Abstract
Introduction
Pregnant South African women with histories of drinking alcohol, abuse by violent partners, depression, and living with HIV are likely to have their post-birth trajectories over 36 months significantly influenced by these risks.
Design
All pregnant women in 24 Cape Town neighborhoods were recruited into a cluster RCT by neighborhood to either: (1) a standard care condition (n=12 neighborhoods, n=594 mothers); or (2) a home-visiting intervention condition (n=12 neighborhoods, n=644 mothers).
Setting/participants
Pregnant women residing in urban, low-income neighborhoods in Cape Town, South Africa.
Intervention
Home visiting included prenatal and postnatal visits by community health workers (Mentor Mothers) focusing on general maternal and child health, HIV/tuberculosis, alcohol use, and nutrition.
Main outcome measures
Mothers were assessed in pregnancy and at 18 and 36 months post birth: 80.6% of mothers completed all assessments between 2009 and 2014 and were included in these analyses performed in 2014. Longitudinal structural equation modeling examined alcohol use, partner violence, and depression at the baseline and 18-month interviews as predictors of maternal outcomes at 36 months post birth.
Results
Relative to standard care, intervention mothers were significantly less likely to report depressive symptoms and more positive quality of life at 36 months. Alcohol use was significantly related to use over time, but was also related to depression and HIV status at each assessment and partner violence at 36 months.
Conclusions
Alcohol, partner violence, and depression are significantly related over time. A home-visiting intervention improved the emotional health of low-income mothers even when depression was not initially targeted.
Introduction
To stem the public health consequences of infectious and chronic diseases in low- and middle-income countries, task shifting from professionals to community health workers (CHWs) is becoming broadly adopted.1,2 South Africa is currently in the process of re-engineering primary health care and will add 65,000 CHWs to their workforce, aiming to increase both the accessibility and quality of care. Yet, CHWs typically address only a single health challenge, for example, to reduce perinatal HIV transmission, low-birth-weight infants, or malnutrition.3–8 Almost all low-income women face multiple health challenges associated with poverty, life histories, and community contexts.9
South Africa has the highest rates of alcohol consumption globally, with high rates of fetal alcohol spectrum disorder in some communities.10–13 Alcohol use is responsible for 60% of automobile accidents, >75% of homicides, and 50% of non-natural deaths; is implicated in 67% of all intimate partner violence (IPV)14; and costs South Africa ZAR 9 billion annually.15 Alcohol use disinhibits sexual behavior, thus associating it with HIV.16,17 Alcohol use is also associated with unplanned pregnancies, and is more common among women whose partners also abuse alcohol.18–20 Alcohol use and abuse in pregnancy contributes to children’s failure to thrive, as homes are less organized and daily family routines more chaotic (E Davis, MJ Rotheram-Borus, M Tomlinson, unpublished work, 2014). Alcohol use also reduces maternal adherence to all health regimens, including HIV medications.21
Poverty is the primary marker associated with IPV.22,23 When poverty is the only predictor, it is not feasible to screen and target women in a specified geographic area, given that 74.4% of South Africans are considered low-income.24 IPV is a problem that can only be identified after an initial violent episode or addressed preventively with public health intervention strategies.
Postpartum depression in South Africa has been found to exceed 30%.25,26 Depression negatively affects children’s social and cognitive development. Depressed mothers have been found to have lower levels of social support.27,28 Depressed women are also more likely to use alcohol frequently and be more resistant to treatment to reduce alcohol use in other countries, though these patterns are not well documented in South Africa.29–31
South Africa has 3.4 million women living with HIV.32 In 2012, 17.9% of South Africans aged 15–49 years, the majority of whom are women of childbearing age, were infected.32 Antenatal testing during pregnancy identifies most women living with HIV; about 99% of pregnant women are tested for HIV in the Western Cape, a region with a relatively strong health infrastructure. About 30% of pregnant women are HIV seropositive; however, interventions to prevent mother to child transmission have reduced perinatal transmission from roughly 30% to about 3%.33–35 Yet, HIV impacts many areas of a woman’s life, including relationships with partners and children, as well as mental and physical health.36,37
Given the high prevalence of each of these maternal risk factors for negative long-term outcomes, we examine low-income mothers in Cape Town, South Africa from pregnancy until 36 months post birth. We examine how the risk factors interact, and how each of these factors is related to demographic and behavioral contexts that are potential avenues for intervention. We hypothesize that participation in a supportive prenatal and postnatal intervention would have long-term benefits for maternal well-being. Paraprofessionals with the same educational and socioeconomic background as the study participants, from nearby neighborhoods, served as the CHW home visitors. The paraprofessionals reflected task shifting from clinics to families’ homes. The CHWs were taught to address multiple risk factors concurrently.
Methods
Three independent teams were involved: assessments (Stellenbosch University), intervention (Philani Programme), and data analyses (University of California, Los Angeles [UCLA]). Participants completed all assessments between 2009 and 2014 and were included in the following analyses performed in 2014.
Study Sample
Six matched sets of four neighborhoods each (n=24 neighborhoods) were identified in low-income, urban areas outside Cape Town, South Africa, based on similarity in size (using aerial maps); number of shebeens (bars) determined by walking and plotting the sites; electricity; access to running water; toilets; and distance to clinic care. Buffer zones were established between neighborhoods. Street intercept surveys regarding duration of living in the neighborhood were conducted on weekends with 20 randomly selected women per neighborhood. Based on these matching procedures, UCLA randomized each neighborhood to either an intervention condition, in which all pregnant women received home visitors from CHWs (called “mentor mothers”), as well as clinic care, or a standard care condition (clinic care).
From May 2009 to September 2010, a total of 12 low-income, urban women were recruited by Stellenbosch University as recruiters, went house to house in one intervention and one control neighborhood to identify all adult pregnant women (aged ≥18 years), and obtained consent for them to be contacted by the assessment team. Only 2% of pregnant women refused participation. We recruited, trained, and certified additional low-income, urban women to serve as interviewers; each interviewer entered participants’ responses to the assessment measures on mobile phones in an hour-long interview. Supervisors monitored and gave feedback on the data quality weekly.
