Abstract
Enterohemorrhagic Escherichia coli (EHEC) strains are well-documented human pathogens and causative agents of diarrheal episodes and hemorrhagic colitis. The serotype O157:H7 is highly virulent and responsible for both outbreaks and sporadic cases of diarrhea. Because antibiotic treatment is contraindicated against this pathogen, development of a human vaccine could be an effective intervention in public health. In our recent Infection and Immunity paper, we applied integrated approaches of in silico genome wide search combined with bioinformatics tools to identify and test O157 vaccine candidates for their protective effect on a murine model of gastrointestinal infection. Using genomic/immunoinformatic approaches that are further described here, we categorized vaccine candidates as high, medium, and low priorities, and demonstrate that some high priority candidates were able to significantly induce Th2 cytokines and reduce EHEC colonization. Using the STRING database, we have recently evaluated the vaccine candidates and predict functional protein interactions, determining whether correlations exist for the development of a multi-subunit vaccine, targeting different pathways against EHEC O157:H7. The overall approach is designed to screen potential vaccine candidates against EHEC; however, the methodology can be quickly applied to many other intestinal pathogens.
Keywords: DNA immunization, enterohemorrhagic E. coli, EHEC, murine model, vaccine
Enterohemorrhagic E. coli (EHEC) O157:H7 is a well-known food-borne pathogen, responsible for human cases of bloody diarrhea and with the potential to progress to hemolytic uremic syndrome (HUS).1 EHEC O157:H7 is the most prevalent serotype worldwide, typically affecting infants and the elderly and which is implicated in outbreaks and/or sporadic cases of diarrhea.2,3 These outbreaks are attributed to the consumption of contaminated food and water, particularly those food products in contact with cattle manure. The study of EHEC pathogenicity reveals that key virulence factors include expression of fimbriae to initially adhere, formation of attaching and effacing (A/E) lesions that facilitate intimate adhesion to the intestinal epithelia and production of Shiga toxins (Stx) which are associated with systemic disease.1,4 Analysis of the human intestinal mucosa revealed a preferential tropism of STEC O157:H7 for the follicle-associated epithelium overlying the distal ileal Peyer's patches,5-7 where the bacteria either cross the intestinal barrier through M cells or causes A/E lesions (Fig. 1).
Figure 1.
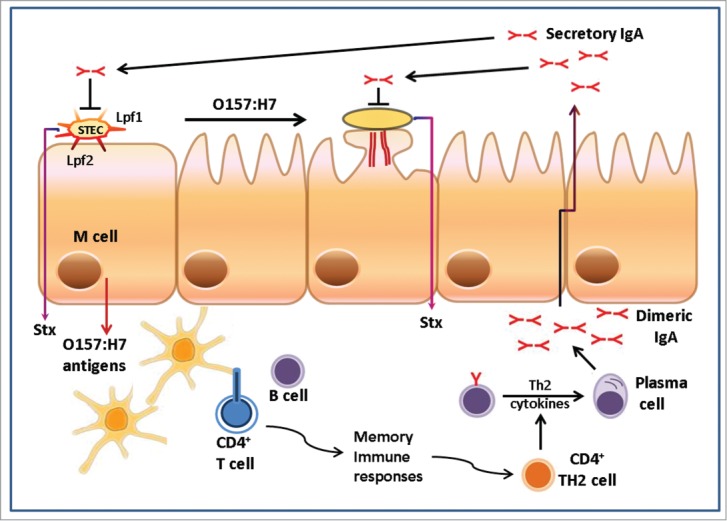
Working model for EHEC O157 colonization and immune responses required to confer protection. The diagram shows human intestinal epithelia displaying an M cell and the first stages of colonization by Shiga toxin-producing E. coli (STEC) or enterohemorrhagic E. coli (EHEC) O157:H7 where bacteria interact with the intestine via the Lpf fimbriae or other adhesins, followed by the formation of type III secretion-mediated A/E lesion. Luminal O157 antigens are transported through the intestinal Peyer's patches where dendritic cells process and present antigens to T cells. CD4+ T cells are stimulated, preferentially influencing induction of IgA-committed B-cells. Concomitant production of antigen and innate immune activation can promote memory immune responses to provide protection against subsequent exposure to the pathogen, ideally by the production of secretory IgA released into the intestinal tract.
