Abstract
The insula has been implicated in salience processing, craving, and interoception, all of which are critical to the clinical manifestations of drug and behavioral addiction. In this fMRI study, we examined resting-state functional connectivity (rsFC) of the insula and its association with Internet gaming characteristics in 74 young adults with Internet gaming disorder (IGD) and 41 age and gender matched healthy control subjects (HCs). In comparison to HCs, IGD subjects (IGDs) exhibited enhanced rsFC between the anterior insula and a network of regions including anterior cingulate cortex (ACC), putamen, angular gyrus, and precuneous, which are involved in salience, craving, self-monitoring, and attention. IGDs also demonstrated significantly stronger rsFC between the posterior insula and postcentral gyrus, precentral gyrus, supplemental motor area, and superior temporal gyrus (STG), which are involved in interoception, movement control, and auditory processing. Furthermore, IGD severity was positively associated with connectivity between the anterior insula and angular gyrus, and STG, and with connectivity between the posterior insula and STG. Duration of Internet gaming was positively associated with connectivity between the anterior insula and ACC. These findings highlight a key role of the insula in manifestation of the core symptoms of IGD and the importance to examine functional abnormalities of the anterior and posterior insula separately in IGDs.
Keywords: functional connectivity, fMRI, insula, Internet gaming disorder, resting-state
Introduction
Internet gaming disorder (IGD) is regarded as a behavioral addiction, defined as compulsive and uncontrolled gaming online, that jeopardizes individuals’ academic performance, interpersonal relationships, and health condition (Petry et al., 2014). Recently, IGD has been included in Section III of the DSM-5 as a topic deserving future studies (American Psychiatric Association, 2013). There are more than 360 million current Internet games players in China, and young adults make up the majority of this population (China Internet Network Information Center, 2014). Since young adults get access to Internet easily and often spend an excessive amount of time on online gaming, they become one of the most susceptible populations to develop IGD (Chou et al., 2005). The development of IGD would have a severe impact on both personal health and productivity.
Researchers have proposed that addiction is associated with structural and functional brain reorganization (Koob and Volkow, 2010). In addition, abnormal interactions between brain regions may contribute to behavioral deficits of addiction-related disorders (Sutherland et al., 2012). Resting-state functional connectivity (rsFC) has emerged as a non-invasive and systems-level approach to assess the interaction between brain areas and provided a powerful tool to identify biological markers of various psychiatric disorders, including addiction (Di Martino et al., 2009; Sutherland et al., 2012). RsFC studies are accumulating to demonstrate impaired interactions of brain networks that underlie reward processing (Gu et al., 2010; Ma et al., 2010; Motzkin et al., 2014), executive control (Janes et al., 2012), self-monitoring and attention (Ding and Lee, 2013; Ma et al., 2011) in substance addiction. An open question thus is whether these altered functional connectivities also manifest in IGD.
To date, relatively few studies have examined the rsFC in individuals with IGD. For example, a previous study focusing on the posterior cingulate cortex reported altered default network rsFC in adolescents with IGD (Ding et al., 2013). In addition, studies showed abnormal rsFC in reward circuits in adolescents with IGD (Hong et al., 2013; Hong et al., 2015), consistent with the findings from substance use disorders. Furthermore, young adults with IGD exhibited lower rsFC in executive control network (ECN) in association with a diminished Stroop effect (Dong et al., 2015). More details of these studies were listed in Table S1.
In addition to widely examined dysfunction in reward system, default mode network (DMN), and ECN in addiction-related disorders, more recently, studies indicated that the insula, the structure that was largely overlooked for a long time in this field, may serve as a key neural substrate in the pathogenesis addiction (Naqvi and Bechara, 2009; Naqvi et al., 2014; Paulus and Stewart, 2014; Sutherland et al., 2013). Smokers with insula damage quit smoking easily and remain abstinent for a longer period of time (Naqvi et al., 2007). Furthermore, insula has been implicated in salience attribution, incentive motivation, cognitive control, and interoception, processes that are critical to the development and maintenance of addiction (Naqvi et al., 2014; Paulus, 2007). Given its central role in cognitive and affective functions, understanding how rsFC of the insula is altered in addiction-related disorders would elucidate neural mechanism underlying addiction and contribute to the development of more effective interventions.