Figure 1 summarizes participant flow through the study. The neighborhoods and pregnant women were highly similar across conditions. The minimum number of pregnant women needed per neighborhood to achieve 80% power to detect a standardized effect size of 0.40 established the sample size. The original sample consisted of 1,238 women. There were 117 dyads in which either the mother or infant died, and these dyads were removed from the study (final n=1,121). The current sample of mothers has 904 women (80.6%) who participated in all assessments at baseline, 18 months, and 36 months.
Figure 1.
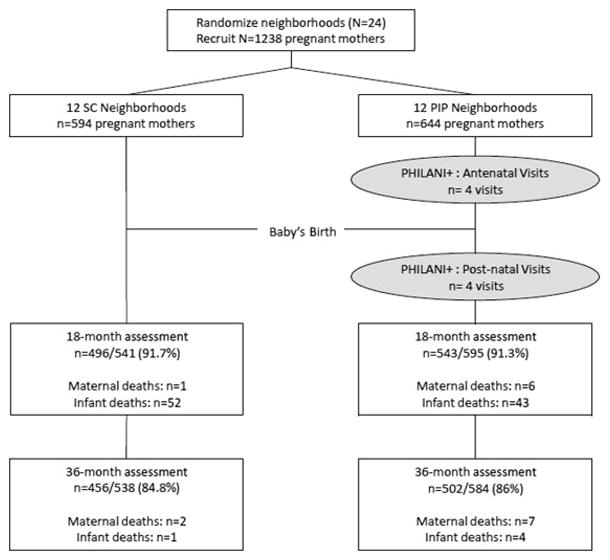
Movement of participants through the RCT at assessment points comparing mothers in a control condition and a home visiting intervention.
Intervention and Control Conditions
Standard clinic care in Cape Town is accessible within 5 km to each neighborhood and provides tuberculosis (TB), HIV, and CD4 testing; partner testing; dual-regimen therapies for people living with HIV; consistent access to milk tins (formula); co-trimoxazole for infants until HIV testing; HIV polymerase chain reaction testing at 6 weeks; and (inconsistent) postnatal visits at 1 week. Standard antenatal care is typically four visits, with well-baby visits post birth. HIV care is offered within antenatal visits for pregnant women and in specialized HIV care clinics post birth.
Each intervention neighborhood had the same services available as in the standard care condition. The Philani Programme implemented and supervised all intervention procedures, building on its existing home-visiting program. Philani recruited 12 CHWs, selected because of their good social and problem-solving skills and success in raising their own healthy children, to make home visits to participants in the 12 intervention neighborhoods. CHWs were trained for 1 month in cognitive–behavioral change strategies and role-playing. They also watched videotapes of common situations they might face. They were trained to provide and apply health information about general maternal and child health, HIV/TB, alcohol use, and nutrition to low-income, urban women’s lives. CHWs were certified and supervised biweekly with random observations of home visits.
The Philani Programme implemented the intervention. CHWs were trained for 1 month on how to document their contacts with mothers using a mobile phone and a paper log system, and on the educational knowledge regarding key health topics: HIV/TB, prevention of mother to child transmission of HIV, alcohol, mental health, breastfeeding, and malnutrition. The second month of training focused on the theory of behavior change (people change slowly over time with small steps, in relationship with opportunities and rewards) and skills to facilitate behavior change: goal setting, problem solving, relaxation, assertiveness, and shaping. CHWs practiced home visits with each other and mock clients, and were certified by supervisors at the end of training. About half of the potential CHWs who entered training were not certified and were eliminated as potential CHWs. The intervention dose (number, length of home visits) delivered by CHWs was monitored on mobile phones, including a time stamp and brief summary report. On average, CHWs made six antenatal visits (SD=3.8), five postnatal visits between birth and 2 months post birth (SD=1.9), and 1.4 visits/month until the children were 18 months old. After 18 months, visits only occurred once every 6 months. Sessions lasted 31 minutes each on average. At each visit, CHWs reported the content of the session on their mobile phone from among the eight targeted topics and if it was a crisis session. Supervisors randomly site visited each CHW twice a month and reviewed charts on an ongoing basis and at monthly in-service training meetings. At monthly in-service trainings, CHWs received refresher training and role-played difficult cases.
Measures
Baseline measures included demographic variables initially used in the analysis, which were maternal age in years, education, having a current partner and monthly income; alcohol use during pregnancy, which was a single-item dichotomous measure (yes=1/no=0) derived from responses to items on alcohol use during pregnancy; and depression and emotional distress, which were assessed with two scales used as indicators of a latent variable representing depression. The ten-item Edinburgh Postnatal Depression Scale (EPDS) asks how a mother has felt in the past 7 days on a 4-point scale ranging from No, not at all to Yes, quite a lot.38 The EPDS was developed to identify women at risk for postpartum depression. Scale items correspond to various clinical depression symptoms, such as guilt, sleep disturbance, low energy, anhedonia, and suicidal ideation. Overall assessment is done by the total score, determined by adding together the scores for each of the ten items. Higher scores indicate more depressive symptoms. The EPDS has been used during the postpartum period, as well as during pregnancy.39,40
The second scale was the GHQ-12, a reliable and sensitive short-form of the General Health Questionnaire. The GHQ-12 assesses the inability to carry out normal functions and the appearance of new and distressing life events,41 with a 4-point scale ranging from Not at all to Much more than usual referring to the past few weeks. Scores were summed; higher scores indicated greater psychological distress.
