Abstract
Introduction
Homeless people have a high burden of cancer risk factors and suboptimal rates of cancer screening, but the epidemiology of cancer has not been well described in this population. We assessed cancer incidence, stage, and mortality in homeless adults relative to general population standards.
Methods
We cross-linked a cohort of 28,033 adults seen at Boston Health Care for the Homeless Program in 2003–2008 to Massachusetts cancer registry and vital registry records. We calculated age-standardized cancer incidence and mortality ratios (SIRs and SMRs). We examined tobacco use among incident cases and estimated smoking-attributable fractions. Trend tests were used to compare cancer stage distributions with those in Massachusetts adults. Analyses were conducted in 2012–2015.
Results
During 90,450 person-years of observation, there were 361 incident cancers (SIR=1.13, 95% CI=1.02, 1.25) and 168 cancer deaths (SMR=1.88, 95% CI=1.61, 2.19) among men, and 98 incident cancers (SIR=0.93, 95% CI=0.76, 1.14) and 38 cancer deaths (SMR=1.61, 95% CI=1.14, 2.20) among women. For both sexes, bronchus and lung cancer was the leading type of incident cancer and cancer death, exceeding Massachusetts estimates more than twofold. Oropharyngeal and liver cancer cases and deaths occurred in excess among men, whereas cervical cancer cases and deaths occurred in excess among women. About one third of incident cancers were smoking-attributable. Colorectal, female breast, and oropharyngeal cancers were diagnosed at more-advanced stages than in Massachusetts adults.
Conclusions
Efforts to reduce cancer disparities in homeless people should include addressing tobacco use and enhancing participation in evidence-based screening.
Introduction
About 2.3–3.5 million people experience homelessness annually in the U.S.1 Homeless people have a high burden of behavioral and environmental risk factors for developing cancer. An estimated 68%–80% are current cigarette smokers,2–7 and 29%–63% consume alcohol at problematic levels.7–14 Dietary inadequacy15–19 and prolonged sun exposure7 are common. Chronic hepatitis C virus20–24 and HIV24–26 infections are disproportionately prevalent among homeless individuals and associated with a higher risk of certain cancer types.
Homeless people may also experience barriers to the diagnosis and treatment of cancer. Many lack health insurance and forego needed medical care.27,28 Competing priorities for managing day-to-day subsistence needs29 may detract from the perceived importance of cancer prevention and screening. Additionally, certain screening tests such as colonoscopy pose numerous logistic challenges in the setting of homelessness. Several studies have documented low rates of colorectal cancer screening among homeless individuals,7,30–32 and completion of cervical cancer7 and breast cancer7,32,33 screening may also be suboptimal in this population. Even when structural barriers to cancer screening are reduced, uptake of certain screening tests may still be lacking,34 perhaps due in part to anticipated discomfort or misperceptions about cancer risk.7
Despite the potential for adverse cancer outcomes, the epidemiology of cancer among homeless people has not been well described. In a prior study of mortality among homeless adults in Boston,35 we found that cancer was the second leading cause of death overall and the leading killer among those aged 45 years and older. Studies of homeless and marginally housed individuals in Canada36 and Sweden37 have also documented a considerable number of cancer deaths. Although these studies found higher cancer mortality rates among homeless adults than in the general population, none examined the epidemiology of cancer incidence and stage at presentation to assess the contributions of excess risk and delays in diagnosis to these mortality disparities. A study of homeless men residing in Glasgow hostels in 1975–1993 estimated cancer incidence relative to less impoverished individuals and found an excess number of lung and upper aerodigestive malignancies,38 pointing toward the potential role of tobacco and alcohol use in elevating the cancer risk in this population. There have been no subsequent studies to confirm these findings in more diverse or contemporary samples of homeless people.
To address this gap in evidence, we cross-linked a cohort of 28,033 homeless adults in Boston to cancer registry and vital registry records to assess cancer incidence, stage at diagnosis, and cancer mortality relative to the Massachusetts general population. Additionally, we examined tobacco use among incident cases and estimated the burden of smoking-attributable cancer.
Methods
Study Population and Setting
We assembled a cohort of all adults aged ≥ 18 years who had an in-person encounter at Boston Health Care for the Homeless Program (BHCHP) between January 1, 2003, and December 31, 2008. We measured observation time in person-years, starting at the date of first contact with BHCHP during the study period and continuing until the date of death or December 31, 2008. BHCHP serves >11,000 individuals annually in >90,000 outpatient medical, oral health, and behavioral health encounters through a network of >70 service sites based in emergency shelters, transitional housing facilities, hospitals, and other social service settings in greater Boston.39,40 Individuals must be homeless to enroll in services at BHCHP, but some patients continue to receive care at the program after they are no longer homeless. We did not have housing status data for the cohort, but unpublished data on BHCHP patients seen in 2011 showed that 16% were housed.
Ascertainment of Cancer Cases
We identified cancer cases in the BHCHP cohort through cross-linkage to cancer incidence files at the Massachusetts Cancer Registry (MCR), a member of the North American Association of Central Cancer Registries and a bureau of the Massachusetts Department of Public Health (MDPH). MCR analysts internally conducted the data linkage using LinkPlus, version 2.0. LinkPlus is a probabilistic record linkage software program that uses expectation maximization algorithms and an array of linkage tools to compute linkage probability scores for possible record pairs based on the level of agreement and relative importance of various personal identifiers.41 Linkages to MCR records were based on first name, middle name (when available), last name, date of birth, and social security number (SSN). There were minimal missing data for the core identifiers in the BHCHP cohort (0% for first name, last name, and birth date; 9% for SSN). Individuals could link to more than one MCR record if they were diagnosed with multiple cancers.