Previous study showed reduced positive rsFC between the putamen and posterior insula in cocaine-addicted individuals (McHugh et al., 2013). Cocaine dependence was associated with greater rsFC of the right anterior insula with dorsomedial prefrontal cortex and inferior frontal gyrus, and greater rsFC of the right middle insula with dorsolateral prefrontal cortex (Cisler et al., 2013). Another study showed that nicotine withdrawal was associated with increased rsFC between the anterior insula and amygdala and DMN, and maladaptive interactions between the insula and other regions could be down-regulated by varenicline and nicotine (Sutherland et al., 2013). Additionally, a recent study examining the amygdala-centered network in IGD demonstrated stronger connectivity of bilateral amygdala and insula in individuals with IGD than controls (Ko et al., 2015). However, there is a dearth of research that systematically investigates the insula-centered rsFC in the context of IGD.
Moreover, previous studies have largely overlooked the fact that the insula is a multimodal brain region, with the anterior insula involved in salience and motivational processes (Cauda et al., 2011; Craig, 2002; Di Martino et al., 2009), and the posterior insula involved in interoception (Craig, 2002; Deen et al., 2011; Naqvi et al., 2014). All of these processes are critical to the development and maintenance of addiction, and examining the rsFC of the anterior and posterior insula separately would help our understanding of the etiology of IGD and develop more effective interventions targeting specific symptoms of IGD. The present study addressed these issues in a large sample of young adults with IGD.
On the basis of afore-mentioned evidence that the anterior insula plays critical roles in salience attribution, attention, and motivational processes (especially craving), through interacting with regions such as anterior cingulate cortex (ACC), DMN, and striatum (Deen et al., 2011; Sutherland et al., 2012; Zhang and Li, 2012a, b), we hypothesized that, in comparison to healthy control subjects (HCs), IGD subjects (IGDs) would demonstrate altered rsFC of the anterior insula with these brain regions.
On the other hand, as the posterior insula mediates interoception, which consists of the receiving and integrating bodily signals and external stimuli to influence ongoing behavior, we hypothesized altered rsFC between the posterior insula and somatosensory cortices in IGDs (Cauda et al., 2011; Deen et al., 2011; Paulus and Stewart, 2014).
Materials and Methods
Participants
Participants were recruited by means of online advertisements and word of mouth. A total of 76 IGDs and 41 HCs were selected through online questionnaire and telephone screening of 701 candidates (546 for IGD group and 155 for HC group). Two subjects in IGD group were excluded due to excessive head motion during scanning, and the final dataset comprised 74 IGDs and 41 HCs for analyses. All participants were right-handed. Given the higher prevalence of IGD in men versus women (Dong et al., 2015; Ko et al., 2009), only male participants were included.
Participants were recruited according to their weekly Internet gaming time and scores on the Chen Internet Addiction Scale (CIAS; Chen et al., 2003), which consists of 26 items on a 4-point Likert scale. Inclusion criteria for IGDs were: (1) a score of 67 or higher on the CIAS (Ko et al., 2009); (2) engagement in Internet gaming for over 14 hours per week for a minimum of one year; and (3) reporting of Internet gaming as their primary online activity. In contrast, HCs demonstrated: (1) a score of 60 or lower on the CIAS; and (2) less than 2 hours per week spent on Internet games (non-gamers also included). Participants who reported current or history of use of illegal substances and gambling (including online gambling) were excluded. Additional exclusion criteria included any history of psychiatric or neurological illness and current use of psychotropic medications, as assessed by a semi-structured personal interview.
Rate of cigarettes and alcohol use were recorded, and the Fagerstrom test for nicotine dependence (FTND; Fagerstrom, 1978) was used to assess nicotine use disorders. Additionally, current status of depression and anxiety were assessed using the Beck Depression Inventory (BDI; Beck et al., 1961) and the Beck Anxiety Inventory (BAI; Beck et al., 1988).
This study was approved by the Institutional Review Board of the State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University. All participants provided written informed consent and were financially compensated for their time.
Scanning procedure
Resting-state fMRI data were collected on a 3.0 T Siemens Trio scanner at Beijing Normal University. Participants were instructed to keep their head still and eyes open during scanning. Earplugs and a head coil with foam pads were used to minimize machine noise and head motion. Scanning parameters were: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 200 × 200 mm2, acquisition matrix = 64 × 64, voxel size = 3.1 × 3.1 × 3.5 mm3, slice = 33, time point = 200. Additionally, a high-resolution T1-wighted scan was acquired to permit functional localization (TR = 2530 ms, TE = 3.39 ms, flip angle = 7°, FOV = 256 × 256 mm2, voxel size = 1 × 1 × 1.33 mm3, slice = 144).