Other baseline measures included a dichotomous HIV-positive serostatus variable (yes=1/no=0) and an IPV variable assessed with four items adapted from Jewkes and colleagues23,42 on women’s experiences of violence. Women were asked four items referring to the past 12 months: if they were slapped or had anything thrown at them; were pushed or shoved; were punched with a fist or another object; or were attacked or threatened with a weapon by their partner. Responses ranged from never (1), once (2), few (3), to many (4).
Eighteen-month variables included alcohol use, which was a latent variable indicated by the following three items: frequency of drinking alcohol (0–9 scale, 0=never to 9=every day); amount of alcohol on drinking days (0–5 scale, 0=none to 5=ten or more drinks); and frequency of three or more drinks per day (0–9 scale, 0=never to 9=every day)43; and emotional distress and IPV, both of which were assessed in the same manner as at baseline.
Three-year outcomes included alcohol use, which was assessed in the same manner as at 18 months; IPV, which was assessed in the same manner as at baseline and 18 months; and positive emotional health, which was assessed with four scales that were scored to indicate greater emotional health and less depression. A summary score was used for each subscale. Scales 1 and 2 are subscales derived from the 36-item Short Form Health Survey,44 the first of which was the Emotional Health subscale and the second the Mental Health subscale. These scale items are scored from 1 to 5 and refer to the past 4 weeks. The third scale was the Hopkins Symptom Checklist Depression Scale, also known as the Brief Symptom Inventory.45 The fourth scale was the EPDS. Finally, a dichotomous variable (yes=1/no=0) indicated if the participant was in the intervention group. Thus, our analysis reflects an intent-to-treat analysis.
Statistical Analysis
The EQS, version 6, structural equations program was used to assess an initial confirmatory factor model and a predictive path model. The initial confirmatory factor analysis assessed the adequacy of the hypothesized measurement model, and the associations among the latent variables and single item variables without imputing any directionality. A directional latent variable path model positioned the baseline variables of age, alcohol use during pregnancy, depression, HIV-positive status, and IPV as predictors of the 18-month intermediate measures of alcohol use, depression, and IPV. These, in turn, predicted the 36-month outcomes of alcohol use, positive emotional health, and IPV. Intervention condition status initially predicted the 18-month intervening variables and the outcome variables. Having a current partner, education, and income were not significantly associated with the other variables of interest and were not included in the models. Nonsignificant paths and covariances were gradually dropped until only significant paths and covariances remained. Paths were added from the baseline variables to the outcome variables based on suggestions from the Lagrange Multiplier test for fit improvement.46
These analyses compare a proposed hypothetical model with a set of actual data. The closeness of the hypothetical model to the empirical data is evaluated statistically through various goodness-of-fit indexes. Goodness of fit was assessed with both maximum likelihood (ML) chi-square and the robust Satorra–Bentler (S–B) chi-square values, the comparative fit index (CFI), robust comparative fit index (RCFI), and the root mean squared error of approximation (RMSEA).47 The robust S–B chi-square was used in addition to normal ML methods because it is appropriate when the data depart from multivariate normality. The multivariate kurtosis estimate was high in the data set (normalized Mardia z-statistic, 333.88). The CFI and RCFI range from 0 to 1 and reflect the improvement in fit of a hypothesized model over a model of complete independence among the measured variables. RCFI values at ≥0.95 are desirable, indicating that the hypothesized model reproduces ≥95% of the covariation in the data. The RMSEA is a measure of lack of fit per degrees of freedom, controlling for sample size, and values <0.06 indicate a relatively good fit between the hypothesized model and the observed data.
Because of random assignment, we did not expect intervention condition membership to be correlated significantly with any baseline predictors, although we examined in the confirmatory factor analysis whether by chance any of the baseline measures were associated significantly with intervention status. If so, we controlled for these pre-existing associations. We also report whether there were significant indirect effects on the 36-month outcomes of any baseline predictor or of intervention status mediated through the 18-month variables. Data were collected between 2009 and 2014 for these longitudinal analyses, which were performed in 2014.
The IRBs of UCLA, Stellenbosch University, and Emory University approved the study, whose methods have previously been published.48
Results
The average age of the women was 26.5 (SD=5.6) years, ranging from 18 to 42 years. Regarding marital status, 43% were single, 20% were living with someone, and 37% were married. Tenth grade was the average education level, 75% did not complete high school, 79% were not employed, and 14% were employed part or full time. Others had temporary jobs or were self-employed. Fifty percent had incomes equal to or under ZAR 2000/month (about $200).
Table 1 reports the means, SDs, ranges, and standardized factor loadings for the measured variables. All measured variables loaded significantly (p<0.001) on their hypothesized latent factors. The fit indexes were highly acceptable (ML chi-square [343, n=904]=955.83, CFI=0.96, RMSEA=0.05, S–B chi-square [343, n=904)=551.43, RCFI=0.97, RMSEA=0.03). No modifications were necessary in this model.
Table 1.
