Abstract
Background & Aims
Celiac disease has been linked to irritable bowel syndrome (IBS)-like symptoms in outpatient clinics. Guidelines recommend that all patients with IBS-like symptoms undergo serologic testing for celiac disease, but there is controversy over whether celiac disease is more prevalent in populations with IBS-like symptoms. We aimed to determine whether positive results from serologic tests for celiac disease are associated with IBS and other functional gastrointestinal disorders (FGIDs) in a large US White population.
Methods
Validated, self-report bowel disease questionnaires (BDQs) were sent to randomly selected cohorts of Olmsted County, Minnesota, residents. In separate protocols, serum samples were collected from more than 47,000 Olmsted County residents without a prior diagnosis of celiac disease; we performed serologic tests for celiac disease on stored serum samples from residents who completed the BDQ. Logistic regression was used to test for the association between serologic markers of celiac disease (positive vs negative) and individual FGIDs.
Results
A total of 3202 subjects completed the BDQ and had serum available for testing. IBS was identified in 13.6% of these subjects (95% confidence interval [CI], 12.4%–14.8%), and any gastrointestinal symptom occurred in 55.2% (95% CI, 53.5%−56.9%). The prevalence of celiac disease by based on serologic markers was 1.0% (95% CI, 0.7%–1.4%). IBS was less prevalent in patients with celiac disease (3%) than patients without celiac disease (14%), though the difference was not statistically significant (odds ratio=0.2; 95% CI, 0.03−1.5). Abdominal pain, constipation, weight loss, and dyspepsia were the most frequent symptom groups in subjects who were seropositive for celiac disease, but none of the gastrointestinal symptoms or disorders was significantly associated with celiac disease serology.
Conclusions
Symptoms indicative of FGIDs and sero-positive celiac disease are relatively common in a US white community. Testing for celiac disease in patients with IBS in the community may not have a significantly increased yield over population-based screening in the US.
Keywords: celiac disease, gastrointestinal symptoms, community study
INTRODUCTION
Celiac disease (CD) is triggered by exposure to gluten in genetically predisposed individuals.1-3 On gluten exposure, these patients can develop inflammation and villous atrophy in the proximal small intestine. 1, 2 Despite wide availability of non-invasive screening tests, more than 80% of CD cases remain undiagnosed.4, 5 Patients with classical CD have malabsorptive symptoms and signs, but this presentation is now uncommon, and many patients have other gastrointestinal (GI) symptoms (non-classic CD), or no symptoms at all (asymptomatic CD). 1, 6 Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders.
Irritable bowel syndrome (IBS) and other functional gastrointestinal disorders (FGIDs) are remarkably prevalent in the United State and worldwide.7 Moreover, patients with IBS report symptoms such as lower abdominal pain, diarrhea, and abdominal bloating or distension, which are also common in patients with CD.8, 9 A recent systematic review 8 suggested that the prevalence of CD in patients with IBS-like symptoms was significantly higher than that in controls without these symptoms. Guidelines 1-3, 10 have recommended that all patients with IBS-like symptoms undergo serologic testing for CD, but whether these recommendations should be applied in the United States is uncertain 11, 12 and most patients are not offered CD testing. For example, Cash, et al.11 observed that the prevalence of CD in patients with nonconstipated IBS was similar to that in controls. Moreover, it remains unknown if CD in the community is linked to FGIDs other than IBS. We aimed to determine if positive results of serologic testing for CD using a highly sensitive and specific assay were associated with IBS and other FGIDs in a large representative US white population. We hypothesized that CD would be linked not only to IBS but also to other FGIDs.
METHODS
In a population based study conducted in Olmsted County, MN, randomly selected subjects were sent a validated GI symptom survey between 1988 and 2009 and had serum tested for CD. This study was approved by the institutional review boards of the Mayo Clinic and the Olmsted Medical Center.