MRI data processing
Data were preprocessed and analyzed using DPABI version 1.2 (http://rfmri.org/dpabi), REST version 1.8 (Song et al., 2011; http://restfmri.net/forum/REST_V1.8), which are both based on SPM8 software package (http://www.fil.ion.ucl.ac.uk/spm).
For preprocessing, the first 10 volumes were discarded to allow the magnetization to approach a dynamic equilibrium and to allow participants get used to the scanning noise. Individual EPI data were slice time corrected. Participants whose head motion exceeding 3.0 mm in translation or 3° in rotation were excluded; two subjects in IGD group were excluded for this reason. We further reduced the confound of head motion with Friston’s 24-parameter model (Friston et al., 1996; Yan et al., 2013). To reduce the effect of physiological artifacts, we covaried signals from cerebrospinal fluid and white matter (Murphy et al., 2009). EPI data were then normalized to the MNI space. A spatial filter of 4 mm full width at half maximum (FWHM) Gaussian kernel was used. Subsequently, a band pass temporal filter (0.01–0.08 Hz) was applied to reduce the low-frequency drifts and high-frequency noise (Kühn and Gallinat, 2015).
To compute the rsFC of the insula, four spherical seed regions of interest (ROIs) (radius = 6 mm) were defined, each corresponding to the left and right ventral anterior insula (MNI coordinates: −33, 13, −7 and 32, 10, −6), and the left and right posterior insula (MNI coordinates: −38, −6, 5 and 35, −11, 6) on the basis of a previous study investigating insula connectivity at resting state (Deen et al., 2011). The average time-series within each seed were regressed against whole brain voxels to generate cross correlation maps. Voxelwise correlation coefficients were converted to Z-score via Fisher’s r-to-Z transformation.
Voxelwise two-sample t-tests on the Z-score maps derived from each seed were performed to compare the anterior and posterior insula-centered rsFC between IGD subjects and HCs. Group difference maps were corrected by means of Monte Carlo simulation combining a height threshold of P < 0.005 with cluster P < 0.05 to result in a family-wise-error rate of 5%. Cluster extents were calculated using DPABI and smoothing kernel was estimated based on the 4D residual. Therefore, a corrected P < 0.005 was achieved using a minimum cluster size of 64–67 voxels according to different smoothing kernel of the statistical maps.
Furthermore, Spearman’s rank correlations were used to assess the relationships between aberrant insula-centered rsFC identified by the two-sample t-tests and Internet gaming characteristics including CIAS score, weekly gaming time, duration of Internet gaming in the IGD group.
Results
Demographic characteristics
Demographic characteristics of 74 IGDs and 41 HCs are shown in Table 1. IGDs and HCs did not differ significantly in age or years of education. Consistent with the inclusion criteria, IGDs had significantly higher CIAS scores than HCs, although they did not differ in Internet use lifetime. Eight of the 41 HCs reported engaging in Internet games occasionally. However, the IGDs spent significantly more time on Internet games weekly than did the subgroup of HCs reporting occasional Internet gaming.
Table 1.
Demographic characteristics of IGD and HC subjects.
| IGDs (n = 74) |
HCs (n = 41) |
t/χ2 value | |
|---|---|---|---|
| mean ± S.D. | mean ± S.D. | ||
| Age | 22.28 ± 1.98 | 23.02 ± 2.09 | −1.89 |
| Years of education | 15.74 ± 1.84 | 16.32 ± 1.71 | −1.64 |
| CIAS | 78.31 ± 8.50 | 43.49 ± 9.64 | 20.05*** |
| Years of Internet use | 8.72 ± 2.84 | 7.83 ± 3.05 | 1.56 |
| Years of Internet gaming | 7.28 ± 3.02 | 5.13 ± 3.09a | 1.92 |
| Time spent on Internet gaming (hours per week) | 25.62 ± 12.89 | 1.19 ± 0.53a | 16.21*** |
| Alcohol use (at least once per month) | 57 | 29 | 0.55 |
| Frequency of alcohol use (per month) | 1.91 ± 1.50 | 1.28 ± 0.70 | 2.67** |
| Cigarette use (at least once per month) | 8 | 0 | 4.76* |
| FTND | 2.29 ± 1.50 | - | - |
| BAI | 5.49 ± 5.48 | 2.61 ± 3.26 | 3.52** |
| BDI | 8.97 ± 5.72 | 2.85 ± 3.64 | 6.97*** |
P < 0.05;
P < 0.01;
P < 0.001.
S.D. = standard deviation; IGD = Internet gaming disorder; HC = healthy control; CIAS = Chen Internet addition scale; FTND = Fagerstrom test for nicotine dependence; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory.
n = 8.