Summary Statistics, Ranges, and Factor Loadings in the Confirmatory Factor Analysis (n=904 Cape Town Mothers)
| Variables (range) | Ma | SD | Factor loading |
|---|---|---|---|
| Baseline | |||
| Age (years) (18–42 years) | 26.53 | 5.63 | — |
| Alcohol use during pregnancy (%) (yes=1, no=0) | 27 | — | |
| Depression | |||
| GHQ (1–36) | 14.69 | 8.62 | 0.95 |
| EPDS (0–30) | 10.72 | 6.91 | 0.85 |
| HIV-positive (%) (yes=1, no=0) | 28 | — | |
| Intimate partner violence (1–4) | |||
| Slap | 1.51 | 0.85 | 0.78 |
| Shove | 1.32 | 0.70 | 0.74 |
| Punch | 1.19 | 0.57 | 0.75 |
| Weapons | 1.07 | 0.36 | 0.42 |
| 18 Months | |||
| Alcohol use | |||
| Alcohol frequency (1–8) | 1.34 | 1.00 | 0.95 |
| Number of drinks (0–5) | 0.19 | 0.60 | 0.86 |
| Frequency of 3 or more drinks (0–7) | 0.38 | 1.03 | 0.97 |
| Depression | |||
| GHQ (1–36) | 9.28 | 8.08 | 0.85 |
| EPDS (0–30) | 6.77 | 7.34 | 0.89 |
| Intimate partner violence (1–4) | |||
| Slap | 1.19 | 0.57 | 0.86 |
| Shove | 1.17 | 0.54 | 0.84 |
| Punch | 1.11 | 0.47 | 0.89 |
| Weapons | 1.05 | 0.29 | 0.55 |
| Year 3 outcomes | |||
| Alcohol use | |||
| Alcohol frequency (1–8) | 1.57 | 1.35 | 0.97 |
| Number of drinks (0–3) | 0.22 | 0.54 | 0.88 |
| Freq. 3 or more drinks (1–8) | 1.42 | 1.18 | 0.88 |
| Positive emotional healthb | |||
| SF-36 EH (6–20) | 18.54 | 2.80 | 0.76 |
| SF-36 Depression (9–30) | 33.79 | 6.83 | 0.87 |
| Hopkins (25–100) | 89.97 | 15.98 | 0.94 |
| EPDS (0–30) | 23.60 | 7.61 | 0.88 |
| Intimate partner violence (1–4) | |||
| Slap | 1.20 | 0.58 | 0.88 |
| Shove | 1.16 | 0.53 | 0.81 |
| Punch | 1.12 | 0.49 | 0.86 |
| Weapons | 1.05 | 0.32 | 0.50 |
| Group membership | |||
| Intervention member (%) (yes=1, no=0) | 52 | — | |
Note: Boldface indicates statistical significance (p≤0.001).
Values are M, unless otherwise noted.
Higher scores indicate better emotional health. Scales reversed where necessary.
EH, Emotional Health subscale; EPDS, Edinburgh Postnatal Depression Scale; GHQ, General Health Questionnaire; SF-36, 36-item Short Form Health Survey.
Correlations among all of the latent and single-item variables are reported in Table 2. Of note, intervention membership was not significantly associated with any baseline variables except for an unexpected significant association with the intervention mothers reporting more depression. This relationship was not significant in the original overall sample (only 80.6% of participants were in these current analyses, i.e., those who completed all assessments). Focusing only on the most highly significant correlations (p≤0.001), having used alcohol during pregnancy was most associated with IPV at baseline (0.26) and again at 36 months (0.21), as well as continued alcohol use at 18 and 36 months (0.38 and 0.40, respectively). Depression at baseline was most associated with concurrent partner violence (0.24), continued depression at 18 months (0.17), and more partner violence (0.12) and less positive emotional health at 36 months (−0.29). HIV-positive status had a significant relationship with more depression at baseline (0.11) and was associated with older age (0.10).
Table 2.
Correlations Among Measured and Latent Variables in Model
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | ||||||||||||
| 1. Age | — | |||||||||||
| 2. Alcohol during pregnancy | –0.11*** | — | ||||||||||
| 3. Depression | 0.01 | 0.12*** | — | |||||||||
| 4. HIV-positive | 0.10*** | 0.06 | 0.11*** | — | ||||||||
| 5. Intimate partner violence | −0.05 | 0.26*** | 0.24*** | 0.01 | — | |||||||
| 18-months | ||||||||||||
| 6. Alcohol use | 0.04 | 0.38*** | 0.04 | 0.09** | 0.19*** | — | ||||||
| 7. Depression | 0.15*** | 0.00 | 0.17*** | 0.08* | 0.13*** | 0.04 | — | |||||
| 8. Intimate partner violence | −0.02 | 0.10** | 0.06 | 0.03 | 0.50*** | 0.19*** | 0.16*** | — | ||||
| 3-Year outcomes | ||||||||||||
| 9. Alcohol use | −0.10** | 0.40*** | 0.06 | 0.04 | 0.20*** | 0.51*** | 0.02 | 0.11** | — | |||
| 10. Positive emotional health | −0.05 | −0.13*** | −0.19*** | −0.06 | −0.14*** | −0.11*** | −0.23*** | −0.11** | −0.21*** | — | ||
| 11. Intimate partner violence | −0.02 | 0.21*** | 0.12*** | 0.04 | 0.22*** | 0.19*** | 0.05 | 0.21*** | 0.29*** | −0.39*** | — | |
| Group membership | ||||||||||||
| 12. Intervention member | 0.04 | 0.00 | 0.07* | −0.02 | −0.02 | −0.03 | 0.03 | 0.04 | −0.02 | 0.07* | 0.02 | — |
Note: N=904. Boldface indicates statistical significance
p≤0.05;
p≤0.01;
p≤0.001.
Baseline IPV was associated with numerous maladaptive behaviors, including alcohol use at 18 and 36 months (0.19 and 0.20, respectively), more depression at 18 months (0.13), and less positive emotional health at 36 months (−0.14). It also was stable over time (0.50 at 18 months and 0.22 at 36 months post birth). Older women reported more depression (0.15) at 18 months. At 18 months, alcohol use was associated with more IPV concurrently (0.19) and at 36 months (0.19). It was also highly associated with continued alcohol use at 36 months (0.51). Depression at 18 months was associated concurrently with more IPV and with less positive emotional health at 36 months (−0.23). IPV during pregnancy was associated with continued IPV at 36 months (0.21). The negative outcomes at 36 months were significantly correlated among themselves, as shown in Table 2. Being in the intervention group was significantly associated with more positive emotional health (0.07, p≤0.05).
The final path model had excellent fit statistics (ML chi-square [380, n=904]=1,007.04, CFI=0.96, RMSEA=0.04 and S–B chi-square [380, n=904]=601.28, RCFI=0.97, RMSEA=0.03). Results of the analysis with all significant paths included are depicted in Figure 2. Nonsignificant paths were dropped gradually. Latent variables are represented by circles; measured variables are depicted in rectangles. Only significant direct effects are shown in Figure 2. The substantial associations among the residuals of the outcome variables are also depicted.
