- 3. Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science 2005; 308:1635-8; PMID:15831718; http://dx.doi.org/ 10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gibbons D, Fleming P, Virasami A, Michel ML, Sebire NJ, Costeloe K, Carr R, Klein N, Hayday A. Interleukin-8 (CXCL8) production is a signatory T cell effector function of human newborn infants. Nat Med 2014; 20:1206-10; PMID:25242415; http://dx.doi.org/ 10.1038/nm.3670 [DOI] [PubMed] [Google Scholar]
- 5. Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity 2008; 29:637-49; PMID:18835196; http://dx.doi.org/ 10.1016/j.immuni.2008.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005; 122:107-18; PMID:16009137; http://dx.doi.org/ 10.1016/j.cell.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 7. Chan YK, Estaki M, Gibson DL. Clinical consequences of diet-induced dysbiosis. Ann Nutr Metab 2013; 63 Suppl 2:28-40; http://dx.doi.org/ 10.1159/000354902 [DOI] [PubMed] [Google Scholar]
- 8. Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012; 4:1095-119; PMID:23016134; http://dx.doi.org/ 10.3390/nu4081095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Myles IA, Fontecilla NM, Janelsins BM, Vithayathil PJ, Segre JA, Datta SK. Parental dietary fat intake alters offspring microbiome and immunity. J Immunol 2013; 191:3200-9; http://dx.doi.org/ 10.4049/jimmunol.1301057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fenton JI, Hord NG, Ghosh S, Gurzell EA. Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2013; 89:379-90; PMID:24183073; http://dx.doi.org/ 10.1016/j.plefa.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T, et al. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion 2008; 77:57-64; PMID:18349539; http://dx.doi.org/ 10.1159/000121412 [DOI] [PubMed] [Google Scholar]
- 12. Jantchou P, Morois S, Clavel-Chapelon F, Boutron-Ruault MC, Carbonnel F. Animal protein intake and risk of inflammatory bowel disease: The E3N prospective study. A J Gastroenterol 2010; 105:2195-201; PMID:20461067; http://dx.doi.org/ 10.1038/ajg.2010.192 [DOI] [PubMed] [Google Scholar]
- 13. John S, Luben R, Shrestha SS, Welch A, Khaw KT, Hart AR. Dietary n-3 polyunsaturated fatty acids and the aetiology of ulcerative colitis: a UK prospective cohort study. Eur J Gastroenterol Hepatol 2010; 22:602-6; PMID:20216220; http://dx.doi.org/ 10.1097/MEG.0b013e3283352d05 [DOI] [PubMed] [Google Scholar]
- 14. Tjonneland A, Overvad K, Bergmann MM, Nagel G, Linseisen J, Hallmans G, Palmqvist R, Sjodin H, Hagglund G, Berglund G, et al. Linoleic acid, a dietary n-6 polyunsaturated fatty acid, and the aetiology of ulcerative colitis: a nested case-control study within a European prospective cohort study. Gut 2009; 58:1606-11; PMID:19628674; http://dx.doi.org/ 10.1136/gut.2008.169078 [DOI] [PubMed] [Google Scholar]
- 15. Turner D, Zlotkin SH, Shah PS, Griffiths AM. Omega 3 fatty acids (fish oil) for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2009:CD006320; PMID:19160277 [DOI] [PubMed] [Google Scholar]
- 16. Matsunaga H, Hokari R, Kurihara C, Okada Y, Takebayashi K, Okudaira K, Watanabe C, Komoto S, Nakamura M, Tsuzuki Y, et al. Omega-3 fatty acids exacerbate DSS-induced colitis through decreased adiponectin in colonic subepithelial myofibroblasts. Inflamm Bowel Dis 2008; 14:1348-57; PMID:18484673; http://dx.doi.org/ 10.1002/ibd.20491 [DOI] [PubMed] [Google Scholar]
- 17. Chapkin RS, Davidson LA, Ly L, Weeks BR, Lupton JR, McMurray DN. Immunomodulatory effects of (n-3) fatty acids: putative link to inflammation and colon cancer. J Nutr 2007; 137:200S-4S; PMID:17182826 [DOI] [PubMed] [Google Scholar]