Fifty-seven of 74 IGDs and 29 of 41 HCs were alcohol drinkers. Although the rates of alcohol use were low in both groups (once a week or less), IGD subjects reported a higher monthly frequency of alcohol use relative to HCs. Eight IGDs and no HCs reported current cigarette smoking. In addition, IGDs scored significantly higher than HCs on the BDI and BAI.
RsFC results
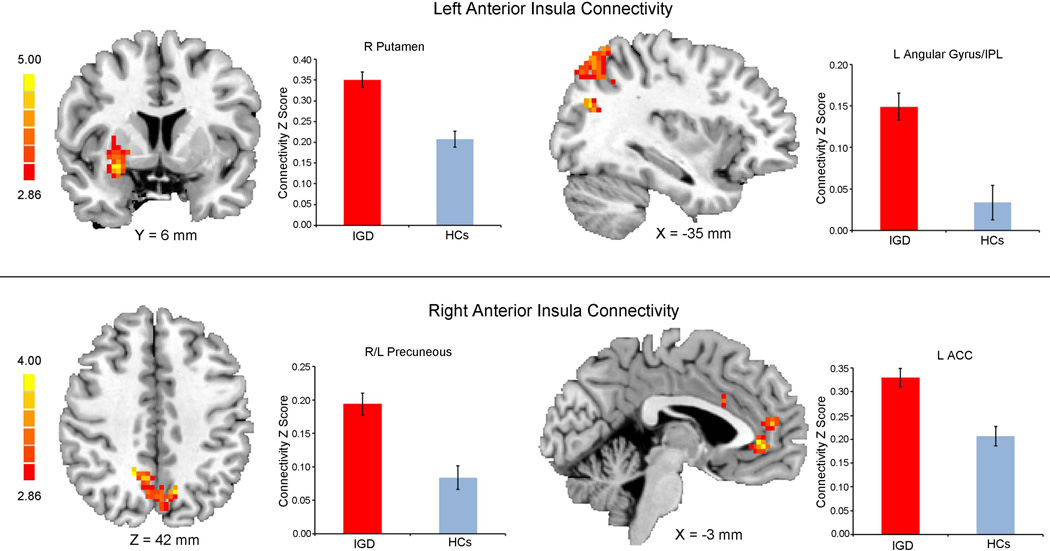
As shown in Table 2, IGDs exhibited generally stronger insula-centered rsFC relative to HCs. Specifically, the left anterior seed showed greater connectivity in IGDs relative to HCs with the right putamen, left angular gyrus, and inferior frontal gyrus (IFG). The right anterior seed demonstrated greater connectivity with the ACC, right middle cingulate gyrus, left angular gyrus, left precuneus, and bilateral superior frontal gyrus in IGDs relative to HCs (Figure 1).
Table 2.
Seed locations and regions showing significant differences in connectivity between IGD and HC subjects
| Seed | Region | Hemisphere | BA | Cluster size |
Peak MNI (mm) | Peak t value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Left AI | Putamen | R | 228 | 27 | 6 | −12 | 5.32 | |
| Angular gyrus | L | 7/39 | 173 | −36 | −72 | 24 | 4.19 | |
| IFG | L | 47 | 80 | −42 | 36 | −6 | 3.69 | |
| IFG | L | 44 | 152 | −39 | 6 | 30 | 4.61 | |
| IFG | R | 45 | 91 | 48 | 21 | 12 | 3.73 | |
| ITG | L | 20 | 70 | −60 | −51 | −15 | 4.12 | |
| MFG | R | 44 | 69 | 42 | 24 | 39 | 3.69 | |
| Right AI | ACC | L | 32 | 118 | −3 | 42 | −3 | 3.66 |
| Angular gyrus | L | 39 | 106 | −57 | −57 | 36 | 3.81 | |
| Precuneus | L/R | 7 | 100 | −12 | −66 | 42 | 4.10 | |
| SFG | R | 8 | 160 | 18 | 18 | 60 | 4.05 | |
| MCG | R | 23/24 | 116 | 15 | −27 | 27 | 4.60 | |
| SFG | L | 32 | 65 | −18 | 21 | 42 | 3.46 | |
| STG | R | 22 | 80 | 48 | −9 | −12 | 3.85 | |
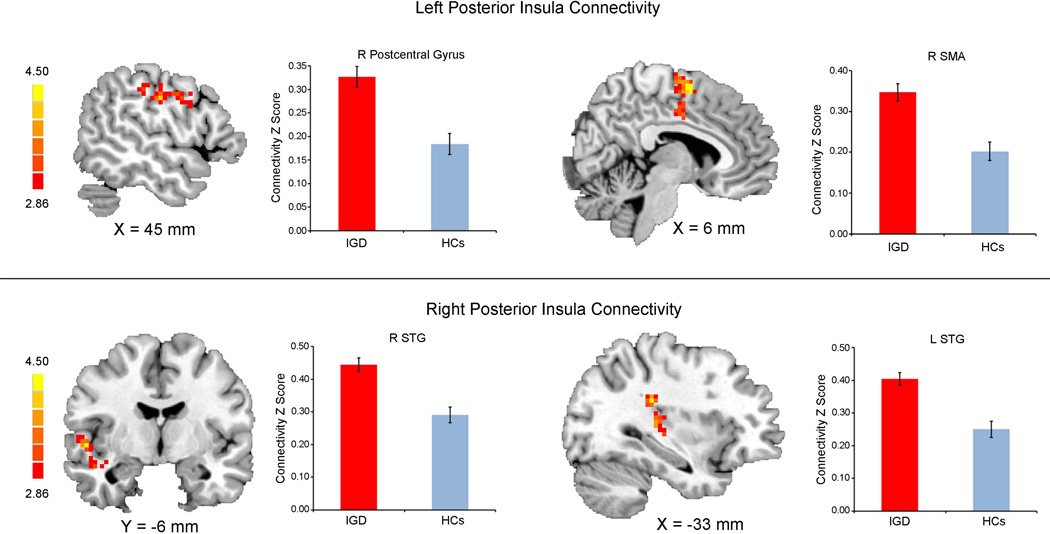
| Left PI | Precentral gyrus/postcentral gyrus | L | 3/6 | 270 | −36 | 3 | 30 | 4.24 |
| Postcentral gyrus | R | 3 | 72 | 45 | −24 | 51 | 4.09 | |
| SMA | R | 6 | 272 | 6 | −3 | 63 | 4.43 | |
| MCG | L | 23/24 | 98 | −15 | −18 | 42 | 4.00 | |
| STG | R | 22 | 90 | 57 | −9 | 6 | 4.48 | |
