Abstract
Diarrhea causes substantial morbidity and mortality in children in low-income countries. Although numerous pathogens cause diarrhea, the etiology of many episodes remains unknown. Serratia marcescens is incriminated in hospital-associated infections, and HIV/AIDS associated diarrhea. We have recently found that Serratia spp. may be found more commonly in the stools of patients with diarrhea than in asymptomatic control children. We therefore investigated the possible enteric pathogenicity of S. marcescens in vitro employing a polarized human colonic epithelial cell (T84) monolayer. Infected monolayers were assayed for bacterial invasion, transepithelial electrical resistance (TEER), cytotoxicity, interleukin-8 (IL-8) release and morphological changes by scanning electron microscopy. We observed significantly greater epithelial cell invasion by S. marcescens compared to Escherichia coli strain HS (p = 0.0038 respectively). Cell invasion was accompanied by reduction in TEER and secretion of IL-8. Lactate dehydrogenase (LDH) extracellular concentration rapidly increased within a few hours of exposure of the monolayer to S. marcescens. Scanning electron microscopy of S. marcescens-infected monolayers demonstrated destruction of microvilli and vacuolization. Our results suggest that S. marcescens interacts with intestinal epithelial cells in culture and induces dramatic alterations similar to those produced by known enteric pathogens.
Keywords: adhesion, Chemokine, cytotoxicity, invasion, pathogenicity, polarized monolayer, T84 cells, Serratia marcescens
Abbreviations
- CFU
colony forming units
- ELISA
enzyme linked immunosorbent assay
- IL-8
interleukin-8
- LDH
lactate dehydrogenase
- TEER
transepithelial electric resistance
- TNFα
Tumor necrotic factor-α
Introduction
Diarrhea causes substantial morbidity and mortality in children in low-income countries.1,2 Although numerous pathogens can cause diarrhea, many episodes do not have a known etiology. Epidemiological research in enteric diseases have shown that a majority of cases of moderate to severe diarrhea among children under the age of 5 y and among hospitalized patient could not be attributed to a specific known pathogen.1,3 Therefore, identification of additional potential enteric pathogens is an important global health priority. In addition, many cases of necrotizing enterocolitis and antibiotic associated diarrhea are without identifiable enteric pathogens. Identification of potentially pathogenic species within the intestinal microbiota is therefore important.
Serratia marcescens is a common enteric bacterium generally thought not to be pathogenic in the gastrointestinal tract.4 However, the organism is capable of causing nosocomial infections in other body systems, most notably respiratory and urinary tract infection, meningitis, bacteremia and different types of wound infection.5-7 Serratia species cause outbreaks in neonatal intensive care units,8,9 including necrotizing enterocolitis, and have been associated with significant morbidity and mortality in children.10 However, recent studies showed that 65% of all Serratia infections were community-based, with S. marcescens being the most commonly isolated species, accounting for 92% of all isolated Serratia.11
Taxonomically, the genus Serratia is classified as a member of the family Enterobacteriaceace, and currently consists of 14 recognized species with 2 identified subspecies.12 S. marcescens is the most commonly isolated Serratia species in human infections.7 It is a widely distributed saprophytic bacterium and causes disease in plants and in a wide range of both invertebrate and vertebrate hosts.7,12 Based on biochemical characteristics, 6 biogroups consisting of 19 biotypes of S. marcescens namely A1 (A1a, A1b); A2/6 (A2a, A2b, A6a, A6b); A3 (A3a, A3b, A3c, A3d); A4 (A4a, A4b); A5/8 (A5, A8a, A8b, A8c); and TCT (TT, TC) have been recognized.13,14 The biogroups consist of red pigmented (A1 and A1/6) and nonpigmented (A3, A4, A5/8 and TCT) serotypes. Infection has been acquired through ingestion of contaminated food, contaminated hospital equipment, or the hands of medical staff.15 S. marcescens can infect numerous sites including urinary,16 respiratory,17 epithelia, muscle and subcutaneous tissues.18 Although non-pigmented biogroups are the most common cause of nosocomial infections, the red pigmented biogroups also cause significant common source outbreaks and cross-infections.15
Like other enteric bacterial pathogens, S. marcescens is capable of producing well known virulence factors such as fimbriae, the RssAB-FlhDC-ShlBA pathway, quorum sensing systems and various secreted enzymes.12,19 The organism has been associated with a potent cytotoxin, ShlA, which in concert with the ShlB protein causes contact-dependent cytotoxicity in eukaryotic cells.20 An extracellular hemolysin, PhlA, with phospholipase activity has also been characterized.21 The PhlA acts upon phospholipids and produces lysophospholipid, which lyses human, horse and sheep red blood cells and HeLa cells.21 A type VI secretion system has also been described in Serratia species,22 although its contribution to virulence is unknown.
The gastrointestinal epithelium deploys multiple innate defense mechanisms against microbial intruders,23 including epithelial integrity and innate immune responses. In addition, human colonic epithelial cells in-vivo and in-vitro can express and release specific cytokines such as IL-8, monocyte chemotactic protein-1 and TNFα in response to infection with invasive strains of bacteria.24 IL-8 has been shown to be a key chemokine in inflammation and bacterial translocation.25 Although pathogens frequently induce or evade these defenses, the effects of commensal bacteria are largely unknown.
In this work, we evaluated S. marcescens in a number of in-vitro assays commonly associated with the behavior of proven enteric pathogens to ascertain pathogenic potential of the bacteria. The effects of S. marcescens infection of polarized T84 monolayers was compared with that of known invasive wild type Shigella flexneri 2a, non-pathogenic Escherichia coli HS and a laboratory strain of Klebsiella oxytoca. Our results demonstrate that S. marcescens commonly considered harmless in the gastrointestinal tract, elicits dramatic changes including inflammation, cytotoxicity, adherence, and invasion.