For those who linked to an MCR record, we used the International Classification of Diseases for Oncology, 3rd Revision (ICD-O-3) code listed in the registry file to categorize the cancer type according to the scheme used by the MCR (Supplemental Table).42 ICD-O-3 is a multi-axial classification system that captures tumor site, histology, and behavior. We used the topography axis code to determine the cancer site, and we used the morphology axis code to delineate the cancer histology. In keeping with MCR protocol, we combined in situ urinary bladder tumors with malignant neoplasms of the urinary bladder.42 Otherwise, we excluded in situ neoplasms and confined all analyses to tumors with malignant behavior (i.e., cancers).
We examined the stage at diagnosis for seven cancer types encompassing the five most common cancers among men and women in the cohort to assess whether homeless people experience delays in diagnosis relative to the general population. We used the Derived Surveillance, Epidemiology, and End Results (SEER) Summary Stage 2000 code listed in the cancer registry file to classify the stage at diagnosis as local (1), regional (2, 3, 4, or 5), distant (7), or unknown (9 or missing).
Among incident cases, we examined tobacco use status at the time of diagnosis for 11 cancer types that the U.S. Surgeon General43 and the International Agency for Research on Cancer44 consider tobacco-related: bronchus and lung, cervix uteri, colon and rectum, esophagus, kidney and renal pelvis, larynx, liver and intrahepatic bile ducts, oral cavity and pharynx, pancreas, stomach, and urinary bladder. In Appendix A, we present the details of a supplemental analysis estimating the burden of incident cancers attributable to tobacco smoking.
Ascertainment of Cancer Deaths
We identified deaths by cross-linking the BHCHP cohort with the MDPH Registry of Vital Records and Statistics death occurrence files for 2003–2008. We used LinkPlus, version 2.0 to perform this linkage based on a probabilistic approach detailed elsewhere35 that was very similar to that used for the MCR linkage. We based causes of death on the ICD-10 underlying cause of death codes in the Massachusetts mortality file. Cancer deaths were those with an ICD-10 underlying cause code in the range C00–C97. We subdivided these deaths by cancer type according to the scheme used by the MCR (Supplemental Table).42
We reclassified deaths due to “malignant neoplasms of ill-defined, secondary, and unspecified sites” (ICD-10 C76–C80; n=15) as well as other vaguely defined sites (n=3) to more-specific site codes when MCR records provided sufficient detail to do so. An additional 15 death records had specific cancer site codes in the underlying cause of death field that differed from the cancer site code in the MCR records. In these instances, we regarded the MCR record as the gold standard (A MacMillan, Massachusetts Cancer Registry, written communication, 2014) and revised the cancer site on the death record to match it. Finally, six cancer deaths could not be linked to an MCR record because the cancer diagnoses were made at a Veterans Affairs facility, and an additional six cancer deaths had not been reported to the MCR. We analyzed these as cancer deaths but not as incident cancer cases because we lacked diagnosis dates. In a sensitivity analysis, we excluded these deaths from our mortality calculations and the findings were unchanged.
Statistical Analysis
We tabulated the overall and site-specific number of incident cancer cases and deaths that occurred on or after the index observation through December 31, 2008. We classified individuals who were diagnosed with cancer prior to their index observation as having a lifetime history of cancer, but we did not count these individuals as incident cases unless they developed a new cancer following the index observation.
We used age-standardized incidence ratios (SIR) and age-standardized mortality ratios (SMRs) to compare the observed numbers of cancer cases and deaths with the numbers expected based on cancer incidence and mortality rates in the general population. To estimate the expected number of incident cases, we multiplied cancer incidence rates in 10-year age bands for the 2004–2008 Massachusetts adult population42 by the observed person-time in the corresponding age bands of the BHCHP cohort and summed this product across all age groups. To estimate the expected number of cancer deaths, we multiplied cancer mortality rates in 10-year age bands for the 2004–2008 Massachusetts adult population45 by the person-time in the corresponding age bands of the BHCHP cohort and summed this product across all age groups. We divided the observed number of events by the expected number of events to calculate the SIR and SMR, and we used “proc stdrate” in SAS, version 9.4 to compute exact 95% Poisson CIs.46 We stratified all SIR and SMR analyses by sex.
Because we revised the SMR numerator of observed deaths caused by certain cancer types to resolve discrepancies with MCR records, we used a correction factor47,48 to adjust the expected number of cancer deaths in the SMR denominator according to similar discrepancies known to occur in the general population.49 Appendix B describes the methods we used to estimate the correction factor for each cancer type and the results of sensitivity analyses performed with and without correction for discrepancies between death records and cancer registry records.
Given the ordinal nature of cancer staging, we used the Cochran–Armitage trend test with exact two-tailed p-values to compare the type-specific cancer stage at diagnosis in BHCHP adults with the stage distributions for Massachusetts adults, which we obtained from the MCR. We excluded cases with an unknown or missing stage as well as cases diagnosed in 2003 because the MCR used a different staging scheme prior to 2004. Where applicable, we combined male and female cases in the stage analysis to improve statistical power. In subgroup analyses, we reassessed the trend tests for colorectal and female breast cancer stage among individuals diagnosed within the age ranges that the U.S. Preventive Services Task Force (USPSTF) recommends screening for these diseases (50–75 years50 and 50–74 years,51 respectively).
We conducted our analyses in 2012–2015 using Microsoft Excel 2003 and 2007 and SAS versions, 9.2–9.4. The Partners Human Research Committee and the MDPH IRB approved this study. In accordance with MCR requirements, we present non-zero counts <5 as “1–4” events to protect participant confidentiality.
Results
Overall, 28,033 adults were followed for a median of 3.3 years, yielding 90,450 person-years of observation. The mean age at cohort entry was 40.5 years, and two thirds of participants were male (Table 1). Forty-three percent were white, 29% were black, and 19% were Hispanic. About 1.3% (n=369) had a history of cancer diagnosed prior to the index observation, most commonly prostate (n=60), bronchus and lung (n=37), colon and rectum (n=36), breast (n=33), and oral cavity and pharynx (n=21) (Table 2).