| STG/PI | L | 13/41 | 203 | −48 | −30 | 9 | 4.41 | |
| STG | R | 41 | 165 | 45 | −24 | 18 | 4.61 | |
| Right PI | STG | R | 22 | 159 | 54 | −6 | −3 | 4.52 |
| STG | L | 41 | 116 | −33 | −33 | 24 | 4.88 | |
IGD = Internet gaming disorder; HC = healthy control; IPL = inferior parietal lobule; ITG = inferior temporal gyrus; IFG = inferior frontal gyrus; MCG = Middle cingulate gyrus; MFG = middle frontal gyrus; SFG = superior frontal gyrus; ACC = anterior cingulate cortex; SMA = supplemental motor area; MCG = middle cingulate gyrus; MTG = middle temporal gyrus; STG = superior temporal gyrus.
Figure 1.
The left posterior seed showed greater connectivity with the bilateral postcentral gyrus, left precentral gyrus, right supplemental motor area (SMA), and superior temporal gyrus (STG), whereas the right posterior seed showed greater connectivity with the bilateral STG in IGDs compared with HCs (Figure 2).
Figure 2.
Relationship between rsFC strength and IGD characteristics
As shown in Table 3, within IGD group, IGD severity (i.e., CIAS score) is positively associated with connectivity between the right anterior insula and left angular gyrus, right STG, and left SFG, between the left posterior insula with adjacent left STG, and between the right posterior insula and right STG. Additionally, years of Internet gaming is positively associated with connectivity between the right anterior insula and ACC. No significant association between weekly Internet gaming time and insula-centered rsFC was observed.
Table 3.
Regions showing significant correlations with Internet gaming characteristics in IGD group.
| Seed | Region | Variable | r | P |
|---|---|---|---|---|
| Right anterior insula | STG | CIAS scores | 0.23 | 0.048 |
| Angular gyrus | CIAS scores | 0.23 | 0.045 | |
| SFG | CIAS scores | 0.25 | 0.029 | |
| ACC | Duration of Internet gaming | 0.33 | 0.002 | |
| Left posterior insula | STG | CIAS scores | 0.27 | 0.021 |
| Right posterior insula | STG | CIAS scores | 0.23 | 0.049 |
IGD = Internet gaming disorder; STG = superior temporal gyrus; SFG = superior frontal gyrus; CIAS = Chen Internet addiction scale.