Figure 2.
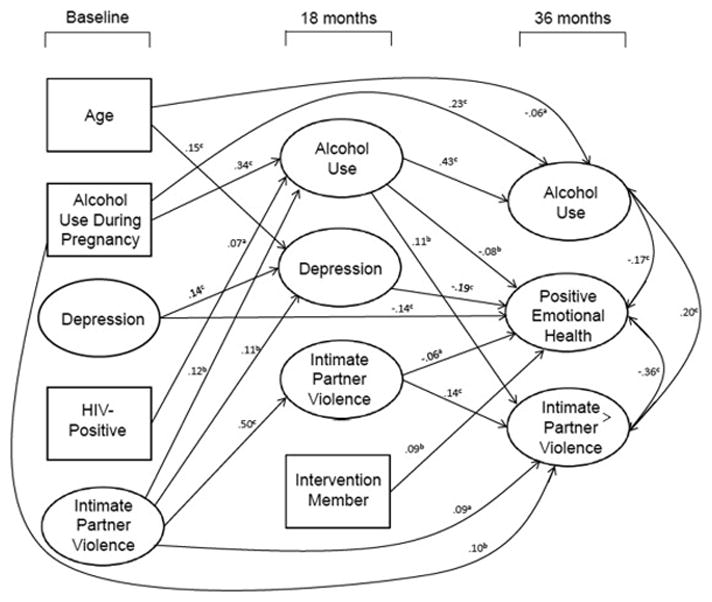
Significant regression paths predicting 3-year outcomes among 904 mothers in Cape Town.
Note: Large circles represent latent variables; rectangles represent single-item indicators. For readability, correlations among the predictors are not shown. Regression coefficients are standardized (ap<0.05; bp<0.01; cp<0.001).
Focusing on the 36-month outcomes, alcohol use was significantly predicted by prior alcohol use at baseline and 18 months, as well as younger age. IPV was predicted by alcohol use at 18 months, IPV at 18 months, alcohol use during pregnancy, and IPV at baseline. Positive emotional health was predicted by less alcohol use, less depression and less IPV at 18 months, less depression at baseline, and by being in the intervention condition. Controlling for the prior association between intervention status and baseline depression significantly enhanced the relationship between better emotional health and intervention participation. The intervention reduced depression even though initially the mothers in this condition were more depressed than those in the control condition.
There also were significant indirect effects of baseline variables on the 36-month outcome variables mediated through the 18-month variables. In addition to its direct effect, alcohol during pregnancy had an indirect effect on alcohol use at 36 months through its effect on alcohol use at 18 months (p<0.001). HIV-positive status and IPV also exerted significant indirect effects on greater alcohol use at 36 months (p<0.05 and p<0.01, respectively), also mediated through alcohol use at 18 months. Positive emotional health was impacted indirectly by baseline variables of less alcohol during pregnancy (p<0.05), less depression (p<0.01), less IPV (p<0.01), and younger age (p<0.001). IPV was impacted indirectly by baseline variables of alcohol during pregnancy (p<0.01) and more IPV (p<0.001).
Discussion
The home-visiting intervention with urban South African mothers was associated with improved maternal emotional health 36 months after their children were born, though the intervention did not initially target reductions in maternal depression or improved maternal emotional health. It is noteworthy that the benefits of the intervention were broader than only a measure of perinatal depression. There were four measures of emotional health included in the latent variable reflecting emotional distress: the EPDS (only depression measure used at baseline in pregnancy), Brief Symptom Inventory, and subscales from the 36-item Short Form Health Survey. The significant intervention impact affects the latent variable, reflecting a much broader measure of well-being compared with only being a depression measure.
This outcome was encouraging given that within this sample, by chance, the intervention condition mothers were more depressed at baseline. This paper focuses only on mothers who participated in all follow-up assessments—in the entire sample, there were no significant baseline differences in depression. Perhaps having a supportive individual in their lives had unanticipated, positive consequences. CHWs encouraged and trained mothers to care for their infants, regardless of their mothers’ feelings or stress. In prior analyses, we found that mothers’ depression did not remit post birth, at 6 or 18 months.4,49 However, we did find that children of depressed mothers maintained a healthier growth pattern, being both heavier and longer than infants of control mothers.4,49 We do not know the mechanism by which maternal depression remits significantly more at 36 months among intervention mothers. However, healthier children are easier to care for, and it is possible that the long-term benefits of the intervention (which basically stopped at about 6 months post birth) accrued over time. This effect is more impressive given one of the few baseline differences was that the intervention mothers were significantly more depressed at baseline.
There is a persistent negative impact associated with IPV over 36 months post birth. IPV is the best predictor of previous partner violence and the effect is maintained across both the 18- and 36-month assessments. It is not clear from our data whether mothers are staying with the same partner or if new partners engage in this negative behavior. Our measures of IPV only focused on violence in the last year, and we did not inquire about rape. Therefore, there may be an even greater relationship between IPV and other risks. However, our measures did refer to significant violent acts compared to measures of violence with the best psychometric properties,50 such as “hurt, insults, threatened, or screamed.”50 Thus, although no measure of IPV has demonstrated ideal psychometric properties,50 our measure does reflect violent acts, with physical contact or weapons involved in each item.
A similarly strong and persistent relationship is found in alcohol use and abuse over time. Mothers who used alcohol while pregnant continued using and abusing alcohol over time. In previous analyses, we found that mothers in the control condition who drank alcohol prior to knowing that they were pregnant (25%), in fact, increased their use threefold during pregnancy.4 Although the intervention reduced alcohol use in pregnancy, drinking resumed post birth. These analyses suggest that drinking is a very stable behavior. A more intensive or group-focused intervention is needed for these mothers to be successful in reducing alcohol use over time.51 This is a disturbing finding, given the pervasive and long-term negative outcomes of alcohol use and abuse. IPV at baseline also predicted more alcohol use, depression, and continued violence at 18 months. It also had significant indirect effects on all three outcomes. This constellation of depression, IPV, and alcohol behaviors must be addressed with a comprehensive approach when designing an intervention.