Prevention of EHEC infections is a significant problem in the public health setting. Up to date, 2 veterinary vaccines are commercially available that have had some success in reducing EHEC O157 colonization in the cattle intestine8,9; however, no human vaccine has been developed yet. Recently, EHEC O157 got further attention due to emergence of a subpopulation of more virulent strains, contributing to severe disease in humans. Despite extensive progress in understanding the virulence mechanisms associated with disease, we still lack an effective prevention approach and novel strategies to treat EHEC infections.10,11 In our Infection and Immunity paper, we utilize a reverse vaccinology approach, methodology that was developed and mastered by Rino Rappuoli and colleagues.12,13 Reverse vaccinology has been extensively used to identify protective and broadly cross reactive vaccine candidates against several pathogens, including extraintestinal pathogenic E. coli.14 Based on these successful studies, we implemented a modified reverse vaccinology approach to identify novel EHEC O157 vaccine candidates. We utilized a combinatorial approach including immunoinformatics along with comparative genomics; followed by validation in an animal model of infection which illustrated that the predicted candidates were able to display protective efficacy. We utilized a DNA vaccination approach because the ability for rapid testing of several vaccine candidates as pools. Also, being EHEC an extracellular intestinal pathogen, our approach sought to induce mucosal immune responses and in the case of DNA vaccines, both humoral and systemic immune responses can be achieved when delivered by the intranasal route. In this addendum article, we provide a continue perspective on the understanding of our candidate screening approach and subsequent evaluation of further methods that help optimizing the selection of candidates for further vaccine development.
In the genomic-based exploration stage to find potential vaccine candidates, it was important to exclude those gene-encoded proteins that were also present in commensal E. coli strains. To ascertain this step, we performed a comparative genome to genome sequence analysis to obtain the list of genes encoding orthologous proteins within the genome of prototype E. coli O157:H7 strains (EDL933 and Sakai), which are not present in non-pathogenic E. coli strains, namely intestinal commensal E. coli HS and K-12 strain MG1655. This analysis res-ulted in a set of 897 orthologous proteins only present in the E. coli O157:H7 strains. Protection from disease in many cases is rendered by eliciting cellular immune response by antigens, mostly secretory or membrane spanning in nature. Therefore, we applied subcellular localization as a first additional filter for further selection and the above original list was trimmed to 65 putatively secreted or outer membrane-associated proteins. This subset of proteins was extensively analyzed for critical features that are found essential in an effective vaccine candidate. We used a variety of prediction programs to study a diverse array of features, such as physicochemical properties, adhesiveness, antigenicity and immunodominant epitopes of proteins. The methodology is further described and depicted in Figure 1 and Table 1 of our Infection and Immunity paper.12
Further, we also used other parameters for elucidation of both B- and T-cell epitopes that were evaluated from the output of prediction algorithms. For B-cell epitope predictors, 3 parameters were calculated: a) total number of epitopes per sequence, b) total score of all epitopes combined per sequence and c) average score of an epitope. Similarly, T-cell epitope predictors include parameters such as: a) total number of high-binding (HB) epitopes, b) total score of HB epitopes, c) percentage of HB epitopes among all epitopes predicted for a given sequence and d) number of human leukocyte antigen alleles covered by the epitopes of a given sequence.15 The combined results with these parameters contributed to the total immunogenicity score that help us to rank the candidate vaccines. It is important to discuss the effect of a couple of parameters in epitope binding: i) the percentage of high-affinity binders among all epitopes per sequence. This parameter is important because many sequences are being predicted with numerous epitopes. However, only few epitopes are actually HB. Therefore, ranking 2 proteins based on mere comparison of number of predicted epitopes could be misleading. It is not just epitopes, but the affinity of them, that in most cases elicit the immune response. ii) HLA (human leukocyte antigen) binding. It is mostly desired to cover a wide range of population for a given vaccine candidate to counteract the MHC polymorphism issue in different ethnicities and frequencies.16