Supplemental analyses
Given the group differences in anxiety, depression and the use of alcohol and tobacco, we conducted additional analyses controlling with scores of BAI and BDI, weekly drinking frequency, and status of tobacco use as covariates. As can be seen in Table S1, group differences in these variables do not explain the main findings of this study.
Discussion
In the current study, IGDs demonstrated enhanced rsFC of the anterior insula with a network of brain regions including ACC, putamen, precuneus and angular gyrus, relative to HCs. We also observed greater rsFC of the posterior insula with somatosensory and sensorimotor cortices in IGDs, compared with HCs. Additionally, correlational analyses indicate that the connectivity between the anterior insula and angular gyrus, SFG, and STG, and that between the posterior insula and STG are positively associated with IGD severity, and connectivity between the anterior insula and ACC is positively associated with duration of Internet gaming in IGDs. The different pattern of altered functional connectivities suggest the importance to examine abnormalities of the anterior and posterior insula separately and their distinct effects on cognitive and affective processes in IGD.
In accord with the neurocircuit model of addiction (Naqvi and Bechara, 2009; Naqvi et al., 2014; Sutherland et al., 2012), we observed greater positive connectivity between the right anterior insula with ACC in IGDs, relative to HCs, in association with duration of Internet gaming in IGDs. The anterior insula is anatomically connected to ACC (Deen et al., 2011; Naqvi et al., 2014), centrally situated in the salience network (Menon, 2011), a circuit critical for switching between DMN and ECN activation in the decision whether to maintain ongoing behavior (Cisler et al., 2013; Sutherland et al., 2012). Enhanced interactions between the right anterior insula and ACC in IGDs may be related to elevated salience of Internet games and related cues at the expense of other activities. The disrupted ACC-insula circuits may in turn render IGDs more prone to being engaged in Internet games.
We also observed stronger connectivity between the anterior insula and angular gyrus, precuneus, and inferior parietal lobule, which are thought to be components of the DMN (Fransson and Marrelec, 2008; Menon, 2011; Zhang and Li, 2012a, b). These findings are paralleled a previous report of enhanced functional connectivity during the presence of smoking cues between the right anterior insula and DMN (bilateral precuneus and left angular gyrus) during exposure to smoking cues in nicotine-dependent smokers (Maria et al., 2014). Furthermore, increased rsFC between the insula and DMN is associated with nicotine withdrawal in abstinent smokers (Sutherland et al., 2013). DMN is involved in self-monitoring, attention, and introspective thoughts (Ma et al., 2011; Raichle et al., 2001), and maladaptive interactions between the insula and DMN have been thought as a key neural marker underlying the development and maintenance of addiction (Sutherland et al., 2012). As mentioned above, the anterior insula serves a monitoring role in modulating dynamic interaction between DMN and ECN. DMN and ECN are two competitively brain networks (Menon, 2011). One is tempted to speculate that enhanced connectivity between anterior insula and DMN would suppress ECN activities, disrupting cognitive control and goal-directed behavior (Dong et al., 2015; Sutherland et al., 2013). Therefore, maladaptive interactions between the anterior insula and DMN may reflect heightened sensitivity to subjectively state of Internet experience, such as arousal and craving for gaming in IGDs. This hypothesis is supported by the findings that IGDs exhibited reduced rsFC in ECN (Dong et al., 2015), which limits their ability to control Internet gaming behaviors and related thought (Ferr et al., 2012).
Another important finding of the current study is that, in comparison to HCs, IGDs demonstrated elevated rsFC between left anterior insula and right putamen, a part of the dorsal striatum. Additionally, such altered connectivity is positively associated with IGD severity. A large body of previous studies in substance addiction highlight dorsal striatum as a core subcortical region involved in craving, compulsive drug seeking and drug taking (Everitt and Robbins, 2005; Koob and Volkow, 2010; Volkow et al., 2006). Naqvi et al. (2007) found that smokers with insula damage quit smoking easily and immediately, and followed a prolonged abstinence. Taken together, these findings suggest a large-scale brain network, including the insula and dorsal striatum, is critical to addictive behavior and the insula may serve as a hub in this network (Naqvi et al., 2007).