Alcohol use and IPV were highly stable over time. Depression is also relatively stable, though the emotional health variable was composed of multiple constructs reflecting mental health status. These behaviors are likely to have lifelong effects on the children over time. The CHWs were only trained to reduce alcohol use, not depression or IPV. The integrated and persistent risk pattern over time indicates the need for CHWs to extend the length of their interactions and expand beyond HIV, nutrition, and alcohol. Existing intervention models are almost all categorical funding streams, with CHWs addressing a single outcome. Families’ lives are characterized by embedded risk “routines” of drinking and IPV. Generalist models of interventions using CHWs are recommended to help families slowly change their routines and risky behaviors with practice and small steps over time. Training CHWs as problem-solving coaches is likely to be a more viable, long-term intervention strategy.
Limitations
These data were collected in the context of an RCT. Fortunately, both the internal and external validity of the overall sample were excellent. Only 2% of pregnant women refused participation in the study and the follow-up rates exceeded 85% at each time point. Unfortunately, we did not include teenage parents—the consent process was considered too complex and problematic to include young women, given that they live with their parents.
Our only informant was the mother; the interviewers did complete physical measures of the child. It would be highly desirable to include fathers, but partnerships were transitory and fathers were not available for inclusion. Future studies on the nature and context of IPV should include multiple informants and, hopefully, partners and men.
Conclusions
Alcohol use, IPV, and depression are quite stable from pregnancy through the first 36 months of children’s lives. Depression appears to remit most often, compared to alcohol or IPV. However, the inter-relationships among these risk factors indicate the importance of CHWs and other interventions to concurrently address these health risks. The CHWs in this project were trained to address alcohol and HIV, but not IPV or depression. Yet, intervention mothers had significantly less depression and more positive mental health on multiple measures of well-being, compared to mothers in the control condition. Future interventions must train CHWs on how to address both IPV and depression and, perhaps, there would be improvements in IPV. In addition, the frequency of visits decreased substantially after 6 months post birth—it is likely that interventions must be sustained over time.
Acknowledgments
This study was funded by National Institute on Alcohol Abuse and Alcoholism grant 1R01AA017104 and supported by NIH grants MH58107, 5P30AI028697, 1R24AA022919, and UL1TR000124. ClinicalTrials.gov registration: NCT00972699.
Footnotes
No financial disclosures were reported by the authors of this paper.
References
- 1.Lewin S, Munabi-Babigumira S, Glenton C, et al. Lay health workers in primary and community health care for maternal and child health and the management of infectious diseases. Cochrane Database Syst Rev. 2010;17(3):CD004015. doi: 10.1002/14651858.CD004015.pub3. http://dx.doi.org/10.1002/14651858.CD004015.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel V, Araya R, Chatterjee S, et al. Treatment and prevention of mental disorders in low-income and middle-income countries. Lancet. 2007;370(9591):991–1005. doi: 10.1016/S0140-6736(07)61240-9. http://dx.doi.org/10.1016/S0140-6736(07)61240-9. [DOI] [PubMed] [Google Scholar]
- 3.Richter L, Rotheram-Borus MJ, van Heerden A, et al. Pregnant women living with HIV (WLH) supported at clinics by peer WLH: a cluster randomized controlled trial. AIDS Behav. 2014;18(4):706–715. doi: 10.1007/s10461-014-0694-2. http://dx.doi.org/10.1007/s10461-014-0694-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Roux IM, Tomlinson M, Harwood JM, et al. Outcomes of home visits for pregnant mothers and their infants: a cluster randomized controlled trial. AIDS. 2013;27(9):1461–1471. doi: 10.1097/QAD.0b013e3283601b53. http://dx.doi.org/10.1097/QAD.0b013e3283601b53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tylleskär T, Jackson D, Meda N, et al. Exclusive breastfeeding promotion by peer counsellors in sub-Saharan Africa (PROMISE-EBF): a cluster-randomised trial. Lancet. 2011;378(9789):420–427. doi: 10.1016/S0140-6736(11)60738-1. http://dx.doi.org/10.1016/S0140-6736(11)60738-1. [DOI] [PubMed] [Google Scholar]
- 6.Alderman H. Improving nutrition through community growth promotion: longitudinal study of the nutrition and early child development program in Uganda. World Dev. 2007;35(8):1376–1389. http://dx.doi.org/10.1016/j.worlddev.2007.04.003. [Google Scholar]
- 7.Alderman H, Hoogeveen H, Rossi M. Preschool nutrition and subsequent schooling attainment: longitudinal evidence from Tanzania. Econ Dev Cult Change. 2008;57(2):239–260. http://dx.doi.org/10.1086/592875. [Google Scholar]
- 8.Alderman H, Ndiaye B, Linnemayr S, et al. Effectiveness of a community-based intervention to improve nutrition in young children in Senegal: a difference in difference analysis. Public Health Nutr. 2009;12(5):667–673. doi: 10.1017/S1368980008002619. http://dx.doi.org/10.1017/S1368980008002619. [DOI] [PubMed] [Google Scholar]
- 9.Rotheram-Borus MJ, Swendeman D, Flannery D. Family wellness, not HIV prevention. AIDS Behav. 2009;13(3):409–413. doi: 10.1007/s10461-008-9515-9. http://dx.doi.org/10.1007/s10461-008-9515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May PA, Gossage JP, Kalberg WO, et al. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev. 2009;15(3):176–192. doi: 10.1002/ddrr.68. http://dx.doi.org/10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- 11.Rendall-Mkosi K, London L, Adnams C, Morojele N, McLoughlin JA, Goldstone C. Fetal alcohol spectrum disorder in South Africa: situational and gap analysis. UNICEF; 2008. www.unicef.org/southafrica/SAF_resources_fetalalcohol.pdf. Accessed XXX. [Google Scholar]
- 12.Viljoen DL, Gossage JP, Brooke L, et al. Fetal alcohol syndrome epidemiology in a South African community: a second study of a very high prevalence area. J Stud Alcohol. 2005;66(5):593–604. doi: 10.15288/jsa.2005.66.593. http://dx.doi.org/10.15288/jsa.2005.66.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren KR, Calhoun FJ, May PA, et al. Fetal alcohol syndrome: an international perspective. Alcohol Clin Exp Res. 2001;25(suppl 1):202S–206S. doi: 10.1097/00000374-200105051-00033. http://dx.doi.org/10.1111/j.1530-0277.2001.tb02397.x. [DOI] [PubMed] [Google Scholar]
- 14.Alcohol Abuse in South Africa. Frontline Fellowship. www.frontline.org.za/index.php?option=com_content&id=466:alcohol-abuse-in-south-africa. Published 2007. Accessed XXX.