Setting
Subjects were all residents of Olmsted County, Minnesota, where approximately 120,000 people live. This population has been repeatedly studied because it is one of the few places in the United States where highly accurate population-based studies can be conducted.13, 14 By 2010 US census data, approximately 89% of the community is white, and Olmsted County has been shown to be representative of U.S. whites across a number of sociodemographic variables. 15, 16 The Rochester Epidemiology Project is a National Institutes of Health funded study that links all providers in the county and provides a unique records linkage system so that accurate enumeration of the population is possible and random, representative samples can be drawn.15, 16 These resources have been utilized to investigate the epidemiology of FGIDs and CD in this part of the United States in previous studies.4, 14, 17
CD Serology
In two prior studies of undiagnosed CD,4, 13 serum samples were obtained from a combined total of more than 47,000 adult (age 18 years and older) Olmsted County residents. In the first previous study, 18,774 specimens were collected between 1995 and 2001 from individuals older than 50 years. 13 After subjects without research authorization and samples with insufficient volume for testing were excluded, 16,886 subjects were screened for CD. The more recent study collected waste blood samples between 2006 and 2011 from healthcare-seeking individuals 18 to 50 years of age.4 Subjects without research authorization or with known CD diagnosed before or around the time serum was drawn were excluded up front, leaving 30,724 young adults who were screened for CD.
Serum was screened for CD using sequential testing with tissue transglutaminase (tTG) immunoglobulin (Ig) A enzyme-linked immunosorbent assay as the initial test (Inova Diagnostics) as previously described. 13 A tTG IgA value less than 2.0 U/mL was interpreted as a negative tTG IgA result, while levels from 2 to less than 4, 4 to less than 10, and 10.0 U/mL or greater were considered “borderline negative”, “weak positive”, and positive, respectively. Only samples with a tTG IgA level 2.0 U/mL or greater were subsequently submitted for a confirmatory test based on the endomysial antibodies (EMA) immunofluorescence assay (Beckman Coulter). The result of EMA immunofluorescence assay was positive if fluorescence was present at titers of 1: 5 or greater as previously reported.18 In the previous study from the adult Swedish general population in the Kalixanda study,19 this sequential testing paradigm, using subsequent EMA testing for positive tTG IgA result, showed a sensitivity of 97% and specificity of 100% for the diagnosis of CD.
Undiagnosed CD was defined by the presence of a tTG IgA level greater than 2.0 U/mL accompanied by a positive EMA test result. Subjects were classified as not having CD if their tTG IgA level was less than 2.0 U/mL (no follow-up EMA test was performed), or if the tTG IgA level was between 2.0 and 4.0 U/mL and the EMA test result was negative. In the case of discordant results in which the tTG IgA level was greater than 4.0 U/mL and the EMA test result was negative, CD status was considered indeterminate. The technologist reading the EMA assay was unaware of the tTG IgA status and nature of the research study.
GI Survey
As part of previous investigations,14, 17, 20 we utilized the enumeration of the Olmsted County population to select age- stratified (5-year intervals) and sex-stratified random samples of Olmsted County adult residents, who were mailed valid self-report symptom questionnaires. Subjects who had denied authorization to use their medical records for research, as required by Minnesota law, were excluded from this study.
The detailed contents for the Talley Bowel Disease Questionnaire (BDQ) including has been described elsewhere. 14, 17, 20 Previous testing has shown this instrument to be reliable, with a median kappa statistic for symptom items of 0.78 (range, 0.52-1.00). It has also been demonstrated to have adequate content, predictive and construct validity.14, 20 The BDQ consists of GI symptoms and the Somatic Symptom Checklist (SSC). The SSC consists of questions about relevant symptoms and illnesses (e.g. headaches, backaches, lethargy, insomnia), and subjects are instructed to indicate how often each occurred (0=not a problem to 4=occurs daily) and how bothersome each was (0=not a problem to 4=extremely bothersome when occurs) during the past year, using separate 5-point scales.21 Of 19,778 valid BDQ forms mailed to adults in the community (11,327 individuals) across three time periods (1988-1994, 2002-2004, and 2008-2009), 11,867 completed forms were returned (from 7,217 individuals), reflecting a survey response rate of 60%. Responders were more likely to be older (mean age, 57.5 vs 54.7 years) and female (53% vs 49%) compared to non-responders.