Results
Adhesion and internalization of S. marcescens in T84 cells
To test whether S. marcescens possesses the ability to adhere to or invade intestinal epithelial cells, we performed an adhesion assay and gentamicin protection assay as previously described26 with S. flexneri, E. coli HS, S. marcescens (ATCC® 274TM) and K. oxytoca. All the bacterial strains tested yielded some recovery of adherent bacteria from the T84 monolayer. S. marcescens exhibited higher recovery than S. flexneri or E. coli HS (Fig. 1A), however, the difference among the strains in adherence to T84 cells was not statistically significant (p = 0.096 and p = 0.726, respectively). We observed recovery of S. marcescens from intestinal epithelial cells after treatment with gentamicin (Fig. 1B); recovery was significantly higher compared to that observed for S. flexneri or E. coli HS (P < 0 .05 and P < 0.005, respectively). Negligible numbers of K. oxytoca were recovered in this assay.
Figure 1.
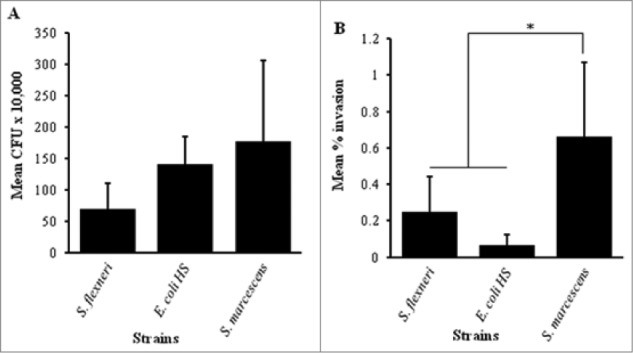
Adhesion and invasion of T84 cell monolayers. T84 cells were seeded in collagen coated 3.0 μm transwell plates and maintained in DMEM-F12 cell culture medium for 5 – 10 d The cells were infected with invasive strain of S. flexneri, Commensal E. coli HS, S. marcescens, or K. oxytoca for 3 hr. Both adhesion (A) and invasion (B) were determined by plating and quantifying CFU as described under materials and methods. The results are compared between the strains. Error bars represent the standard deviation calculated from the means of colony counts estimated from 3 independent experiments done in triplicate. Adhesion to T84 cells (A) was not significantly different when compared among the strains. The recovery of S. marcescens from the invasion assay (B) was significantly higher compared to S. flexneri or E. coli HS (*, P < 0 .05 by ANOVA with post hoc correction).
S. marcescens disrupts transepithelial electric resistance (TEER) of a T84 cell monolayer
The transepithelial electric resistance (TEER) of epithelial cell monolayers has been shown to diminish upon infection with enteric pathogens.27 We assessed the TEER of polarized T84 monolayers before and after addition of Serratia; experiments comprised 3 hours of infection followed by 1 hr post-infection recovery period. The mean percent change in TEER in T84 monolayers infected with S. marcescens dropped by 63% (Fig. 2A) from the initial value (P < 0 .0001), and was significantly lower than monolayers infected with E. coli HS or K. oxytoca, or compared with uninfected monolayers (P < 0 .0001). To characterize the effects of different biotypes of S. marcescens on intestinal barrier function, we measured the TEER of polarized T84 intestinal epithelial cell monolayers infected with different strains of S. marcescens compared with the positive control, enteroaggregative E. coli (EAEC) strain wt 042.28,29 We observed marked reduction in TEER by all the Serratia strains tested (Fig. 2B). The change was statistically significant compared with uninfected cells (P < 0 .0001).
Figure 2.
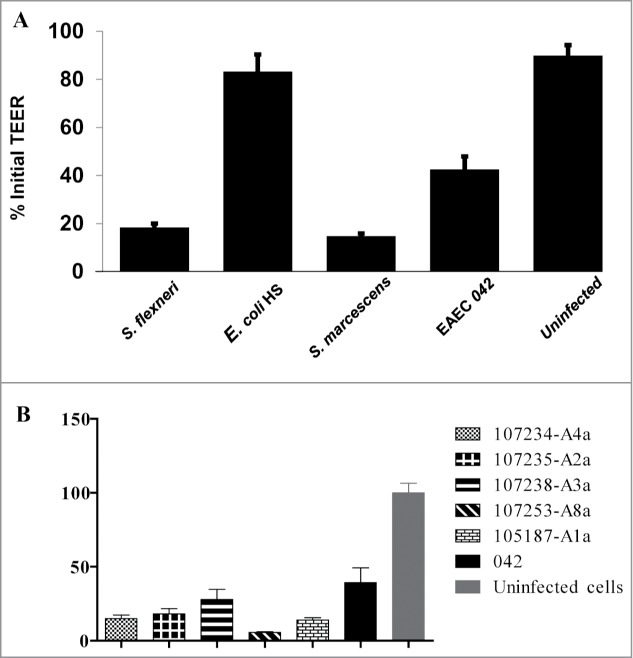
A.Comparison of the ability of S. marcescens and other enteric bacteria to reduce TEER in polarized T84 cell monolayers. T84 cells were seeded in collagen coated 3.0 μm transwell plates and maintained in DMEM-F12 cell culture medium for 5 – 10 d The cells were infected with invasive S. flexneri, commensal E. coli HS, S. marcescens, or EAEC strain 042 for 3 hour, washed and followed for an additional 18 hr as described in materials and methods. TEER was measured before infection and after infection using Evom-2 Vohmmeter. Uninfected cells were used as negative control. Data are means percent change ± standard deviation of the means from triplicate readings of 3 independent experiments. S. marcescens and S. flexneri were significantly different at P < 0 .0001 from negative controls. 2B: Comparison of the ability of different strains S. marcescens to reduce TEER in polarized T84 cell monolayers. T84 cells were seeded in collagen coated 3.0 μm transwell plates and infected as described under Figure 2A with different strains including E. coli 042. Data are means percent change ± standard deviation of the means from triplicate readings of 3 independent experiments. *** All S. marcescens were significantly different at P < 0 .0001 from uninfected cells.