- 15.Alcohol abuse costs R9bn a year. News24. www.news24.com/SouthAfrica/Politics/Alcohol-abuse-puts-R9bn-dent-in-economy-20110905. Published September 5, 2011. Accessed XXX.
- 16.Morojele NK, Kachieng’a MA, Mokoko E, et al. Alcohol use and sexual behaviour among risky drinkers and bar and shebeen patrons in Gauteng province, South Africa. Soc Sci Med. 2006;62(1):217–227. doi: 10.1016/j.socscimed.2005.05.031. http://dx.doi.org/10.1016/j.socscimed.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Bryant KJ. Expanding research on the role of alcohol consumption and related risks in the prevention and treatment of HIV/AIDS. Subst Use Misuse. 2006;41(10–12):1465–1507. doi: 10.1080/10826080600846250. http://dx.doi.org/10.1080/10826080600846250. [DOI] [PubMed] [Google Scholar]
- 18.Morojele NK, Kachieng’a MA, Nkoko MA, et al. Perceived effects of alcohol use on sexual encounters among adults in South Africa. Afr J Drug Alcohol Stud. 2004;3(1):1–20. [Google Scholar]
- 19.Choi KW, Abler LA, Watt MH, et al. Drinking before and after pregnancy recognition among South African women: the moderating role of traumatic experiences. BMC Pregnancy Childbirth. 2014;14(1):97. doi: 10.1186/1471-2393-14-97. http://dx.doi.org/10.1186/1471-2393-14-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naimi TS, Lipscomb LE, Brewer RD, Gilbert BC. Binge drinking in the preconception period and the risk of unintended pregnancy: implications for women and their children. Pediatrics. 2003;111(5 Pt 2):1136–1141. [PubMed] [Google Scholar]
- 21.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. J Acquir Immune Defic Syndr. 2009;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. http://dx.doi.org/10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jewkes R. Intimate partner violence: causes and prevention. Lancet. 2002;359(9315):1423–1429. doi: 10.1016/S0140-6736(02)08357-5. http://dx.doi.org/10.1016/S0140-6736(02)08357-5. [DOI] [PubMed] [Google Scholar]
- 23.Jewkes R, Levin J, Penn-Kekana L. Risk factors for domestic violence: findings from a South African cross-sectional study. Soc Sci Med. 2002;55(9):1603–1617. doi: 10.1016/s0277-9536(01)00294-5. http://dx.doi.org/10.1016/S0277-9536(01)00294-5. [DOI] [PubMed] [Google Scholar]
- 24.Personal Income Estimates for South Africa. Bureau of Market Research: Information Blurb 2011/06. UNISA; 2011. www.unisa.ac.za/contents/faculties/ems/docs/BMR_info_blurp_407.pdf. Published 2011. Accessed XXX. [Google Scholar]
- 25.Rochat TJ, Richter LM, Doll HA, Buthelezi NP, Tomkins A, Stein A. Depression among pregnant rural South African women undergoing HIV testing. JAMA. 2006;295(12):1373–1378. doi: 10.1001/jama.295.12.1376. http://dx.doi.org/10.1001/jama.295.12.1376. [DOI] [PubMed] [Google Scholar]
- 26.Cooper PJ, Tomlinson M, Swartz L, Woolgar M, Murray L, Molteno C. Post-partum depression and the mother-infant relationship in a South African peri-urban settlement. Br J Psychiatry. 1999;175(6):554–558. doi: 10.1192/bjp.175.6.554. http://dx.doi.org/10.1192/bjp.175.6.554. [DOI] [PubMed] [Google Scholar]
- 27.Cairney J, Boyle M, Offord DR, Racine Y. Stress, social support and depression in single and married mothers. Soc Psychiatry Psychiatr Epidemiol. 2003;38(8):442–449. doi: 10.1007/s00127-003-0661-0. http://dx.doi.org/10.1007/s00127-003-0661-0. [DOI] [PubMed] [Google Scholar]
- 28.Brown GW, Andrews B, Harris T, Adler Z, Bridge L. Social support, self-esteem and depression. Psychol Med. 1986;16(4):813–831. doi: 10.1017/s0033291700011831. http://dx.doi.org/10.1017/S0033291700011831. [DOI] [PubMed] [Google Scholar]
- 29.Hanna EZ, Faden VB, Dufour MC. The motivational correlates of drinking, smoking, and illicit drug use during pregnancy. J Subst Abuse. 1994;6(2):155–167. doi: 10.1016/s0899-3289(94)90181-3. http://dx.doi.org/10.1016/S0899-3289(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 30.Meschke LL, Holl JA, Messelt S. Assessing the risk of fetal alcohol syndrome: understanding substance use among pregnant women. Neurotoxicol Teratol. 2003;25(6):667–674. doi: 10.1016/j.ntt.2003.07.004. http://dx.doi.org/10.1016/j.ntt.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor MJ, Whaley SE. Health care provider advice and risk factors associated with alcohol consumption following pregnancy recognition. J Stud Alcohol. 2006;67(1):22–31. doi: 10.15288/jsa.2006.67.22. http://dx.doi.org/10.15288/jsa.2006.67.22. [DOI] [PubMed] [Google Scholar]
- 32.South Africa: HIV and AIDS estimates. UNAIDS; www.unaids.org/en/regionscountries/countries/southafrica. Published 2013. Accessed XXX. [Google Scholar]