Study Population
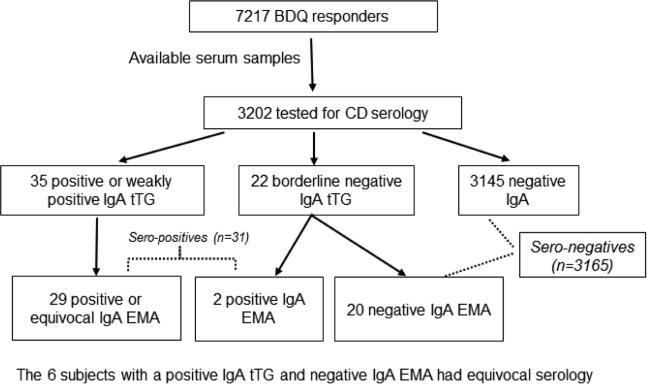
Of 47,610 individuals who had serologic testing for CD and 7,217 individuals who responded to GI symptom surveys, we identified an overlap of 3,202 subjects for this study, who had both questionnaire data and serum analyses available (Figure).
Figure 1.
Flow Chart for the Current Study Population. BDQ indicates Bowel Disease Questionnaire; CD, celiac disease; EMA, endomysial antibodies; IgA, immunoglobulin A; tTG, tissue transglutaminase.
Compared to BDQ responders without serum analyses, this group was older on average at the time of BDQ response (mean, 61 [range, 20-94] years), but had a similar proportion of women (52%). Of 3,202 subjects tested, the proportion with IBS was 13.6% (95% confidence interval [CI] 12.4%-14.8%) and the proportion with any GI symptom was 55.2% (95% CI, 53.5%-56.9%).
Statistical Analyses
Descriptive statistics on patient demographics, clinical characteristics and BDQ responses are presented as mean (standard deviation [SD]) or count (percentage) as appropriate. As some symptom definitions could not be measured across all versions of the BDQ, percentages were calculated on the basis of the observed denominators available. The variability of an observed proportion (e.g., sero-positive) was conveyed by an “exact” CI as computed based on the method of Clopper and Pearson.22
To test for an association with having positive CD serology, each candidate risk factor was entered in a logistic regression model where serology status was the response variable. To control for confounding, each effect was then re-estimated from a second set of models in which age, sex and time between BDQ and serum collection were included as adjusting covariates. Odds ratios (with 95% CI) are provided to summarize the risk of sero-positivity across levels of the candidate risk factor (e.g., in subjects indicated as having a particular GI symptom relative to those not having the symptom). As zero counts in categorical predictors are problematic for model convergence, the odds ratios for any BDQ symptom that was unseen in the small group of sero-positive subjects was instead derived using the Firth correction and is reported along with profile likelihood-based 95% CIs.23 All analyses were performed using SAS statistical software (version 9.3, SAS Institute). A significance level of 0.05 was used, and all tests were two-sided.
RESULTS
Serology Test
Among 3,202 subjects tested, the tTG IgA titer was negative in 3,167 (98.9%), weakly positive in 15 (0.5%), and positive in 20 (0.6%) (Table 1). Fifty-seven individuals (1.8% of total) who tested positive, weakly positive, or borderline negative for tTG IgA underwent confirmatory EMA testing. When the final composite serology was estimated from this two-stage testing, 31 subjects were found to be sero-positive for CD, and another 6 subjects had serology that was equivocal and thus were excluded from further analyses (Figure). In the remaining 3,196 individuals, the proportion with sero-positive (undiagnosed) CD in our study sample is 1.0% (95% CI, 0.7% - 1.4%).