S. marcescens infection induces elevated IL-8 secretion from T84 cells
Intestinal epithelial cells are known to express pro-inflammatory cytokines including IL-8, monocyte chemotactic protein-1 and TNFα upon interaction with many enteric pathogens.24 In addition IL-8 has been described as a major cytokine produced by intestinal epithelial cells when invaded by bacteria.25 We therefore examined IL-8 secretion by epithelial cells following S. marcescens exposure, compared with 3 other intestinal bacteria. Basolateral supernatants of T84 cell monolayers were collected after 3 h of exposure to each bacterial species and examined for presence of IL-8. There was significantly higher secretion of IL-8 (mean 426.6 pg/ml) in T84 intestinal epithelial monolayers infected with S. marcescens (Fig. 3A) compared to each of the 3 other enteric bacteria tested and to the uninfected cell monolayers (P < 0 .0001). We examined IL-8 secretion by epithelial cells following exposure to various strains of S. marcescens, and compared with E. coli HS and uninfected cells (Fig. 3B). All strains induced release of IL-8 greater than negative controls.
Figure 3.
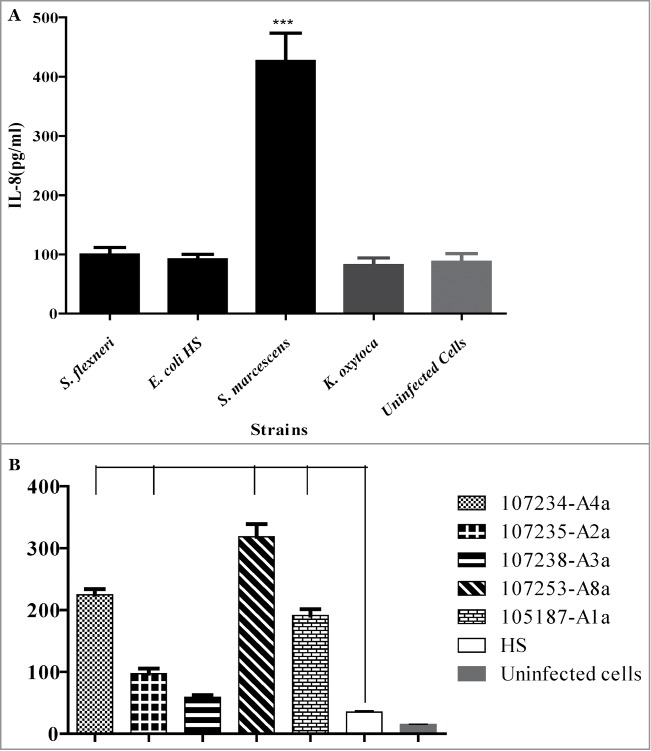
A.IL-8 release by T84 cells upon infection with intestinal bacteria. T84 cells were infected with invasive S. flexneri, Commensal E. coli HS, S. marcescens, or K. oxytoca for 3 hour and basolateral supernatants collected for assay of pro-inflammatory cytokine IL-8 by ELISA as described under materials and methods. Uninfected cells were used as negative control. Data are means ± standard deviation of the means from triplicate readings of 3 independent experiments. ***S. marcescens was significantly different at P < 0 .0001 from each of the other bacteria tested. 3B: IL-8 release by T84 cells upon infection with different strains of S. marcescens. T84 cells were infected with different strains of S. marcescens and IL-8 released from the cells determined as described under Figure 3A. Data are means ± standard deviation of the means from triplicate readings of 3 independent experiments. ***, (P < 0 .0001) and **, (P < 0 .001).
S. marcescens induces release of LDH
Polarized T84 cell monolayers were infected with S. marcescens, S. flexneri, E. coli HS or K. oxytoca individually; basolateral supernatants were collected after 3 and 6 hrs post-infection, and LDH was measured by immunoassay (Fig. 4). We found that LDH release from cells infected with S. marcescens was more than 3 times greater than that observed for each of the other comparisons (P < 0 .0001). We also found that supernatants from cells infected with S. marcescens after 6 hrs of infection appeared to be 10-fold greater than the concentrations measured at 3 hrs (P < 0 .001), and more than 60 times greater than that induced by the other 3 enteric bacteria tested (P < 0 .0001).
Figure 4.