Table 1.
Characteristics of the Study Cohort
| All (N=28,033) | Male (N=18,612) | Female (N=9,421) | |
|---|---|---|---|
| Age at cohort entry | |||
| Mean (SD) | 40.5 (12.4) | 42.7 (11.7) | 36.1 (12.6) |
| 18–24 years, N (%) | 3,491 (12.5) | 1,320 (7.1) | 2,171 (23.0) |
| 25–34 years, N (%) | 5,874 (21.0) | 3,320 (17.8) | 2,554 (27.1) |
| 35–44 years, N (%) | 7,930 (28.3) | 5,663 (30.4) | 2,267 (24.1) |
| 45–54 years, N (%) | 7,131 (25.4) | 5,483 (29.5) | 1,648 (17.5) |
| 55–64 years, N (%) | 2,796 (10.0) | 2,217 (11.9) | 579 (6.2) |
| 65–74 years, N (%) | 644 (2.3) | 500 (2.7) | 144 (1.5) |
| 75–84 years, N (%) | 149 (0.5) | 100 (0.5) | 49 (0.5) |
| ≥85 years, N (%) | 18 (0.1) | 9 (0.1) | 9 (0.1) |
| Sex, N (%) | |||
| Male | 18,612 (66.4) | 18,612 (100) | - |
| Female | 9,421 (33.6) | - | 9,421 (100) |
| Race/ethnicity, N (%) | |||
| White, non-Hispanic | 11,912 (42.5) | 8,476 (45.5) | 3,436 (36.5) |
| Black, non-Hispanic | 8,066 (28.8) | 5,262 (28.3) | 2,804 (29.8) |
| Hispanic | 5,301 (18.9) | 3,239 (17.4) | 2,062 (21.9) |
| Other/unknown | 2,754 (9.8) | 1,635 (8.8) | 1,119 (11.9) |
Table 2.
Number of Individuals With a History of Cancer Diagnosed Prior to the Index Visit.
| Cancer site | N |
|---|---|
| All sites | 369 |
| Prostate | 60 |
| Bronchus and lung | 37 |
| Colon and rectum | 36 |
| Breast | 33 |
| Oral cavity and pharynx | 21 |
| Non-Hodgkin lymphoma | 17 |
| Urinary bladder | 17 |
| Testis | 14 |
| Kidney and renal pelvis | 12 |
| Melanoma | 12 |
| Cervix uteri | 11 |
| Larynx | 10 |
| Leukemia | 10 |
| Thyroid | 10 |
| Hodgkin lymphoma | 7 |
| Liver and intrahepatic bile ducts | 7 |
| Corpus uteri and uterus NOS | 5 |
| Multiple myeloma | 5 |
| Brain and other nervous system | 1–4 |
| Esophagus | 1–4 |
| Ovary | 1–4 |
| Pancreas | 1–4 |
| Stomach | 1–4 |
| Other and unknown sites | 35 |
Note: Cell sizes of 1–4 are suppressed for confidentiality.
NOS, not otherwise specified
Four hundred fifty-nine incident cancers occurred in 446 individuals. There were 361 incident cancers among men (SIR=1.13, 95% CI=1.02, 1.25) and 98 incident cancers among women (SIR=0.93, 95% CI=0.76, 1.14) (Table 3). The leading types of incident cancer among men were bronchus and lung (n=85, SIR= 2.30, 95% CI=1.84, 2.84), prostate (n=59, SIR=0.63, 95% CI=0.48, 0.81), colon and rectum (n=36, SIR=1.24, 95% CI=0.87, 1.71), liver and intrahepatic bile duct (n=34, SIR=4.31, 95% CI=2.99, 6.02), and oral cavity and pharynx (n=25, SIR=2.03, 95% CI=1.31, 3.00). Among women, the most common incident cancers were bronchus and lung (n=23, SIR=2.23, 95% CI=1.41, 3.35), breast (n=21, SIR=0.59, 95% CI=0.37, 0.91), cervix uteri (n=10, SIR=4.42, 95% CI=2.12, 8.12), colon and rectum (n=7, SIR=0.99, 95% CI=0.40, 2.03), and oral cavity and pharynx (n=5, SIR=3.34, 95% CI=1.08, 7.79).
Table 3.