Table 1.
Demographic and Somatic Characteristics of Subjects with Sero-positive CD Compared with Sero-negative Subjects
| Serology negative (n=3,165) | Serology positive (n=31) | OR (95% CI) | |
|---|---|---|---|
| Age at time of survey, mean (SD), years | 61.2±15.3 | 60.4±14.0 | 1.0 (0.8-1.2)† |
| Female sex, No. (%) | 1664 (53%) | 14 (45%) | 0.7 (0.4-1.5) |
| Absolute years between survey and serologic testing, mean (SD) | 5.3±3.4 | 4.1±3.1 | 0.9 (0.8-1.0) |
| SSC items, mean (SD)‡ | |||
| Headaches | 0.70±0.94 | 0.32±0.63 | 0.5 (0.3-1.1) |
| Backaches | 1.05±1.17 | 1.16±1.05 | 1.1 (0.8-1.5) |
| Insomnia | 0.78±1.04 | 0.86±1.11 | 1.1 (0.7-1.6) |
| General stiffness | 1.16±1.25 | 1.27±1.26 | 1.1 (0.8-1.5) |
| Dizziness | 0.27±0.68 | 0.09±0.25 | 0.5 (0.2-1.6) |
| Weakness | 0.30±0.79 | 0.11±0.31 | 0.6 (0.2-1.6) |
| Aggregate SSC score, mean (SD)* | 0.74±0.62 | 0.68±0.52 | 0.9 (0.4-1.7) |
CD, celiac disease; CI, confidence interval; OR, odds ratio; SSC, somatic symptom checklist
OR for age expressed per 10-year increase
Excluding those with missing data, descriptive statistics of SSC item and aggregate scores are based on subsets ranging from 2,270 to 2,294 sero-negative subjects (depending on the item) and 22 sero-positive subjects.
Interquartile range of aggregate SSC score in the general population was 0.18-0.77.38
GI Symptoms and FGIDs
The association of sero-positive (undiagnosed) CD with GI symptoms and FGIDs is summarized in Table 2. Any GI symptoms occurred in 55% of the sero-negative group, compared to in 45% of the sero-positive group. Abdominal pain (19%), constipation (13%), and weight loss (13%) were the most common symptoms in the sero-positive group, though these frequencies were not significantly higher than those in sero-negative subjects. Interestingly, diarrhea and IBS were each reported in only 1 of 31 sero-positive subjects. Other symptoms were also infrequent in the sero-positive group (though evaluation showed these negative associations were not statistically significant), including 5% with bloating, 3% with gastroesophageal reflux disease, and 0% with abdominal distension. None of the other GI symptoms or disorders was significantly associated with CD serology. For brevity, only unadjusted results are shown in Table 2, though analyses adjusting for age, sex and time between BDQ and serum collection produced similar results.
Table 2.
Distribution of Reported Symptoms in Subjects with Sero-positive CD vs Sero-negative Subjects
| GI Symptom | Serology negative (n=3,165) | Serology positive (n=31) | OR (95% CI)* |
|---|---|---|---|
| Abdominal pain | 803 / 3,165 (25%) | 6 / 31 (19%) | 0.7 (0.3-1.7) |
| Constipation | 270 / 3,165 (9%) | 4 / 31 (13%) | 1.6 (0.6-4.6) |
| Diarrhea | 283 / 3,155 (9%) | 1 / 31 (3%) | 0.3 (0.05-2.5) |
| Vomiting | 21 / 2,816 (1%) | 0 / 27 (0%) | 2.4 (0.02-17.9)^ |
| Weight loss | 116 / 1,944 (6%) | 2 / 16 (13%) | 2.3 (0.5-10.0) |
| IBS | 433 / 3,165 (14%) | 1 / 31 (3%) | 0.2 (0.03-1.5) |
| Dyspepsia | 149 / 3,089 (5%) | 2 / 29 (7%) | 1.5 (0.3-6.2) |
| GERD | 526 / 3,079 (17%) | 1 / 29 (3%) | 0.2 (0.02-1.3) |
| Difficulty swallowing | 300 / 2,633 (11%) | 1 / 25 (4%) | 0.3 (0.04-2.4) |
| Bloating | 412 / 1,808 (23%) | 1 / 22 (5%) | 0.2 (0.02-1.2) |
| Abdominal distension | 327 / 2,391 (14%) | 0 / 21 (0%) | 0.1 (<0.01-1.1)^ |
| Any GI symptoms | 1753 / 3,165 (55%) | 14 / 31 (45%) | 0.7 (0.3-1.4) |
CD, celiac disease; GI, gastrointestinal; OR, odds ratio; CI, confidence interval; IBS, irritable bowel syndrome; GERD, gastroesophageal reflux disease.