Consistent with our hypothesis, we found that the posterior insula demonstrated greater functional connectivity with somatosensory and sensorimotor cortices, including postcentral gyrus, precentral gyrus, SMA, cingulate gyrus, and STG in IGDs relative to HCs. The posterior insula is a critical site for interoception, somatosensory processing, and movement execution (Deen et al., 2011; Di Martino et al., 2009; Naqvi et al., 2014), and it is anatomically and functionally connected to primary and secondary motor and somatosensory cortices (Cauda et al., 2011; Craig, 2002; Deen et al., 2011). Maladaptive interactions between the posterior inusla and postcentral gyrus among IGD subjects may reflect abnormality in receiving, processing, and integrating body-relevant signals to guide ongoing behavior (Cauda et al., 2011; Paulus and Stewart, 2014). Furthermore, the precentral gyrus, SMA, middle cingulate gyrus, and STG control movement and process auditory signals (Howard et al., 2000; Yalachkov et al., 2010). While gaming online, players need to attend to sounds and control their avatars skillfully to achieve actions, such as dodging enemies and selecting weapons (Bavelier et al., 2011). Therefore, it is plausible that excessive gaming altered rsFC between posterior insula and sensorimotor cortices. Altogether, these findings also indicate the importance to distinguish anterior and posterior insula abnormality in IGD.
Our study has identified two separate functional networks centered on the anterior and posterior insula that have gone awry in IGDs. These findings may help develop more effective interventions for IGD. For example, pharmacological interventions targeting circuits including the anterior insula and dorsal striatum may be effective in attenuating craving in individuals with IGD. Moreover, real-time fMRI feedback (Li et al., 2012) can also be used to normalize the interactions between the anterior or posterior insula with other regions according to specific IGD-related symptoms.
Several limitations of the current study should be considered. First, we only recruited young male adults in this study. Although this population is particularly at risk for IGD (Chou et al., 2005; Ko et al., 2009), the current findings should be considered as specific to young adult males with IGD, and future studies should verify our findings in female subjects and in subjects with other age groups. Another limitation is that these findings are cross-sectional and longitudinal studies are needed to determine whether altered insula connectivity preceded the development of IGD or were the consequences of excessively gaming. Finally, functional connectivity between brain regions is correlational and the direction of activity between nodes cannot be determined only using rsFC approach (Gu et al., 2010).
In summary, the present study is the first to assess rsFC of the anterior and posterior insula in IGDs. IGDs demonstrated enhanced anterior insula-centered rsFC with a network of brain regions implicated in salience, self-monitoring, and craving, accompanied by enhanced rsFC of the posterior insula with somatosensory and sensorimotor cortices. These findings highlight the key and distinct roles of the anterior and posterior insula underlying core symptoms of IGD, consistent with current neuropathological models of addiction (Li and Sinha, 2008), and indicate the importance to examine abnormalities of the anterior and posterior insula separately in individuals with IGD.
Supplementary Material
Acknowledgements
This study was supported by the National Natural Science Foundation of China (No. 31170990 and No. 81100992), the fundamental research funds for the central universities (No. 2012WYB01), and a NIH grant (No. K02DA026990). Dr. Zang is partly supported by “Qian Jiang Distinguished Professor” program.
Footnotes
The address where the work was carried out: 3.0 T Siemens Trio scanner at State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University
The authors declare no competing financial interests.
Authors Contribution
JTZ and XYF were responsible for the study concept and design. ZJS, LL, LJW, and BL contributed to the acquisition of MRI data. JTZ, YWY, and YFZ assisted with data analysis and interpretation of findings. YWY drafted the manuscript. JTZ, CSRL, YFZ, and XYF provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for publication.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- Bavelier D, Green CS, Han DH, Renshaw PF, Merzenich MM, Gentile DA. Brains on video games. Nat Rev Neurosci. 2011;12:763–768. doi: 10.1038/nrn3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psych. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiat. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- China Internet Network Information Center. Statistics Report about the Development of Internet Network in China. Beijing: China Internet Network Information Center; 2014. [Google Scholar]
- Chen S, Weng L, Su Y, Wu H, Yang P. Development of a Chinese Internet addiction scale and its psychometric study. Chin J Psychol. 2003;45:279–294. [Google Scholar]