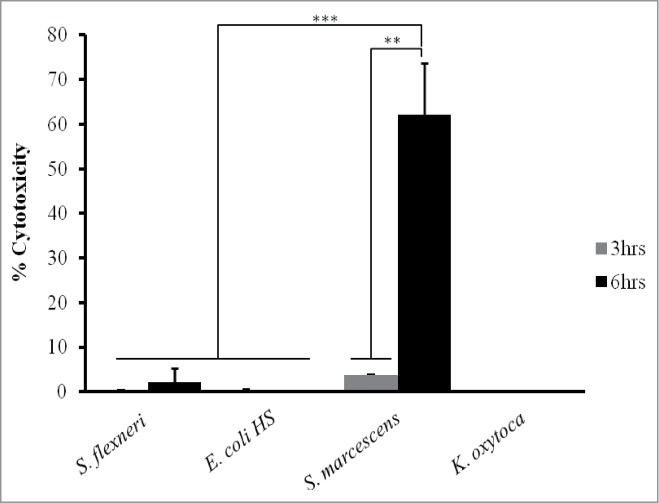
Cytotoxicity as determined by LDH release over time in T84 cells infected with S. marcescens and other enteric bacteria. T84 cells were infected with invasive strain of S. flexneri, Commensal E. coli HS, S. marcescens, or K. oxytoca for 3 hr (gray bars) and 6 hrs (black bars). Thereafter, basolateral supernatants were collected at each time point for LDH testing by ELISA as described under materials and methods. LDH released from cells infected with S. marcescens was more than 3 times greater than that observed for each of the other comparisons (***, P<0 .0001) and tenfold greater than the concentration measured at 3 hrs (**, P<0 .001). Data are means ± standard deviation of the means from triplicate readings of 3 independent experiments.
Effect of S. marcescens infection on morphology of T84 cells
To investigate the effect of S. marcescens infection on intestinal epithelial cell morphology, we infected non-polarized T84 cell monolayers with bacteria at a multiplicity of infection of 100 for 3 hrs and examined the monolayers under scanning electron microscopy. A distinctive change in cellular morphology was observed in cells infected with S. marcescens compared with cells infected with S. flexneri or E. coli HS. Cell monolayers infected with S. marcescens manifested close adherence of bacteria and loss of microvilli. We observed a distinct “halo” phenomenon around adhering bacteria (white arrow in Fig. 5C), which was not observed with either of the other bacteria.
Figure 5.
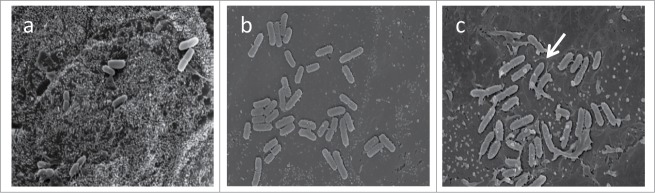
Effect of S. marcescens on T84 cell monolayer after 3 hours of infection. T84 cells were infected with bacteria for 3 hour and fixed with paraformaldehide solution overnight at 4°C as described under materials and methods. The cells were prepared and examined using JSM-6400 scanning microscope. (A) T84 monolayer infected with E. coli HS; (B) monolayer infected with S. flexneri strain 2457T; (C) monolayer infected with S. marcescens. White arrow indicates typical “halo” surrounding S. serratia bacteria.
Discussion
Using human colon-derived polarized epithelial (T84) cells we have demonstrated that S. marcescens has the potential for adhesion, invasion, cytotoxicity, perturbation of intestinal barrier function, cytokine release, and alteration of cellular morphology. Previous studies have shown that S. marcescens is capable of adhering to bladder epithelial cells, and have identified a secreted pore-forming cytolysin ShlA as an important factor mediating internalization of S marcescens and lysis of epithelial cells32,33; the ability of S marcescens to adhere to and invade intestinal epithelial cells has not been reported to our knowledge. Tight adhesion increases the proximity of the bacteria with the cell, possibly bringing the cell bound cytotoxin in close contact with the cell, thereby enhancing host-cell invasion. This is supported by published results demonstrating that adherent but non-cytolytic mutant strain of S. marcescens showed no cell invasion.33 In this study, S. marcescens exhibited more abundant adherence and significantly greater invasiveness for the epithelial cells than nonpathogenic E. coli HS controls.
The integrity of the epithelial monolayer is sustained by tight cell-cell junctions, and many bacterial pathogens target tight junctions by perturbing this structure.34 For some pathogens, previous studies have demonstrated that reduction in TEER is correlated with significant decrease in tight junction protein expression and increased permeability, thus allowing translocation of virus and bacteria across the mucosa.35 Similarly, apical application of bacteria has been shown to induce opening of the paracellular pathway and transmigration of polymorphonuclear leucocytes, which in turn facilitate pathogen invasion.36 We report that infection with S. marcescens drastically decreased TEER of T84 cell monolayers, although the mechanism of this effect is unknown.