For brevity, only unadjusted ORs and 95% CIs from univariable logistic regression models are shown, though the results from models adjusting for age, sex and time between Bowel Disease Questionnaire and serum collection were similar
Due to a zero count among the 31 sero-positive subjects, the OR was instead derived using the Firth correction along with profile likelihood-based 95% CIs
DISCUSSION
In this large, overlapping set created from a large population-based cohort of BDQ responders and a convenience sample with stored serum, the prevalence of undiagnosed CD was 1.0% (95% CI, 0.7%-1.4%), which is consistent with other epidemiologic data in the United States. 24 We found that GI symptoms are relatively common in subjects with undiagnosed CD but comparable to those of adults without CD. We also found that sero-positivity for CD was not significantly associated with increased FGIDs including IBS and functional dyspepsia. These results may have important management and screening implications.
Thought be a rare disease in the past, 17 CD is recognized to be an increasing threat to public health. 4, 25 Early detection of CD, with introduction of a gluten free diet, is likely to alter the consequences of CD. Thus, several guidelines have suggested that screening testing be recommended for people at increased risk for CD, including those who have first-degree relatives with CD and those with type 1 diabetes, autoimmune thyroid disease, or autoimmune liver disease. 3, 6, 10 Notably, IBS is also listed as one of the risk conditions for CD in a number of current guidelines. 3, 6, 10
A meta-analysis 8 found that prevalence of biopsy-proven CD in cases meeting diagnostic criteria for IBS was more than 4-fold higher than that in controls without IBS. However, the data are based on cases series and case-control studies in secondary or tertiary clinic populations from different countries with a relatively high background prevalence of CD (as high as 4%). Thus, these results should be cautiously interpreted. In the present population-based study, we did not observe any significant differences in the prevalence of GI symptoms between sero-positive CD patients and sero-negative individuals. Moreover, we did not observe any significant association between sero-positive CD and FGIDs including IBS. Few population based studies have evaluated the association between CD and IBS, but all26, 27 failed to show any significant relationship between CD and IBS. In contrast, several studies 28, 29 based on data from secondary or tertiary centers have shown a higher prevalence of CD in subjects with IBS-like symptoms compared to that in controls without symptoms. Similarly, in other FGIDs including dyspepsia, the prevalence of CD has been reported to be higher than that in controls.30, 31 However, because these studies were conducted in the secondary or referred population, unrecognized selection bias may account for these findings. Moreover, other studies including meta-analysis did not find significant associations between CD and dyspepsia.27, 32
The present findings might be influenced by the small number of cases, which is likely to increase the chance of a type II error; however, our results are consistent with other population-based observations. 26, 27 Moreover, a large clinic based study by Cash et al.11, which evaluated the prevalence of biopsy confirmed CD among patients with non-constipated IBS, showed that the prevalence of CD in these patients was 0.41%, nearly identical to that in observed in healthy controls (0.44%). In another large Swedish pediatric study, Rosen et al.9 found that GI symptoms were as common among children with screen-detected CD as among children without CD. Other data also indicate that the majority of patients with undetected CD do not have typical GI symptoms, such as diarrhea, abdominal pain, and weight loss, which makes CD more challenging to clinically diagnose.33 Moreover, Katz et al.12 showed in a North American population, that GI symptoms did not predict the presence of CD. In a Swedish endoscopic population-based study, Walker et al.19 also demonstrated a lack of association of bowel disease symptoms with CD sero-positivity or histologic severity. Consistency with these findings, our study also found that the frequency of GI symptoms in subjects with undiagnosed CD did not exceed that in subjects from a general population who did not have this disease. CD can present in many disguises and the absence of classic symptoms does not exclude the diagnosis.12, 33 Moreover, gastroenterologists may encounter undetected CD in patients while investigating other GI disorders and patients with incidentally detected CD may benefit from a gluten free diet.34 Further, primary care providers should be aware of the risk of CD, particularly in those with a family history of CD, or diseases such as type 1 diabetes, iron deficiency, or osteoporosis. 1, 11-13