In addition to epitope analysis, our candidates were also ranked on the basis of number of HLA alleles that a given protein sequence binds. For this, all HLA alleles for each of the HB epitopes were collected and a final compiled list was prepared to count for the number of alleles recognized. Similarly, to know the importance of few physicochemical properties used in the prediction, we included the ‘adhesion’ property in our vaccine approach. The ability to adhere to host surfaces by microbial pathogens is the most critical step in a successful colonization and a key property to block by secreted antibodies.17,18 Higher the probability of adherence of a given protein along with other high-scored parameters provides an extra edge to that protein compared to others with similar scores but low adherence property. These parameters help in refining the selection of fewer candidates to be validated in our in vivo model of infection. Following the combined informatics analysis and ranking of all the properties evaluated, candidates were divided as High Priority (HP, 17 candidates), Medium Priority (MP, 10 candidates) and Low Priority (LP, 38 candidates) (Table 3 in Infection and Immunity paper).15 In support of our analysis, the HP group includes several proteins that we and others have previously identified as important virulence factors of EHEC O157:H7 as well as other proteins not previously identified in virulence but which were consider important immunogens.18-23
Several studies in murine models of EHEC infection have emphasized the importance of mucosal delivery routes as a promising way to induce immune responses offering a combination of protection and blockage of EHEC intestinal colonization, mainly through expression of secretory IgA (sIgA)24 (Fig. 1). One limitation that we have observed in several vaccine studies is the small number of antigens that can be evaluated during in vivo experiments and; therefore, prior investigations have focused mainly on the analysis of key virulence-associated factors of EHEC O157:H7. However, in our vaccine selection approach, we overcame the bias of assaying only a limited number of O157 factors by using the identified priority candidates combined with a DNA vaccination approach. In our published study, we randomly selected 3 candidates from each priority group (31, 56.2 and 12 for HP; 43, 16 and 9 for MP; and 51, 49.1 and 49.2 for LP) and cloned them into the pVAX-1 vector.15 During the cloning process, large DNA sequences were divided in coding sequences with a maximum length of 1 kb. These constructs were given intranasally as a mix in their respective groups with scheduled priming and 2 boosts along with cholera toxin as an adjuvant.
We used intranasal DNA vaccination to evaluate the ability of our candidates to provide EHEC mucosal immune responses, mainly induction of sIgA that could reduce bacterial colonization in the murine intestine. We observed that the mice receiving either pools of HP and MP were able to induce EHEC humoral immune responses, both in serum and intestinal fecal samples, presenting higher levels of EHEC sera IgG and fecal sIgA. The production of sIgA by the HP pool group correlated with a reduction in colonization; and in contrast, the MP pool was also able to increase serum IgG levels but could not reduce EHEC colonization after challenge. In contrast, the LP pool failed to provide protection. We extended our study by screening individual HP candidates (pVAX-12, pVAX-31, and pVAX-56.2) and their ability to stimulate intestinal mucosa humoral responses. Despite having a discreet increased in fecal Igs, we observed that pVAX31 and pVAX-12 could not demonstrate any protective effect against EHEC colonization. However, pVAX-56.2 (encoding the C-terminal fragment of the type III secretion structural protein EscC) induced high serum IgG and fecal sIgA titers and caused reduction of EHEC shedding and colonization. Nevertheless, it appeared that the combination pool with the 3 HP candidates produce an additive immune response effect with a subsequent reduction in the degree of colonization. Based on the fact that effector CD4+ T-cell also plays an important role in protection and long lasting immune response (Fig. 1), we assessed the expression of various inflammatory cytokines in serum samples. Analysis of serum from HP-vaccinated animals revealed the induction of an inflammatory response, with mixed Th1 and Th2 cytokines, is reminiscent to responses previously reported.25 However, our findings showed 11 elevated cytokines, including Th1 cytokines IL-1β, and TNF-α; Th2 cytokines IL-3, IL-5, IL-6 and IL-10; the chemokines MIP-1α, MIP-1β, eotaxin, KC and MCP-1 and the growth factor GM-CSF. Pro-inflammatory cytokines such as IL-1β, TNF-α and IL-6 were increased at an early stage of immune response and play a central role in the host defense mechanism. The increased levels of Th2 cytokines (IL-3, IL-5, IL-6, IL-10, but not IL-4) in HP immunized mice indicated that Th2-dominated response account for subsequent generation of the humoral antibody response.