Intestinal epithelial cells act as sentinels in intestinal infection,38 mounting early innate immune responses against foreign substances via pro-inflammatory cytokine production. Recent studies suggest that IL-8 produced by the intestinal epithelial cells plays a central role in the initial control of infection by recruiting polymorphonuclear leukocytes, and transmigration of these cells to the epithelial lining. The polymorphonuclear leukocytes contribute to bacterial killing, often at the expense of tissue destruction.25 Thus IL-8 appears to be an essential chemokine in Shigella transepithelial translocation. We therefore investigated the ability of S. marcescens to induce IL-8 responses in intestinal epithelial cell monolayers. Whereas all the enteric bacteria tested induced some secretion of IL-8 from T84 cells (at levels less than 100 pg/ml), S. marcescens induced more than 3 times the levels of IL-8 secretion. S. marcescens strains A4a, A8a, A1a and A2a induced more pronounced IL-8 secretion suggesting high potential of causing inflammation in the intestinal tract compared to S. marcescens strains A3a and E. coli HS. Thus, our results are in concurrence with previous epidemiologic findings which attributed most frequently occurring nosocomial S. marcescens infection to biogroups A4, A5/8, and A2/6,7,13 with A2b isolated from infants (mostly from feces) in a neonatal ward.7
S. marcescens has been reported to have a cytotoxin/cytolysin and a type VI secretion system that exports a non-diffusable cytolysin.32 These effects have not been demonstrated on intestinal cells, which represent the typical habitat of Serratia in Homo sapiens. S. marcescens produced a rapid rise in LDH levels from the infected cells within 3 hr of infection, and levels were elevated tenfold at 6 hr of infection, suggesting rapid destruction of the epithelial cells. The cytotoxic activity may be attributable to the secreted hemolysin/cytotoxin ShlA previously reported.32 The cytolytic effect may contribute to the observed invasiveness and pro-inflammatory ability in our system. Of note, we also observed detachment of epithelial cell monolayers and rounding of cells (data not shown). Observations of infected monolayer by using SEM confirmed these data. The cytolytic effect apparently included disruption of the microvilli, shedding of the microvilli and vacuole formation on the epithelial cells. This is consistent with previous studies which demonstrated pore-formation, extended vacuolation and cell lysis induced by secreted cytolysin ShlA on bladder epithelial cells.33 A similar cytolysin excreted by Haemophilus ducreyi was shown to enhance invasion of human epithelial cells and contributes to evasion of immune responses.38 Thus, the disruption of the microvillus layer, vacuolation and possibly lysis of the host-cell elicited by S. marcescens in this study may enable the bacteria to penetrate the tissue layer. Similarly, previous studies demonstrated that clinical isolates of K. oxytoca induce antibiotic-associated hemorrhagic colitis and a high proportion of stool isolates tested were cytotoxin positive.39,40 The cytotoxin caused rounding, fragmentation and detachment of HEp-2 cells from the substratum which may precede cell death. In the current study, the K. oxytoca strain used exhibited no cytotoxicity suggesting that the cytotoxin production might be strain specific.
In conclusion, the current study provides evidence that S. marcescens is a potential enteric pathogen. Epidemiologic studies should consider possible association between this organism and diarrhea on non-diarrheal pathogenic states, and in other pathogenic states such as necrotizing enterocolitis.
Materials and Methods
Bacterial strains and growth conditions
The bacterial strains used in this study are summarized in Table 1. Bacteria were routinely cultured on Luria-Bertani (LB) agar (American Bioanalytical) except S. flexneri which was grown on LB agar with Congo red; all were incubated at 37°C aerobically for 18–24 hrs. A single colony of the bacteria was picked and inoculated in 10 ml of LB broth (American Bioanalytical) and incubated as above in a shaking incubator. Ten microliters of a stationary phase culture was used to inoculate 10 ml of LB broth, and the bacteria grown in a shaking incubator for approximately 3 hours at 37°C aerobically to mid-exponential phase and used to infect the T84 cells.
Table 1.
Bacterial strains used in the study
| Strain | Species | Serotype | Biotype | Red pigment | References |
|---|---|---|---|---|---|
| ATCC 274 | Serratia marcescens | O:6 | A2a | yes | American Type Culture Collection, 45,46 |
| 107234 | Serratia marcescens | O5:H1 | A4a | yes | Pasteur Institute |
| 107235 | Serratia marcescens | O6:H3 | A2a | no | Pasteur Institute |
| 107238 | Serratia marcescens | O9:H11 | A3a | no | Pasteur Institute |
| 107253 | Serratia marcescens | O25:H12 | A8a | no | Pasteur Institute |
| 105187 | Serratia marcescens | O28:H2 | A1a | yes | Pasteur Institute |
| 2457T | Shigella flexneri | 2a | NA | NA | 44 |
| ? | Klesiella oxytoca | ? | NA | NA | |
| 042 | Escherichia coli | O44:H18 | NA | NA | 47 |
| HS | Escherichia coli | O9 | NA | NA | 48 |
Growth and maintenance of cell culture
Human colonic epithelial (T84) cells (ATCC CCL-248) were seeded into 75 cm2 tissue culture flasks and routinely maintained as described previously41 with slight modification. Briefly, the T84 cells were maintained in Dulbecco's Modified Eagle Medium-F-12 medium (DMEM/F12) (1:1 mix; Sigma-Aldrich) supplemented with 10% fetal bovine serum (Sigma-Aldrich), 1% L-Glutamine (Sigma-Aldrich), 50 U/mL penicillin and 50 mg/mL streptomycin (Sigma-Aldrich) at 37°C in 5% CO2 atmosphere. Fresh medium was replenished every 2 d When the monolayer reached 80–90% confluent, the cells were detached from the bottom of the flask using 0.25% trypsin-EDTA (GIBCO, Life Technologies) and split 1:10 into new flasks. The T84 cells between passages 4 and 15 were seeded at a density of 3 × 105 cells/mL onto collagen-coated, 12-mm polycarbonate Costar tissue culture Transwell permeable support inserts filters with 3.0 μm pore size (Corning Inc.) and grown for 5 to 10 days, during which time fresh medium in both chambers was replenished as indicated above.
Measurement of TEER
The TEER was used to monitor the integrity of the epithelial monolayer as previously described30,42 using an epithelial ohmeter resistance reader (World Precision Instruments). Monolayers were considered polarized and used for experiment when resistance was equal to or greater than 1,300 Ω/cm2. TEER was also measured before infection (time zero) and 1 hr after recovery (time t) following the antibiotic protection assay (see below for full description). TEER was then expressed as the ratio of TEER at time t in relation to the initial value (time zero) for each experiment. Statistical analysis was performed to compare the means percent change in experimental and medium control groups.