Cost effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1% and becomes the dominant strategy when the prevalence exceeds 8%.35 However, we cannot confirm whether or not CD testing is a cost effective approach in our population. Recently, there has been increasing public attention on the role of gluten free diet in IBS, with some studies36, 37 suggesting a benefit. For example, Vazquez-Roque et al.37 demonstrated that patients with diarrhea-predominant IBS who are HLA-DQ2/8 positive benefit from a gluten free diet in terms of improved small bowel permeability. In contrast, those who lack gluten-related genes may be responding to other food components, such as poorly absorbed short-chain carbohydrates.
A potential limitation of this study was that we did not confirm the presence of CD by endoscopy and small bowel biopsy in our subjects and controls. However, the sequential serologic approach (tTG IgA positive and EMA positive) has shown an excellent correlation with the histologic results on duodenal biopsy,19 and thus misclassification bias should have been minimized. Since we did not test for IgA deficiency, patients with selective IgA deficiency and undetected CD could have been missed in this study. Further patients with symptomatic CD are more likely to be tested and diagnosed; thus, the prevalence of CD in subjects reporting GI symptoms could be underestimated. However, the proportion of reporting GI symptoms in our study population was consistent with previous studies in Olmsted County residents.20, 38 Thus, this potential bias seems unlikely to be a major issue. Our data should be generalizable to the US white population but may not be generalized to the whole U.S. population because the racial composition of this community is predominantly white.15 The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races.5 Responder bias is possible because we were not able to include members of the community who did not respond to the survey, nor were we able to include the portion of responders who did not have a serum sample available for CD testing. However, other data using BDQ data found that demographic imbalances were not evidence of systematic bias, given that the rates of having actual GI conditions were not found to significantly differ between non-responders and responders. 39 We know that GI symptoms based on self-rated responses were not necessarily measured near the time the serum was collected (only 18% were separated by less than 1 year, and nearly half [48%] had more than 5 years in between), though attempts to control for this possible confounding via regression adjustment appeared to make little difference in the symptom effects.
In conclusion, symptoms indicative of FGIDs and serologic CD are relatively common in a representative US white community. In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community. Our results suggest that testing for CD in IBS will not have a significantly increased yield over population-based serologic screening.
Acknowledgments
Grant Support: This study was made possible in part by use of the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01 AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported in part by research grant R01-DK57892 (JAM) from the National Institutes of Health, US Public Health Service.
Abbreviations
- CD
celiac disease
- EMA
endomysial antibodies
- GI
gastrointestinal
- FGIDs
functional gastrointestinal disorders
- BDQ
bowel disease questionnaire
- Ig
immunoglobulin
- SSC
Somatic Symptom Checklist
- tTG
tissue transglutaminase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Talley and Mayo Clinic have licensed the Talley Bowel Disease Questionnaire. The remaining authors disclose no conflicts.