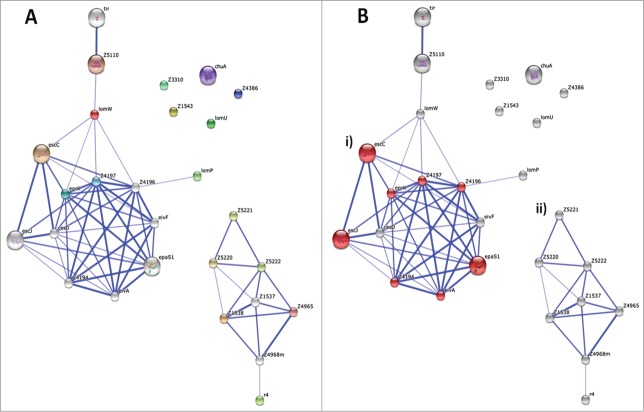
After our publication,15 we have further analyzed the interactive network of 17 HP proteins using the Search Tool for the Retrieval of Interacting Genes (STRING) database,26 choosing EHEC O157:H7 as the reference organism. This database is used to execute interaction predictions done by different algorithms that depend on genome sequence information. It also provides cluster networks and structural information, including homology models, data updates and connectivity and integration with third-party resources. These features can be used to predict functional protein interactions and determine whether correlations exist for the development of multi-subunit vaccines. In our interaction analysis, the network was allowed to expand by automatic addition of interacting partners (shown as white nodes) using a threshold of 0.9 confidence score for interactions (Fig. 2A). The threshold used is highly stringent allowing only 10 extra interactions to the existing 17 HP candidates set. The obtained network was explored for enrichment of pathways based on KEGG database, showing 2 functional modules. One of them includes components of the bacterial secreted factors consisting of 8 proteins with FDR p-value of 1.94e−8 (Fig. 2Bi). Five of the proteins from this module represent HP proteins. A separate cluster consisted of 6 HP proteins representing fimbrial/pilin proteins (Fig. 2Bii). This additional approach has allow us to intensify our effort to further characterize the stockpile of immunogens that can be used to develop a multi-subunit vaccine targeting either different pathways (e.g., secreted and fimbrial proteins) or multiple points in one pathway against EHEC O157:H7. Currently, we are evaluating possible combination of these candidates as well as characterizing the efficacy of the rest of the HP proteins.
Figure 2.
Protein-protein interaction network of 17 HP proteins. (A) Using STRING database, network mapping was prepared. Thickness of line shows the confidence of the interaction. White nodes depict the added proteins by extended interactions. (B) Functionally enriched pathway ‘bacterial secreted proteins’ are shown by red color nodes, cluster (i). In the case of cluster (ii) this shows many fimbrial proteins. For 5 of HP proteins, no interaction could be obtained with high stringency.
Besides the strategic analysis and identification of vaccine candidates in our study, the ultimate goal in our DNA vaccine is the expression of the antigens of interest in the appropriate anatomical location (e.g., intestinal lumen) which can cause that the antigen is getting presented to the host immune system, inducing protective responses due to the immunogenic epitope regions present in the candidates. It has been observed in multiple cases that a set of immunodominant epitopes is sufficient to generate an adequate protective immune response, which is an approach preferred instead of using the entire pathogen or a full-length protein difficult to purify. The ensemble of epitopes which may contain T- or B-cell epitopes or a combination of both, it is an ideal way to create a multivalent vaccine. This approach has led to the development of epitope-driven or subunit vaccines against hepatitis B, influenza and HIV vaccines.27–29 However, screening potential epitopes in a genome-wide approach could be daunting task to achieve. Fortunately, recent developments in immunoinformatics have dramatically reduced the time and effort in this screening phase. Whole genomes from multiple pathogenic E. coli strains can be scanned, compared and yet identification of pathogen-specific candidates can be achieved in few weeks as compared to months or years. In our recent publication, we leveraged the availability of the following tools: a) complete genomes from pathogenic and non-pathogenic E. coli strains, b) reliable software programs to screen the features of a good vaccine candidate, and c) computational power.