Bacterial adhesion and invasion assays
Infection of T84 cells was performed as previously described26 with modification. One day before infection, polarized T84 cells were incubated with DMEM/F12 without antibiotic. Overnight LB broth cultures of bacteria were standardized in DMEM/F12 without antibiotic to an optical density of 600 nm (OD600) of 0.60 ± 0.02, which is equal to approximately 1 × 109 CFU/mL. Bacterial samples at multiplicity of infection of 100 in 200 μL of the medium without antibiotic were administered on the apical surface of each T84 cell monolayers grown on transwell inserts. Infection experiments in duplicate were incubated for 3 hrs at 37°C in 5% CO2, at the end of which time the bacteria were aspirated. Supernatants were collected from the basolateral side of the wells and stored at 4°C and −20°C for LDH cytotoxicity and IL-8 cytokines assays, respectively. Thereafter, the cells were washed 3 times with PBS. One set of the cells was lysed with 500 μL of 1% triton X-100 and the lysates diluted 1:1000 in sterile PBS and plated on LB agar medium for CFU count to determine the bacterial association with the cells.26,42 For gentamicin protection assays,31 fresh DMEM-F-12 with 100 μg/ml gentamicin (Sigma-Aldrich) or 500 μg/ml amikacin (Sigma-Aldrich), chosen according to the susceptibility of the test bacteria, was added to the apical and basolateral chambers of the second set of cells and incubated as above for 1 hr to kill extracellular bacteria. After extensive washing (3 times) to remove the antibiotic, the intracellular bacteria were released by lysis with 1% Triton X-100 as described above. The cell lysates were diluted 1:10 in sterile PBS and plated on LB agar medium for CFU count to determine invasion.43 Data were expressed as CFU/well for each monolayer. Adhesion was defined as the difference between the number of associating bacterial CFU and the number of internalized bacterial CFU as previously described,41 while the percent invasion was defined as the number of internalized bacterial CFU (invasion) divided by the associating bacterial CFU (bacterial association) expressed as percentage. The results for each experiment were presented as means of an assay performed in triplicate and independently repeated 3 times.
Interleukin-8 enzyme-linked immonosorbent assay
Supernatants from each infection were collected and IL-8 protein levels were determined by enzyme linked immunosorbent assay (ELISA) using human IL-8 ELISA kit (Invitogen) according to the manufacturer's instructions. Supernatants from uninfected cells were used as negative controls and each sample assessed in duplicate.
Lactate dehydrogenase assay
The T84 monolayers were infected for various time periods, and medium was aspirated. The medium was then centrifuged to remove viable bacteria. Cytotoxicity was tested using lactate dehydrogenase (LDH) kit (BioVision) according to manufacturer's instructions.
Scanning electron microscopy
Bacterial strains at multiplicity of infection of 100 were cultured for 3 hrs with T84 cell monolayers grown on 12-mm glass coverslips. Culture medium was removed, and cells fixed in 0.1 M cacodylate buffer (pH 7.4) containing 2.5% paraformaldehyde and 2% glutaraldehyde, rinsed, postfixed in 1% osmium in 0.1 M cacodylate buffer, and then dehydrated in a graded series of ethanol mixtures and treated with hexamethyldisilane. After drying, cover slips were coated with gold and examined using a JEOL JSM-6400 scanning electron microscope (JOEL Ltd).
Data analysis
Results were expressed as means and standard deviation (±SD) of individual experiments performed in triplicate (n) times. Comparisons between mean values were performed by one-way analysis of variance (ANOVA) using SAS computer software (Version 9.1, SAS Institute Inc.), and P-values less than 0.05 were considered statistically significant.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to acknowledge the University of Virginia, Departments of Pediatrics and all the staff in Dr. Nataro's research laboratory for their support and ideas when performing the experiments; Dr. Stacey Guillot and staff at the advanced microscopy facility for imaging services and Dr. James B. Kaper for laboratory support.
Funding
This study was supported by a grant from the Bill and Melinda Gates Foundation.
References
- 1. Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209-22; PMID:23680352; http://dx.doi.org/ 10.1016/S0140-6736(13)60844-2 [DOI] [PubMed] [Google Scholar]
- 2. Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 1999; 77:651-66; PMID:10516787 [PMC free article] [PubMed] [Google Scholar]
- 3. Nair GB, Ramamurthy T, Bhattacharya MK, Krishnan T, Ganguly S, Saha DR, Rajendran K, Manna B, Ghosh M, Okamoto K, et al. Emerging trends in the etiology of enteric pathogens as evidenced from an active surveillance of hospitalized diarrhoeal patients in Kolkata, India. Gut Pathog 2010; 2:4; PMID:20525383; http://dx.doi.org/ 10.1186/1757-4749-2-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Almuneef MA, Baltimore RS, Farrel PA, Reagan-Cirincione P, Dembry LM. Molecular typing demonstrating transmission of gram-negative rods in a neonatal intensive care unit in the absence of a recognized epidemic. Clin Infect Dis 2001; 32:220-7; PMID:11170911; http://dx.doi.org/ 10.1086/318477 [DOI] [PubMed] [Google Scholar]