- 18. Morampudi V, Bhinder G, Wu X, Dai C, Sham HP, Vallance BA, Jacobson K. DNBS/TNBS colitis models: providing insights into inflammatory bowel disease and effects of dietary fat. J Visualized Exp 2014:e51297; PMID:24637969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ghosh S, Molcan E, Decoffe D, Dai C, Gibson DL. Diets rich in n-6 PUFA induce intestinal microbial dysbiosis in aged mice. Br J Nutr 2013:1-9. [DOI] [PubMed] [Google Scholar]
- 20. Innis SM, Dai C, Wu X, Buchan AM, Jacobson K. Perinatal lipid nutrition alters early intestinal development and programs the response to experimental colitis in young adult rats. A J Physiol Gastrointest Liver Physiol 2010; 299:G1376-85; PMID:20864654; http://dx.doi.org/ 10.1152/ajpgi.00258.2010 [DOI] [PubMed] [Google Scholar]
- 21. Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol 2007; 5:e177; PMID:17594176; http://dx.doi.org/ 10.1371/journal.pbio.0050177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Baker J, Brown K, Rajendiran E, Yip A, DeCoffe D, Dai C, Molcan E, Chittick SA, Ghosh S, Mahmoud S, et al. Medicinal lavender modulates the enteric microbiota to protect against Citrobacter rodentium-induced colitis. Am J Physiol Gastrointest Liver Physiol 2012; 303:G825-36; PMID:22821949; http://dx.doi.org/ 10.1152/ajpgi.00327.2011 [DOI] [PubMed] [Google Scholar]
- 23. Wopereis H, Oozeer R, Knipping K, Belzer C, Knol J. The first thousand days - intestinal microbiology of early life: establishing a symbiosis. Pediatr Allergy Immunol 2014; 25:428-38; PMID:24899389 [DOI] [PubMed] [Google Scholar]
- 24. Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr 1999; 69:1035S-45S; PMID:10232646 [DOI] [PubMed] [Google Scholar]
- 25. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, et al. A core gut microbiome in obese and lean twins. Nature 2009; 457:480-4; PMID:19043404; http://dx.doi.org/ 10.1038/nature07540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444:1022-3; PMID:17183309; http://dx.doi.org/ 10.1038/4441022a [DOI] [PubMed] [Google Scholar]
- 27. Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6:e280; PMID:19018661; http://dx.doi.org/ 10.1371/journal.pbio.0060280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anuradha DE, Saraswathi K, Gogate A. Anaerobic bacteraemia: a review of 17 cases. J Postgraduate Med 1998; 44:63-6; PMID:10703573 [PubMed] [Google Scholar]
- 29. Baron EJ. Bilophila wadsworthia: a unique Gram-negative anaerobic rod. Anaerobe 1997; 3:83-6; PMID:16887567; http://dx.doi.org/ 10.1006/anae.1997.0075 [DOI] [PubMed] [Google Scholar]
- 30. MacEachern P, Giannoccaro JP, Elsayed S, Read RR, Laupland KB. A rare case of pleuropulmonary infection and septic shock associated with Enterococcus faecium endocarditis. J Infect 2005; 50:84-8; PMID:15603848; http://dx.doi.org/ 10.1016/j.jinf.2003.11.005 [DOI] [PubMed] [Google Scholar]
- 31. Patterson JE, Sweeney AH, Simms M, Carley N, Mangi R, Sabetta J, Lyons RW. An analysis of 110 serious enterococcal infections. Epidemiology, antibiotic susceptibility, and outcome. Medicine 1995; 74:191-200; PMID:7623654; http://dx.doi.org/ 10.1097/00005792-199507000-00003 [DOI] [PubMed] [Google Scholar]
- 32. Brook I. Clinical review: bacteremia caused by anaerobic bacteria in children. Critical Care 2002; 6:205-11; PMID:12133179; http://dx.doi.org/ 10.1186/cc1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brook I. Bacteremia due to anaerobic bacteria in newborns. J Perinatol 1990; 10:351-6; PMID:2277280 [PubMed] [Google Scholar]