Together, we have further optimized a prototype of ‘genome mining to vaccine candidates’ approach, based on the original reverse vaccinology method,13 combining the above-described methodology and then validating the predictions in the animal model. In our experience, the entire methodology can be easily applied to any vaccine study, for any pathogenic organism, in which the genome sequence (fully annotated or not) is available. Overall, our publication in Infection and Immunity demonstrated that the antigen selection strategy was successful because the reverse vaccinology approach, integrating bioinformatics and experimental validations, performed by the subtraction of proteins found in intestinal commensal or K-12 E. coli strains that produced a pool of EHEC candidates with high potential to encode protective immunogens.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol 2004; 2:123-40; PMID:15040260; http://dx.doi.org/ 10.1038/nrmicro818 [DOI] [PubMed] [Google Scholar]
- 2. Karmali MA, Gannon V, Sargeant JM. Verocytotoxin-producing Escherichia coli (VTEC). Vet Microbiol 2010; 140:360-70; PMID:19410388; http://dx.doi.org/ 10.1016/j.vetmic.2009.04.011 [DOI] [PubMed] [Google Scholar]
- 3. Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000–2006. Clin Infect Dis 2009; 49:1480-5; PMID:19827953; http://dx.doi.org/ 10.1086/644621 [DOI] [PubMed] [Google Scholar]
- 4. Carvalho HM, Teel LD, Goping, G O’Brien AD. A three-dimensional tissue culture model for the study of attach and efface lesion formation by enteropathogenic and enterohaemorrhagic Escherichia coli. Cell Microbiol 2005; 7:1771-81; PMID:16309463; http://dx.doi.org/ 10.1111/j.1462-5822.2004.00594.x [DOI] [PubMed] [Google Scholar]
- 5. Fitzhenry RJ, Pickard DJ, Hartland EL, Reece S, Dougan G, Phillips AD, Frankel G. Intimin type influences the site of human intestinal mucosal colonisation by enterohaemorrhagic Escherichia coli O157:H7. Gut 2002; 50:180-5; PMID:11788556; http://dx.doi.org/ 10.1136/gut.50.2.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chong Y, Fitzhenry R, Heuschkel R, Torrente F, Frankel G, Phillips AD. Human intestinal tissue tropism in Escherichia coli O157:H7- initial colonization of terminal ileum and Peyer's patches and minimal colonic adhesion ex vivo. Microbiology 2007; 153:794-802; PMID:17322200; http://dx.doi.org/ 10.1099/mic.0.2006/003178-0 [DOI] [PubMed] [Google Scholar]
- 7. Fitzhenry R, Dahan S, Torres AG, Chong Y, Heuschkel R, Murch SH, Thomson M, Kaper JB, Frankel G, Phillips AD. Long polar fimbriae and tissue tropism in Escherichia coli O157:H7. Microbes Infect 2006; 8:1741-9; PMID:16815722; http://dx.doi.org/ 10.1016/j.micinf.2006.02.012 [DOI] [PubMed] [Google Scholar]
- 8. Varela NP, Dick P, Wilson J. Assessing the existing information on the efficacy of bovine vaccination against Escherichia coli O157:H7–a systematic review and meta-analysis. Zoonoses Public Health 2013; 60:253-68; PMID:22856462; http://dx.doi.org/ 10.1111/j.1863-2378.2012.01523.x [DOI] [PubMed] [Google Scholar]
- 9. Snedeker KG, Campbell M, Sargeant JM. A systematic review of vaccinations to reduce the shedding of Escherichia coli O157 in the faeces of domestic ruminants. Zoonoses Public Health 2012; 59:126-38; PMID:21824378; http://dx.doi.org/ 10.1111/j.1863-2378.2011.01426.x [DOI] [PubMed] [Google Scholar]
- 10. Orth D, Grif K, Zimmerhackl LB, Würzner R. Prevention and treatment of enterohemorrhagic Escherichia coli infections in humans. Expert Rev Anti-Infective Ther 2008; 6:101-8; PMID:18251667; http://dx.doi.org/ 10.1586/14787210.6.1.101 [DOI] [PubMed] [Google Scholar]
- 11. Melton-Celsa A, Mohawk K, Teel L, O’Brien A. Pathogenesis of Shiga-toxin producing Escherichia coli. Curr Top Microbiol Immunol 2012; 357:67-103; PMID:21915773 [DOI] [PubMed] [Google Scholar]
- 12. Pizza M, Scarlato V, Masignani V, Giuliani MM, Aricò B, Comanducci M, Jennings GT, Baldi L, Bartolini E, Capecchi B, et al. Identification of vaccine candidates against serogroup B meningococcus by whole-genome sequencing. Science 2000; 287:1816-20; PMID:10710308; http://dx.doi.org/ 10.1126/science.287.5459.1816 [DOI] [PubMed] [Google Scholar]
- 13. Rappuoli R. Reverse vaccinology, a genome-based approach to vaccine development. Vaccine 2001; 19:2688-91; PMID:11257410; http://dx.doi.org/ 10.1016/S0264-410X(00)00554-5 [DOI] [PubMed] [Google Scholar]