Incident Cancer Cases and Deaths in the BHCHP Cohort, With Age-Standardized Incidence and Mortality Ratios
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer site/type | Incident cases | SIR (95% CI) | Deaths | SMR (95% CI) | Incident cases | SIR (95% CI) | Deaths | SMR (95% CI) |
| All sites | 361 | 1.13 (1.02–1.25) | 168 | 1.88 (1.61–2.19) | 98 | 0.93 (0.76–1.14) | 38 | 1.61 (1.14–2.20) |
| Brain and other nervous system | 5 | 0.94 (0.31–2.20) | 1–4 | 0.60 (0.07–2.15) | 0 | -- | 0 | -- |
| Breast | 0 | -- | 0 | -- | 21 | 0.59 (0.37–0.91) | 5 | 1.07 (0.35–2.50) |
| Bronchus and lung | 85 | 2.30 (1.84–2.84) | 61 | 2.39 (1.83–3.08) | 23 | 2.23 (1.41–3.35) | 14 | 2.31 (1.26–3.88) |
| Cervix uteri | N/A | -- | N/A | -- | 10 | 4.42 (2.12–8.12) | 1–4 | 6.01 (1.24–17.6) |
| Colon and rectum | 36 | 1.24 (0.87–1.71) | 19 | 2.37 (1.43–3.70) | 7 | 0.99 (0.40–2.03) | 1–4 | 1.61 (0.33–4.72) |
| Corpus uteri and uterus NOS | N/A | -- | N/A | -- | 1–4 | 0.54 (0.15–1.38) | 1–4 | 1.29 (0.03–7.17) |
| Esophagus | 10 | 1.51 (0.73–2.78) | 8 | 1.82 (0.79–3.59) | 0 | -- | 0 | -- |
| Hodgkin lymphoma | 1–4 | 0.37 (0.01–2.07) | 0 | -- | 0 | -- | 0 | -- |
| Kidney and renal pelvis | 10 | 0.69 (0.33–1.26) | 0 | -- | 1–4 | 0.43 (0.01–2.40) | 1–4 | 2.90 (0.07–16.2) |
| Larynx | 8 | 2.07 (0.90–4.09) | 1–4 | 3.13 (0.85–8.00) | 0 | -- | 0 | -- |
| Leukemia | 6 | 0.82 (0.30–1.79) | 1–4 | 1.28 (0.35–3.28) | 0 | -- | 0 | -- |
| Liver and intrahepatic bile ducts | 34 | 4.31 (2.99–6.02) | 22 | 4.35 (2.73–6.59) | 1–4 | 3.58 (0.43–12.9) | 0 | -- |
| Melanoma | 8 | 0.47 (0.20–0.92) | 1–4 | 1.77 (0.48–4.53) | 1–4 | 0.29 (0.03–1.04) | 0 | -- |
| Multiple myeloma | 1–4 | 1.08 (0.29–2.76) | 1–4 | 1.29 (0.16–4.67) | 1–4 | 1.47 (0.04–8.21) | 1–4 | 3.50 (0.09–19.5) |
| Non-Hodgkin lymphoma | 10 | 0.72 (0.35–1.33) | 1–4 | 0.33 (0.01–1.85) | 1–4 | 0.31 (0.01–1.73) | 0 | -- |
| Oral cavity and pharynx | 25 | 2.03 (1.31–3.00) | 9 | 2.37 (1.08–4.49) | 5 | 3.34 (1.08–7.79) | 1–4 | 2.81 (0.07–15.7) |
| Ovary | N/A | -- | N/A | -- | 1–4 | 0.63 (0.08–2.29) | 1–4 | 1.57 (0.19–5.66) |
| Pancreas | 11 | 1.64 (0.82–2.94) | 9 | 1.62 (0.74–3.07) | 1–4 | 1.24 (0.15–4.48) | 1–4 | 1.61 (0.20–5.83) |
| Prostate | 59 | 0.63 (0.48–0.81) | 1–4 | 0.92 (0.25–2.36) | N/A | -- | N/A | -- |
| Stomach | 8 | 1.60 (0.69–3.15) | 1–4 | 1.42 (0.39–3.63) | 1–4 | 2.75 (0.33–9.95) | 1–4 | 2.42 (0.06–13.5) |
| Testis | 0 | -- | 0 | -- | N/A | -- | N/A | -- |
| Thyroid | 1–4 | 0.29 (0.03–1.03) | 0 | -- | 5 | 0.52 (0.17–1.21) | 0 | -- |
| Urinary bladder | 17 | 0.93 (0.54–1.49) | 1–4 | 1.29 (0.35–3.30) | 1–4 | 0.53 (0.01–2.97) | 1–4 | 3.11 (0.08–17.3) |
Note: Cell sizes of 1–4 are suppressed for confidentiality.
BHCHP, Boston Health Care for the Homeless Program; SIR, standardized incidence ratio; SMR, standardized mortality ratio; NOS, not otherwise specified
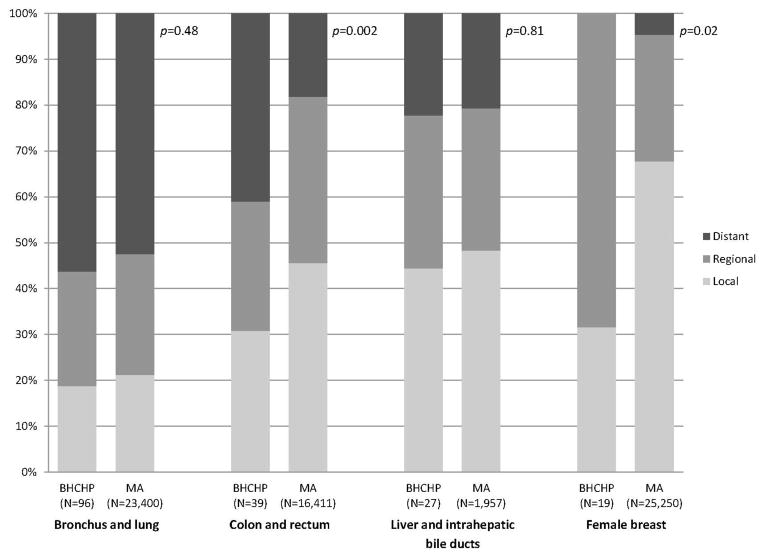
BHCHP adults were diagnosed with colon and rectum (p=0.002), female breast (p=0.02), and oral cavity and pharynx (p<0.001) cancers at significantly later stages than Massachusetts adults (Figure 1). Forty-one percent of colorectal cancers and 43% of oropharyngeal cancers had distant metastases at presentation. Although no breast cancer cases were distant at diagnosis, 68% were regionally advanced. The trends for colorectal cancer stage (p=0.001) and breast cancer stage (p=0.03) remained significant among those diagnosed within the recommended screening age ranges. There were no significant stage differences between BHCHP and Massachusetts adults for bronchus and lung (p=0.48), prostate (p=1.00), liver and intrahepatic bile duct (p=0.81), and cervical (p=0.62) cancers.
Figure 1.