Specific Author Contributions: Alberto Rubio-Tapia, Rok Seon Choung, G. Richard Locke III, Nicholas J. Talley, and Joseph A. Murray participated in the design, analysis, and writing of the manuscript. Brian D. Lahr provided the statistical analysis and input on design.
REFERENCES
- 1.Ludvigsson JF, Leffler DA, Bai JC, et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62:43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656–76. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murch S, Jenkins H, Auth M, et al. Joint BSPGHAN and Coeliac UK guidelines for the diagnosis and management of coeliac disease in children. Arch Dis Child. 2013;98:806–11. doi: 10.1136/archdischild-2013-303996. [DOI] [PubMed] [Google Scholar]
- 4.Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137:88–93. doi: 10.1053/j.gastro.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choung RS, Ditah IC, Nadeau AM, et al. Trends and racial/ethnic disparities in gluten-sensitive problems in the United States: findings from the National Health and Nutrition Examination Surveys from 1988 to 2012. Am J Gastroenterol. 2015;110:455–61. doi: 10.1038/ajg.2015.8. [DOI] [PubMed] [Google Scholar]
- 6.Ludvigsson JF, Rubio-Tapia A, van Dyke CT, et al. Increasing incidence of celiac disease in a North American population. Am J Gastroenterol. 2013;108:818–24. doi: 10.1038/ajg.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choung RS, Locke GR., 3rd Epidemiology of IBS. Gastroenterol Clin North Am. 2011;40:1–10. doi: 10.1016/j.gtc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Ford AC, Chey WD, Talley NJ, et al. Yield of diagnostic tests for celiac disease in individuals with symptoms suggestive of irritable bowel syndrome: systematic review and meta-analysis. Arch Intern Med. 2009;169:651–8. doi: 10.1001/archinternmed.2009.22. [DOI] [PubMed] [Google Scholar]
- 9.Rosen A, Sandstrom O, Carlsson A, et al. Usefulness of symptoms to screen for celiac disease. Pediatrics. 2014;133:211–8. doi: 10.1542/peds.2012-3765. [DOI] [PubMed] [Google Scholar]
- 10.Husby S, Koletzko S, Korponay-Szabo IR, et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–60. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 11.Cash BD, Rubenstein JH, Young PE, et al. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology. 2011;141:1187–93. doi: 10.1053/j.gastro.2011.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz KD, Rashtak S, Lahr BD, et al. Screening for celiac disease in a North American population: sequential serology and gastrointestinal symptoms. The American journal of gastroenterology. 2011;106:1333–9. doi: 10.1038/ajg.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godfrey JD, Brantner TL, Brinjikji W, et al. Morbidity and mortality among older individuals with undiagnosed celiac disease. Gastroenterology. 2010;139:763–9. doi: 10.1053/j.gastro.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talley NJ, Phillips SF, Wiltgen CM, et al. Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–79. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 15.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87:1202–13. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc. 2012;87:151–60. doi: 10.1016/j.mayocp.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Talley NJ, Valdovinos M, Petterson TM, et al. Epidemiology of celiac sprue: a community-based study. Am J Gastroenterol. 1994;89:843–6. [PubMed] [Google Scholar]
- 18.Chorzelski TP, Beutner EH, Sulej J, et al. IgA anti-endomysium antibody. A new immunological marker of dermatitis herpetiformis and coeliac disease. Br J Dermatol. 1984;111:395–402. doi: 10.1111/j.1365-2133.1984.tb06601.x. [DOI] [PubMed] [Google Scholar]
- 19.Walker MM, Murray JA, Ronkainen J, et al. Detection of celiac disease and lymphocytic enteropathy by parallel serology and histopathology in a population-based study. Gastroenterology. 2010;139:112–9. doi: 10.1053/j.gastro.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Talley NJ, Zinsmeister AR, Van Dyke C, et al. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991;101:927–34. doi: 10.1016/0016-5085(91)90717-y. [DOI] [PubMed] [Google Scholar]