- 14. Moriel DG, Bertoldi I, Spagnuolo A, Marchi S, Rosini R, Nesta B, Pastorello I, Corea VA, Torricelli G, Cartocci E, et al. Identification of protective and broadly conserved vaccine antigens from the genome of extraintestinal pathogenic. Escherichia coli. Proc Natl Acad Sci U S A 2010; 107:9072-7; PMID:20439758; http://dx.doi.org/ 10.1073/pnas.0915077107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. García-Angulo VA, Kalita A, Kalita M, Lozano L, Torres A. G. Comparative genomics and immunoinformatics approach for the identification of vaccine candidates for enterohemorrhagic Escherichia coli O157:H7. Infect Immun 2014; 82:2016-26; PMID:24595137; http://dx.doi.org/ 10.1128/IAI.01437-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics 2006; 7:153; PMID:16545123; http://dx.doi.org/ 10.1186/1471-2105-7-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Antão EM, Wieler LH, Ewers C. Adhesive threads of extraintestinal pathogenic Escherichia coli. Gut Pathog 2009; 1:22; PMID:Can't; http://dx.doi.org/ 10.1186/1757-4749-1-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torres AG, Zhou X, Kaper JB. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect Immun 2005; 73:18-29; PMID:15618137; http://dx.doi.org/ 10.1128/IAI.73.1.18-29.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Farfan MJ, Torres AG. Molecular mechanisms that mediate colonization of Shiga toxin-producing Escherichia coli strains. Infect Immun 2012; 80:903-13; PMID:22144484; http://dx.doi.org/ 10.1128/IAI.05907-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Torres AG, Payne SM. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol 1997; 23:825-33; PMID:9157252; http://dx.doi.org/ 10.1046/j.1365-2958.1997.2641628.x [DOI] [PubMed] [Google Scholar]
- 21. Lathem WW, Grys TE, Witowski SE, Torres AG, Kaper JB, Tarr PI, Welch RA. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol Microbiol 2002; 45:277-88; PMID:12123444; http://dx.doi.org/ 10.1046/j.1365-2958.2002.02997.x [DOI] [PubMed] [Google Scholar]
- 22. Torres AG, Giron JA, Perna NT, Burland V, Blattner FR, Avelino-Flores F, Kaper JB. Identification and characterization of lpfABCC’DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect Immun 2002; 70:5416-27; PMID:12228266; http://dx.doi.org/ 10.1128/IAI.70.10.5416-5427.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Torres AG, Kanack KJ, Tutt CB, Popov V, Kaper JB. Characterization of the second long polar (LP) fimbriae of Escherichia coli O157:H7 and distribution of LP fimbriae in other pathogenic E. coli strains. FEMS Microbiol Lett 2004; 238:333-44; PMID:15358418 [DOI] [PubMed] [Google Scholar]
- 24. Garcia-Angulo VA, Kalita A, Torres AG. Advances in the development of enterohemorrhagic Escherichia coli vaccines using murine models of infection. Vaccine 2013; 31:3229-35; PMID:23707170; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cai K, Gao X, Li T, Hou X, Wang Q, Liu H, Xiao L, Tu W, Liu Y, Shi J, et al. Intragastric immunization of mice with enterohemorrhagic Escherichia coli O157:H7 bacterial ghosts reduces mortality and shedding and induces a Th2-type dominated mixed immune response. Can J Microbiol 2010; 56:389-98; PMID:20555401; http://dx.doi.org/ 10.1139/W10-025 [DOI] [PubMed] [Google Scholar]
- 26. Szklarczyk D, Franceschini A, Kuhn M, Simonovic M, Roth A, Minguez P, Doerks T, Stark M, Muller J, Bork P, et al. The STRING database in 2011: functional interaction networks of proteins, globally integrated and scored. Nucleic Acids Res 2011; 39:D561-8; PMID:21045058; http://dx.doi.org/ 10.1093/nar/gkq973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Szmuness W, Stevens CE, Oleszko WR, Goodman A. Passive-active immunization against hepatitis B: immunogenicity studies in adult Americans. Lancet 1981; 1:575-7; PMID:6110818; http://dx.doi.org/ 10.1016/S0140-6736(81)92030-4 [DOI] [PubMed] [Google Scholar]
- 28. Ben-Yedidia T, Arnon R. Epitope-based vaccine against influenza. Expert Rev Vaccines 2007; 6:939-48; PMID:18034655; http://dx.doi.org/ 10.1586/14760584.6.6.939 [DOI] [PubMed] [Google Scholar]
- 29. De Groot AS, Bosma A, Chinai N, Frost J, Jesdale BM, Gonzalez MA, Martin W, Saint-Aubin C. From genome to vaccine: in silico predictions, ex vivo verification. Vaccine 2001; 19:4385-95; PMID:11483263; http://dx.doi.org/ 10.1016/S0264-410X(01)00145-1 [DOI] [PubMed] [Google Scholar]