- 33.The 2012 National Antenatal Sentinel HIV & Herpes Simplex Type-2 Prevalence Survey in South Africa. National Department of Health; http://www.health-e.org.za/wp-content/uploads/2014/05/ASHIVHerp_Report2014_22May2014.pdf. Published 2013. Accessed XXX. [Google Scholar]
- 34.National PMTCT Accelerated Plan (A-Plan) Status Report. National Department of Health; 2010. www.sarrahsouthafrica.org/LinkClick.aspx?fileticket=Qt5CxiOrHFc%3D&tabid=2326. Accessed XXX. [Google Scholar]
- 35.Goga A, Dinh T, Jackson D. Evaluation of the Effectiveness of the National Prevention o9f Mother-to-Child Transmission (PMTCT) Programme on Infant HIV Measured at Six Weeks Postpartum in South Africa. Medical Research Council; www.mrc.ac.za/healthsystems/SAPMTCTE2010.pdf. Published 2012. Accessed XXX. [Google Scholar]
- 36.Rotheram-Borus MJ, Robin L, Reid HM, Draimin BH. Parent adolescent conflict and stress when parents are living with AIDS. Fam Process. 1998;37(1):83–94. doi: 10.1111/j.1545-5300.1998.00083.x. http://dx.doi.org/10.1111/j.1545-5300.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 37.Rotheram-Borus MJ, Lee MB, Gwadz M, Draimin BH. An intervention for parents with AIDS and their adolescent children. Am J Public Health. 2001;91(8):1294–1302. doi: 10.2105/ajph.91.8.1294. http://dx.doi.org/10.2105/AJPH.91.8.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. http://dx.doi.org/10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 39.Bergink V, Kooistra L, Lambregtse-van den Berg MP, et al. Validation of the Edinburgh Depression Scale during pregnancy. J Psychosom Res. 2011;70(4):385–389. doi: 10.1016/j.jpsychores.2010.07.008. http://dx.doi.org/10.1016/j.jpsychores.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Chibanda D, Mangezi W, Tshimanga M, et al. Validation of the Edinburgh Postnatal Depression Scale among women in a high HIV prevalence area in urban Zimbabwe. Arch Womens Ment Health. 2010;13(3):201–206. doi: 10.1007/s00737-009-0073-6. http://dx.doi.org/10.1007/s00737-009-0073-6. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg DP, Williams P. A User’s Guide to the General Health Questionnaire. Windsor: NFER-Nelson; 1988. [Google Scholar]
- 42.Jewkes RK, Levin JB, Penn-Kekana LA. Gender inequalities, intimate partner violence and HIV preventive practices: findings of a South African cross-sectional study. Soc Sci Med. 2003;56(1):125–134. doi: 10.1016/s0277-9536(02)00012-6. http://dx.doi.org/10.1016/S0277-9536(02)00012-6. [DOI] [PubMed] [Google Scholar]
- 43.Dawson D, Grant B, Stinson F. The AUDIT-C: screening for alcohol use disorders and risk drinking in the presence of other psychiatric disorders. Compr Psychiatry. 2005;46(6):405–416. doi: 10.1016/j.comppsych.2005.01.006. http://dx.doi.org/10.1016/j.comppsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 44.Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2(3):217–227. doi: 10.1002/hec.4730020305. http://dx.doi.org/10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- 45.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behav Sci. 1974;19(1):1–15. doi: 10.1002/bs.3830190102. http://dx.doi.org/10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 46.Chou CP, Bentler PM. Model modification in covariance structure modeling: a comparison among likelihood ratio, Lagrange multiplier, and Wald tests. Multivariate Behav Res. 1990;25(1):115–136. doi: 10.1207/s15327906mbr2501_13. http://dx.doi.org/10.1207/s15327906mbr2501_13. [DOI] [PubMed] [Google Scholar]
- 47.Hu L-T, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equat Model. 1999;6(1):1–55. http://dx.doi.org/10.1080/10705519909540118. [Google Scholar]
- 48.Rotheram-Borus MJ, le Roux IM, Tomlinson M, et al. Philani Plus (+): A mentor mother community health worker home visiting program to improve maternal and infants’ outcomes. Prev Sci. 2011;12(4):372–388. doi: 10.1007/s11121-011-0238-1. http://dx.doi.org/10.1007/s11121-011-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rotheram-Borus MJ, Tomlinson M, Le Roux I, et al. A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. PLoS One. 2014;9(10):e105934. doi: 10.1371/journal.pone.0105934. http://dx.doi.org/10.1371/journal.pone.0105934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabin RF, Jennings JM, Campbell JC, Bair-Merritt MH. Intimate partner violence screening tools. Am J Prev Med. 2009;36(5):439–445. doi: 10.1016/j.amepre.2009.01.024. http://dx.doi.org/10.1016/j.amepre.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rendall-Mkosi K, Morojele N, London L, Moodley S, Singh C, Girdler-Brown B. A randomized controlled trial of motivational interviewing to prevent risk for an alcohol-exposed pregnancy in the Western Cape, South Africa. Addiction. 2013;108(4):725–732. doi: 10.1111/add.12081. http://dx.doi.org/10.1111/add.12081. [DOI] [PubMed] [Google Scholar]