- 21.Attanasio V, Andrasik F, Blanchard EB, et al. Psychometric properties of the SUNYA revision of the Psychosomatic Symptom Checklist. J Behav Med. 1984;7:247–57. doi: 10.1007/BF00845390. [DOI] [PubMed] [Google Scholar]
- 22.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 23.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 24.Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–92. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 25.Riddle MS, Murray JA, Porter CK. The incidence and risk of celiac disease in a healthy US adult population. Am J Gastroenterol. 2012;107:1248–55. doi: 10.1038/ajg.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanders DS, Patel D, Stephenson TJ, et al. A primary care cross-sectional study of undiagnosed adult coeliac disease. Eur J Gastroenterol Hepatol. 2003;15:407–13. doi: 10.1097/00042737-200304000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Agreus L, Svardsudd K, Tibblin G, et al. Endomysium antibodies are superior to gliadin antibodies in screening for coeliac disease in patients presenting supposed functional gastrointestinal symptoms. Scand J Gastroenterol. 2000;18:105–10. doi: 10.1080/028134300750018990. [DOI] [PubMed] [Google Scholar]
- 28.Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–8. doi: 10.1016/S0140-6736(01)06581-3. [DOI] [PubMed] [Google Scholar]
- 29.Cristofori F, Fontana C, Magista A, et al. Increased prevalence of celiac disease among pediatric patients with irritable bowel syndrome: a 6-year prospective cohort study. JAMA Pediatr. 2014;168:555–60. doi: 10.1001/jamapediatrics.2013.4984. [DOI] [PubMed] [Google Scholar]
- 30.Bardella MT, Minoli G, Ravizza D, et al. Increased prevalence of celiac disease in patients with dyspepsia. Arch Intern Med. 2000;160:1489–91. doi: 10.1001/archinte.160.10.1489. [DOI] [PubMed] [Google Scholar]
- 31.Ozaslan E, Akkorlu S, Eskioglu E, et al. Prevalence of silent celiac disease in patients with dyspepsia. Dig Dis Sci. 2007;52:692–7. doi: 10.1007/s10620-006-9453-1. [DOI] [PubMed] [Google Scholar]
- 32.Ford AC, Ching E, Moayyedi P. Meta-analysis: yield of diagnostic tests for coeliac disease in dyspepsia. Aliment Pharmacol Ther. 2009;30:28–36. doi: 10.1111/j.1365-2036.2009.04008.x. [DOI] [PubMed] [Google Scholar]
- 33.Green PH. The many faces of celiac disease: clinical presentation of celiac disease in the adult population. Gastroenterology. 2005;128:S74–8. doi: 10.1053/j.gastro.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Kurppa K, Paavola A, Collin P, et al. Benefits of a gluten-free diet for asymptomatic patients with serologic markers of celiac disease. Gastroenterology. 2014;147:610–617. e1. doi: 10.1053/j.gastro.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 35.Spiegel BM, DeRosa VP, Gralnek IM, et al. Testing for celiac sprue in irritable bowel syndrome with predominant diarrhea: a cost-effectiveness analysis. Gastroenterology. 2004;126:1721–32. doi: 10.1053/j.gastro.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Wahnschaffe U, Schulzke JD, Zeitz M, et al. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–50. doi: 10.1016/j.cgh.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903–911. e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choung RS, Locke GR, 3rd, Zinsmeister AR, et al. Psychosocial distress and somatic symptoms in community subjects with irritable bowel syndrome: a psychological component is the rule. Am J Gastroenterol. 2009;104:1772–9. doi: 10.1038/ajg.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choung RS, Locke GR, Schleck CD, et al. A low response rate does not necessarily indicate non-response bias in gastroenterology survey research: a population-based study. J Public Health. 2013;21:87–95. [Google Scholar]