Stage at diagnosis of selected cancer types in the BHCHP cohort and Massachusetts adults.
BHCHP, Boston Health Care for the Homeless Program; MA, Massachusetts
Notes: p-values are for the Cochran-Armitage trend test. Stage comparisons for prostate (p=1.00), oral cavity and pharynx (p<0.001), and cervical (p=0.62) cancers are not shown for confidentiality reasons because of small counts for certain stages. Stages are based on the Derived SEER Summary Stage 2000 code, where local=1, regional=2, 3, 4, or 5, and distant=7.
Eighty-eight percent of incident bronchus and lung cancers, 83% of incident oral cavity and pharynx cancers, and 75% of all 11 tobacco-related cancer types combined occurred in current smokers. An estimated 88% (95% CI=81%, 91%) of lung cancer cases and 60% (95% CI=45%, 71%) of oropharyngeal cancer cases were smoking-attributable. In total, about 157 (95% CI=147, 166) cancer cases were smoking-attributable, representing 34% (95% CI=32%, 36%) of all incident cancers in the BHCHP cohort. Appendix A contains the full results of the tobacco analysis.
There were 168 cancer deaths among men (SMR=1.88, 95% CI=1.61, 2.19) and 38 cancer deaths among women (SMR=1.61, 95% CI=1.14, 2.20). The leading causes of cancer death among men were bronchus and lung (n=61, SMR=2.39, 95% CI=1.83, 3.08), liver and intrahepatic bile duct (n=22; SMR=4.35, 95% CI=2.73, 6.59), colon and rectum (n=19, SMR=2.37, 95% CI=1.43, 3.70), oral cavity and pharynx (n=9, SMR=2.37, 95% CI=1.08, 4.49), and pancreas (n=9, SMR=1.62, 95% CI=0.74, 3.07) cancers. Among women, the leading causes of cancer death were bronchus and lung (n=14, SMR=2.31, 95% CI=1.26, 3.88) and breast (n=5, SMR=1.07, 95% CI=0.35, 2.50) cancers. Although fewer in number, deaths due to cervical cancer significantly exceeded the expected number (SMR=6.01, 95% CI=1.24, 17.6).
Discussion
In this study of more than 28,000 currently and formerly homeless adult clinic patients in Boston, men had a significantly higher cancer incidence rate and both sexes had significantly higher cancer mortality rates than expected based on Massachusetts general population estimates.
The excess burden of lung and oropharyngeal cancer in the BHCHP cohort is consistent with prior studies of homeless and marginally housed people in Scotland38 and Canada.36 We have previously estimated that more than 90% of lung cancer deaths in this cohort were tobacco-attributable.52 The current study extends these findings in estimating that 88% of incident lung cancer cases and about one third of all incident cancer cases were attributable to tobacco smoking. These findings suggest that interventions to reduce tobacco use among homeless people should be a pillar of primary prevention efforts. Additionally, the USPSTF now recommends lung cancer screening with computed tomography in 55- to 80-year-old adults with recent extensive smoking histories53; our prior analyses of cigarette smoking among homeless people2 suggests that more than half in this age range might qualify for screening. Although the USPSTF has determined that there is insufficient evidence to recommend for or against oral cancer screening,54 this is a low-cost and low-risk examination that can be readily performed in a medical, dental, or community setting55–57 and should be strongly considered in this high-risk population with a heavy burden of advanced oropharyngeal cancer.
Although the incidence of colorectal cancer was not significantly elevated in comparison to the general population, BHCHP patients were diagnosed with colorectal cancer at a significantly later stage, contributing to excess colorectal cancer mortality among men. BHCHP women had a significantly lower incidence of breast cancer than Massachusetts women, but this did not translate into lower breast cancer mortality, likely due in part to the large proportion presenting with non-localized disease. Though these findings are limited by small sample sizes, they are concordant with a similar discrepancy in breast cancer incidence and mortality observed among African American women,58 who are over-represented in this and other homeless samples relative to the general population. Although BHCHP maintains a close relationship with hospitals that offer comprehensive cancer screening services and has a medical respite facility where patients can prepare for colonoscopies, the advanced stage of breast and colorectal cancer diagnoses in the study cohort suggests the need for additional strategies to promote screening for these and other cancers. Patient-level interventions might include educational initiatives targeting cancer knowledge, attitudes, and beliefs; navigator programs to assist in attending offsite cancer screening appointments; and the provision of incentives for screening participation. Health system interventions might include initiatives to enhance provider counseling, as well as same-day or flexible scheduling models to improve the accessibility of cancer screening services.
Limitations
Study participants were patients of a large HCH program in Boston. This could have enriched the cohort with individuals at higher risk of cancer but likely presents a best-case scenario with respect to stage at diagnosis given the clinical resources of BHCHP and the high prevalence of health insurance coverage among homeless people in Boston. The accuracy of death certificates has been questioned,59 but they appear to be reliable for selected cancer types49,60 and we were able to verify them against cancer registry records. Additionally, decedents in this cohort underwent autopsy at a considerably higher rate than decedents in the Massachusetts general population.35 Finally, we were unable to discern the potential role that differences in cancer treatment might have had on the excess cancer mortality seen in the study sample.
Conclusions
This cohort of homeless adults in Boston had a high burden of tobacco-related cancer and was diagnosed with screen-detectable malignancies at a later stage than Massachusetts residents. Prevention efforts should focus on reducing tobacco use and enhancing completion of cancer screening.
Supplementary Material
Acknowledgments
This study was supported by award K23DA034008 to Dr. Baggett from the National Institute on Drug Abuse at the NIH, and by award 122269-IRG-12-070-01-IRG to Dr. Baggett from the American Cancer Society. The sponsors had no role in any aspect of this study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or American Cancer Society. At the time of the study, Dr. Bharel was also affiliated with the Boston Health Care for the Homeless Program and Division of General Internal Medicine, Massachusetts General Hospital.