- 5. Buffet-Bataillon S, Rabier V, Betremieux P, Beuchee A, Bauer M, Pladys P, Le Gall E, Cormier M, Jolivet-Gougeon A. Outbreak of Serratia marcescens in a neonatal intensive care unit: contaminated unmedicated liquid soap and risk factors. J Hosp Infect 2009; 72:17-22; PMID:19246120; http://dx.doi.org/ 10.1016/j.jhin.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 6. Friedman ND, Kotsanas D, Brett J, Billah B, Korman TM. Investigation of an outbreak of Serratia marcescens in a neonatal unit via a case-control study and molecular typing. Am J Infect Control 2008; 36:22-8; PMID:18241732; http://dx.doi.org/ 10.1016/j.ajic.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 7. Grimont F. and Grimont PAD. The Genus Serratia. In The Prokaryotes. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, eds. Volume 6. 2006: pp. 219-244. ISBN: 978-0-387-25496-8. [Google Scholar]
- 8. Adamson V, Mitt P, Pisarev H, Metsvaht T, Telling K, Naaber P, Maimets M. Prolonged outbreak of Serratia marcescens in Tartu University Hospital: a case-control study. BMC Infect Dis 2012; 12:S; PMID:NOT_FOUND; http://dx.doi.org/ 10.1186/1471-2334-12-281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fleisch F, Zimmermann-Baer U, Zbinden R, Bischoff G, Arlettaz R, Waldvogel K, Nadal D, Ruef C. Three consecutive outbreaks of Serratia marcescens in a neonatal intensive care unit. Clin Infect Dis 2002; 34:767-73; PMID:11830800; http://dx.doi.org/ 10.1086/339046 [DOI] [PubMed] [Google Scholar]
- 10. Berthelot P, Grattard F, Amerger C, Frery MC, Lucht F, Pozzetto B, Fargier P. Investigation of a nosocomial outbreak due to Serratia marcescens in a maternity hospital. Infect Control Hosp Epidemiol 1999; 20:233-6; PMID:10219872; http://dx.doi.org/ 10.1086/501617 [DOI] [PubMed] [Google Scholar]
- 11. Laupland KB, Parkins MD, Gregson DB, Church DL, Ross T, Pitout JD. Population-based laboratory surveillance for Serratia species isolates in a large Canadian health region. Eur J Clin Microbiol Infect Dis 2008; 27:89-95; PMID:17960436; http://dx.doi.org/ 10.1007/s10096-007-0400-7 [DOI] [PubMed] [Google Scholar]
- 12. Mahlen SD. Serratia infections: from military experiments to current practice. Clin Microbiol Rev 2011; 24:755-91; PMID:21976608; http://dx.doi.org/ 10.1128/CMR.00017-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grimont PA, Grimont F. Biotyping of Serratia marcescens and its use in epidemiological studies. J Clin Microbiol 1978; 8:73-83; PMID:353073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grimont PA, Grimont F, Le Minor S, Davis B, Pigache F. Compatible results obtained from biotyping and serotyping in Serratia marcescens. J Clin Microbiol 1979; 10:425-32; PMID:393712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farmer JJ, 3rd, Davis BR, Hickman FW, Presley DB, Bodey GP, Negut M, Bobo RA. Detection of Serratia outbreaks in hospital. Lancet 1976; 2:455-9; PMID:73753; http://dx.doi.org/ 10.1016/S0140-6736(76)92539-3 [DOI] [PubMed] [Google Scholar]
- 16. Marre R, Hacker J, Braun V. The cell-bound hemolysin of Serratia marcescens contributes to uropathogenicity. Microb Pathog 1989; 7:153-6; PMID:2687613; http://dx.doi.org/ 10.1016/0882-4010(89)90034-X [DOI] [PubMed] [Google Scholar]
- 17. Albers MJ, Mouton JW, Tibboel D. Colonization and infection by Serratia species in a paediatric surgical intensive care unit. J Hosp Infect 2001; 48:7-12; PMID:11358465; http://dx.doi.org/ 10.1053/jhin.2001.0939 [DOI] [PubMed] [Google Scholar]
- 18. Egebo K, Toft P, Jakobsen CJ. Contamination of central venous catheters. The skin insertion wound is a major source of contamination. J Hosp Infect 1996; 32:99-104; PMID:8666769; http://dx.doi.org/ 10.1016/S0195-6701(96)90051-1 [DOI] [PubMed] [Google Scholar]
- 19. Hejazi A, Falkiner FR. Serratia marcescens. J Med Microbiol 1997; 46:903-12; PMID:9368530; http://dx.doi.org/ 10.1099/00222615-46-11-903 [DOI] [PubMed] [Google Scholar]
- 20. Kurz CL, Chauvet S, Andres E, Aurouze M, Vallet I, Michel GP, Uh M, Celli J, Filloux A, De Bentzmann S, Steinmetz I, Hoffmann JA, Finlay BB, Gorvel JP, Ferrandon D, Ewbank JJ. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. Embo J 2003; 22:1451-60; PMID:12660152; http://dx.doi.org/ 10.1093/emboj/cdg159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shimuta K, Ohnishi M, Iyoda S, Gotoh N, Koizumi N, Watanabe H. The hemolytic and cytolytic activities of Serratia marcescens phospholipase A (PhlA) depend on lysophospholipid production by PhlA. BMC Microbiol 2009; 9:261; PMID:20003541; http://dx.doi.org/ 10.1186/1471-2180-9-261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murdoch SL, Trunk K, English G, Fritsch MJ, Pourkarimi E, Coulthurst SJ. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol 2011; 193:6057-69; PMID:21890705; http://dx.doi.org/ 10.1128/JB.05671-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim M, Ashida H, Ogawa M, Yoshikawa Y, Mimuro H, Sasakawa C. Bacterial interactions with the host epithelium. Cell Host Microbe 2010; 8:20-35; PMID:20638639; http://dx.doi.org/ 10.1016/j.chom.2010.06.006 [DOI] [PubMed] [Google Scholar]