- Chou C, Condron L, Belland JC. A review of the research on Internet addiction. Educ Psychol Rev. 2005;17:363–388. [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD. Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiat Res-Neuroim. 2013;213:39–46. doi: 10.1016/j.pscychresns.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A, Shehzad Z, Kelly C, Roy AK, Gee DG, Uddin LQ, Gotimer K, Klein DF, Castellanos FX, Milham MP. Relationship between cingulo-insular functional connectivity and autistic traits in neurotypical adults. Am J Psychiat. 2009;166:891–899. doi: 10.1176/appi.ajp.2009.08121894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding W, Sun J, Sun Y, Zhou Y, Li L, Xu J, Du Y. Altered default network resting-state functional connectivity in adolescents with Internet gaming addiction. PLoS ONE. 2013;8:e59902. doi: 10.1371/journal.pone.0059902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Lee S-W. Changes of functional and effective connectivity in smoking replenishment on deprived heavy smokers: a resting-state FMRI study. PLoS ONE. 2013;8:e59331. doi: 10.1371/journal.pone.0059331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G, Lin X, Potenza MN. Decreased functional connectivity in an executive control network is related to impaired executive function in Internet gaming disorder. Prog Neuro-Psychoph. 2015;57:76–85. doi: 10.1016/j.pnpbp.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Fagerstrom KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Farr OM, Hu S, Zhang S, Li C-S. Decreased saliency processing as a neural measure of Barratt impulsivity in healthy adults. Neuoimage. 2012;63:1070–1077. doi: 10.1016/j.neuroimage.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: Evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med. 1996;35:346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S-B, Zalesky A, Cocchi L, Fornito A, Choi E-J, Kim H-H, Suh J-E, Kim C-D, Kim J-W, Yi S-H. Decreased functional brain connectivity in adolescents with internet addiction. PLoS ONE. 2013;8:e57831. doi: 10.1371/journal.pone.0057831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MA, Volkov IO, Mirsky R, Garell PC, Noh MD, Granner M, Damasio H, Steinschneider M, Reale RA, Hind JE, Brugge JF. Auditory cortex on the human posterior superior temporal gyrus. J Comp Neurol. 2000;416:79–92. doi: 10.1002/(sici)1096-9861(20000103)416:1<79::aid-cne6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick BDB, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depen. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. Brains online: structural and functional correlates of habitual Internet use. Addict Biol. 2015;20:415–422. doi: 10.1111/adb.12128. [DOI] [PubMed] [Google Scholar]
- Ko C-H, Yen J-Y, Chen S-H, Yang M-J, Lin H-C, Yen C-F. Proposed diagnostic criteria and the screening and diagnosing tool of Internet addiction in college students. Compr Psychiat. 2009;50:378–384. doi: 10.1016/j.comppsych.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacol. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C-S, Sinha R. Inhibitory control and emotional stress regulation: neuroimaging edidence for frontal-limbic dysfunction in psycho-stimulant addition. Neurosci Biobehav Rev. 2008;32:581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hartwell KJ, Borckardt J, Prisciandaro JJ, Saladin ME, Morgan PS, Johnson KA, LeMatty T, Brady KT, George MS. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict Biol. 2013;18:739–748. doi: 10.1111/j.1369-1600.2012.00449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu X-M, Li N, Wang C-X, Zhang H, Qian R-B, Xu H-S, Hu X, Zhang D-R. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS ONE. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang C-X, Zhang H, Jiang X-F, Xu H-S, Fu X-M, Hu X, Zhang D-R. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maria MS, Megan M, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, George MS. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol. 2014 doi: 10.1111/adb.12124. Available at: http://onlinelibrary.wiley.com/doi/10.1111/adb.12124/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Ab. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Baskin-Sommers A, Newman JP, Kiehl KA, Koenigs M. Neural correlates of substance abuse: reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp. 2014;35:4282–4292. doi: 10.1002/hbm.22474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP. Decision-making dysfunctions in psychiatry—altered homeostatic processing? Science. 2007;318:602–606. doi: 10.1126/science.1142997. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76:342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Rehbein F, Gentile DA, Lemmens JS, Rumpf HJ, Mößle T, Bischof G, Tao R, Fung DS, Borges G. An international consensus for assessing internet gaming disorder using the new DSM-5 approach. Addiction. 2014;109:1399–1406. doi: 10.1111/add.12457. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X-W, Dong Z-Y, Long X-Y, Li S-F, Zuo X-N, Zhu C-Z, He Y, Yan C-G, Zang Y-F. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiat. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Childress A-R, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci. 2006;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee C-Y, Zhao Z, Yap P-T, Wu G, Shi F, Price T, Du Y, Xu J, Zhou Y, Shen D. Disrupted Brain Functional Network in Internet Addiction Disorder: A Resting-State Functional Magnetic Resonance Imaging Study. PloS ONE. 2014;9:e107306. doi: 10.1371/journal.pone.0107306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behav Brain Res. 2010;207:215–222. doi: 10.1016/j.bbr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N, Castellanos FX, Milham MP. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage. 2013;76:183–201. doi: 10.1016/j.neuroimage.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012a;59:3548–3562. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li C-SR. Task-related, low-frequency task-residual, and resting state activity in the default mode network brain regions. Front Psychol. 2012b;3:172. doi: 10.3389/fpsyg.2012.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.