We thank Annie MacMillan, MPH, at the Massachusetts Cancer Registry for linking the Boston Health Care for the Homeless Program cohort to the Massachusetts Cancer Registry database, for providing cancer stage data for the Massachusetts adult population, and for her assistance in working with cancer registry data. This paper does not represent the work or views of Ms. MacMillan or the Massachusetts Cancer Registry.
Footnotes
No financial disclosures were reported by the authors of this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burt MR. Helping America’s Homeless: Emergency Shelter or Affordable Housing? Washington, D.C: Urban Institute; 2001. [Google Scholar]
- 2.Baggett TP, Rigotti NA. Cigarette smoking and advice to quit in a national sample of homeless adults. Am J Prev Med Aug. 2010;39(2):164–172. doi: 10.1016/j.amepre.2010.03.024. http://dx.doi.org/10.1016/j.amepre.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 3.Connor SE, Cook RL, Herbert MI, Neal SM, Williams JT. Smoking cessation in a homeless population: there is a will, but is there a way? J Gen Intern Med. 2002;17(5):369–372. doi: 10.1046/j.1525-1497.2002.10630.x. http://dx.doi.org/10.1007/s11606-002-0042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snyder LD, Eisner MD. Obstructive lung disease among the urban homeless. Chest. 2004;125(5):1719–1725. doi: 10.1378/chest.125.5.1719. http://dx.doi.org/10.1378/chest.125.5.1719. [DOI] [PubMed] [Google Scholar]
- 5.Szerlip MI, Szerlip HM. Identification of cardiovascular risk factors in homeless adults. Am J Med Sci. 2002;324(5):243–246. doi: 10.1097/00000441-200211000-00002. http://dx.doi.org/10.1097/00000441-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Tsai J, Rosenheck RA. Smoking among chronically homeless adults: prevalence and correlates. Psychiatr Serv. 2012;63(6):569–576. doi: 10.1176/appi.ps.201100398. [DOI] [PubMed] [Google Scholar]
- 7.Chau S, Chin M, Chang J, et al. Cancer risk behaviors and screening rates among homeless adults in Los Angeles County. Cancer Epidemiol Biomarkers Prev. 2002;11(5):431–438. [PubMed] [Google Scholar]
- 8.North CS, Eyrich-Garg KM, Pollio DE, Thirthalli J. A prospective study of substance use and housing stability in a homeless population. Soc Psychiatry Psychiatr Epidemiol. 2010;45(11):1055–1062. doi: 10.1007/s00127-009-0144-z. http://dx.doi.org/10.1007/s00127-009-0144-z. [DOI] [PubMed] [Google Scholar]
- 9.Koegel P, Burnam MA, Farr RK. The prevalence of specific psychiatric disorders among homeless individuals in the inner city of Los Angeles. Arch Gen Psychiatry. 1988;45(12):1085–1092. doi: 10.1001/archpsyc.1988.01800360033005. http://dx.doi.org/10.1001/archpsyc.1988.01800360033005. [DOI] [PubMed] [Google Scholar]
- 10.Glasser I, Zywiak WH. Homelessness and substance misuse: a tale of two cities. Subst Use Misuse. 2003;38(3–6):551–576. doi: 10.1081/ja-120017385. http://dx.doi.org/10.1081/JA-120017385. [DOI] [PubMed] [Google Scholar]
- 11.Bassuk EL, Buckner JC, Perloff JN, Bassuk SS. Prevalence of mental health and substance use disorders among homeless and low-income housed mothers. Am J Psychiatry. 1998;155(11):1561–1564. doi: 10.1176/ajp.155.11.1561. http://dx.doi.org/10.1176/ajp.155.11.1561. [DOI] [PubMed] [Google Scholar]
- 12.Robertson MJ, Zlotnick C, Westerfelt A. Drug use disorders and treatment contact among homeless adults in Alameda County, California. Am J Public Health. 1997;87(2):221–228. doi: 10.2105/ajph.87.2.221. http://dx.doi.org/10.2105/AJPH.87.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Breakey WR, Fischer PJ, Kramer M, et al. Health and mental health problems of homeless men and women in Baltimore. JAMA. 1989;262(10):1352–1357. http://dx.doi.org/10.1001/jama.1989.03430100086034. [PubMed] [Google Scholar]
- 14.Burt MR, Aron LY, Douglas T, et al. Homelessness: Programs and the People They Serve: Findings of the National Survey of Homeless Assistance Providers and Clients: Technical Report. Washington, D.C: U.S. Department of Housing and Urban Development, Office of Policy Development and Research; 1999. [Google Scholar]
- 15.Baggett TP, Singer DE, Rao SR, et al. Food insufficiency and health services utilization in a national sample of homeless adults. J Gen Intern Med. 2011;26(6):627–634. doi: 10.1007/s11606-011-1638-4. http://dx.doi.org/10.1007/s11606-011-1638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luder E, Ceysens-Okada E, Koren-Roth A, Martinez-Weber C. Health and nutrition survey in a group of urban homeless adults. J Am Diet Assoc. 1990;90(10):1387–1392. [PubMed] [Google Scholar]
- 17.Drake MA. The nutritional status and dietary adequacy of single homeless women and their children in shelters. Public Health Rep. 1992;107(3):312–319. [PMC free article] [PubMed] [Google Scholar]
- 18.Wolgemuth JC, Myers-Williams C, Johnson P, Henseler C. Wasting malnutrition and inadequate nutrient intakes identified in a multiethnic homeless population. J Am Diet Assoc. 1992;92(7):834–839. [PubMed] [Google Scholar]
- 19.Gelberg L, Stein JA, Neumann CG. Determinants of undernutrition among homeless adults. Public Health Rep. 1995;110(4):448–454. [PMC free article] [PubMed] [Google Scholar]