- 24. Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Invest 1995; 95:55-65; PMID:7814646; http://dx.doi.org/ 10.1172/JCI117676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sansonetti PJ, Arondel J, Huerre M, Harada A, Matsushima K. Interleukin-8 controls bacterial transepithelial translocation at the cost of epithelial destruction in experimental shigellosis. Infect Immun 1999; 67:1471-80; PMID:10024597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCormick BA, Colgan SP, Delp-Archer C, Miller SI, Madara JL. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol 1993; 123:895-907; PMID:8227148; http://dx.doi.org/ 10.1083/jcb.123.4.895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun 2010; 78:4958-64; PMID:20823198; http://dx.doi.org/ 10.1128/IAI.00580-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nataro JP, Hicks S, Phillips AD, Vial PA, Sears CL. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun 1996; 64:4761-8; PMID:8890237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nataro JP, Steiner T, Guerrant RL. Enteroaggregative Escherichia coli. Emerg Infect Dis 1998; 4:251-61; PMID:9621195; http://dx.doi.org/ 10.3201/eid0402.980212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Edwards LA, Bajaj-Elliott M, Klein NJ, Murch SH, Phillips AD. Bacterial-epithelial contact is a key determinant of host innate immune responses to enteropathogenic and enteroaggregative Escherichia coli. PLoS One 2011; 6:e27030; PMID:22046438; http://dx.doi.org/ 10.1371/journal.pone.0027030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elsinghorst EA. Measurement of invasion by gentamicin resistance. Methods Enzymol 1994; 236:405-20; PMID:7968625; http://dx.doi.org/ 10.1016/0076-6879(94)36030-8 [DOI] [PubMed] [Google Scholar]
- 32. Hertle R, Hilger M, Weingardt-Kocher S, Walev I. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect Immun 1999; 67:817-25; PMID:9916096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hertle R, Schwarz H. Serratia marcescens internalization and replication in human bladder epithelial cells. BMC Infect Dis 2004; 4:16; PMID:15189566; http://dx.doi.org/ 10.1186/1471-2334-4-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guttman JA, Finlay BB. Tight junctions as targets of infectious agents. Biochim Biophys Acta 2009; 1788:832-41; PMID:19059200; http://dx.doi.org/ 10.1016/j.bbamem.2008.10.028 [DOI] [PubMed] [Google Scholar]
- 35. Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 2010; 6:e1000852; PMID:20386714; http://dx.doi.org/ 10.1371/journal.ppat.1000852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perdomo JJ, Gounon P, Sansonetti PJ. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J Clin Invest 1994; 93:633-43; PMID:7906696; http://dx.doi.org/ 10.1172/JCI117015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Waterhouse CC, Joseph RR, Winsor GL, Lacombe TA, Stadnyk AW. Monocyte chemoattractant protein-1 production by intestinal epithelial cells in vitro: a role for p38 in epithelial chemokine expression. J Interferon Cytokine Res 2001; 21:223-30; PMID:11359653; http://dx.doi.org/ 10.1089/107999001750169853 [DOI] [PubMed] [Google Scholar]
- 38. Wood GE, Dutro SM, Totten PA. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect Immun 1999; 67:3740-9; PMID:10417132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoffmann KM, Deutschmann A, Weitzer C, Joainig M, Zechner E, Hogenauer C, Hauer AC. Antibiotic-associated hemorrhagic colitis caused by cytotoxin-producing Klebsiella oxytoca. Pediatrics 2010; 125:e960-3; PMID:20194278; http://dx.doi.org/ 10.1542/peds.2009-1751 [DOI] [PubMed] [Google Scholar]
- 40. Joainig MM, Gorkiewicz G, Leitner E, Weberhofer P, Zollner-Schwetz I, Lippe I, Feierl G, Krause R, Hinterleitner T, Zechner EL, Hogenauer C. Cytotoxic effects of Klebsiella oxytoca strains isolated from patients with antibiotic-associated hemorrhagic colitis or other diseases caused by infections and from healthy subjects. J Clin Microbiol 2010; 48:817-24; PMID:20053860; http://dx.doi.org/ 10.1128/JCM.01741-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCormick BA, Miller SI, Carnes D, Madara JL. Transepithelial signaling to neutrophils by salmonellae: a novel virulence mechanism for gastroenteritis. Infect Immun 1995; 63:2302-9; PMID:7768613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sakaguchi T, Kohler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 2002; 4:367-81; PMID:12067320; http://dx.doi.org/ 10.1046/j.1462-5822.2002.00197.x [DOI] [PubMed] [Google Scholar]
- 43. McCormick BA, Siber AM, Maurelli AT. Requirement of the Shigella flexneri virulence plasmid in the ability to induce trafficking of neutrophils across polarized monolayers of the intestinal epithelium. Infect Immun 1998; 66:4237-43; PMID:9712773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hume EB, Willcox MD, Sweeney DF, Holden BA. An examination of the clonal variants of Serratia marcescens that infect the eye during contact lens wear. J Med Microbiol 1996; 45:127-32; PMID:8683548; http://dx.doi.org/ 10.1099/00222615-45-2-127 [DOI] [PubMed] [Google Scholar]
- 45. Rius N, Sole M, Francia A, Loren JG. Buffering capacity of pigmented and nonpigmented strains of serratia marcescens. Appl Environ Microbiol 1994; 60:2152-4; PMID:16349300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nataro JP, Baldini MM, Kaper JB, Black RE, Bravo N, Levine MM. Detection of an adherence factor of enteropathogenic Escherichia coli with a DNA probe. J Infect Dis 1985; 152:560-5; PMID:2863319; http://dx.doi.org/ 10.1093/infdis/152.3.560 [DOI] [PubMed] [Google Scholar]
- 47. Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1978; 1:1119-22; PMID:77415; http://dx.doi.org/ 10.1016/S0140-6736(78)90299-4 [DOI] [PubMed] [Google Scholar]