- 34. Ghosh S, Dai C, Brown K, Rajendiran E, Makarenko S, Baker J, Ma C, Halder S, Montero M, Ionescu VA, et al. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. A J Physiol Gastrointest Liver Physiol 2011; 301:G39-49; PMID:21454446; http://dx.doi.org/ 10.1152/ajpgi.00509.2010 [DOI] [PubMed] [Google Scholar]
- 35. Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Microbes and Health Sackler Colloquium: Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 2011; 108:4578-85; http://dx.doi.org/ 10.1073/pnas.1000081107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Donohoe DR, Garge N, Zhang X, Sun W, O'Connell TM, Bunger MK, Bultman SJ. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab 2011; 13:517-26; PMID:21531334; http://dx.doi.org/ 10.1016/j.cmet.2011.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatric Res 2011; 70:406-10; PMID:21705959; http://dx.doi.org/ 10.1203/PDR.0b013e31822a59f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Das UN. Essential fatty acids and their metabolites could function as endogenous HMG-CoA reductase and ACE enzyme inhibitors, anti-arrhythmic, anti-hypertensive, anti-atherosclerotic, anti-inflammatory, cytoprotective, and cardioprotective molecules. Lipids Health Dis 2008; 7:37; PMID:18922179; http://dx.doi.org/ 10.1186/1476-511X-7-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther 1999; 83:217-44; PMID:10576293; http://dx.doi.org/ 10.1016/S0163-7258(99)00026-1 [DOI] [PubMed] [Google Scholar]
- 40. James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr 2000; 71:343S-8S; PMID:10617994 [DOI] [PubMed] [Google Scholar]
- 41. Calder PC. N-3 polyunsaturated fatty acids and inflammation: from molecular biology to the clinic. Lipids 2003; 38:343-52; PMID:12848278; http://dx.doi.org/ 10.1007/s11745-003-1068-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chambers CA, Allison JP. Costimulatory regulation of T cell function. Curr Opin Cell Biol 1999; 11:203-10; PMID:10209159; http://dx.doi.org/ 10.1016/S0955-0674(99)80027-1 [DOI] [PubMed] [Google Scholar]
- 43. Ferrucci L, Cherubini A, Bandinelli S, Bartali B, Corsi A, Lauretani F, Martin A, Andres-Lacueva C, Senin U, Guralnik JM. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab 2006; 91:439-46; PMID:16234304; http://dx.doi.org/ 10.1210/jc.2005-1303 [DOI] [PubMed] [Google Scholar]
- 44. Guo X, Xia X, Tang R, Zhou J, Zhao H, Wang K. Development of a real-time PCR method for Firmicutes and Bacteroidetes in faeces and its application to quantify intestinal population of obese and lean pigs. Lett Appl Microbiol 2008; 47:367-73; PMID:19146523; http://dx.doi.org/ 10.1111/j.1472-765X.2008.02408.x [DOI] [PubMed] [Google Scholar]
- 45. Delroisse JM, Boulvin AL, Parmentier I, Dauphin RD, Vandenbol M, Portetelle D. Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res 2008; 163:663-70; PMID:19216105; http://dx.doi.org/ 10.1016/j.micres.2006.09.004 [DOI] [PubMed] [Google Scholar]
- 46. Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, Antonopoulos DA, Jabri B, Chang EB. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012; 487:104-8; PMID:22722865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kang S, Denman SE, Morrison M, Yu Z, Dore J, Leclerc M, McSweeney CS. Dysbiosis of fecal microbiota in Crohn's disease patients as revealed by a custom phylogenetic microarray. Inflamm Bowel Dis 2010; 16:2034-42; PMID:20848492; http://dx.doi.org/ 10.1002/ibd.21319 [DOI] [PubMed] [Google Scholar]
- 48. Walter J, Tannock GW, Tilsala-Timisjarvi A, Rodtong S, Loach DM, Munro K, Alatossava T. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl Environ Microbiol 2000; 66:297-303; PMID:10618239; http://dx.doi.org/ 10.1128/AEM.66.1.297-303.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]