- 20.Strehlow AJ, Robertson MJ, Zerger S, et al. Hepatitis C among clients of health care for the homeless primary care clinics. J Health Care Poor Underserved. 2012;23(2):811–833. doi: 10.1353/hpu.2012.0047. http://dx.doi.org/10.1353/hpu.2012.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nyamathi AM, Dixon EL, Robbins W, et al. Risk factors for hepatitis C virus infection among homeless adults. J Gen Intern Med. 2002;17(2):134–143. doi: 10.1046/j.1525-1497.2002.10415.x. http://dx.doi.org/10.1046/j.1525-1497.2002.10415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai RA, Rosenheck RA, Agnello V. Prevalence of Hepatitis C virus infection in a sample of homeless veterans. Soc Psychiatry Psychiatr Epidemiol. 2003;38(7):396–401. doi: 10.1007/s00127-003-0639-y. [DOI] [PubMed] [Google Scholar]
- 23.Gelberg L, Robertson MJ, Arangua L, et al. Prevalence, distribution, and correlates of hepatitis C virus infection among homeless adults in Los Angeles. Public Health Rep. 2012;127(4):407–421. doi: 10.1177/003335491212700409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klinkenberg WD, Caslyn RJ, Morse GA, et al. Prevalence of human immunodeficiency virus, hepatitis B, and hepatitis C among homeless persons with co-occurring severe mental illness and substance use disorders. Compr Psychiatry. 2003;44(4):293–302. doi: 10.1016/S0010-440X(03)00094-4. http://dx.doi.org/10.1016/S0010-440X(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 25.Robertson MJ, Clark RA, Charlebois ED, et al. HIV seroprevalence among homeless and marginally housed adults in San Francisco. Am J Public Health. 2004;94(7):1207–1217. doi: 10.2105/ajph.94.7.1207. http://dx.doi.org/10.2105/AJPH.94.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotnick C, Zerger S. Survey findings on characteristics and health status of clients treated by the federally funded (US) Health Care for the Homeless Programs. Health Soc Care Community. 2009;17(1):18–26. doi: 10.1111/j.1365-2524.2008.00793.x. http://dx.doi.org/10.1111/j.1365-2524.2008.00793.x. [DOI] [PubMed] [Google Scholar]
- 27.Baggett TP, O’Connell JJ, Singer DE, Rigotti NA. The unmet health care needs of homeless adults: a national study. Am J Public Health. 2010;100(7):1326–1333. doi: 10.2105/AJPH.2009.180109. http://dx.doi.org/10.2105/AJPH.2009.180109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kushel MB, Vittinghoff E, Haas JS. Factors associated with the health care utilization of homeless persons. JAMA. 2001;285(2):200–206. doi: 10.1001/jama.285.2.200. http://dx.doi.org/10.1001/jama.285.2.200. [DOI] [PubMed] [Google Scholar]
- 29.Gelberg L, Gallagher TC, Andersen RM, Koegel P. Competing priorities as a barrier to medical care among homeless adults in Los Angeles. Am J Public Health. 1997;87(2):217–220. doi: 10.2105/ajph.87.2.217. http://dx.doi.org/10.2105/AJPH.87.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lebrun-Harris LA, Baggett TP, Jenkins DM, et al. Health Status and Health Care Experiences Among Homeless Patients in Federally Supported Health Centers: Findings from the 2009 Patient Survey. Health Serv Res. 2013;48(3):992–1017. doi: 10.1111/1475-6773.12009. http://dx.doi.org/10.1111/1475-6773.12009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asgary R, Garland V, Jakubowski A, Sckell B. Colorectal cancer screening among the homeless population of New York City shelter-based clinics. Am J Public Health. 2014;104(7):1307–1313. doi: 10.2105/AJPH.2013.301792. http://dx.doi.org/10.2105/AJPH.2013.301792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Long HL, Tulsky JP, Chambers DB, et al. Cancer screening in homeless women: attitudes and behaviors. J Health Care Poor Underserved. 1998;9(3):276–292. doi: 10.1353/hpu.2010.0070. http://dx.doi.org/10.1353/hpu.2010.0070. [DOI] [PubMed] [Google Scholar]
- 33.Asgary R, Garland V, Sckell B. Breast Cancer Screening Among Homeless Women of New York City Shelter-Based Clinics. Womens Health Issues. 2014;24(5):529–534. doi: 10.1016/j.whi.2014.06.002. http://dx.doi.org/10.1016/j.whi.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Bharel M, Casey C, Wittenberg E. Disparities in cancer screening: acceptance of Pap smears among homeless women. J Womens Health (Larchmt) 2009;18(12):2011–2016. doi: 10.1089/jwh.2008.1111. http://dx.doi.org/10.1089/jwh.2008.1111. [DOI] [PubMed] [Google Scholar]
- 35.Baggett TP, Hwang SW, O’Connell JJ, et al. Mortality among homeless adults in Boston: shifts in causes of death over a 15-year period. JAMA Intern Med. 2013;173(3):189–195. doi: 10.1001/jamainternmed.2013.1604. http://dx.doi.org/10.1001/jamainternmed.2013.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang SW, Wilkins R, Tjepkema M, O’Campo PJ, Dunn JR. Mortality among residents of shelters, rooming houses, and hotels in Canada: 11 year follow-up study. BMJ. 2009;339:b4036. doi: 10.1136/bmj.b4036. http://dx.doi.org/10.1136/bmj.b4036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beijer U, Andreasson S, Agren G, Fugelstad A. Mortality and causes of death among homeless women and men in Stockholm. Scand J Public Health. 2011;39(2):121–127. doi: 10.1177/1403494810393554. http://dx.doi.org/10.1177/1403494810393554. [DOI] [PubMed] [Google Scholar]
- 38.Lamont DW, Toal FM, Crawford M. Socioeconomic deprivation and health in Glasgow and the west of Scotland--a study of cancer incidence among male residents of hostels for the single homeless. J Epidemiol Community Health. 1997;51(6):668–671. doi: 10.1136/jech.51.6.668. http://dx.doi.org/10.1136/jech.51.6.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boston Health Care for the Homeless Program. [Accessed February 29, 2012];2010 www.bhchp.org.
- 40.O’Connell JJ, Oppenheimer SC, Judge CM, et al. The Boston Health Care for the Homeless Program: a public health framework. Am J Public Health. 2010;100(8):1400–1408. doi: 10.2105/AJPH.2009.173609. http://dx.doi.org/10.2105/AJPH.2009.173609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.CDC. Registry Plus: Link Plus Users Guide, Version 2.0. Atlanta, GA: U.S. CDC, Cancer Division; 2007. [Google Scholar]
- 42.Gershman ST Massachusetts Cancer Registry. Cancer Incidence and Mortality in Massachusetts 2004–2008: Statewide Report. Massachusetts Department of Public Health, Bureau of Health Information, Statistics, Research, and Evaluation; Aug, 2011. [Google Scholar]
- 43.U.S. DHHS. A Report of the Surgeon General. Atlanta, GA: U.S. DHHS, CDC, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2014. The Health Consequences of Smoking: 50 Years of Progress. [Google Scholar]
- 44.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Volume 100E: Personal Habits and Indoor Combustions. Lyon: International Agency for Research on Cancer; 2012. Tobacco Smoking; pp. 43–211. [PMC free article] [PubMed] [Google Scholar]
- 45.CDC, National Center for Health Statistics. Underlying Cause of Death 1999–2011 on CDC WONDER Online Database, released 2014. [Accessed September 23, 2014];Data are from the Multiple Cause of Death Files, 1999–2011, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://wonder.cdc.gov/ucd-icd10.html.
- 46.The STDRATE Procedure. SAS/STAT 13.2 User’s Guide. Cary, NC: SAS Institute Inc; 2014. [Google Scholar]
- 47.Hoel DG, Ron E, Carter R, Mabuchi K. Influence of death certificate errors on cancer mortality trends. J Natl Cancer Inst. 1993;85(13):1063–1068. doi: 10.1093/jnci/85.13.1063. http://dx.doi.org/10.1093/jnci/85.13.1063. [DOI] [PubMed] [Google Scholar]
- 48.Ron E, Carter R, Jablon S, Mabuchi K. Agreement between death certificate and autopsy diagnoses among atomic bomb survivors. Epidemiology. 1994;5(1):48–56. doi: 10.1097/00001648-199401000-00009. http://dx.doi.org/10.1097/00001648-199401000-00009. [DOI] [PubMed] [Google Scholar]
- 49.German RR, Fink AK, Heron M, et al. The accuracy of cancer mortality statistics based on death certificates in the United States. Cancer Epidemiol. 2011;35(2):126–131. doi: 10.1016/j.canep.2010.09.005. http://dx.doi.org/10.1016/j.canep.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 50.U S. Preventive Services Task Force. Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. http://dx.doi.org/10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 51.U S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151(10):716–726. W-236. doi: 10.7326/0003-4819-151-10-200911170-00008. http://dx.doi.org/10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 52.Baggett TP, Chang Y, Singer DE, et al. Tobacco-, Alcohol-, and Drug-Attributable Deaths and Their Contribution to Mortality Disparities in a Cohort of Homeless Adults in Boston. Am J Public Health. 2014:e1–e9. doi: 10.2105/AJPH.2014.302248. (epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. doi: 10.7326/M13-2771. http://dx.doi.org/10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 54.Moyer VA. Screening for oral cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(1):55–60. doi: 10.7326/M13-2568. http://dx.doi.org/10.7326/M13-2568. [DOI] [PubMed] [Google Scholar]
- 55.Collins J, Freeman R. Homeless in North and West Belfast: an oral health needs assessment. Br Dent J. 2007;202(12):E31. doi: 10.1038/bdj.2007.473. http://dx.doi.org/10.1038/bdj.2007.473. [DOI] [PubMed] [Google Scholar]
- 56.Moore CE, Durden F. Head and neck cancer screening in homeless communities: HEAL (Health Education, Assessment, and Leadership) J Natl Med Assoc. 2010;102(9):811–816. doi: 10.1016/s0027-9684(15)30678-7. [DOI] [PubMed] [Google Scholar]
- 57.Carter JM, Winters RD, Lipin R, Lookabaugh S, Cai D, Friedlander PL. A faith- and community-based approach to identifying the individual at risk for head and neck cancer in an inner city. Laryngoscope. 2013;123(6):1439–1443. doi: 10.1002/lary.23981. http://dx.doi.org/10.1002/lary.23981. [DOI] [PubMed] [Google Scholar]
- 58.CDC. Vital signs: racial disparities in breast cancer severity--United States, 2005–2009. MMWR Morb Mortal Wkly Rep. 61(45):922–926. [PubMed] [Google Scholar]
- 59.Ravakhah K. Death certificates are not reliable: revivification of the autopsy. South Med J. 2006;99(7):728–733. doi: 10.1097/01.smj.0000224337.77074.57. http://dx.doi.org/10.1097/01.smj.0000224337.77074.57. [DOI] [PubMed] [Google Scholar]
- 60.Doria-Rose VP, Marcus PM. Death certificates provide an adequate source of cause of death information when evaluating lung cancer mortality: an example from the Mayo Lung Project. Lung Cancer. 2009;63(2):295–300. doi: 10.1016/j.lungcan.2008.05.019. http://dx.doi.org/10.1016/j.lungcan.2008.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.